Identification of Floral Resources Used by the Stingless Bee Melipona beecheii for Honey Production in Different Regions of the State of Campeche, Mexico
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sample Collection Sites
2.3. Palynological Analysis
2.4. Honey Botanical Origin
2.5. Ecological Analysis
3. Results
3.1. Honey Botanical Origin
3.1.1. Southeast
3.1.2. Northeast
3.1.3. West
3.1.4. North
3.2. Ecological Parameters
3.3. Principal Component Analysis (PCA)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grüter, C. Stingless Bees; Springer International Publishing: Cham, Switzerland, 2020; Volume 109, pp. 1182–1186. [Google Scholar]
- Bueno, F.G.B.; Kendall, L.; Alves, D.A.; Tamara, M.L.; Heard, T.; Latty, T.; Gloag, R. Stingless bee floral visitation in the global tropics and subtropics. Glob. Ecol. Conserv. 2023, 43, e02454. [Google Scholar] [CrossRef]
- Donkersley, P.; Rhodes, G.; Pickup, R.W.; Jones, K.C.; Wilson, K. Honeybee Nutrition Is Linked to Landscape Composition. Ecol. Evol. 2014, 4, 4195–4206. [Google Scholar] [CrossRef] [PubMed]
- Roulston, T.A.H.; Cane, J.H. The effect of diet breadth and nesting ecology on body size variation in bees (Apiformes). J. Kans. Entomol. Soc. 2000, 73, 129–142. [Google Scholar]
- Toledo-Hernández, E.; Peña-Chora, G.; Hernández-Velázquez, V.M.; Lormendez, C.C.; Toribio-Jiménez, J.; Romero-Ramírez, Y.; Léon-Rodríguez, R. The stingless bees (Hymenoptera: Apidae: Meliponini): A review of the current threats to their survival. Apidologie 2022, 53, 8. [Google Scholar] [CrossRef]
- Araújo, E.D.; Costa, M.; Chaud-Netto, J.; Fowler, H.G. Body size and flight distance in stingless bees (Hymenoptera: Meliponini): Inference of flight range and possible ecological implications. Braz. J. Biol. 2004, 64, 563–568. [Google Scholar] [CrossRef]
- Cab-Baqueiro, S.; Ferrera-Cerrato, R.; Quezada-Euán, J.J.; Moo-Valle, H.; Vargas-Díaz, A.A. Nesting substrates and nest density of stingless bees in the Petenes Biosphere Reserve, Mexico. Acta Biol. Colomb. 2022, 27, 61–69. [Google Scholar]
- Requier, F.; Leonhardt, S.D. Beyond flowers: Including non-floral resources in bee conservation schemes. J. Insect Conserv. 2020, 24, 5–16. [Google Scholar] [CrossRef]
- Cairns, C.E.; Villanueva-Gutiérrez, R.; Koptur, S.; Bray, D.B. Bee populations, forest disturbance, and Africanization in Mexico. Biotropica 2005, 37, 686–692. [Google Scholar] [CrossRef]
- Ellis, E.A.; Hernández-Gómez, I.U.; Romero-Montero, J.A. Processes and causes of forest cover change in the Yucatan Peninsula. Ecosistemas 2017, 26, 101–111. [Google Scholar] [CrossRef]
- Villanueva-G, R.; Roubik, D.W.; Colli-Ucán, W. Extinction of Melipona beecheii and traditional beekeeping in the Yucatán peninsula. Bee World 2005, 86, 35–41. [Google Scholar] [CrossRef]
- Moo-Huchin, V.M.; Sauri-Duch, E.; Moo-Huchin, M.; Estrada-León, R.J.; Estrada-Mota, I.; Pérez-Pacheco, E. Antioxidant Activity of stingless bee honey. In Stingless Bee’s Honey from Yucatán: Culture, Traditional Uses and Nutraceutical Potential; Ortiz-Vázquez, E.L., Ruiz Ruiz, J.C., Magaña Ortiz, D.I., Ramon Sierra, J.M., Eds.; Nova Science Publishers: New York, NY, USA, 2016; pp. 77–90. [Google Scholar]
- Pat-Fernández, L.A.; Anguebes Franceschi, F.; Pat Fernández, J.M.; Hernández-Bahena, P.; Ramos-Reyes, R. Condition and perspectives of meliponiculture in mayan communities at Los Petenes biosphere reserve in Campeche, Mexico. Estud. Cult. Maya 2018, 52, 227–254. [Google Scholar] [CrossRef]
- Quezada-Euán, J.J.G. Taxonomy and diversity of the stingless bees. In Stingless Bees of Mexico; Quezada-Euán, J.J.G., Ed.; Springer: Cham, Switserland, 2018; pp. 1–40. [Google Scholar]
- Antonini, Y.; Martins, P.R. The value of tree species (Caryocar brasiliense) for a stingless bee Melipona quadrifasciata quarifaciata. J. Insect Conserv. 2003, 7, 167–174. [Google Scholar] [CrossRef]
- Van Drunen, S.G.; Linton, J.E.; Kuwahara, G.; Norris, D.R. Flower plantings promote insect pollinator abundance and wild bee richness in Canadian agricultural landscapes. J. Insect Conserv. 2022, 26, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, J.T.F.; Costa, L.; Zappi, D.C.; Batista, W.F., Jr.; da Lopes, K.S.; de Alves, R.C.O.; de Romeiro, L.A.; da Silva, E.F.; Carreira, L.M.M.; Rodrigues, T.M.; et al. Melissopalynology reveals the foraging preferences of the stingless bee Melipona seminigra pernigra Moure & Kerr 1950 (Apidae: Meliponini) in cangas of Serra dos Carajás, southeastern Amazonia. Biota Neotrop. 2021, 21, e20201004. [Google Scholar]
- Leal-Ramos, D.A.; León-Sánchez, D.L.E. Antagonism of Apis mellifera and Melipona beecheii for the sources of feeding. Rev. Cub. Cienc. For. 2013, 1, 102–109. [Google Scholar]
- Alvarez-Suarez, J.M.; Giampieri, F.; Brenciani, A.; Mazzoni, L.; Gasparrini, M.; González-Paramás, A.M.; Battino, M. Apis melifera vs. Melipona beecheii cuban polifloral honeys: A comparison based on their physicochemical parameters, chemical composition and biological properties. LWT-Food Sci. Technol. 2018, 87, 272–279. [Google Scholar]
- Villanueva-Gutiérrez, R.; Roubik, D.W.; Colli-Ucán, W.; Tuz-Novelo, M. The value of plants for the mayan stingless honey bee Melipona beecheii (Apidae: Meliponini): A pollen-based study in the Yucatán peninsula, Mexico. In Pot-Pollen in Stingless Bee Melittology; Vit, P., Pedro, S., Roubik, D., Eds.; Springer: Cham, Switzerland, 2018; pp. 67–76. [Google Scholar]
- Sánchez-Chino, X.M.; Jiménez-Martínez, C.; Ramírez-Arriaga, E.; Martínez-Herrera, J.; Corzo-Rios, L.J.; Godínez, G.L.M. Antioxidant and metal chelating activities of honeys of Melipona beecheii and Frieseomelitta nigra from Tabasco, Mexico. Tip Rev. Espec. Cienc. Quím. Biol. 2019, 22, 1–7. [Google Scholar]
- López-Roblero, E.; Espinosa, C.T.; López, J.A.G.; Grajales, J.C.; Quiroz-García, D.L. Floral resources collected by four native bees species in southern Mexico. Grana 2021, 60, 57–68. [Google Scholar] [CrossRef]
- Ramírez-Arriaga, E.; Pacheco-Palomo, K.G.; Moguel-Ordoñez, Y.B.; Zepeda García Moreno, M.; Godínez-García, L.M. Angiosperm resources for stingless bees (Apidae, Meliponini): A pot-pollen melittopalynological study in the Gulf of Mexico. In Pot-Pollen in Stingless Bee Melittology; Vit, P., Pedro, S., Roubik, D., Eds.; Springer: Cham, Switzerland, 2018; pp. 111–130. [Google Scholar]
- CONABIO. Portal de Información Geográfica—CONABIO. Available online: http://www.conabio.gob.mx/informacion/gis/ (accessed on 7 September 2023).
- Jones, G.D.; Bryant, V.M., Jr. The use of ETOH for the dilution of honey. Grana 2004, 43, 174–182. [Google Scholar] [CrossRef]
- Erdtman, G. The acetolysis method a revised description. Sven. Bot. Tidskr. 1960, 54, 561–564. [Google Scholar]
- Kisser, J. Bemerkungen zum Einschluss in Glycerin-Gelatine. Z Wiss Mikrosk Mikrosk Tecch 1935, 51, 372–374. [Google Scholar]
- Alfaro-Bates, R.G.; González-Acereto, J.A.; Ortiz-Díaz, J.J.; Viera-Castro, F.A.; Burgos-Pérez, A.I.; Martínez-Hernández, E.; Ramírez-Arriaga, E. Caracterización Palinológica de las Mieles de la Península de Yucatán, 1st ed.; Universidad Autónoma de Yucatán: Mérida, Mexico, 2010; 156p. [Google Scholar]
- Roubik, D.W.; Moreno, P.J.E. Pollen and Spores of Barro Colorado Island. Kew Bull. 1991, 47, 791. [Google Scholar] [CrossRef]
- Sánchez-Dzib, Y.D.L.A.; Sosa-Nájera, S.; Lozano-García, M.D.S. Pollen morphology of species from the tropical forest of the Candelaria River Basin, Campeche. Bol. Soc. Bot. 2009, 84, 83–104. [Google Scholar]
- Louveaux, J.; Maurizio, A.; Vorwohl, G. Methods of melissopalynology. Bee World 1978, 59, 139–157. [Google Scholar] [CrossRef]
- Margalef, R. Homage to Evelyn Hutchison, or why is there an upper limit to diversity. Trans. Conn. Acad. Arts. Sci. 1972, 44, 211–235. [Google Scholar]
- Simpson, E.H. Measurement of diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- da Luz, C.F.P.; Fidalgo, A.O.; Silva, S.A.; Rodríguez, S.S.; Nocelli, R.C.F. Comparative floral preferences in nectar and pollen foraging by Scaptotrigona postica (Latreille 1807) in two different biomes in São Paulo (Brazil). Grana 2019, 58, 200–226. [Google Scholar] [CrossRef]
- Hanifa, H.S.; Sartiami, D.; Priawandiputra, W.; Buchori, D. Characteristics of apiculture and meliponiculture in Banten province, Indonesia: Profile of beekeepers, bee and pollen diversity. IOP Conf. Ser. Environ. Earth Sci. 2021, 948, 12050. [Google Scholar] [CrossRef]
- Pinto, R.S.; Silva, A.G.; Rêgo, M.M.C.; Albuquerque, P.M.C. Pollen analysis of the post-emergence residue of Euglossa bees (Apidae: Euglossini) nesting in an urban fragment. Sociobiology 2019, 66, 88–96. [Google Scholar] [CrossRef]
- Ramalho, M.; Kleinert-Giovannini, A.; Imperatriz-Fonseca, V.L. Important bee plants for stingless bees (Melipona and Trigonini) and africanized honeybees (Apis mellifera) in neotropical habitats: A review. Apidologie 1990, 21, 469–488. [Google Scholar] [CrossRef]
- Carvalho, C.A.L.; Moreti, A.D.C.; Marchini, L.C.; Alves, R.D.O.; Oliveira, P.C.F. Pollen spectrum of honey of “Uruçu” bee (Melipona scutellaris Latreille, 1811). Rev. Bras. Biol. 2001, 61, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.C.L.; Rêgo, M.M.C.; Carreira, L.M.M.; Albuquerque, P.M.C. Pollen spectrum of honey of Tiúba (Melipona fasciculata Smith, 1854, Hymenoptera, Apidae). Acta Amaz. 2011, 41, 183–190. [Google Scholar] [CrossRef]
- Matos, V.R.; Santos, F.A.R. Pollen in honey of Melipona scutellaris L. (Hymenoptera: Apidae) in an Atlantic rainforest area in Bahia, Brazil. Palynology 2017, 41, 144–156. [Google Scholar] [CrossRef]
- Espinoza-Toledo, C.; Vázquez-Ovando, A.; Santos, R.T.D.L.; López-García, A.; Albores-Flores, V.; Grajales-Conesa, J. Stingless bee honeys from Soconusco, Chiapas: A complementary approach. Rev. Biol. Trop. 2018, 66, 1536–1546. [Google Scholar] [CrossRef]
- de Novais, J.S.; Absy, M.L. Melissopalynological records of honeys from Tetragonisca angustula (Latreille, 1811) in the lower amazon, Brazil: Pollen spectra and concentration. J. Apic. Res. 2015, 54, 11–29. [Google Scholar] [CrossRef]
- Ahmad, F.; Anwar, F.; Hira, S. Review on medicinal importance of fabaceae family. Pharmacologyonline 2016, 3, 151–157. [Google Scholar]
- Villaseñor, J.L. Diversidad y distribución de las Magnoliophuya de Mexico. Interciencia 2003, 28, 160–167. [Google Scholar]
- Villanueva-Gutiérrez, R.; Moguel-Ordóñez, Y.B.; Echazarreta, G.C.M.; Arana, L.G. Monofloral honeys in the Yucatán Peninsula, Mexico. Grana 2009, 48, 214–223. [Google Scholar] [CrossRef]
- Báez, C.G.; Zamora-Crescencio, P.; Hernández-Mundo, S.C. Structure and floristic composition of deciduous forest of Mucuychacan, Campeche, Mexico. For. Veracruzana 2012, 14, 9–16. [Google Scholar]
- Zamora-Crescencio, P.; Domínguez-Carrasco, M.D.R.; Villegas, P.; Gutiérrez-Báez, C.; Manzanero-Acevedo, L.A.; Ortega-Haas, J.J.; Puc-Chávez, R. Floristic composition and structure of the secondary vegetation in northern Campeche, Mexico. Bol. Soc. Bot. Mex. 2011, 89, 27–35. [Google Scholar]
- Carreón-Santos, R.J.; Valdez-Hernández, J.I. Estructura y diversidad arbórea de vegetación secundaria derivada de una selva mediana subperennifolia en Quintana Roo. Rev. Chapingo Ser. Cienc. For. Ambiente 2014, 20, 119–130. [Google Scholar]
- Zamora, L.G.; Beukelman, K.; van den Berg, B.; Arias, M.L.; Umaña, E.; Aguilar, I.; Gross, N. The antimicrobial activity and microbiological safety of stingless bee honeys from Costa Rica. J. Apic. Res. 2014, 53, 503–513. [Google Scholar] [CrossRef]
- Bohn, J.L.; Diemont, S.A.; Gibbs, J.P.; Stehman, S.V.; Vega, J.M. Implications of mayan agroforestry for biodiversity conservation in the Calakmul biosphere reserve, Mexico. Agrofor. Syst. 2014, 88, 269–285. [Google Scholar] [CrossRef]
- Martínez-Vásquez, E.; Vázquez-Garcia, V. Impacto f the transgenic soy expansión on corn and honey production in Campeche, Mexico. Rev. Latinoam. Estud. Socioamb. 2019, 26, 173–190. [Google Scholar]
- Roubik, D.W.; Villanueva-Gutierrez, R. Invasive africanized honey bee impact on native solitary bees: A pollen resource and trap nest analysis. Biol. J. Linn. Soc. 2009, 98, 152–160. [Google Scholar] [CrossRef]
- Momose, K.; Yumoto, T.; Nagamitsu, T.; Kato, M.; Nagamasu, H.; Sakai, S.; Harrison, R.; Itioka, T.; Hamid, A.; Inoue, T. Pollination biology in a lowland dipterocarp forest in Sarawak, Malaysia. Characteristics of the plant-pollinator community in a lowland dipterocarp forest. Am. J. Bot. 1998, 85, 1477–1501. [Google Scholar] [CrossRef]
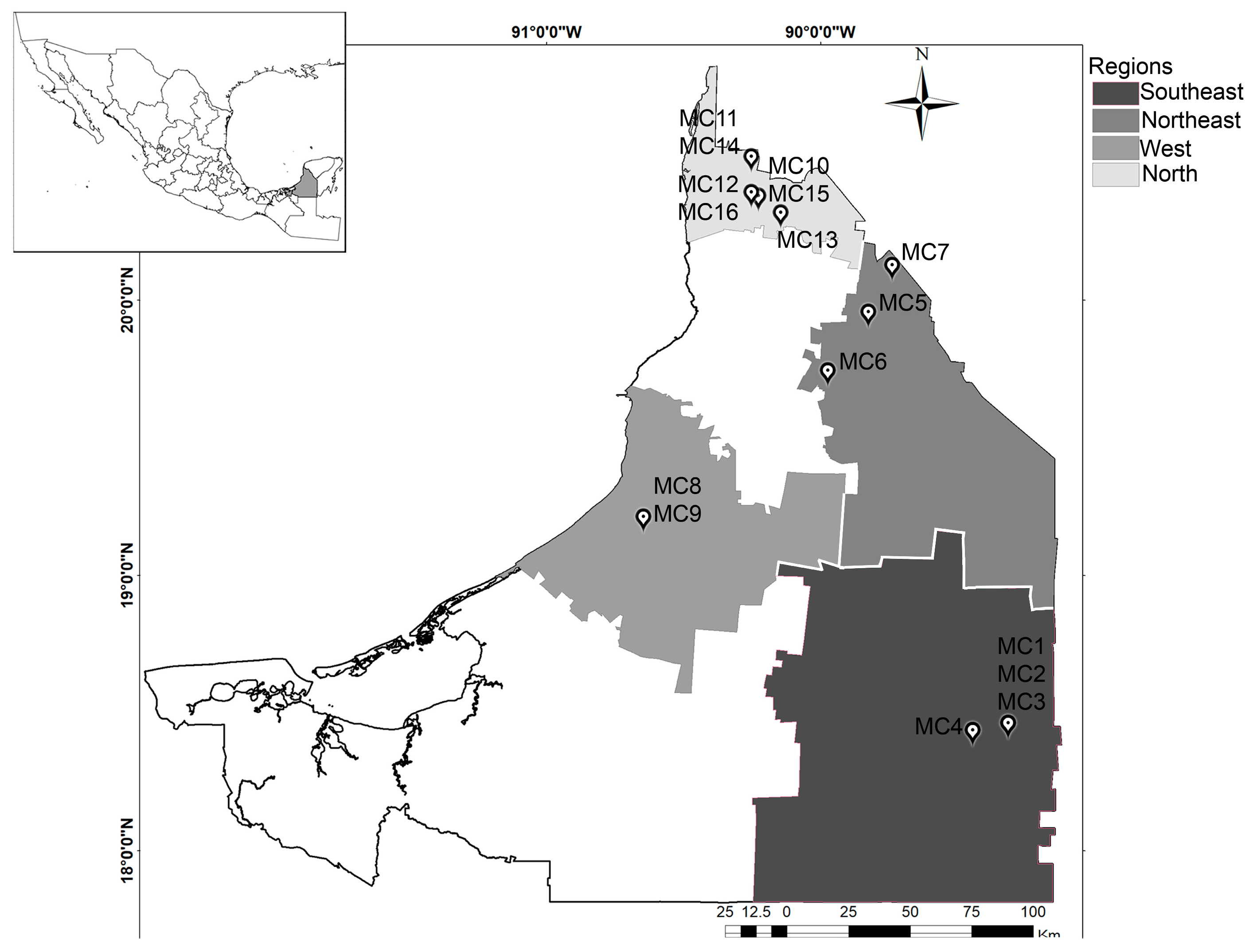




| Regions | Locality | Sample | Vegetation Type |
|---|---|---|---|
| Southeast | |||
| 20 de Noviembre, Calakmul | MC1 | Medium sub-evergreen forest | |
| 20 de Noviembre, Calakmul | MC2 | Medium sub-evergreen forest | |
| 20 de Noviembre, Calakmul | MC3 | Medium sub-evergreen forest | |
| La lucha I, Calakmul | MC4 | Medium sub-evergreen forest | |
| Northeast | |||
| Xcalot Akal, Hopelchén | MC5 | Medium sub-deciduous forest | |
| Ich ek, Hopelchén | MC6 | Medium sub-deciduous forest | |
| San Antonio, Hopelchén | MC7 | Medium sub-deciduous forest | |
| West | |||
| Sihochac, Champotón | MC8 | Medium sub-deciduous forest | |
| Sihochac, Champotón | MC9 | Medium sub-deciduous forest | |
| North | |||
| Pucnachén, Calkiní | MC10 | Medium deciduous forest | |
| Tankuché, Calkiní | MC11 | Medium deciduous forest | |
| Santa María, Calkiní | MC12 | Medium deciduous forest | |
| Sahcabchén, Calkiní | MC13 | Medium deciduous forest | |
| Tankunché, Calkiní | MC14 | Medium deciduous forest | |
| Pucnachén, Calkiní | MC15 | Medium deciduous forest | |
| Santa María, Calkiní | MC16 | Medium deciduous forest |
| Taxa | Mayan Name | Stratum | MC1 | MC2 | MC3 | MC4 | MC5 | MC6 | MC7 | MC8 | MC9 | MC10 | MC11 | MC12 | MC13 | MC14 | MC15 | MC16 | PR (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acanthaceae | |||||||||||||||||||
| Avicennia germinans | Ta’abché | A | 1 | 0.2 | 13 | ||||||||||||||
| Amaranthaceae | |||||||||||||||||||
| Alternanthera ramosissima | Zakmuul | H | 11.2 | 0.8 | 13 | ||||||||||||||
| Asteraceae | |||||||||||||||||||
| Asteraceae sp. | H | 2.3 | 6 | ||||||||||||||||
| Chaptalia nutans | H | 0.6 | 6 | ||||||||||||||||
| Viguiera dentata | Taj (tajonal) | H | 7.2 | 18.5 | 0.8 | 18.7 | 1.8 | 0.2 | 38 | ||||||||||
| Boraginaceae | |||||||||||||||||||
| Boraginaceae | 24 | 6 | |||||||||||||||||
| Brassicaceae | |||||||||||||||||||
| Arabideae sp. | H | 4 | 0.2 | 13 | |||||||||||||||
| Burseraceae | |||||||||||||||||||
| Bursera simaruba | Chakaj | A | 3.4 | 50 | 0.2 | 2.4 | 4.9 | 5.1 | 5.5 | 0.9 | 35.4 | 20.9 | 0.2 | 18.6 | 2.6 | 7.4 | 88 | ||
| Protium copal | Sak chakaj | A | 9 | 0.2 | 2 | 19 | |||||||||||||
| Cactaceae | |||||||||||||||||||
| Cactaceae sp. | H | 0.2 | 6 | ||||||||||||||||
| Combretaceae | |||||||||||||||||||
| Bucida buceras | Pucté | A | 0.8 | 6 | |||||||||||||||
| Convulvulaceae | |||||||||||||||||||
| Ipomea sp. | H | 0.2 | 6 | ||||||||||||||||
| Cyclanthaceae | |||||||||||||||||||
| Carludovica palmata | Guano | H | 0.4 | 6 | |||||||||||||||
| Euphorbiaceae | |||||||||||||||||||
| Croton sp. | S | 2.1 | 18.4 | 13 | |||||||||||||||
| Euphorbiaceae | 1.9 | 6 | |||||||||||||||||
| Fabaceae | |||||||||||||||||||
| Acacia sp. | S | 0.2 | 6 | ||||||||||||||||
| Acacia collinsii | Subin | S | 0.7 | 6 | |||||||||||||||
| Caesalpinia gaumeri | Kitim che’ | A | 2.2 | 0.6 | 13 | ||||||||||||||
| Gliricidia sepium | A | - | - | 6.6 | 3.4 | 13 | |||||||||||||
| Leucaena leucocephala | Waaxim | A | 3.7 | 1.5 | 2.6 | 2.9 | 4.2 | 9 | 2 | - | 44 | ||||||||
| Lonchocarpus longistylus | Baal che’ | A | 3.6 | 2.3 | 3.9 | 1.2 | 6.4 | 22 | 2.9 | - | 0.9 | 19.6 | 47.2 | 31.3 | 1.2 | 0.2 | 16.8 | 24.9 | 94 |
| Lonchocarpus xuul | K’an xu’ul | A | 1.2 | 0.6 | 13 | ||||||||||||||
| Mimosa bahamensis | Káatsim blanco | A | 1.9 | 4.5 | 1.2 | 1.3 | 1 | 6.8 | 27.5 | 0.9 | 0.4 | 0.9 | 5.7 | 3.2 | 26.6 | 81 | |||
| Mimosa pigra | Je’ beech’ | S | 25.6 | 6 | |||||||||||||||
| Mimosa pudica | Múuts’il xiiw | H | 0.4 | 4.2 | 0.4 | 0.4 | 1.4 | 1.3 | 0.6 | 7.8 | 50 | ||||||||
| Piscidia piscipula | Ja’abin | S | 20.6 | 78.1 | 24.7 | 26 | 1 | 0.2 | 2.2 | 0.9 | 4.2 | 4.9 | 47.3 | 3.2 | 10.1 | 81 | |||
| Senna atomaria | A | 4.9 | 6 | ||||||||||||||||
| Senna pallida | Ch’iilib mich | S | 6.4 | 27.9 | 58.8 | 15.4 | 3 | 31 | |||||||||||
| Senna racemosa | K’an lool | A | 10 | 27.9 | 6.9 | 13.6 | 27.6 | 66.7 | 26.8 | 40 | 12.1 | 0.2 | 1.7 | 15.8 | 1.7 | 19.4 | 88 | ||
| Senna villosa | Saal che’ | S | 5.9 | 62.1 | 6.3 | 19 | |||||||||||||
| Gesneriaceae | |||||||||||||||||||
| Achimenes palmata | H | 0.2 | 6 | ||||||||||||||||
| Malvaceae | |||||||||||||||||||
| Luehea speciosa | K’an kaat | A | 0.4 | 6 | |||||||||||||||
| Waltheria communis | H | 5.3 | 2.2 | 34.5 | 7.4 | 25 | |||||||||||||
| Waltheria rotundifolia | H | 1.6 | 1.8 | 1 | 19 | ||||||||||||||
| Meliaceae | |||||||||||||||||||
| Meliaceae sp. | 0.8 | 6 | |||||||||||||||||
| Myrtaceae | |||||||||||||||||||
| Eugenia axillaris | S | 6.5 | 6.6 | 7.3 | 6.3 | 0.9 | 2.2 | 0.7 | 1.7 | 0.4 | - | 0.8 | 0.6 | 0.6 | 75 | ||||
| Eugenia foetida | Sak loob | A | 1.5 | 6 | |||||||||||||||
| Pimienta dioica | Boox pool | A | 44.7 | 3.1 | 0.4 | 19 | |||||||||||||
| Psidium guajava | Pichi | S | 0.2 | 6 | |||||||||||||||
| Nictaginaceae | |||||||||||||||||||
| Pisonia aculeata | Béeb | H | 3.9 | 6 | |||||||||||||||
| Onagraceae | |||||||||||||||||||
| Ludwigia octovalvis | Máaskab che’ | H | 2.2 | 1.6 | 2.6 | 19 | |||||||||||||
| Poligoncaceae | |||||||||||||||||||
| Coccoloba manzanillensis | A | 4.7 | 0.6 | 13 | |||||||||||||||
| Neomillspaughia emarginata | Sak iitsa’ | S | 0.4 | 3.7 | 1.5 | 1 | 1.2 | 31 | |||||||||||
| Poligonaceae sp. | A | 0.3 | 17.6 | 1.7 | 19 | ||||||||||||||
| Primulaceae | |||||||||||||||||||
| Jacquinia aurantiaca | S | 0.4 | 6 | ||||||||||||||||
| Rubiaceae | |||||||||||||||||||
| Psychotria nervosa | K’aanan | S | 3.9 | 6 | |||||||||||||||
| Sapindaceae | |||||||||||||||||||
| Sapindacea sp. | H | 13.9 | 1 | 13 | |||||||||||||||
| Serjania goniocarpa | Chak sik’iix le’ | H | 2.4 | 0.8 | 13 | ||||||||||||||
| Serjania lundellii | buy aak’ | H | 0.8 | 2.2 | 13 | ||||||||||||||
| Thouinia paucidentata | k’an chuunup | A | 2.3 | 4.4 | 13 | ||||||||||||||
| Sapotaceae | |||||||||||||||||||
| Sideroxylon foetidissimum | Sibul | A | 1.4 | 6 | |||||||||||||||
| SOLANACEAE | |||||||||||||||||||
| Cestrum nocturnum | k’an chuunuk | S | 4.2 | 6 | |||||||||||||||
| Solanacea sp. | S | 12.7 | 65 | ||||||||||||||||
| Solanum lanceifolium | H | 1.1 | 6.4 | 13 | |||||||||||||||
| Solanum lanceolatum | Sikil múuch | S | 4.6 | 6 | |||||||||||||||
| Solanum nudum | Boox kúuts | S | - | 3.3 | 5.5 | 1.3 | 9 | ||||||||||||
| Solanum tridynamun | Kóon ya’ax iik | H | 4.6 | 6 | |||||||||||||||
| Unidentified sp.s | |||||||||||||||||||
| sp. 1 | 1.2 | 6 | |||||||||||||||||
| sp. 2 | 0.2 | 6 | |||||||||||||||||
| sp. 3 | 0.9 | 6 | |||||||||||||||||
| sp. 4 | 2.6 | 6 | |||||||||||||||||
| sp. 5 | 3.3 | 6 | |||||||||||||||||
| sp. 6 | 1.2 | 6 | |||||||||||||||||
| sp. 7 | 0.6 | 6 | |||||||||||||||||
| sp. 8 | 6.6 | 6 | |||||||||||||||||
| sp. 9 | 0.2 | 6 | |||||||||||||||||
| sp. 10 | 3.7 | 6 | |||||||||||||||||
| sp. 11 | 0.6 | 6 | |||||||||||||||||
| sp. 12 | 1.1 | 6 | |||||||||||||||||
| Total | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
León-Canul, R.A.; Chalé-Dzul, J.B.; Vargas-Díaz, A.A.; Ortiz-Díaz, J.J.; Durán-Escalante, K.C.; Carrillo-Ávila, E.; Santillán-Fernández, A. Identification of Floral Resources Used by the Stingless Bee Melipona beecheii for Honey Production in Different Regions of the State of Campeche, Mexico. Diversity 2023, 15, 1218. https://doi.org/10.3390/d15121218
León-Canul RA, Chalé-Dzul JB, Vargas-Díaz AA, Ortiz-Díaz JJ, Durán-Escalante KC, Carrillo-Ávila E, Santillán-Fernández A. Identification of Floral Resources Used by the Stingless Bee Melipona beecheii for Honey Production in Different Regions of the State of Campeche, Mexico. Diversity. 2023; 15(12):1218. https://doi.org/10.3390/d15121218
Chicago/Turabian StyleLeón-Canul, Román Alberto, Juan Bautista Chalé-Dzul, Arely Anayansi Vargas-Díaz, Juan Javier Ortiz-Díaz, Kelly Cristina Durán-Escalante, Eugenio Carrillo-Ávila, and Alberto Santillán-Fernández. 2023. "Identification of Floral Resources Used by the Stingless Bee Melipona beecheii for Honey Production in Different Regions of the State of Campeche, Mexico" Diversity 15, no. 12: 1218. https://doi.org/10.3390/d15121218
APA StyleLeón-Canul, R. A., Chalé-Dzul, J. B., Vargas-Díaz, A. A., Ortiz-Díaz, J. J., Durán-Escalante, K. C., Carrillo-Ávila, E., & Santillán-Fernández, A. (2023). Identification of Floral Resources Used by the Stingless Bee Melipona beecheii for Honey Production in Different Regions of the State of Campeche, Mexico. Diversity, 15(12), 1218. https://doi.org/10.3390/d15121218






