The Diversity of Larvae with Multi-Toothed Stylets from About 100 Million Years Ago Illuminates the Early Diversification of Antlion-like Lacewings
Abstract
1. Introduction
2. Material and Methods
2.1. Material
2.2. Imaging and Documentation
2.3. Shape Analysis
- -
- -
- -
- -
3. Results
3.1. Short Descriptions of New Specimens of Macleodiella
- (1)
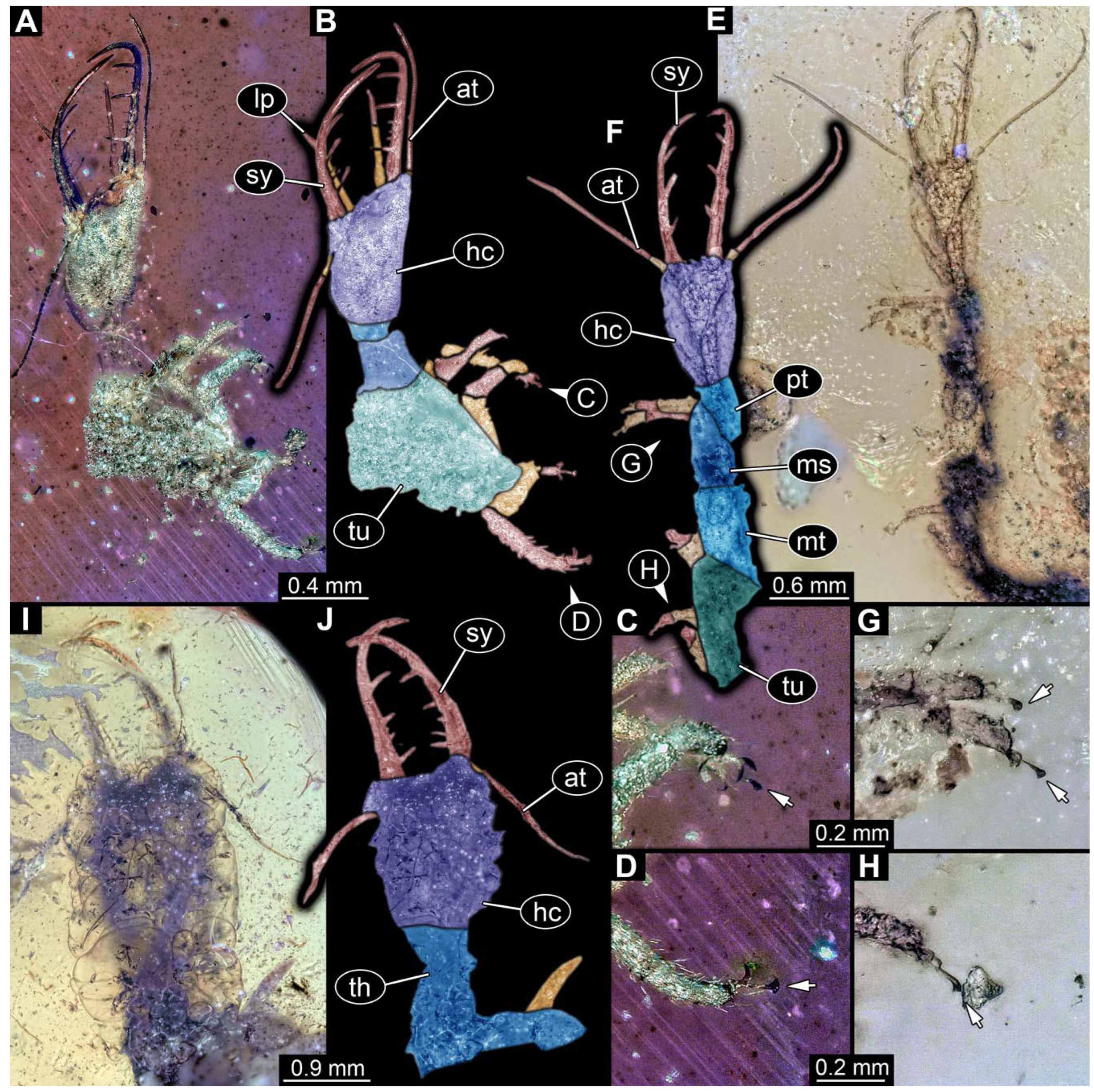
- Specimen 0812 (PED 1068) is poorly visible due to dirt in the amber. Visible in dorsal view (Figure 1I). Body with a distinct head and trunk. Head with forward-projecting mouthparts, the stylets, and antero-laterally projecting antennae (Figure 1J) Each stylet has three larger teeth, and two smaller teeth between the proximal tooth and the middle tooth are recognizable. Antennae are incompletely preserved. Only the head and anterior parts of the trunk (parts of the thorax) are preserved; the further posterior part is outside of the amber piece.
- (2)
- Specimen 0813 (PED 1334) is visible in dorsal view (Figure 1E). Body with a distinct head and trunk. Head with forward-projecting mouthparts, the stylets, and antero-laterally projecting antennae (Figure 1F). In each stylet, three larger teeth are recognizable. Antennae elongate and longer than stylets. Anterior trunk segments (thorax) with pairs of locomotory appendages (legs). Legs have trumpet-shaped attachment structures distally (empodia; Figure 1G,H). The posterior part of the trunk (abdomen) is missing.
- (3)
- Specimen 0814 (PED 2274) is well preserved and accessible from both sides, dorsal (Figure 2B,C) and ventral (Figure 2A). Body with a distinct head and trunk. Head with forward-projecting mouthparts, the stylets, and labial palps, and antero-laterally projecting antennae. In each stylet three larger teeth are recognizable. Labial palps are elongate and shorter than stylets, with at least two elements each; the distal element is slightly leaf-shaped. Antennae elongate and longer than stylets. The first trunk segment (prothorax) is elongate. Anterior trunk segments (thorax) with pairs of locomotory appendages (legs). Legs have trumpet-shaped attachment structures distally (empodia; Figure 2D). The posterior part of the trunk (abdomen) has at least eight units: seven segments and a trunk end (most likely several conjoined segments). The abdomen is slender and elongate, roughly cone-shaped.
- (4)
- Specimen 0815 (PED 2292) is incompletely preserved. Visible in dorsal view (Figure 1A,B). Body with a distinct head and trunk. Head with forward projecting mouthparts, the stylets and labial palps, and antero-laterally projecting antennae (Figure 1A,B). Each stylet has three larger teeth, and three smaller teeth between the proximal tooth and the middle tooth are recognisable. Labial palps are elongate and shorter than stylets, with at least two elements each. Antennae are elongate and longer than stylets. Anterior trunk segments (thorax) with pairs of locomotory appendages (legs). Legs have trumpet-shaped attachment structures distally (empodia; Figure 1C,D). The posterior part of the trunk (abdomen) is missing.
- (5)
- The amber piece PED 1885 includes the remains of several specimens (Figure 3). Specimen 0816 (Figure 3A) is more completely preserved, allowing us to recognise the subdivision into head, anterior trunk (thorax), and posterior trunk (abdomen). Head with stylets and antennae. Specimen 0817 (Figure 3F) is also more complete, allowing us to recognise the head (with stylets and labial palp) and trunk. Other remains are collectively addressed (“specimen 0818”); remains include four mandibles (Figure 3B–E), meaning that these are at least the remains of two specimens but could also be the remains of up to four specimens. The piece therefore includes the remains of four to six specimens.
3.2. Short Descriptions of Specimens of Superfang Type 1
- (6)
- (7)
- Specimen 0843 (BUB 3392) is relatively well preserved in dorsal view (Figure 5A,B). Body with a distinct head and trunk. Head with forward-projecting mouthparts, the stylets and labial palps, and antero-laterally projecting antennae. Stylets are partly damaged and incomplete, each with eight teeth preserved (Figure 5D); laterally, they bear short setae. The second distal tooth is the longest. Labial palps are elongate and shorter than stylets, with at least two elements each. Antennae elongate and longer than stylets. Anterior trunk segments (thorax) with pairs of locomotory appendages (legs). Legs have trumpet-shaped attachment structures distally (empodia; Figure 5C). The posterior part of the trunk (abdomen) is slender and elongate, roughly cone-shaped. Head, trunk, and legs bear numerous shorter setae; abdomen has especially long setae (Figure 5E).
- (8)
- Specimen 0844 (BUB 3398) is relatively well preserved in dorsal (Figure 6A,B) and ventral views (Figure 6D). Body with a distinct head and trunk. Head with forward-projecting mouthparts, the stylets and labial palps, and antero-laterally projecting antennae. Stylets are partly damaged, with eight teeth; proximo-medially with some setae; laterally bearing short setae. The second distal tooth is the longest (Figure 6C). Labial palps are elongate, shorter than stylets, with at least two elements each (Figure 6F). Antennae elongate and longer than stylets. Anterior trunk segments (thorax) with pairs of locomotory appendages (legs). Legs have trumpet-shaped attachment structures distally (empodia; Figure 6E). The posterior part of the trunk (abdomen) is slender, elongate, and roughly cone-shaped; the posterior end seems to be turned downward. The head, trunk, and legs bear numerous shorter setae; the abdomen has especially long setae.
- (9)
- Specimen 0845 (PED 0372) is relatively well preserved in dorsal (Figure 7A) and ventral views (Figure 7B,C). Body with a distinct head and trunk. Head with forward-projecting mouthparts, the stylets and labial palps, and antero-laterally projecting antennae. Stylets are partly damaged, with eight teeth; proximo-medially with some setae; laterally bearing short setae. The second distal tooth is the longest (Figure 7E). Labial palps are elongate and shorter than stylets, with at least two elements each. Antennae elongate longer than stylets. Anterior trunk segments (thorax) with pairs of locomotory appendages (legs). Legs have trumpet-shaped attachment structures distally (empodia; Figure 7D). The posterior part of the trunk (abdomen) is not preserved. The head, trunk, and legs bear numerous shorter setae; the abdomen has especially long setae.
- (10)
- Specimen 0846 (PED 0658) is relatively well preserved in dorsal view (Figure 8A,B). Body with a distinct head and trunk. Head with forward-projecting mouthparts, the stylets and labial palps, and antero-laterally projecting antennae. Stylets largely outside the amber. Labial palps are elongate and shorter than stylets, with at least two elements each (Figure 8D). Antennae elongate longer than stylets. Anterior trunk segments (thorax) with pairs of locomotory appendages (legs). Legs have trumpet-shaped attachment structures distally (empodia; Figure 8C). Posterior part of trunk (abdomen) stouter, roughly cone-shaped. The head, trunk, and legs bear numerous shorter setae; the abdomen has especially long setae.
- (11)
- Specimen 0848 (PED 1165) is relatively well preserved in dorsal view (Figure 8E,F). Body with a distinct head and trunk. Head with lateral projections with eyes (Figure 8H); head also with forward projecting mouthparts, the stylets and labial palps, and antero-laterally projecting antennae. Stylets distally outside the amber, proximally with at least four shorter teeth. Labial palps elongate shorter than stylets, with at least two elements each (Figure 8G). Antennae elongate longer than stylets. Anterior trunk segments (thorax) with pairs of locomotory appendages (legs). Posterior part of trunk (abdomen) stouter, roughly cone-shaped (Figure 8I). The head, trunk, and legs bear numerous shorter setae; the abdomen has especially long setae.
- (12)
- Specimen 0849 (PED 2255) is relatively well preserved in latero-dorsal (Figure 9C) and latero-ventral views (Figure 9A,B). Body with a distinct head and trunk. Head with forward-projecting mouthparts, the stylets and labial palps, and antero-laterally projecting antennae. Stylets with eight teeth; proximo-medially with some setae. Two distal teeth are the longest. Labial palps are elongate and shorter than stylets. Antennae elongate and longer than stylets. Anterior trunk segments (thorax) with pairs of locomotory appendages (legs). Legs have trumpet-shaped attachment structures distally (empodia; Figure 9D). The posterior part of the trunk (abdomen) is damaged.
- (13)
- Specimen 0850 (PED 2708) is relatively well preserved in ventral view (Figure 10A,B). Body with a distinct head and trunk. Head with forward-projecting mouthparts, the stylets and labial palps, and antero-laterally projecting antennae. Stylets with eight teeth. The second distal tooth is the longest (Figure 10C). Labial palps are not well preserved. Antennae elongate and longer than stylets. Anterior trunk segments (thorax) with pairs of locomotory appendages (legs) and distal parts outside the amber (Figure 10D). The posterior part of the trunk (abdomen) is outside the amber.
- (14)
3.3. Short Descriptions of Specimens of Superfang Type 2
- (15)
- Specimen 0861 (PED 0361) is incompletely preserved (Figure 11A,B). Mostly head with forward-projecting mouthparts and stylets. Stylets with six teeth. Distal teeth are significantly longer than proximal ones. In addition, distal parts of locomotory appendages (legs) are preserved. Legs have trumpet-shaped attachment structures distally (empodia; Figure 11C).
- (16)
- Specimen 0862 (PED 0088) is incompletely preserved (Figure 12A–C). Most likely representing exuvia; the main body is strongly crumpled. Forwardly projecting mouthparts, the stylets and labial palps, and antero-laterally projecting antennae are better preserved. Stylets with six teeth (Figure 12E). Distal teeth are significantly longer than proximal ones, with a few setae medio-proximally and numerous laterally (Figure 12E). Labial palps are elongate and shorter than stylets. Antennae are elongate and longer than stylets. In addition, parts of locomotory appendages (legs) are better preserved. Legs have trumpet-shaped attachment structures distally (empodia; Figure 12D).
- (17)
- Specimen 0863 (PED 0112) is incompletely preserved (Figure 11D,E). Most likely representing exuvia; the main body is strongly crumpled. Forwardly projecting mouthparts, the stylets and labial palps, and antero-laterally projecting antennae are better preserved. Stylets with six teeth. Distal teeth are significantly longer than proximal ones (Figure 11F), with a few setae medio-proximally and latero-proximally. Labial palps are elongate and shorter than stylets. Antennae are elongate and longer than stylets. In addition, parts of locomotory appendages (legs) are better preserved. Legs have trumpet-shaped attachment structures distally (empodia; Figure 11G,H).
- (18)
- Specimen 0864 (PED 0054) is incompletely preserved (Figure 13A,B). Originally more complete, but damaged during transport (Figure 13C). Most likely representing exuvia; the main body is strongly crumpled. Head with antero-lateral eyes (Figure 13F) and forward-projecting mouthparts, the stylets and labial palps, and antero-laterally projecting antennae is better preserved. Stylets with few teeth. Distal teeth are significantly longer than proximal ones, with a few setae medio-proximally and latero-proximally. Labial palps are elongate and shorter than stylets (Figure 13E). Antennae are elongate and longer than stylets. In addition, parts of locomotory appendages (legs) are better preserved. Legs have trumpet-shaped attachment structures distally (empodia; Figure 13D). Trunk ends are slender with prominent setae (Figure 13G).
- (19)
- Specimen 0865 (PED 0339) is incompletely preserved (Figure 14A,B). Most likely representing exuvia; the main body is strongly crumpled. Forwardly projecting mouthparts, the stylets and labial palps, and antero-laterally projecting antennae are better preserved. Stylets with six teeth (Figure 14D). Distal teeth are significantly longer than proximal ones (Figure 14D), with few setae medio-proximally. Labial palps are elongate and shorter than stylets. Antennae are elongate, longer than stylets, with at least three distinct elements (Figure 14D). In addition, parts of locomotory appendages (legs) are better preserved. Legs have trumpet-shaped attachment structures distally (empodia; Figure 14C).
- (20)
- Specimen 0866 (PED 0394) is incompletely preserved (Figure 15A,B,D). Most likely representing exuvia; the main body is strongly crumpled. Forwardly projecting mouthparts, the stylets and labial palps, and antero-laterally projecting antennae are better preserved. Stylets with six teeth. Distal teeth are significantly longer than proximal ones, with a few setae medio-proximally and more laterally. Labial palps are elongate and shorter than stylets. Antennae are elongate and longer than stylets, with at least three distinct elements (Figure 15B). In addition, parts of locomotory appendages (legs) are better preserved. Legs have trumpet-shaped attachment structures distally (empodia; Figure 15C,E,F).
- (21)
- Specimen 0867 (PED 0510) is incompletely preserved (Figure 16A,B). Most likely representing exuvia; the main body is strongly crumpled. Forwardly projecting mouthparts, the stylets and labial palps, and antero-laterally projecting antennae are better preserved. Stylets with six teeth. Distal teeth are significantly longer than proximal ones, with a few setae medio-proximally and more laterally. Labial palps are elongate and shorter than stylets. Antennae elongate and longer than stylets. In addition, parts of locomotory appendages (legs) are better preserved. Legs have trumpet-shaped attachment structures distally (empodia; Figure 16C).
- (22)
- Specimen 0868 (PED 0630) is incompletely preserved (Figure 16D,E). Most likely representing exuvia; the main body is strongly crumpled. Forwardly projecting mouthparts, the stylets and labial palps, and antero-laterally projecting antennae are better preserved. Stylets with six teeth. Distal teeth are significantly longer than proximal ones, with one distinct socketed seta proximally. Labial palps are elongate and shorter than stylets. Antennae elongate and longer than stylets. In addition, parts of locomotory appendages (legs) are better preserved. Legs have trumpet-shaped attachment structures distally (empodia; Figure 16F).
- (23)
- Specimen 0869 (PED 0657) is incompletely preserved (Figure 17A,B). Most likely representing exuvia; the main body is strongly crumpled. Forwardly projecting mouthparts, the stylets and labial palps, and antero-laterally projecting antennae are better preserved. Stylets with at least five teeth (Figure 17C). Distal teeth are significantly longer than proximal ones, with one distinct socketed seta proximally. Labial palps are elongate and shorter than stylets. Antennae elongate and longer than stylets. In addition, parts of locomotory appendages (legs) are better preserved. Trunk with bulbous projections bearing longer setae (Figure 17D).
- (24)
- Specimen 0870 (PED 0671) is incompletely preserved (Figure 18A,B). Most likely representing exuvia; the main body is strongly crumpled. Forwardly projecting mouthparts, the stylets and labial palps, and antero-laterally projecting antennae are better preserved. Stylets with at least five teeth. The distal teeth are significantly longer than the proximal ones, with setae between the teeth. Labial palps are elongate and shorter than stylets. Antennae elongate and longer than stylets. In addition, parts of locomotory appendages (legs) are better preserved. Legs have trumpet-shaped attachment structures distally.
- (25)
- Specimen 0871 (PED 0739) is incompletely preserved (Figure 18C,D). Most likely representing exuvia; the main body is slightly crumpled. Forwardly projecting mouthparts, the stylets and labial palps, and antero-laterally projecting antennae are better preserved. Stylets with at least five teeth. Distal teeth are significantly longer than proximal ones; with setae between the teeth, there are more setae laterally. Labial palps are elongate and shorter than stylets. Antennae elongate and longer than stylets. In addition, parts of locomotory appendages (legs) are better preserved. Legs have trumpet-shaped attachment structures distally (Figure 18E).
- (26)
- Specimen 0872 (PED 0784) is incompletely preserved (Figure 19A,B). Most likely representing exuvia; the main body is strongly crumpled. Forwardly projecting mouthparts, the stylets and labial palps, and antero-laterally projecting antennae are better preserved. Stylets with six teeth. The distal teeth are significantly longer than the proximal ones, with setae between the teeth. Labial palps are elongate and shorter than stylets. Antennae elongate and longer than stylets. In addition, parts of locomotory appendages (legs) are better preserved. Legs have trumpet-shaped attachment structures distally (Figure 19C).
- (27)
- Specimen 0873 (PED 0963) is incompletely preserved (Figure 19D,E). Most likely representing exuvia; the main body is strongly crumpled. Forwardly projecting mouthparts, the stylets and labial palps, and antero-laterally projecting antennae are better preserved. Stylets with six teeth. Distal teeth are significantly longer than proximal ones, with setae between the teeth and more setae laterally. Labial palps are elongate and shorter than stylets. Antennae are elongate, shorter than stylets, and most likely incomplete. In addition, parts of locomotory appendages (legs) are preserved.
- (28)
- Specimen 0874 (PED 1034) is incompletely preserved (Figure 20A,B). Most likely representing exuvia; the main body is strongly crumpled. Head with antero-lateral projection, bearing eyes (Figure 20C). Forwardly projecting mouthparts, the stylets and labial palps, and antero-laterally projecting antennae are better preserved. Stylets with at least five teeth (Figure 20E). The distal teeth are significantly longer than the proximal ones, with setae between the teeth. Labial palps are elongate and shorter than stylets. Antennae are elongate, shorter than stylets, and most likely incomplete. In addition, parts of locomotory appendages (legs) are preserved (Figure 20D).
- (29)
- Specimen 0876 (Weiterschan BuB 9) is incompletely preserved (Figure 21C,D). Most likely representing exuvia; the main body is strongly crumpled. Head with forward-projecting mouthparts, the stylets and labial palps, and antero-laterally projecting antennae is better preserved. Stylets with six teeth. Distal teeth are significantly longer than proximal ones, with setae between the teeth; laterally with numerous setae. Labial palps are elongate and shorter than stylets. Antennae are elongate, shorter than stylets, and most likely incomplete. In addition, parts of locomotory appendages (legs) are preserved. Legs have trumpet-shaped attachment structures distally (Figure 21E).
- (30)
- Specimen 0877 (PED 1110) is incompletely preserved (Figure 22A,B). Most likely representing exuvia; the main body is strongly crumpled. Head with forward-projecting mouthparts, the stylets and labial palps and antero-laterally projecting antennae is better preserved. Stylets with few teeth. Antennae elongate and longer than stylets. In addition, parts of locomotory appendages (legs) are preserved.
- (31)
- Specimen 0878 (PED 1242) is incompletely preserved (Figure 22C,D). Most likely representing exuvia; the main body is strongly crumpled. The head with forward-projecting mouthparts, the stylets, and antero-laterally projecting antennae is better preserved. Stylets with few teeth. Antennae elongate and longer than stylets. In addition, parts of locomotory appendages (legs) are preserved.
- (32)
- Specimen 0879 (PED 1255) is incompletely preserved (Figure 22E,F). Mostly head preserved, with forward projecting mouthparts, the stylets and labial palps, and antero-laterally projecting antennae better preserved. Stylets with six teeth and numerous setae laterally. Labial palps are elongate and shorter than stylets. Antennae elongate and longer than stylets.
- (33)
- Specimen 0880 (PED 1293) is incompletely preserved (Figure 23A,B). Most likely representing exuvia; the main body is strongly crumpled. Head with forward-projecting mouthparts, the stylets and labial palps and antero-laterally projecting antennae is better preserved. Stylets with six teeth; one prominent seta proximally. Distal teeth are significantly longer than proximal ones; laterally, there are numerous setae. Labial palps are elongate and shorter than stylets (Figure 23C). Antennae are elongate, shorter than stylets, and most likely incomplete. In addition, parts of locomotory appendages (legs) are preserved. Legs have trumpet-shaped attachment structures distally (Figure 23D).
- (34)
- Specimen 0931 (PED 1327) is incompletely preserved (Figure 24A–C). Most likely representing exuvia; the main body is strongly crumpled. The head with forward-projecting mouthparts, the stylets, and antero-laterally projecting antennae is better preserved. Stylets with six teeth (Figure 24D). Distal teeth are significantly longer than proximal ones. Antennae elongate and longer than stylets. In addition, parts of locomotory appendages (legs) are preserved. Legs have trumpet-shaped attachment structures distally (Figure 24E).
- (35)
- Specimen 0932 (PED 1367) is incompletely preserved (Figure 24F,G). Mostly head with forward-projecting mouthparts, the stylets, and antero-laterally projecting antennae better preserved. Stylets have at least five teeth (Figure 24H); laterally, they have numerous setae. Antennae are elongate, shorter than stylets, and most likely incomplete.
- (36)
- Specimen 0933 (PED 1409) is incompletely preserved (Figure 25A,B). Most likely representing exuvia; the main body is strongly crumpled. The head with forward-projecting mouthparts, the stylets, and antero-laterally projecting antennae is better preserved. Stylets with six teeth. Distal teeth are significantly longer than proximal ones; laterally, there are numerous setae. Antennae elongate and longer than stylets. In addition, parts of locomotory appendages (legs) are preserved. Legs have trumpet-shaped attachment structures distally (Figure 25C,D).
- (37)
- Specimen 0934 (PED 1610) is poorly preserved (Figure 25E,F). Head hardly recognisable, mostly stylets apparent. Stylets with at least five teeth.
- (38)
- Specimen 0935 (PED 1739) is incompletely preserved (Figure 21A,B). Mostly head with forward projecting mouthparts, the stylets and labial palps, and antero-laterally projecting antennae preserved. Stylets with six teeth. Distal teeth are significantly longer than proximal ones. Labial palps are elongate and shorter than stylets. Antennae are elongate, shorter than stylets, and most likely incomplete.
- (39)
- Specimen 0936 (PED 1810) is incompletely preserved (Figure 25G). Most likely representing exuvia; the main body is strongly crumpled. The head with forward-projecting mouthparts, the stylets, and antero-laterally projecting antennae is better preserved. Stylets are broken, with few teeth. Antennae are elongate but incomplete. In addition, parts of locomotory appendages (legs) are preserved.
- (40)
- Specimen 0937 (PED 1822) is incompletely preserved (Figure 21I). Most likely representing exuvia; main body strongly crumpled; amber in addition very dirty. Head with forward-projecting mouthparts, the stylets and labial palps, and antero-laterally projecting antennae. Antennae elongate and longer than stylets. In addition, parts of locomotory appendages (legs) are preserved.
- (41)
- Specimen 0938 (PED 1971) is incompletely preserved (Figure 26A,B). Most likely representing exuvia; the main body is strongly crumpled. Head with forward-projecting mouthparts, the stylets, is better preserved. Stylets with at least five teeth. Distal teeth are significantly longer than proximal ones. In addition, parts of locomotory appendages (legs) are preserved.
- (42)
- Specimen 0939 (PED 2190) is incompletely preserved (Figure 26C,D). Most likely representing exuvia; the main body is strongly crumpled. Head with forward-projecting mouthparts, the stylets, is better preserved. In addition, parts of locomotory appendages (legs) are preserved. Legs have trumpet-shaped attachment structures distally (Figure 26E).
- (43)
- Specimen 0940 (BUB 3381) is incompletely preserved (Figure 27A,B). Most likely representing exuvia; the main body is strongly crumpled. Head with forward-projecting mouthparts, the stylets and labial palps, and antero-laterally projecting antennae is better preserved. Stylets with six teeth (Figure 27C). Distal teeth are significantly longer than proximal ones; setae between teeth; also laterally, with numerous setae. Labial palps are elongate and shorter than stylets. Antennae are elongate, longer than stylets, with at least three elements (Figure 27E). In addition, parts of locomotory appendages (legs) are preserved. Legs have trumpet-shaped attachment structures distally (Figure 27D).
- (44)
- Specimen 0941 (BUB 3371) is incompletely preserved (Figure 26F,G). Most likely representing exuvia; the main body is strongly crumpled. The head with forward-projecting mouthparts, the stylets, and antero-laterally projecting antennae is better preserved. Stylets are broken with few teeth. Antennae are elongate but incomplete. In addition, parts of locomotory appendages (legs) are preserved.
- (45)
- Specimen 0943 (PED 2633) is incompletely preserved (Figure 21F,G). Most likely representing exuvia; the main body is strongly crumpled. Head with forward-projecting mouthparts, the stylets and labial palps, and antero-laterally projecting antennae is better preserved. Stylets with six teeth. Distal teeth are significantly longer than proximal ones, with setae between the teeth; laterally with numerous setae. Labial palps are elongate and shorter than stylets. Antennae are elongate, shorter than stylets, and most likely incomplete. In addition, parts of locomotory appendages (legs) are preserved. Legs have trumpet-shaped attachment structures distally (Figure 21H).
3.4. Short Descriptions of Partly Unclear Specimens, Most Likely Superfang Type 2
- (46)
- Specimen 0847 (PED 0716) is incompletely preserved (Figure 28E,F). Most likely representing exuvia; the main body is strongly crumpled. The head with forward-projecting mouthparts, the stylets, and antero-laterally projecting antennae is better preserved. Stylets are broken off distally. Antennae incomplete. In addition, parts of locomotory appendages (legs) are preserved. Legs have trumpet-shaped attachment structures distally (Figure 28G).
- (47)
- Specimen 0875 (PED 0586) is incompletely preserved (Figure 28A,B). Most likely representing exuvia; the main body is strongly crumpled. Head with eyes (Figure 28C), forward projecting mouthparts, the stylets and labial palps, and antero-laterally projecting antennae is better preserved. Stylets with six teeth; stylets deformed. Distal teeth are significantly longer than proximal ones; setae between teeth; also laterally with numerous setae. Labial palps elongate and shorter than stylets. Antennae elongate. In addition, parts of locomotory appendages (legs) are preserved. Legs have trumpet-shaped attachment structures distally (Figure 28D).
3.5. A Short Description of a New Specimen of Kuafupolydentes hui
- (48)
- Specimen 0903 (PED 2335) is incompletely preserved in dorsal (Figure 29C) and ventral views (Figure 29A,B). Mostly head with antero-lateral projections with eyes. Also, forward-projecting mouthparts, the stylets, and antero-laterally projecting antennae are better preserved. Stylets with few blunt teeth (Figure 29A); laterally with numerous setae. Antennae are elongate, shorter than stylets, with two (or three?) elements each.
3.6. Short Descriptions of Specimens of a New Type of Larva: 4–5 Teeth Type
- (49)
- Specimen 0855 (PED 0476) is incompletely preserved (Figure 30A,B). Most likely representing exuvia; main body crumpled. Head broad; with eyes, forward-projecting mouthparts, the stylets, and antero-laterally projecting antennae better preserved. Stylets with four teeth; numerous setae between teeth; also laterally numerous setae. Antennae are elongate but short and thin (filiform). In addition, parts of locomotory appendages (legs) are preserved. Legs distally with claws (Figure 30C).
- (50)
- Specimen 0856 (PED 1326) is incompletely preserved (Figure 30D,E). Most likely representing exuvia; main body crumpled. Head broad, with forward-projecting mouthparts, the stylets, and antero-laterally projecting antennae better preserved. Stylets with four teeth; numerous setae between teeth; also laterally numerous setae. Antennae are elongate but short and thin (filiform). In addition, parts of locomotory appendages (legs) are preserved. Legs distally with claws (Figure 30F).
- (51)
- Specimen 0857 (PED 1337) is poorly preserved (Figure 30G,H). Most likely representing exuvia; main body crumpled. Head broad, with forward-projecting mouthparts and stylets. Stylets with few teeth preserved; laterally with some setae. In addition, parts of locomotory appendages (legs) are recognisable.
- (52)
- Specimen 0858 (Weiterschan BuB 30) is incompletely preserved (Figure 31A). Most likely representing exuvia; main body crumpled. Head broad, with forward-projecting mouthparts, the stylets. Stylets with four teeth; numerous setae between teeth; also laterally numerous setae (Figure 31C,D). In addition, parts of locomotory appendages (legs) are preserved. Legs distally with claws (Figure 31B).
- (53)
- Specimen 0942 (PED 2599) is incompletely preserved (Figure 31E,F). Mostly head with forward-projecting mouthparts, the stylets. Stylets with five teeth.
- (54)
- Specimen 0944 (Weiterschan BuB 1) is incompletely preserved (Figure 31G,H). Mostly head with forward-projecting mouthparts, the stylets. Stylets are distally broken off.
3.7. Morphospace of the Head Capsule
3.8. Morphospace of the Head and Stylets with Teeth
3.9. Morphospace of the Stylets with Teeth
4. Discussion
4.1. Identity of the Specimens
4.2. A New Detail of Macleodiella-Type Larvae
4.3. New Details on Superfang Type 1 Larvae
4.4. New Details on Superfang Type 2 Larvae
4.5. A New Type of Larva: 4–5 Teeth
4.6. Tokogenetic Relationships
4.7. Diversity of Shapes through Time
4.8. Convergent Evolution
4.9. Resilience in Lacewing Larvae
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dunn, R.R. Modern insect extinctions, the neglected majority. Conserv. Biol. 2005, 19, 1030–1036. [Google Scholar] [CrossRef]
- Hallmann, C.A.; Sorg, M.; Jongejans, E.; Siepel, H.; Hofland, N.; Schwan, H.; Stenmans, W.; Müller, A.; Sumser, H.; Hörren, T.; et al. More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS ONE 2017, 12, e0185809. [Google Scholar] [CrossRef] [PubMed]
- Hallmann, C.A.; Ssymank, A.; Sorg, M.; de Kroon, H.; Jongejans, E. Insect biomass decline scaled to species diversity: General patterns derived from a hoverfly community. Proc. Natl. Acad. Sci. USA 2021, 118, e2002554117. [Google Scholar] [CrossRef] [PubMed]
- Forister, M.L.; Pelton, E.M.; Black, S.H. Declines in insect abundance and diversity: We know enough to act now. Conserv. Sci. Pract. 2019, 1, e80. [Google Scholar] [CrossRef]
- Goulson, D. The insect apocalypse, and why it matters. Curr. Biol. 2019, 29, R967–R971. [Google Scholar] [CrossRef] [PubMed]
- Seibold, S.; Gossner, M.M.; Simons, N.K.; Blüthgen, N.; Müller, J.; Ambarlı, D.; Ammer, C.; Bauhus, J.; Fischer, M.; Habel, J.C. Arthropod decline in grasslands and forests is associated with landscape-level drivers. Nature 2019, 574, 671–674. [Google Scholar] [CrossRef]
- Almond, R.E.; Grooten, M.; Peterson, T. Living Planet Report 2020—Bending the Curve of Biodiversity Loss; World Wildlife Fund: Gland, Switzerland, 2020. [Google Scholar]
- van der Sluijs, J.P. Insect decline, an emerging global environmental risk. Curr. Opin. Environ. Sustain. 2020, 46, 39–42. [Google Scholar] [CrossRef]
- Wagner, D.L. Insect declines in the Anthropocene. Ann. Rev. Entomol. 2020, 65, 457–480. [Google Scholar] [CrossRef]
- Fenoglio, M.S.; Calviño, A.; González, E.; Salvo, A.; Videla, M. Urbanisation drivers and underlying mechanisms of terrestrial insect diversity loss in cities. Ecol. Entomol. 2021, 46, 757–771. [Google Scholar] [CrossRef]
- Schachat, S.R.; Labandeira, C.C. Are insects heading toward their first mass extinction? Distinguishing turnover from crises in their fossil record. Ann. Entomol. Soc. Am. 2021, 114, 99–118. [Google Scholar] [CrossRef]
- Wagner, D.L.; Grames, E.M.; Forister, M.L.; Berenbaum, M.R.; Stopak, D. Insect decline in the Anthropocene: Death by a thousand cuts. Proc. Natl. Acad. Sci. USA 2021, 118, e2023989118. [Google Scholar] [CrossRef] [PubMed]
- Weisser, W.; Blüthgen, N.; Staab, M.; Achury, R.; Müller, J. Experiments are needed to quantify the main causes of insect decline. Biol. Lett. 2023, 19, 20220500. [Google Scholar] [CrossRef] [PubMed]
- Schowalter, T.D.; Noriega, J.A.; Tscharntke, T. Insect effects on ecosystem services—Introduction. Basic Appl. Ecol. 2018, 26, 1–7. [Google Scholar] [CrossRef]
- Dangles, O.; Casas, J. Ecosystem services provided by insects for achieving sustainable development goals. Ecosyst. Serv. 2019, 35, 109–115. [Google Scholar] [CrossRef]
- Morimoto, J. Addressing global challenges with unconventional insect ecosystem services: Why should humanity care about insect larvae? People Nat. 2020, 2, 582–595. [Google Scholar] [CrossRef]
- Grimaldi, D.; Engel, M.S. Evolution of the Insects; Cambridge University Press: Cambridge, MA, USA, 2005; pp. 1–755. [Google Scholar]
- Huber, J.T. Biodiversity of Hymenoptera. In Insect Biodiversity: Science and Society; Fottitt, R.G., Adler, P.H., Eds.; Wiley Blackwell: Hoboken, NJ, USA, 2017; pp. 419–461. [Google Scholar]
- Peters, R.S.; Krogmann, L.; Mayer, C.; Donath, A.; Gunkel, S.; Meusemann, K.; Kozlov, A.; Podsiadlowski, L.; Petersen, M.; Lanfear, R.; et al. Evolutionary history of the Hymenoptera. Curr. Biol. 2017, 27, 1013–1018. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, L.; Shih, C.; Gao, T.; Ren, D. Hymenoptera—Sawflies and Wasps. In Rhythms of Insect Evolution; Ren, D., Shih, C.K., Gao, T., Yao, Y., Wang, Y., Eds.; Wiley Blackwell: Hoboken, NJ, USA, 2019; pp. 429–496. [Google Scholar] [CrossRef]
- Lawrence, J.F.; Ślipiski, A.; Seago, A.E.; Thayer, M.K.; Newton, A.F.; Marvaldi, A.E. Phylogeny of the Coleoptera based on morphological characters of adults and larvae. Ann. Zool. 2011, 61, 1–217. [Google Scholar] [CrossRef]
- Yuan, M.L.; Zhang, Q.L.; Zhang, L.; Guo, Z.L.; Liu, Y.J.; Shen, Y.Y.; Shao, R. High-level phylogeny of the Coleoptera inferred with mitochondrial genome sequences. Mol. Phyl. Evol. 2016, 104, 99–111. [Google Scholar] [CrossRef]
- McKenna, D.D.; Shin, S.; Ahrens, D.; Balke, M.; Beza-Beza, C.; Clarke, D.J.; Donath, A.; Escalona, H.E.; Friedrich, F.; Letsch, H.; et al. The evolution and genomic basis of beetle diversity. Proc. Natl. Acad. Sci. USA 2019, 116, 24729–24737. [Google Scholar] [CrossRef]
- Timmermans, M.J.; Lees, D.C.; Simonsen, T.J. Towards a mitogenomic phylogeny of Lepidoptera. Mol. Phyl. Evol. 2014, 79, 169–178. [Google Scholar] [CrossRef]
- Goldstein, P.Z. Diversity and significance of Lepidoptera: A phylogenetic perspective. In Insect Biodiversity: Science and Society; Fottitt, R.G., Adler, P.H., Eds.; Wiley Blackwell: Hoboken, NJ, USA, 2017; pp. 463–495. [Google Scholar]
- Mitter, C.; Davis, D.R.; Cummings, M.P. Phylogeny and evolution of Lepidoptera. Ann. Rev. Entomol. 2017, 62, 265–283. [Google Scholar] [CrossRef]
- Yeates, D.K.; Wiegmann, B.M.; Courtney, G.W.; Meier, R.; Lambkin, C.; Pape, T. Phylogeny and systematics of Diptera: Two decades of progress and prospects. Zootaxa 2007, 1668, 565–590. [Google Scholar] [CrossRef]
- Lambkin, C.L.; Sinclair, B.J.; Pape, T.; Courtney, G.W.; Skevington, J.H.; Meier, R.; Yeates, D.K.; Blagoderov, V.; Wiegmann, B.M. The phylogenetic relationships among infraorders and superfamilies of Diptera based on morphological evidence. Syst. Entomol. 2013, 38, 164–179. [Google Scholar] [CrossRef]
- Courtney, G.W.; Pape, T.; Skevington, J.H.; Sinclair, B.J. Biodiversity of Diptera. In Insect Biodiversity: Science and Society; Fottitt, R.G., Adler, P.H., Eds.; Wiley Blackwell: Hoboken, NJ, USA, 2017; pp. 229–278. [Google Scholar]
- Carpenter, F.M. The geological history and evolution of insects. Am. Sci. 1953, 41, 256–270. [Google Scholar]
- Yang, A.S. Modularity, evolvability, and adaptive radiations: A comparison of the hemi- and holometabolous insects. Evol. Dev. 2001, 3, 59–72. [Google Scholar] [CrossRef]
- Mayhew, P.J. Why are there so many insect species? Perspectives from fossils and phylogenies. Biol. Rev. 2007, 82, 425–454. [Google Scholar] [CrossRef]
- Benefer, C.; Andrew, P.; Blackshaw, R.; Ellis, J.; Knight, M. The spatial distribution of phytophagous insect larvae in grassland soils. Appl. Soil Ecol. 2010, 45, 269–274. [Google Scholar] [CrossRef]
- Haug, J.T. Why the term “larva” is ambiguous, or what makes a larva? Acta Zool. 2020, 101, 167–188. [Google Scholar] [CrossRef]
- Aspöck, U.; Aspöck, H. Kamelhälse, Schlammfliegen, Ameisenlöwen. Wer sind sie? (Insecta: Neuropterida: Raphidioptera, Megaloptera, Neuroptera). Stapfia 1999, 60, 1–34. [Google Scholar]
- Aspöck, U.; Aspöck, H. Verbliebene Vielfalt vergangener Blüte. Zur Evolution, Phylogenie und Biodiversität der Neuropterida (Insecta: Endopterygota). Denisia 2007, 20, 451–516. [Google Scholar]
- Aspöck, U.; Plant, J.D.; Nemeschkal, H.L. Cladistic analysis of Neuroptera and their systematic position within Neuropterida (Insecta: Holometabola: Neuropterida: Neuroptera). Syst. Entomol. 2001, 26, 73–86. [Google Scholar] [CrossRef]
- Aspöck, U.; Haring, E.; Aspöck, H. The phylogeny of the Neuropterida: Long lasting and current controversies and challenges (Insecta: Endopterygota). Arthrop. Syst. Phyl. 2012, 70, 119–129. [Google Scholar] [CrossRef]
- Winterton, S.L.; Hardy, N.B.; Wiegmann, B.M. On wings of lace: Phylogeny and Bayesian divergence time estimates of Neuropterida (Insecta) based on morphological and molecular data. Syst. Entomol. 2010, 35, 349–378. [Google Scholar] [CrossRef]
- Winterton, S.L.; Lemmon, A.R.; Gillung, J.P.; Garzon, I.J.; Badano, D.; Bakkes, D.K.; Breitkreuz, L.C.V.; Engel, M.; Moriarty, E.M.; Liu, X.; et al. Evolution of lacewings and allied orders using anchored phylogenomics (Neuroptera, Megaloptera, Raphidioptera). Syst. Entomol. 2018, 43, 330–354. [Google Scholar] [CrossRef]
- Engel, M.S.; Winterton, S.L.; Breitkreuz, L.C. Phylogeny and evolution of Neuropterida: Where have wings of lace taken us? Ann. Rev. Entomol. 2018, 63, 531–551. [Google Scholar] [CrossRef] [PubMed]
- Vasilikopoulos, A.; Misof, B.; Meusemann, K.; Lieberz, D.; Flouri, T.; Beutel, R.G.; Niehuis, O.; Wappler, T.; Rust, J.; Peters, R.S.; et al. An integrative phylogenomic approach to elucidate the evolutionary history and divergence times of Neuropterida (Insecta: Holometabola). BMC Evol. Biol. 2020, 20, 64. [Google Scholar] [CrossRef]
- Oswald, J.D. Lacewing Digital Library, Research Publication No. 1. Available online: http://lacewing.tamu.edu/SpeciesCatalog/Main (accessed on 3 December 2023).
- Haug, C.; Braig, F.; Haug, J.T. Quantitative analysis of lacewing larvae over more than 100 million years reveals a complex pattern of loss of morphological diversity. Sci. Rep. 2023, 13, 6127. [Google Scholar] [CrossRef] [PubMed]
- MacLeod, E.G. A Comparative Morphological Study of the Head Capsule and Cervix of Larval Neuroptera (Insecta). Ph.D. Thesis, Harvard University, Cambridge, MA, USA, 1964. [Google Scholar]
- New, T.R. Neuroptera. In The Insects of Australia: A Textbook for Students and Research Workers; Commonwealth Scientific and Industrial Research Organisation, Ed.; Melbourne University Press: Melbourne, Australia, 1991; Volume 2, pp. 525–542. [Google Scholar]
- Tauber, C.A.; Tauber, M.J.; Albuquerque, G.S. Neuroptera:(lacewings, antlions). In Encyclopedia of Insects; Elsevier: Amsterdam, The Netherlands, 2009; pp. 695–707. [Google Scholar]
- Beutel, R.G.; Friedrich, F.; Yang, X.-K.; Ge, S.-Q. Insect Morphology and Phylogeny: A Textbook for Students of Entomology; Walter de Gruyter: Berlin, Germany, 2013. [Google Scholar]
- Zimmermann, D.; Randolf, S.; Aspöck, U. From chewing to sucking via phylogeny–From sucking to chewing via ontogeny: Mouthparts of Neuroptera. In Insect Mouthparts, Zoological Monographs 5; Krenn, H.W., Ed.; Springer: Berlin, Germany, 2019; pp. 361–385. [Google Scholar]
- Badano, D.; Aspöck, U.; Aspöck, H.; Cerretti, P. Phylogeny of Myrmeleontiformia based on larval morphology (Neuropterida: Neuroptera). Syst. Entomol. 2017, 42, 94–117. [Google Scholar] [CrossRef]
- MacLeod, E.G. The Neuroptera of the Baltic Amber. I. Ascalaphidae, Nymphidae, and Psychopsidae. Psyche 1970, 77, 147–180. [Google Scholar] [CrossRef]
- Larsson, S.G. Baltic Amber: A Palaeobiological Study; Scandinavian Science Press: Klampenborg, Denmark, 1978; pp. 1–192. [Google Scholar]
- Weitschat, W.; Wichard, W. Atlas der Pflanzen und Tiere im Baltischen Bernstein; Friedrich Pfeil: Munich, Germany, 1998. [Google Scholar]
- Scheven, J. Bernstein-Einschlüsse: Eine Untergegangene Welt Bezeugt die Schöpfung. Erinnerungen an die Welt vor der Sintflut; Kuratorium Lebendige Vorwelt e.V.: Hofheim, Germany, 2004. [Google Scholar]
- Wichard, W.; Gröhn, C.; Seredszus, F. Aquatic Insects in Baltic Amber; Kessel: Remagen, Germany, 2009. [Google Scholar]
- Ohl, M. Aboard a spider—A complex developmental strategy fossilized in amber. Naturwiss 2011, 98, 453–456. [Google Scholar] [CrossRef]
- Gröhn, C. Einschlüsse im Baltischen Bernstein; Wachholtz Verlag-Murmann Publishers: Kiel, Germany, 2015. [Google Scholar]
- Haug, J.T.; Baranov, V.; Schädel, M.; Müller, P.; Gröhn, C.; Haug, C. Challenges for understanding lacewings: How to deal with the incomplete data from extant and fossil larvae of Nevrorthidae? (Neuroptera). Fragm. Entomol. 2020, 52, 137–167. [Google Scholar] [CrossRef]
- Haug, J.T.; van der Wal, S.; Gröhn, C.; Hoffeins, C.; Hoffeins, H.-W.; Haug, C. Diversity and fossil record of larvae of three groups of lacewings with unusual ecology and functional morphology: Ithonidae, Coniopterygidae and Sisyridae. Palaeontol. Electron. 2022, 25, a14. [Google Scholar] [CrossRef] [PubMed]
- Haug, J.T.; Kiesmüller, C.; Haug, G.T.; Haug, C.; Hörnig, M.K. A fossil aphidlion preserved together with its prey in 40 million-year-old Baltic amber. Palaeobiodiv. Palaeoenvir. 2023, 103, 155–163. [Google Scholar] [CrossRef]
- Mengel, L.; Linhart, S.; Haug, G.T.; Weiterschan, T.; Müller, P.; Hoffeins, C.; Hoffeins, H.-W.; Baranov, V.; Haug, C.; Haug, J.T. The morphological diversity of dragon lacewing larvae (Nevrorthidae, Neuroptera) changed more over geological time scales than anticipated. Insects 2023, 14, 749. [Google Scholar] [CrossRef]
- Poinar, G.O.; Poinar, R. The Amber Forest: A Reconstruction of a Vanished World; Princeton University Press: Princeton, NJ, USA, 1999. [Google Scholar]
- Engel, M.S.; Grimaldi, D.A. The neuropterid fauna of Dominican and Mexican amber (Neuropterida: Megaloptera, Neuroptera). Am. Mus. Nov. 2007, 3587, 1–58. [Google Scholar] [CrossRef]
- Haug, C.; Haug, G.T.; Baranov, V.A.; Solórzano-Kraemer, M.M.; Haug, J.T. An owlfly larva preserved in Mexican amber and the Miocene record of lacewing larvae. Bol. Soc. Geol. Mex. 2021, 73, A271220. [Google Scholar] [CrossRef]
- Grimaldi, D.A. A diverse fauna of Neuropterodea in amber from the Cretaceous of New Jersey. In Studies on Fossils in Amber, with Particular Reference to the Cretaceous of New Jersey; Grimaldi, D.A., Ed.; Backhuys Publishers: Leiden, The Netherlands, 2000; pp. 259–303. [Google Scholar]
- Grimaldi, D.A.; Engel, M.S.; Nascimbene, P.C. Fossiliferous Cretaceous amber from Myanmar (Burma): Its rediscovery, biotic diversity, and paleontological significance. Am. Mus. Nov. 2002, 3361, 1–71. [Google Scholar] [CrossRef]
- Engel, M.S.; Grimaldi, D.A. Diverse Neuropterida in Cretaceous amber, with particular reference to the paleofauna of Myanmar (Insecta). Nov. Suppl. Entomol. 2008, 20, 1–86. [Google Scholar]
- Xia, F.; Yang, G.; Zhang, Q.; Shi, G.; Wang, B. Amber: Life through Time and Space; Science Press: Beijing, China, 2015. [Google Scholar]
- Wang, B.; Xia, F.; Engel, M.S.; Perrichot, V.; Shi, G.; Zhang, H.; Chen, J.; Jarzembowski, E.A.; Wappler, T.; Rust, J. Debris-carrying camouflage among diverse lineages of Cretaceous insects. Sci. Adv. 2016, 2, e1501918. [Google Scholar] [CrossRef]
- Wichard, W. Family Nevrorthidae (Insecta, Neuroptera) in mid-Cretaceous Burmese amber. Palaeodiversity 2017, 10, 1–6. [Google Scholar] [CrossRef][Green Version]
- Liu, X.; Zhang, W.; Winterton, S.L.; Breitkreuz, L.C.; Engel, M.S. Early morphological specialization for insect-spider associations in Mesozoic lacewings. Curr. Biol. 2016, 26, 1590–1594. [Google Scholar] [CrossRef]
- Liu, X.; Shi, G.; Xia, F.; Lu, X.; Wang, B.; Engel, M.S. Liverwort mimesis in a Cretaceous lacewing larva. Curr. Biol. 2018, 28, 1475–1481. [Google Scholar] [CrossRef]
- Liu, H.; Luo, C.; Jarzembowski, E.A.; Xiao, C. Acanthochrysa langae gen. et sp. nov., a new lacewing larva (Neuroptera: Chrysopoidea) from mid-Cretaceous Kachin amber. Cretac. Res. 2022, 133, 105146. [Google Scholar] [CrossRef]
- Makarkin, V.N. Re-description of Grammapsychops lebedevi Martynova, 1954 (Neuroptera: Psychopsidae) with notes on the Late Cretaceous psychopsoids. Zootaxa 2018, 4524, 581–594. [Google Scholar] [CrossRef]
- Badano, D.; Engel, M.S.; Basso, A.; Wang, B.; Cerretti, P. Diverse Cretaceous larvae reveal the evolutionary and behavioural history of antlions and lacewings. Nature Comm. 2018, 9, 3257. [Google Scholar] [CrossRef] [PubMed]
- Badano, D.; Fratini, M.; Maugeri, L.; Palermo, F.; Pieroni, N.; Cedola, A.; Haug, J.T.; Weiterschan, T.; Velten, J.; Mei, M.; et al. X-ray microtomography and phylogenomics provide insights into the morphology and evolution of an enigmatic Mesozoic insect larva. Syst. Entomol. 2021, 46, 672–684. [Google Scholar] [CrossRef]
- Haug, J.T.; Müller, P.; Haug, C. The ride of the parasite: A 100-million-year old mantis lacewing larva captured while mounting its spider host. Zool. Lett. 2018, 4, 31. [Google Scholar] [CrossRef] [PubMed]
- Haug, C.; Herrera-Flórez, A.F.; Müller, P.; Haug, J.T. Cretaceous chimera—An unusual 100-million-year old neuropteran larva from the “experimental phase” of insect evolution. Palaeodiversity 2019, 12, 1–11. [Google Scholar] [CrossRef]
- Haug, J.T.; Müller, P.; Haug, C. A 100-million-year old predator: A fossil neuropteran larva with unusually elongated mouthparts. Zool. Lett. 2019, 5, 29. [Google Scholar] [CrossRef]
- Haug, J.T.; Müller, P.; Haug, C. A 100-million-year old slim insectan predator with massive venom-injecting stylets-a new type of neuropteran larva from Burmese amber. Bull. Geosci. 2019, 94, 431–440. [Google Scholar] [CrossRef]
- Haug, G.T.; Haug, C.; Pazinato, P.G.; Braig, F.; Perrichot, V.; Gröhn, C.; Müller, P.; Haug, J.T. The decline of silky lacewings and morphological diversity of long-nosed antlion larvae through time. Palaeontol. Electron. 2020, 23, a39. [Google Scholar] [CrossRef] [PubMed]
- Haug, J.T.; Pazinato, P.G.; Haug, G.T.; Haug, C. Yet another unusual new type of lacewing larva preserved in 100-million-year old amber from Myanmar. Riv. Ital. Palaeontol. Strat. 2020, 126, 821–832. [Google Scholar] [CrossRef]
- Haug, G.T.; Baranov, V.; Wizen, G.; Pazinato, P.G.; Müller, P.; Haug, C.; Haug, J.T. The morphological diversity of long-necked lacewing larvae (Neuroptera: Myrmeleontiformia). Bull. Geosci. 2021, 96, 431–457. [Google Scholar] [CrossRef]
- Haug, G.T.; Haug, C.; Haug, J.T. The morphological diversity of spoon-winged lacewing larvae and the first possible fossils from 99 million-year-old Kachin amber, Myanmar. Palaeodiversity 2021, 14, 133–152. [Google Scholar] [CrossRef]
- Haug, J.T.; Baranov, V.; Müller, P.; Haug, C. New extreme morphologies as exemplified by 100 million-year-old lacewing larvae. Sci. Rep. 2021, 11, 20432. [Google Scholar] [CrossRef]
- Haug, J.T.; Haug, G.T.; Zippel, A.; van der Wal, S.; Müller, P.; Gröhn, C.; Wunderlich, J.; Hoffeins, C.; Hoffeins, H.-W.; Haug, C. Changes in the morphological diversity of larvae of lance lacewings, mantis lacewings and their closer relatives over 100 million years. Insects 2021, 12, 860. [Google Scholar] [CrossRef]
- Haug, C.; Posada Zuluaga, V.; Zippel, A.; Braig, F.; Müller, P.; Gröhn, C.; Weiterschan, T.; Wunderlich, J.; Haug, G.T.; Haug, J.T. The morphological diversity of antlion larvae and their closest relatives over 100 million years. Insects 2022, 13, 587. [Google Scholar] [CrossRef]
- Haug, C.; Zippel, A.; Hassenbach, C.; Haug, G.T.; Haug, J.T. A split-footed lacewing larva from about 100-million-year-old amber indicates a now extinct hunting strategy for neuropterans. Bull. Geosci. 2022, 97, 453–464. [Google Scholar] [CrossRef]
- Haug, G.T.; Haug, C.; van der Wal, S.; Müller, P.; Haug, J.T. Split-footed lacewings declined over time: Indications from the morphological diversity of their antlion-like larvae. PalZ 2022, 96, 29–50. [Google Scholar] [CrossRef]
- Haug, J.T.; Linhart, S.; Haug, G.T.; Gröhn, C.; Hoffeins, C.; Hoffeins, H.-W.; Müller, P.; Weiterschan, T.; Wunderlich, J.; Haug, C. The diversity of aphidlion-like larvae over the last 130 million years. Insects 2022, 13, 336. [Google Scholar] [CrossRef] [PubMed]
- Haug, J.T.; Kay, L.T.; Haug, G.T.; Kyaw, N.T.; Haug, C.; Hörnig, M.K. A hatching aphidlion-like lacewing larva in 100 million years old Kachin amber. Insect Sci. 2023, 30, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Flórez, A.F.; Braig, F.; Haug, C.; Neumann, C.; Wunderlich, J.; Hörnig, M.K.; Haug, J.T. Identifying the oldest larva of a myrmeleontiformian lacewing—A morphometric approach. Acta Palaeontol. Pol. 2020, 65, 235–250. [Google Scholar] [CrossRef]
- Hörnig, M.K.; Kiesmüller, C.; Müller, P.; Haug, C.; Haug, J.T. A new glimpse on trophic interactions of 100-million-year old lacewing larvae. Acta Palaeontol. Pol. 2020, 65, 777–786. [Google Scholar] [CrossRef]
- Zippel, A.; Kiesmüller, C.; Haug, G.T.; Müller, P.; Weiterschan, T.; Haug, C.; Hörnig, M.K.; Haug, J.T. Long-headed predators in Cretaceous amber—Fossil findings of an unusual type of lacewing larva. Palaeoentomology 2021, 4, 475–498. [Google Scholar] [CrossRef]
- Luo, C.; Liu, H.; Jarzembowski, E.A. High morphological disparity of neuropteran larvae during the Cretaceous revealed by a new large species. Geol. Mag. 2022, 159, 954–962. [Google Scholar] [CrossRef]
- Haug, J.T.; Haug, C. 100 million-year-old straight-jawed lacewing larvae with enormously inflated trunks represent the oldest cases of extreme physogastry in insects. Sci. Rep. 2022, 12, 12760. [Google Scholar] [CrossRef]
- Haug, J.T.; Haug, C. Oldest record of a dustywing-type larva in about 100-million-year-old amber. Palaeodiversity 2023, 16, 141–150. [Google Scholar] [CrossRef]
- Haug, J.T.; Haug, C. New details of the enigmatic 100 million years old antlion-like larvae of Ankyloleon (Myrmeleontiformia, Neuroptera). Eur. J. Taxon. 2023, 908, 135–154. [Google Scholar] [CrossRef]
- Hassenbach, C.; Buchner, L.; Haug, G.T.; Haug, C.; Haug, J.T. An expanded view on the morphological diversity of long-nosed antlion larvae further supports a decline of silky lacewings in the past 100 million years. Insects 2023, 14, 170. [Google Scholar] [CrossRef]
- Perrichot, V. Environnements Paraliques à Ambre et à Végétaux du Crétacé Nord-Aquitain (Charentes, Sud-Ouest de la France). Ph.D. Thesis, Université Rennes 1, Rennes, France, 2003. Available online: https://tel.archives-ouvertes.fr/tel-00011639/file/Memoire_GS_Web.pdf (accessed on 6 December 2023).
- Pérez-de la Fuente, R.; Delclòs, X.; Peñalver, E.; Speranza, M.; Wierzchos, J.; Ascaso, C.; Engel, M.S. Early evolution and ecology of camouflage in insects. Proc. Natl. Acad. Sci. USA 2012, 109, 21414–21419. [Google Scholar] [CrossRef]
- Pérez-de la Fuente, R.; Delclòs, X.; Peñalver, E.; Engel, M.S. A defensive behavior and plant-insect interaction in Early Cretaceous amber–the case of the immature lacewing Hallucinochrysa diogenesi. Arthropod Struct. Dev. 2016, 45, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Pérez-de la Fuente, R.; Engel, M.S.; Delclòs, X.; Peñalver, E. Straight-jawed lacewing larvae (Neuroptera) from Lower Cretaceous Spanish amber, with an account on the known amber diversity of neuropterid immatures. Cretac. Res. 2020, 106, 104200. [Google Scholar] [CrossRef]
- Pérez-de la Fuente, R.; Peñalver, E.; Azar, D.; Engel, M.S. A soil-carrying lacewing larva in Early Cretaceous Lebanese amber. Sci. Rep. 2018, 8, 16663. [Google Scholar] [CrossRef] [PubMed]
- Pérez-de la Fuente, R.; Engel, M.S.; Azar, D.; Peñalver, E. The hatching mechanism of 130-million-year-old insects: An association of neonates, egg shells and egg bursters in Lebanese amber. Palaeontology 2019, 62, 547–559. [Google Scholar] [CrossRef]
- Wang, B.; Shi, G.; Xu, C.; Spicer, R.A.; Perrichot, V.; Schmidt, A.R.; Feldberg, K.; Heinrichs, J.; Chény, C.; Pang, H.; et al. The mid-Miocene Zhangpu biota reveals an outstandingly rich rainforest biome in East Asia. Sci. Adv. 2021, 7, eabg0625. [Google Scholar] [CrossRef] [PubMed]
- Machado, R.J.; Gillung, J.P.; Winterton, S.L.; Garzón-Orduña, I.J.; Lemmon, A.R.; Lemmon, E.M.; Oswald, J.D. Owlflies are derived antlions: Anchored phylogenomics supports a new phylogeny and classification of Myrmeleontidae (Neuroptera). Syst. Entomol. 2019, 44, 418–450. [Google Scholar] [CrossRef]
- Monserrat, V.J. Nuevos datos sobre algunas especies de Nemopteridae y Crocidae (Insecta: Neuroptera). Heteropt. Rev. Entomol. 2008, 8, 1–33. [Google Scholar] [CrossRef]
- Jones, J.R. Total-evidence phylogeny of the owlflies (Neuroptera, Ascalaphidae) supports a new higher-level classification. Zool. Scr. 2019, 48, 761–782. [Google Scholar] [CrossRef]
- Prost, A.; Popov, A. A first comprehensive inventory of Ascalaphidae, Palparidae, and Myrmeleontidae (Insecta: Neuroptera) of Northeastern Nigeria with description of two new species and an overview of genus Bankisus Navás. Hist. Nat. Bulg. 2021, 43, 51–77. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, Y.; Labandeira, C.C.; Shih, C.; Ren, D. Mesozoic lacewings from China provide phylogenetic insight into evolution of the Kalligrammatidae (Neuroptera). BMC Evol. Biol. 2014, 14, 126. [Google Scholar] [CrossRef]
- Hu, J.; Lu, X.; Wang, B.; Liu, X. Taxonomic notes on Babinskaiidae from the Cretaceous Burmese amber, with the description of a new species (Insecta, Neuroptera). ZooKeys 2018, 748, 31. [Google Scholar] [CrossRef]
- Lu, X.; Wang, B.; Liu, X. New Cretaceous antlion-like lacewings promote a phylogenetic reappraisal of the extinct myrmeleontoid family Babinskaiidae. Sci. Rep. 2021, 11, 16431. [Google Scholar] [CrossRef]
- Cruickshank, R.D.; Ko, K. Geology of an amber locality in the Hukawng Valley, northern Myanmar. J. Asian Earth Sci. 2003, 21, 441–455. [Google Scholar] [CrossRef]
- Shi, G.; Grimaldi, D.A.; Harlow, G.E.; Wang, J.; Wang, J.; Yang, M.; Lei, W.; Li, Q.; Li, X. Age constraint on Burmese amber based on U–Pb dating of zircons. Cretac. Res. 2012, 37, 155–163. [Google Scholar] [CrossRef]
- Yu, T.; Kelly, R.; Mu, L.; Ross, A.; Kennedy, J.; Broly, P.; Xia, F.; Zhang, H.; Wang, B.; Dilcher, D. An ammonite trapped in Burmese amber. Proc. Natl. Acad. Sci. USA 2019, 116, 11345–11350. [Google Scholar] [CrossRef] [PubMed]
- Dunne, E.M.; Raja, N.B.; Stewens, P.P.; Zaw, K. Ethics, law, and politics in palaeontological research: The case of Myanmar amber. Commun. Biol. 2022, 5, 1023. [Google Scholar] [CrossRef]
- Haug, C.; Reumer, J.W.F.; Haug, J.T.; Arillo, A.; Audo, D.; Azar, D.; Baranov, V.; Beutel, R.; Charbonnier, S.; Feldmann, R.; et al. Comment on the letter of the Society of Vertebrate Paleontology (SVP) dated 21 April 2020 regarding “Fossils from conflict zones and reproducibility of fossil-based scientific data”: The importance of private collections. PalZ 2020, 94, 413–429. [Google Scholar] [CrossRef]
- Haug, J.T.; Azar, D.; Ross, A.; Szwedo, J.; Wang, B.; Arillo, A.; Baranov, V.; Bechteler, J.; Beutel, R.; Blagoderov, V.; et al. Comment on the letter of the Society of Vertebrate Paleontology (SVP) dated 21 April 2020 regarding “Fossils from conflict zones and reproducibility of fossil-based scientific data”: Myanmar amber. PalZ 2020, 94, 431–437. [Google Scholar] [CrossRef]
- Haug, C.; Tun, K.L.; Mon, T.L.; Hin, W.W.; Haug, J.T. The strange holometabolan beak larva from about 100 million years old Kachin amber was physogastric and possibly wood-associated. Palaeoentomology 2023, 6, 372–384. [Google Scholar] [CrossRef]
- Ghosh, C.C. XXVII. Entomological notes. Croce filipennis, Westw. J. Bombay Nat. Hist. Soc. 1910, 20, 530–532. [Google Scholar]
- Imms, A.D. X. Contributions to a knowledge of the structure and biology of some Indian Insects—I. On the life-history of Croce filipennis, Westw. (Order Neuroptera, Fam. Hemerobiidæ). Trans. Linn. Soc. London 2nd Ser. Zool. 1911, 11, 151–160. [Google Scholar] [CrossRef][Green Version]
- Navás, L. Once Neurópteros nuevos españoles. Bol. Soc. Entomol. España 1919, 2, 48–56. [Google Scholar]
- Withycombe, C.L. XIII. Systematic notes on the Crocini (Nemopteridae), with descriptions of new genera and species. Trans. R. Entomol. Soc. London 1923, 71, 269–287. [Google Scholar] [CrossRef]
- Pierre, F. Morphologie, milieu biologique et comportement de trois Crocini nouveaux du Sahara nord-occidental (Planipennes, Nemopteridae). Ann. Soc. Entomol. France 1952, 119, 1–22. [Google Scholar] [CrossRef]
- Mansell, M.W. The Crocinae of southern Africa (Neuroptera: Nemopteridae). 2. The genus Concroce Tjeder. J. Entomol. Soc. South. Africa 1981, 44, 91–106. [Google Scholar]
- Mansell, M.W. The Crocinae of southern Africa (Neuroptera: Nemopteridae). 3. The genus Tjederia Mansell, with keys to the southern African Crocinae. J. Entomol. Soc. 1981, 44, 245–257. [Google Scholar]
- Mansell, M.W. A revision of the Australian Crocinae (Neuroptera: Nemopteridae). Austral. J. Zool. 1983, 31, 607–627. [Google Scholar] [CrossRef]
- Monserrat, V.J. Pterocroce capillaris (Klug, 1836) en Europa (Neur., Plan., Nemopteridae). Neuropt. Internat. 1983, 2, 109–128. [Google Scholar]
- Monserrat, V.J. Estadios larvarios de los neurópteros ibéricos I: Josandreva sazi (Neur. Plan., Nemopteridae). Speleon 1983, 26, 39–51. [Google Scholar]
- Satar, A.; Suludere, Z.; Candan, D.; Canbulat, S. Morphology and surface structure of eggs and first instar larvae of Croce schmidti (Navás, 1927) (Neuroptera: Nemopteridae). Zootaxa 2007, 1554, 49–55. [Google Scholar] [CrossRef]
- Zhang, W.W. Frozen Dimensions. The Fossil Insects and Other Invertebrates in Amber; Chongqing University Press: Chongqing, China, 2017. [Google Scholar]
- Brauer, F.M. Beiträge zur Kenntniss des inneren Baues und der Verwandlung der Neuropteren. Verh. Zool.-Bot. Ver. 1854, 4, 463–472. [Google Scholar]
- McClendon, J.F. The life history of Ulula hyalina Latreille. Am. Nat. 1902, 36, 421–429. [Google Scholar] [CrossRef]
- Van der Weele, H.W. Ascalaphiden. Collections Zoologiques du Baron Edm. de Selys Longchamps. Cat. Syst. Descr. 1908, 8, 1–326. [Google Scholar]
- Withycombe, C.L. XV. Some Aspects of the Biology and Morphology of the Neuroptera. With special reference to the immature stages and their possible phylogenetic significance. Trans. R. Entomol. Soc. London 1925, 72, 303–411. [Google Scholar] [CrossRef]
- Stitz, H. Planipennia. In Biologie der Tiere Deutschlands; Lfg. 33, Teil 35; Schultze, P., Ed.; Borntraeger: Berlin, Germany, 1931; pp. 67–304. [Google Scholar]
- Townsend, L.H. Lacewings and their allies. Sci. Mon. 1939, 48, 350–357. [Google Scholar]
- Principi, M.M. Contributi allo studio dei Neurotteri italiani. II. Myrmeleon inconspicuus Ramb. ed Euroleon nostras Fourcroy. Boll. Istit. Entomol. R. Univ. Studi Bologna 1943, 14, 131–192. [Google Scholar]
- Peterson, A. Larvae of Insects. An Introduction to Nearctic Species. Part II. Coleoptera, Diptera, Neuroptera, Siphonaptera, Mecoptera, Trichoptera. Larvae of Insects. An Introduction to Nearctic Species; Edward Brothers: Columbus, OH, USA, 1957; pp. 1–416. [Google Scholar]
- Aspöck, H.; Aspöck, U. Synopsis der Systematik, Ökologie und Biogeographie der Neuropteren Mitteleuropas im Spiegel der Neuropteren-Fauna von Linz und Oberösterreich, sowie Bestimmungs-Schlüsel für die mitteleuropäischen Neuropteren und Beschreibung von Coniopteryx lentiae nov. spec. Naturkundl. Jahrb. Stadt 1964, 1964, 127–282. [Google Scholar]
- Riek, E.F. Neuroptera (lacewings). In The Insects of Australia; Melbourne University Press: Melbourne, Australia, 1970; pp. 472–494. [Google Scholar]
- Henry, C.S. Some aspects of the external morphology of larval owlflies (Neuroptera: Ascalaphidae), with particular reference to Ululodes and Ascalopterynx. Psyche 1976, 83, 1–31. [Google Scholar] [CrossRef]
- Penny, N.D. Neuroptera of the Amazon Basin. Part 3 Ascalaphidae. Acta Amazon. 1981, 11, 605–651. [Google Scholar] [CrossRef][Green Version]
- Kamiya, A.; Ando, H. External Morphogenesis of the Embryo of Ascalaphus ramburi (Neuroptera, Ascalaphidae). In Recent Advances in Insect Embryology in Japan; Ando, H., Miya, K., Eds.; ISEBU Co., Ltd.: Tsukuba, Japan, 1985; pp. 203–213. [Google Scholar]
- Ábrahám, L.; Papp, Z. Preliminary report on the larva of Myrmecaelurus zigan Aspöck, Aspöck et Hölzel, 1980 (Planipennia: Myrmeleonidae). Fol. Hist.-Nat. Mus. Matra. 1990, 15, 37–42. [Google Scholar]
- Dayvault, R.D.; Codington, L.A.; Kohls, D.; Hawes, W.D.; Ott, P.M.; Behnke, D. Fossil insects and spiders from three locations in the Green River Formation of the Piceance Creek Basin, Colorado. In The Green River Formation in Piceance Creek and Eastern Uinta Basins Field Trip; Averett, W.R., Ed.; Geological Society: Grand Junction, CO, USA, 1995; pp. 97–115. [Google Scholar]
- Satar, A.; Suludere, Z.; Canbulat, S.; Oezbay, C. Rearing the larval stages of Distoleon tetragrammicus (Fabricius, 1798) (Neuroptera, Myrmeleontidae) from egg to adult, with notes on their behaviour. Zootaxa 2006, 1371, 57–64. [Google Scholar] [CrossRef]
- Satar, A.; Tusun, S.; Bozdogan, H. Third instars larvae of Gepus gibbosus Hölzel, 1968 (Neuroptera: Myrmeleontindae). Zootaxa 2014, 3793, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Satar, A.; Tusun, S.; Aykut, M. Morphology and surface structure of third instar larvae of Solter ledereri Navás, 1912 (Neuroptera: Myrmeleontidae) from Turkey. Entomol. News 2014, 124, 67–72. [Google Scholar] [CrossRef]
- Nicoli Aldini, R. Observations on the larval morphology of the antlion Myrmeleon bore (Tjeder, 1941) (Neuroptera Myrmeleontidae) and its life cycle in the Po Valley (northern Italy). Ann. Mus. Civ. Stor. Nat. Ferrara 2007, 8, 59–66. [Google Scholar]
- Beutel, R.G.; Friedrich, F.; Aspöck, U. The larval head of Nevrorthidae and the phylogeny of Neuroptera (Insecta). Zool. J. Linn. Soc. 2010, 158, 533–562. [Google Scholar] [CrossRef]
- Pantaleoni, R.A.; Cesaroni, C.; Nicoli Aldini, R. Myrmeleon mariaemathildae n. sp.: A new Mediterranean pit-building antlion (Neuropterida Myrmeleontidae). Bull. Insectol. 2010, 63, 91–98. [Google Scholar]
- Badano, D. The Larvae of European Myrmeleontidae and Ascalaphidae (Neuroptera). Ph.D. Dissertation, Università degli Studi di Sassari, Sassari, Italy, 2012. [Google Scholar]
- Acevedo, F.; Monserrat, V.J.; Badano, D. Comparative description of larvae of the European species of Distoleon Banks: D. annulatus (Klug, 1834) and D. tetragrammicus (Fabricius, 1798) (Neuroptera, Myrmeleontidae). Zootaxa 2013, 3721, 488–494. [Google Scholar] [CrossRef]
- Devetak, D.; Klokočovnik, V.; Lipovšek, S.; Bock, E.; Leitinger, G. Larval morphology of the antlion Myrmecaelurus trigrammus (Pallas, 1771) (Neuroptera, Myrmeleontidae), with notes on larval biology. Zootaxa 2013, 3641, 491–500. [Google Scholar] [CrossRef]
- Miller, R.B.; Stange, L.A. A revision of the genus Eremoleon Banks (Neuroptera: Myrmeleontidae: Nemoleontini). Insecta Mundi 2016, 495, 1–111. [Google Scholar]
- Matsuno, S. A non-destructive method for observation of body-surface fine structure of ethanol-preserved insect larvae. Jpn. J. Environ. Entomol. Zool. 2017, 28, 1–4. [Google Scholar]
- Gupta, A.; Badano, D. Larval morphology and life history of Ascalaphus dicax Walker, 1853 (Neuroptera: Myrmeleontidae, Ascalaphinae). Fragm. Entomol. 2021, 53, 1–8. [Google Scholar] [CrossRef]
- Lin, Y.H.; Liao, J.R.; Ko, C.C. Larval morphology of pit-building antlions of the tribe Myrmeleontini (Neuroptera, Myrmeleontidae) from Taiwan. Zool. Stud. 2021, 60, 39. [Google Scholar] [CrossRef]
- Lehnert, M.S.; Lanba, A.; Reiter, K.E.; Fonseca, R.J.; Minninger, J.; Hall, B.; Huff, W. Mouthpart adaptations of antlion larvae facilitate prey handling and fluid feeding in sandy habitats. J. Exp. Biol. 2022, 225, jeb244220. [Google Scholar] [CrossRef]
- Acevedo Ramos, F.; Monserrat, V.J. Setae and sensilla in the Iberian Myrmeleon Linnaeus, 1767 larvae (Insecta, Neuroptera: Myrmeleontidae). Rev. Brasil. Entomol. 2022, 66, e20220066. [Google Scholar] [CrossRef]
- Hévin, N.M.C.; Kergoat, G.J.; Clamens, A.L.; Le Ru, B.; Mansell, M.W.; Michel, B. Evolution, systematics and historical biogeography of Palparini and Palparidiini antlions (Neuroptera: Myrmeleontidae): Old origin and in situ diversification in Southern Africa. Syst. Entomol. 2023, 48, 600–617. [Google Scholar] [CrossRef]
- Froggatt, W.W. Australian Insects; William Brooks & Company Ltd.: Sydney, Australia, 1907; pp. 1–449. [Google Scholar]
- Tillyard, R.J. The Insects of Australia and New Zealand; Angus and Robertson: Sydney, Australia, 1926; pp. 1–560. [Google Scholar]
- Ross, E.S. Insects Close Up: A Pictorial Guide for the Photographer and Collector Featuring 125 Photographs and Drawings; University of California Press: Los Angeles, CA, USA, 1953; pp. 1–80. [Google Scholar]
- Weidner, H. Einige interessante Insektenlarven aus der Bernsteininklusen-Sammlung des Geologischen Staatsinstituts Hamburg (Odonata, Coleoptera, Megaloptera, Planipennia). Mitt. Geol. Staatsinst. Hamburg 1958, 27, 50–68. [Google Scholar]
- New, T.R. The larva of Nymphes Leach (Neuroptera: Nymphidae). Neuropt. Internat. 1982, 2, 79–84. [Google Scholar]
- New, T.R. Some early stages of Osmylops (Neuroptera: Nymphidae). Syst. Entomol. 1983, 8, 121–126. [Google Scholar] [CrossRef]
- Gepp, J. Erforschungsstand der Neuropteren. Larven der Erde (mit einem Schlüssel zur Larvaldiagnose der Familien, einer Übersicht von 340 Beschriebenen Larven und 600 Literaturzitaten). In Progress in World’s Neuropteroloy; J. Gepp: Melrose Park, IL, USA, 1984; pp. 183–239. Available online: https://www.zobodat.at/pdf/MONO-ENT-NEURO_MEN1_0183-0239.pdf (accessed on 6 December 2023).
- New, T.R.; Lambkin, K.J. The larva of Norfolius (Neuroptera: Nymphidae). Syst. Entomol. 1989, 14, 93–98. [Google Scholar] [CrossRef]
- Jefferies, R.P.S. The origin of chordates: A methodological essay. In The Origin of Major Invertebrate Groups; House, M.R., Ed.; Systematics Association Special; Academic Press: London, UK, 1979; Volume 12, pp. 443–447. [Google Scholar]
- Donoghue, P.C. Saving the stem group—A contradiction in terms? Paleobiology 2005, 31, 553–558. [Google Scholar] [CrossRef]
- Kuhl, F.P.; Giardina, C.R. Elliptic Fourier features of a closed contour. Comput. Graph. Image Process. 1982, 18, 236–258. [Google Scholar] [CrossRef]
- Bonhomme, V.; Picq, S.; Gaucherel, C.; Claude, J. Momocs: Outline Analysis—Using R. J. Stat. Softw. 2014, 56, 1–24. [Google Scholar] [CrossRef]
- Braig, F.; Haug, J.T.; Schädel, M.; Haug, C. A new thylacocephalan crustacean from the Upper Jurassic lithographic limestones of southern Germany and the diversity of Thylacocephala. Palaeodiversity 2019, 12, 69–87. [Google Scholar] [CrossRef][Green Version]
- Braig, F.; Haug, C.; Haug, J.T. Phenotypic variability in the shield morphology of wild- vs. lab-reared eumalacostracan larvae. Nauplius 2023, 31, e2023004. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 30 October 2023).
- Iwata, H.; Ukai, Y. SHAPE: A computer program package for quantitative evaluation of biological shapes based on elliptic Fourier descriptors. J. Hered. 2002, 93, 384–385. [Google Scholar] [CrossRef] [PubMed]
- Guillerme, T. disparity: A modular R package for measuring disparity. Meth. Ecol. Evol. 2018, 9, 1755–1763. [Google Scholar] [CrossRef]
- Guillerme, T.; Puttick, M.N.; Marcy, A.E.; Weisbecker, V. Shifting spaces: Which disparity or dissimilarity measurement best summarize occupancy in multidimensional spaces? Ecol. Evol. 2020, 10, 7261–7275. [Google Scholar] [CrossRef]
- Hörnig, M.K.; Haug, C.; Müller, P.; Haug, J.T. Not quite social—Possible cases of gregarious behaviour of immatures of various lineages of Insecta preserved in 100-million-year-old amber. Bull. Geosci. 2022, 97, 69–87. [Google Scholar] [CrossRef]
- Henry, C.S. Eggs and rapagula of Ululodes and Ascaloptynx (Neuroptera: Ascalaphidae): A comparative study. Psyche 1972, 79, 1–22. [Google Scholar] [CrossRef]
- Labandeira, C.C.; Yang, Q.; Santiago-Blay, J.A.; Hotton, C.L.; Monteiro, A.; Wang, Y.-J.; Goreva, Y.; Shih, C.K.; Siljeström, S.; Rose, T.R.; et al. The evolutionary convergence of mid-Mesozoic lacewings and Cenozoic butterflies. Proc. R. Soc. B Biol. Sci. 2016, 283, 20152893. [Google Scholar] [CrossRef]
- Baranov, V.A.; Schädel, M.; Haug, J.T. Fly palaeo-evo-devo: Immature stages of bibionomorphan dipterans in Baltic and Bitterfeld amber. PeerJ 2019, 7, e7843. [Google Scholar] [CrossRef] [PubMed]
- Haug, C.; Pérez-de la Fuente, R.; Baranov, V.; Haug, G.T.; Kiesmüller, C.; Zippel, A.; Hörnig, M.K.; Haug, J.T. The first fossil record of a mantis lacewing pupa, and a review of pupae in Mantispidae and their evolutionary significance. Riv. Ital. Paleoentol. Strat. 2023, 129, 185–205. [Google Scholar] [CrossRef] [PubMed]
- Haug, C.; Haug, G.T.; Kiesmüller, C.; Haug, J.T. Convergent evolution and convergent loss in the grasping structures of immature earwigs and aphidlion-like larvae as demonstrated by about 100-million-year-old fossils. Swiss J. Palaeonetol. 2023, 142, 21. [Google Scholar] [CrossRef]
- Tuculescu, R.; Topoff, H.; Wolfe, S. Mechanisms of pit construction by antlion larvae. Ann. Entomol. Soc. Am. 1975, 68, 719–720. [Google Scholar] [CrossRef]
- Lucas, J.R. The biophysics of pit construction by antlion larvae (Myrmeleon, Neuroptera). Anim. Behav. 1982, 30, 651–664. [Google Scholar] [CrossRef]
- Hauber, M.E. Variation in pit size of antlion (Myrmeleon carolinus) larvae: The importance of pit construction. Physiol. Entomol. 1999, 24, 37–40. [Google Scholar] [CrossRef]
- Scharf, I.; Ovadia, O. Factors influencing site abandonment and site selection in a sit-and-wait predator: A review of pit-building antlion larvae. J. Insect Behav. 2006, 19, 197–218. [Google Scholar] [CrossRef]
- Humeau, A.; Rougé, J.; Casas, J. Optimal range of prey size for antlions. Ecol. Entomol. 2015, 40, 776–781. [Google Scholar] [CrossRef]
- Badano, D.; Pantaleoni, R.A. The larvae of European Myrmeleontidae (Neuroptera). Zootaxa 2014, 3762, 1–71. [Google Scholar] [CrossRef]
- Badano, D.; Makris, C.; John, E.; Hadjiconstantis, M.; Sparrow, D.; Sparrow, R.; Thomas, B.; Devetak, D. The antlions of Cyprus: Review and new reports (Neuroptera: Myrmeleontidae). Fragm. Entomol. 2018, 50, 95–102. [Google Scholar] [CrossRef]
- Tauber, C.A.; Tauber, M.J. An unusual chrysopid larva: Identification, description, and taxonomic implications. Ann. Entomol. Soc. Am. 2013, 106, 729–740. [Google Scholar] [CrossRef]
- Tauber, C.A.; DeLeón, T.; Lopez Arroyo, J.I.; Tauber, M.J. Ceraeochrysa placita (Neuroptera: Chrysopidae): Generic characteristics of larvae, larval descriptions, and life cycle. Ann. Entomol. Soc. Am. 1998, 91, 608–618. [Google Scholar] [CrossRef]
- Tauber, C.A.; Tauber, M.J.; Albuquerque, G.S. Debris-carrying in larval Chrysopidae: Unraveling its evolutionary history. Ann. Entomol. Soc. Am. 2014, 107, 295–314. [Google Scholar] [CrossRef]
- Naeem, S. Species redundancy and ecosystem reliability. Conserv. Biol. 1998, 12, 39–45. [Google Scholar] [CrossRef]
- Kang, S.; Ma, W.; Li, F.Y.; Zhang, Q.; Niu, J.; Ding, Y.; Han, F.; Sun, X. Functional redundancy instead of species redundancy determines community stability in a typical steppe of Inner Mongolia. PLoS ONE 2015, 10, e0145605. [Google Scholar] [CrossRef] [PubMed]
- Morelli, F.; Tryjanowski, P. The dark side of the “redundancy hypothesis” and ecosystem assessment. Ecol. Complex. 2016, 28, 222–229. [Google Scholar] [CrossRef]
- Liu, H.; Beutel, R.G.; Makarov, K.V.; Jarzembowski, E.A.; Xiao, C.; Luo, C. The first larval record of Migadopinae (Coleoptera: Adephaga: Carabidae) from mid-Cretaceous Kachin amber, northern Myanmar. Cretac. Res. 2023, 142, 105413. [Google Scholar] [CrossRef]
- Liu, H.; Makarov, K.V.; Jarzembowski, E.A.; Xiao, C.; Luo, C. Cretoloricera electra gen. et sp. nov., the oldest record of Loricerini (Coleoptera: Adephaga: Carabidae: Loricerinae) from mid-Cretaceous Kachin amber. Cretac. Res. 2023, 148, 105540. [Google Scholar] [CrossRef]
- Rosová, K.; Prokop, J.; Hammel, J.U.; Beutel, R.G. The earliest evidence of Omophroninae (Coleoptera: Carabidae) from mid-Cretaceous Kachin amber and the description of a larva of a new genus. Arthropod Syst. Phyl. 2023, 81, 689–704. [Google Scholar] [CrossRef]

































Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braig, F.; Popp, T.; Zippel, A.; Haug, G.T.; Linhart, S.; Müller, P.; Weiterschan, T.; Haug, J.T.; Haug, C. The Diversity of Larvae with Multi-Toothed Stylets from About 100 Million Years Ago Illuminates the Early Diversification of Antlion-like Lacewings. Diversity 2023, 15, 1219. https://doi.org/10.3390/d15121219
Braig F, Popp T, Zippel A, Haug GT, Linhart S, Müller P, Weiterschan T, Haug JT, Haug C. The Diversity of Larvae with Multi-Toothed Stylets from About 100 Million Years Ago Illuminates the Early Diversification of Antlion-like Lacewings. Diversity. 2023; 15(12):1219. https://doi.org/10.3390/d15121219
Chicago/Turabian StyleBraig, Florian, Timo Popp, Ana Zippel, Gideon T. Haug, Simon Linhart, Patrick Müller, Thomas Weiterschan, Joachim T. Haug, and Carolin Haug. 2023. "The Diversity of Larvae with Multi-Toothed Stylets from About 100 Million Years Ago Illuminates the Early Diversification of Antlion-like Lacewings" Diversity 15, no. 12: 1219. https://doi.org/10.3390/d15121219
APA StyleBraig, F., Popp, T., Zippel, A., Haug, G. T., Linhart, S., Müller, P., Weiterschan, T., Haug, J. T., & Haug, C. (2023). The Diversity of Larvae with Multi-Toothed Stylets from About 100 Million Years Ago Illuminates the Early Diversification of Antlion-like Lacewings. Diversity, 15(12), 1219. https://doi.org/10.3390/d15121219





