Amphipods in Mediterranean Marine and Anchialine Caves: New Data and Overview of Existing Knowledge
Abstract
1. Introduction
2. Materials and Methods
2.1. Amphipod Sampling
2.2. Literature Review
2.3. Ecological Characterization of the Species and Data Analysis
3. Results
3.1. Research Effort Overview
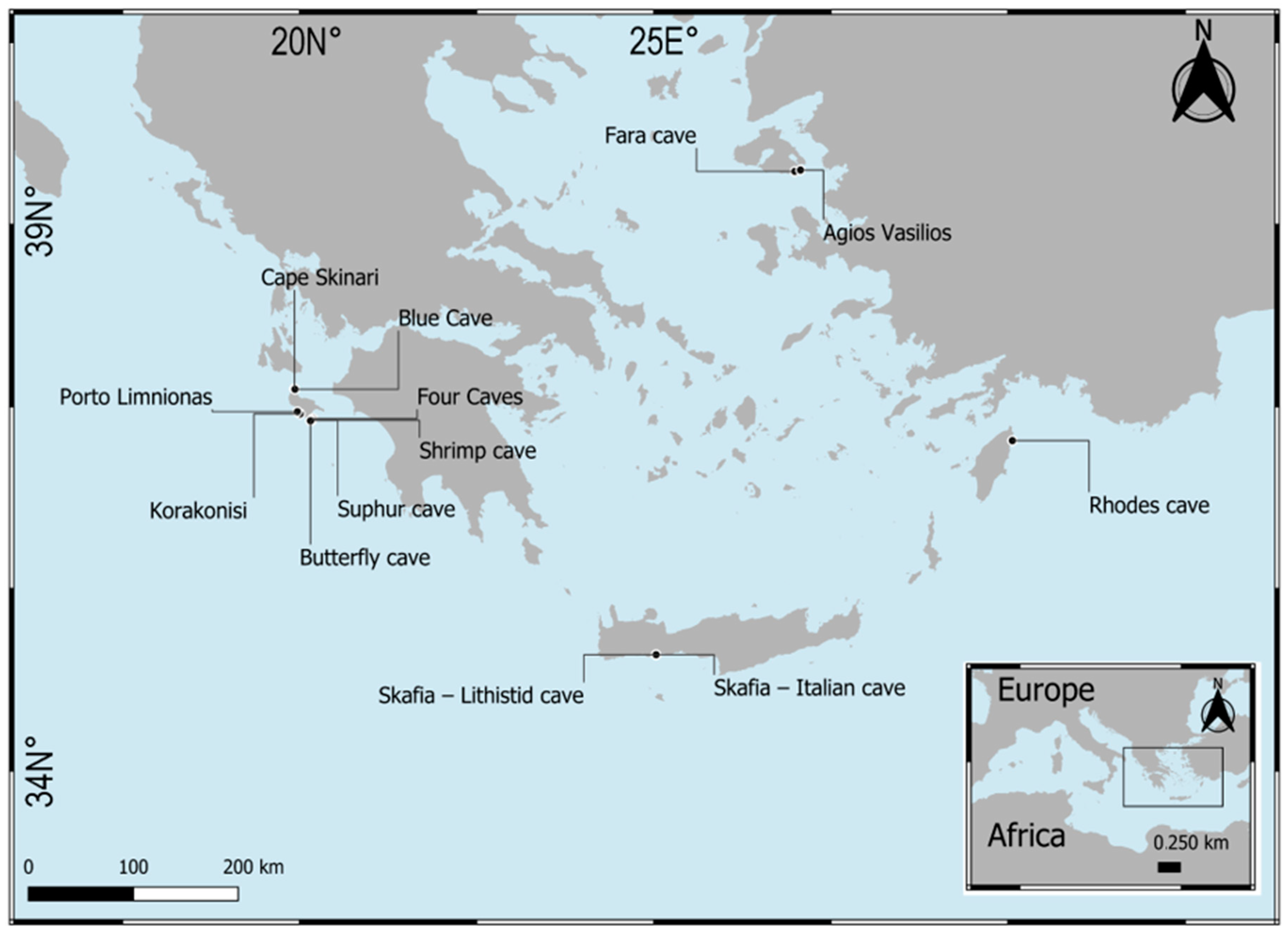
3.2. New Data from Marine Caves of the Eastern Mediterranean Sea
3.3. Amphipod Diversity in Mediterranean Marine Caves
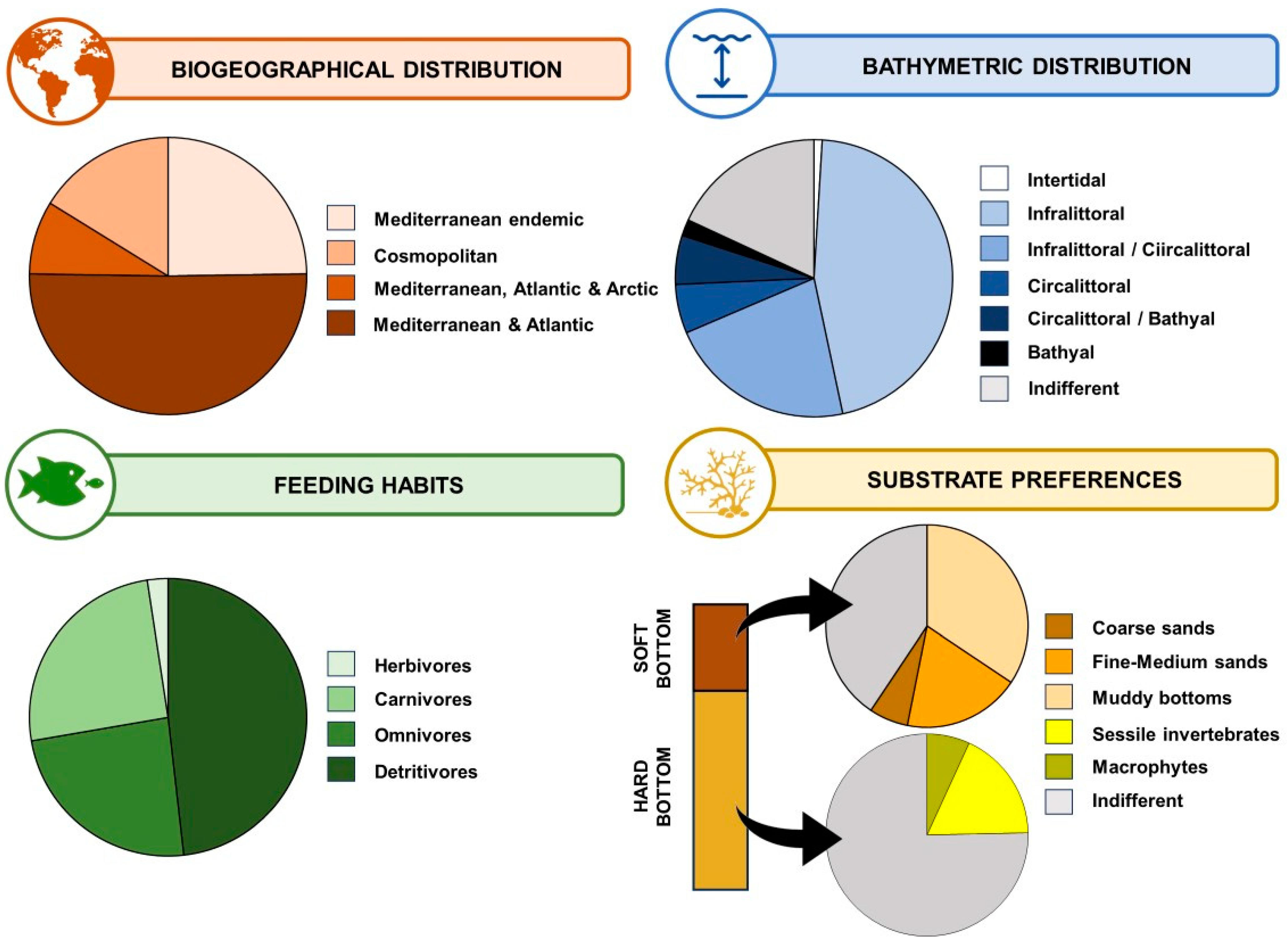
3.4. Regional, Zoogeographic and Ecological Patterns for Marine Cave Amphipods
3.5. Amphipod Diversity in Mediterranean Anchialine Caves
4. Discussion
4.1. Marine Caves
4.2. Anchialine Cave Fauna
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Y.Y.; Edgar, G.J.; Fox, R.J. The nature and ecological significance of epifaunal communities within marine ecosystems. Oceanogr. Mar. Biol. 2021, 59, 585–720. [Google Scholar]
- Taylor, R.B. Density, biomass and productivity of animals in four subtidal rocky reef habitats: The importance of small mobile invertebrates. Mar. Ecol. Prog. Ser. 1998, 172, 37–51. [Google Scholar] [CrossRef]
- Cowles, A.; Hewitt, J.E.; Taylor, R.B. Density, biomass and productivity of small mobile invertebrates in a wide range of coastal habitats. Mar. Ecol. Prog. Ser. 2009, 384, 175–185. [Google Scholar] [CrossRef]
- Fraser, K.M.; Lefcheck, J.S.; Ling, S.D.; Mellin, C.; Stuart-Smith, R.D.; Edgar, G.J. Production of mobile invertebrate communities on shallow reefs from temperate to tropical seas. Proc. R. Soc. B 2020, 287, 20201798. [Google Scholar] [CrossRef] [PubMed]
- Fraser, K.M.; Stuart-Smith, R.D.; Ling, S.D.; Heather, F.J.; Edgar, G.J. Taxonomic composition of mobile epifaunal invertebrate assemblages on diverse benthic microhabitats from temperate to tropical reefs. Mar. Ecol. Prog. Ser. 2020, 640, 31–43. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Cooper, P.; Fulton, C.J.; Fox, R.J. Quantifying epifaunal secondary production within tropical macroalgal meadows: Seasonality and sensitivity to canopy structure. Limnol. Oceanogr. 2021, 66, 4267–4284. [Google Scholar] [CrossRef]
- Arfianti, T.; Costello, M.J. Global biogeography of marine amphipod crustaceans: Latitude, regionalization, and beta diversity. Mar. Ecol. Prog. Ser. 2020, 638, 83–94. [Google Scholar] [CrossRef]
- Arfianti, T.; Wilson, S.; Costello, M.J. Progress in the discovery of amphipod crustaceans. PeerJ 2018, 6, e5187. [Google Scholar] [CrossRef]
- Sánchez-Jerez, P.; Cebrián, C.B.; Ramos-Esplá, A.A. Comparison of the epifauna spatial distribution in Posidonia oceanica, Cymodocea nodosa and unvegetated bottoms: Importance of meadow edges. Acta Oecol. 1999, 20, 391–405. [Google Scholar] [CrossRef]
- Moreira, J.; Lourido, A.; Troncoso, J. Diversity and distribution of peracarid crustaceans in shallow subtidal soft bottoms at the Ensenada de Baiona (Galicia, NW Spain). Crustaceana 2008, 81, 1069–1089. [Google Scholar] [CrossRef]
- Commito, J.A.; Como, S.; Grupe, B.M.; Dow, W.E. Species diversity in the soft-bottom intertidal zone: Biogenic structure, sediment, and macrofauna across mussel bed spatial scales. J. Exp. Mar. Biol. Ecol. 2008, 366, 70–81. [Google Scholar] [CrossRef]
- Guerra-García, J.M.; Cabezas Rodríguez, M.P.; Baeza-Rojano Pageo, E.; Izquierdo, D.; Corzo, J.; Ros, M.; Sánchez, J.A.; Dugo-Cota, A.; Flores-León, M.; Soler Hurtado, M.M. Abundance patterns of macrofauna associated to marine macroalgae along the Iberian Peninsula. Zool. Baetica 2011, 22, 3–17. [Google Scholar]
- De Clippele, L.H.; Buhl-Mortensen, P.; Buhl-Mortensen, L. Fauna associated with cold water gorgonians and sea pens. Cont. Shelf Res. 2015, 105, 67–78. [Google Scholar] [CrossRef]
- Coolen, J.W.; Van Der Weide, B.; Cuperus, J.; Blomberg, M.; Van Moorsel, G.W.; Faasse, M.A.; Bos, O.G.; Degraer, S.; Lindeboom, H.J. Benthic biodiversity on old platforms, young wind farms, and rocky reefs. ICES J. Mar. Sci. 2020, 77, 1250–1265. [Google Scholar] [CrossRef]
- Dauby, P.; Nyssen, F.; De Broyer, C. Amphipods as food sources for higher trophic levels in the Southern Ocean: A synthesis. Antarct. Biol. Glob. Context 2003, 189, 129–134. [Google Scholar]
- Cook, K.; Vanderklift, M.A.; Poore, A.G. Strong effects of herbivorous amphipods on epiphyte biomass in a temperate seagrass meadow. Mar. Ecol. Prog. Ser. 2011, 442, 263–269. [Google Scholar] [CrossRef]
- Gutow, L.; Poore, A.G.; Díaz Poblete, M.A.; Villalobos, V.; Thiel, M. Small burrowing amphipods cause major damage in a large kelp. Proc. R. Soc. B 2020, 287, 20200330. [Google Scholar] [CrossRef]
- Mavraki, N.; Coolen, J.W.; Kapasakali, D.A.; Degraer, S.; Vanaverbeke, J.; Beermann, J. Small suspension-feeding amphipods play a pivotal role in carbon dynamics around offshore man-made structures. Mar. Environ. Res. 2022, 178, 105664. [Google Scholar] [CrossRef]
- Kiljunen, M.; Peltonen, H.; Lehtiniemi, M.; Uusitalo, L.; Sinisalo, T.; Norkko, J.; Kunnasranta, M.; Torniainen, J.; Rissanen, A.J.; Karjalainen, J. Benthic-pelagic coupling and trophic relationships in northern Baltic Sea food webs. Limnol. Oceanogr. 2020, 65, 1706–1722. [Google Scholar] [CrossRef]
- Guerra-García, J.M.; Tierno de Figueroa, J.M.; Navarro-Barranco, C.; Ros, M.; Sánchez-Moyano, J.E.; Moreira, J. Dietary analysis of the marine Amphipoda (Crustacea: Peracarida) from the Iberian Peninsula. J. Sea Res. 2014, 85, 508–517. [Google Scholar] [CrossRef]
- Navarro-Barranco, C.; Ros, M.; Tierno de Figueroa, J.M.; Guerra-García, J.M. Marine crustaceans as bioindicators: Amphipods as case study. In The Natural History of Crustacean; Fisheries and Aquaculture; Lovrich, G., Thiel, M., Eds.; Oxford University Press: Oxford, UK, 2020; Volume 9, pp. 435–462. [Google Scholar]
- Gerovasileiou, V.; Bianchi, C.N. Mediterranean marine caves: A synthesis of current knowledge. Oceanogr. Mar. Biol. 2021, 59, 1–87. [Google Scholar]
- Gerovasileiou, V.; Voultsiadou, E. Marine caves of the Mediterranean Sea: A sponge biodiversity reservoir within a biodiversity hotspot. PLoS ONE 2012, 7, e39873. [Google Scholar] [CrossRef] [PubMed]
- Gerovasileiou, V.; Voultsiadou, E. Mediterranean marine caves as biodiversity reservoirs: A preliminary overview. In Proceedings of the 1st Mediterranean Symposium on the Conservation of Dark Habitats, Portorož, Slovenia, 31 October 2014; Langar, H., Bouafif, C., Ouerghi, A., Eds.; RAC/SPA: Tunis, Tunisia, 2014; pp. 45–50. [Google Scholar]
- Zabala, M.; Riera, T.; Gili, J.M.; Barange, M.; Lobo, A.; Peñuelas, J. Water flow, trophic depletion, and benthic macrofauna impoverishment in a submarine cave from the Western Mediterranean. Mar. Ecol. 1989, 10, 271–287. [Google Scholar] [CrossRef]
- Fichez, R. Decrease in allochthonous organic inputs in dark submarine caves, connection with lowering in benthic community richness. Hydrobiologia 1990, 207, 61–69. [Google Scholar] [CrossRef]
- Bussotti, S.; Terlizzi, A.; Fraschetti, S.; Belmonte, G.; Boero, F. Spatial and temporal variability of sessile benthos in shallow Mediterranean marine caves. Mar. Ecol. Prog. Ser. 2006, 325, 109–119. [Google Scholar] [CrossRef]
- Navarro-Barranco, C.; Guerra-García, J.M.; Sánchez-Tocino, L.; Jiménez-Prada, P.; Cea, S.; García-Gómez, J.C. Soft-bottom diversity patterns in marine caves; lessons from crustacean community. J. Exp. Mar. Biol. Ecol. 2013, 446, 22–28. [Google Scholar] [CrossRef]
- Navarro-Barranco, C. Faunistic and Ecological Study of the Amphipods Inhabiting Submarine Caves in the Southern Iberian Peninsula. Ph.D. Thesis, University of Seville, Seville, Spain, 2015. [Google Scholar]
- Thornber, C.; Jones, E.; Thomsen, M. Epibiont-marine macrophyte assemblages. In Marine Macrophytes as Foundation Species; Ólafsson, E., Ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 43–65. [Google Scholar]
- Bussotti, S.; Di Franco, A.; Francour, P.; Guidetti, P. Fish assemblages of Mediterranean marine caves. PLoS ONE 2015, 10, e0122632. [Google Scholar] [CrossRef]
- Bianchi, C.N.; Gerovasileiou, V.; Morri, C.; Froglia, C. Distribution and ecology of decapod crustaceans in Mediterranean marine caves: A review. Diversity 2022, 14, 176. [Google Scholar] [CrossRef]
- Romano, E.; Bergamin, L.; Parise, M. Benthic foraminifera as environmental indicators in Mediterranean marine caves: A review. Geosciences 2022, 12, 42. [Google Scholar] [CrossRef]
- Ruffo, S. Hadzia minuta n. sp. (Hadziidae) e Salentinella gracillima n. gen. n. sp. (Gammaridae) nuovi Anphipodi troglobi dell’ltalia meridionale. B. Soc. Nat. Nap. 1947, 56, 1–11. [Google Scholar]
- Ruffo, S. Contributo alla conoscenza degli anfipodi delle grotte sottomarine. PSZN 1959, 30, 402–416. [Google Scholar]
- Ruffo, S. Gli anfipodi delle acque sotterranee italiane. Biogeographia 1982, 7, 139–169. [Google Scholar] [CrossRef]
- Riedl, R. Biologie der Meereshölen; Parey: Hamburg, Germany, 1966; 636p. [Google Scholar]
- Sket, B. Ecology of the mixohaline hypogean fauna along the Yugoslav coasts. Stygologia 1986, 2, 317–338. [Google Scholar]
- Stock, J.H. The concept of “anchialine” reconsidered. Stygologia 1986, 2, 90–92. [Google Scholar]
- Sket, B. The Adriatic coast as the cradle of anchihaline (anchialine) ecology. Nat. Croat. 2012, 21 (Suppl. 1), 91–94. [Google Scholar]
- Ledoyer, M. Note sur la faune vagile des grottes sous-marines obscures. Rapp. Réun Cons. Int. Explor. Mer. 1965, 18, 121–124. [Google Scholar]
- Ledoyer, M. Ecologie de la faune vagile des biotopes méditerranéens accessibles en scaphandre autonome. Rec. Trav. Stat. Mar. Endoume 1966, 40, 103–149. [Google Scholar]
- True, M.A. Etude quantitative de quatre peuplements sciaphiles sur substrat rocheux dans la région marseillaise. Bull. Inst. Océanogr. Monaco 1970, 69, 1–48. [Google Scholar]
- Schellenberg, A. Subterrane Amphipoden korsikanischer Biotope. Archiv. Hydrobiol. 1950, 44, 325–332. [Google Scholar]
- Navarro Barranco, C.; Guerra García, J.M.; Sánchez Tocino, L.; García Gómez, J.C. Amphipods from marine cave sediments of the southern Iberian Peninsula: Diversity and ecological distribution. Sci. Mar. 2014, 78, 415–424. [Google Scholar] [CrossRef]
- Navarro-Barranco, C.; Guerra-García, J.M.; Sánchez-Tocino, L.; Florido, M.; García-Gómez, J.C. Amphipod community associated with invertebrate hosts in a Mediterranean marine cave. Mar. Biodivers. 2016, 46, 105–112. [Google Scholar] [CrossRef]
- Carbonell, J. Crustacis de les Iles Medes. In El Sistemes Natural de les Illes Medes; Ros, J., Olivella, I., Gili, J.M., Eds.; Institut d’Estid Catalans: Barcelona, Spain, 1984; pp. 505–528. [Google Scholar]
- Gerovasileiou, V.; Chintiroglou, C.; Vafidis, D.; Koutsoubas, D.; Sini, M.; Dailianis, T.; Issaris, Y.; Akritopoulou, E.; Dimarchopoulou, D.; Voultsiadou, E. Census of biodiversity in marine caves of the Eastern Mediterranean Sea. Mediterr. Mar. Sci. 2015, 16, 245–265. [Google Scholar] [CrossRef]
- Chintiroglou, C.; Koukouras, A. A population of the sea anemone Anemonia viridis (Forskal, 1775) and its associated flora and fauna in the North Aegean Sea. Int. Rev. Ges Hydrobiol. Hydrogr. 1992, 77, 483–495. [Google Scholar] [CrossRef]
- Sanfilippo, R.; Rosso, A.; Guido, A.; Gerovasileiou, V. Serpulid communities from two marine caves in the Aegean Sea, eastern Mediterranean. J. Mar. Biol. Assoc. UK 2017, 97, 1059–1068. [Google Scholar] [CrossRef]
- Rosso, A.; Gerovasileiou, V.; Sanfilippo, R.; Guido, A. Bryozoan assemblages from two submarine caves in the Aegean Sea (Eastern Mediterranean). Mar. Biod. 2019, 49, 707–726. [Google Scholar] [CrossRef]
- Bitner, M.A.; Gerovasileiou, V. Taxonomic composition and assemblage structure of brachiopods from two submarine caves in the Aegean Sea, Eastern Mediterranean. Eur. Zool. J. 2021, 88, 316–327. [Google Scholar] [CrossRef]
- Martinez, A.; Anicic, N.; Calvaruso, S.; Sanchez, N.; PuppienI, L.; Sforzi, T.; Zaupa, S.; Alvarez, F.; Brankovits, D.; Gąsiorowski, L.; et al. A new insight into the Stygofauna Mundi: Assembling a global dataset for aquatic fauna in subterranean environments. In ARPHA Conference Abstracts; Pensoft Publishers: Sofía, Bulgaria, 2018; Volume 1, p. e29514. [Google Scholar]
- Horton, T.; Lowry, J.; De Broyer, C.; Bellan-Santini, D.; Copilas-Ciocianu, D.; Corbari, L.; Costello, M.J.; Daneliya, M.; Dauvin, J.-C.; Fišer, C.; et al. World Amphipoda Database. Amphipoda. Accessed through: World Register of Marine Species. Available online: https://www.marinespecies.org (accessed on 28 August 2023).
- Gerovasileiou, V.; Martínez García, A.; Álvarez Noguera, F.; Boxshall, G.; Humphreys, W.F.; Jaume, D.; Becking, L.E.; Muricy, G.; van Hengstum, P.J.; Yamasaki, H.; et al. World Register of Marine Cave Species (WoRCS). 2023. Available online: https://www.marinespecies.org/worcs (accessed on 28 August 2023).
- Iliffe, T.M.; Kornicker, L.S. Worldwide diving discoveries of living fossil animals from the depths of anchialine and marine caves. Smithson. Contrib. Mar. Sci. 2009, 38, 269–280. [Google Scholar]
- Ruffo, S. The Amphipoda of the Mediterranean: Parts 1–4. Bull. Inst. Océanogr. 1982, 13, 1–959. [Google Scholar]
- Conradi, M.; López-Gónzalez, P.J. The benthic Gammaridea (Crustacea, Amphipoda) fauna of Algeciras Bay (Strait of Gibraltar): Distributional ecology and some biogeographical considerations. Helgoland Mar. Res. 1999, 53, 2–8. [Google Scholar] [CrossRef]
- Guerra-García, J.M.; Cabezas, P.; Baeza-Rojano, E.; Espinosa, F.; García-Gómez, J.C. Is the north side of the Strait of Gibraltar more diverse than the south side? A case study using the intertidal peracarids (Crustacea: Malacostraca) associated to the seaweed Corallina elongata. J. Mar. Biol. Assoc. UK 2009, 89, 387–397. [Google Scholar] [CrossRef]
- Lantzouni, M.; Voultsiadou, E.; Chintiroglou, C. Preliminary observations on amphipod assemblages associated with Mytilus galloprovincialis Lamarck beds from Thermaikos Gulf (Aegean Sea). Rapp. Réun Cons. Int. Explor. Mer. 1998, 35, 458–459. [Google Scholar]
- Conradi, M.; López-González, P.J.; Cervera, J.L.; García-Gómez, J.C. Seasonality and spatial distribution of peracarids associated with the bryozoan Bugula neritina in Algeciras Bay, Spain. J. Crustac. Biol. 2000, 20, 334–349. [Google Scholar] [CrossRef]
- Guerra-García, J.M. Habitat use of the caprellidea (crustacea: Amphipoda) from Ceuta, North Africa. Ophelia 2001, 55, 27–38. [Google Scholar] [CrossRef]
- Ortiz, M.; Jimeno, A. Contribución al conocimiento de los Anfípodos (Gammaridea) de Ibiza, islas Baleares. Graellsia 2003, 59, 97–99. [Google Scholar] [CrossRef][Green Version]
- Voultsiadou, E.; Pyrounaki, M.M.; Chintiroglou, C. The habitat engineering tunicate Microcosmus sabatieri (Roule, 1885) and its associated peracarid epifauna. Estuar. Coast. Shelf Sci. 2007, 74, 197–204. [Google Scholar] [CrossRef]
- Çinar, M.E.; Katağan, T.; Koçak, F.; Öztürk, B.; Ergen, Z.; Kocatas, A.; Özcan, T. Faunal assemblages of the mussel Mytilus galloprovincialis in and around Alsancak Harbour (Izmir Bay, eastern Mediterranean) with special emphasis on alien species. J. Mar. Syst. 2008, 71, 1–17. [Google Scholar] [CrossRef]
- Carvalho, S.; Cúrdia, J.; Pereira, F.; Guerra-García, J.M.; Santos, M.N.; Cunha, M.R. Biodiversity patterns of epifaunal assemblages associated with the gorgonians Eunicella gazella and Leptogorgia lusitanica in response to host, space and time. J. Sea Res. 2014, 85, 37–47. [Google Scholar] [CrossRef]
- Terrón-Sigler, A.; Peñalver-Duque, P.; León-Muez, D.; Torre, F.E. Spatio-temporal macrofaunal assemblages associated with the endangered orange coral Astroides calycularis (Scleractinia: Dendrophylliidae). Aquat. Biol. 2014, 21, 143–154. [Google Scholar] [CrossRef]
- Gavira-O’Neill, K.; Guerra-García, J.M.; Moreira, J.; Ros, M. Mobile epifauna of the invasive bryozoan Tricellaria inopinata: Is there a potential invasional meltdown? Mar. Biod 2018, 48, 1169–1178. [Google Scholar] [CrossRef]
- Gavira O’Neill, K.; Moreira, J.; Guerra García, J.M. Variaciones estacionales de la fauna vágil asociada a Ectopleura crocea (Cnidaria, Hydrozoa) en el puerto de El Rompido (Huelva). Zool. Baetica 2015, 26, 43–68. [Google Scholar]
- Ponti, M.; Grech, D.; Mori, M.; Perlini, R.A.; Ventra, V.; Panzalis, P.A.; Cerrano, C. The role of gorgonians on the diversity of vagile benthic fauna in Mediterranean rocky habitats. Mar. Biol. 2016, 163, 120. [Google Scholar] [CrossRef]
- Bertocci, I.; Badalamenti, F.; Brutto, S.L.; Mikac, B.; Pipitone, C.; Schimmenti, E.; Musco, L. Reducing the data-deficiency of threatened European habitats: Spatial variation of sabellariid worm reefs and associated fauna in the Sicily Channel, Mediterranean Sea. Mar. Environ. Res. 2017, 130, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Saenz-Arias, P.; Navarro-Barranco, C.; Guerra-García, J.M. Seguimiento temporal de la comunidad de anfípodos (Crustacea, Peracarida) asociada al briozoo Bugula neritina en el puerto deportivo de La Alcaidesa (La Línea). Almoraima 2020, 53, 183–194. [Google Scholar]
- Sedano, F.; Navarro-Barranco, C.; Guerra-García, J.M.; Espinosa, F. From sessile to vagile: Understanding the importance of epifauna to assess the environmental impacts of coastal defence structures. Estuar. Coast. Shelf Sci. 2020, 235, 106616. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N. PRIMER v6: User Manual/Tutorial. Plymouth: PRIMER-E; Plymouth Marine Laboratory: Plymouth, UK, 2006. [Google Scholar]
- Scipione, B.; Taramelli, E.; Fresi, E.; Cinelli, F.; Mazzella, L. Distribuzione delle biocenosi bentoniche lungo un gradiente di luce in una grotta marina superficiale: Anfipodi. Mem. Biol. Mar. Ocenaogr. 1981, 11, 1–16. [Google Scholar]
- Navarro-Barranco, C.; Guerra-García, J.M.; Sánchez-Tocino, L.; García-Gómez, J.C. Soft-bottom crustacean assemblages in Mediterranean marine caves: The cave of Cerro Gordo (Granada, Spain) as case study. Helgoland Mar. Res. 2012, 66, 567–576. [Google Scholar] [CrossRef]
- Bellan-Santini, D.; Ledoyer, M. Inventaire des amphipodes gammariens récolectés dans la région de Marseille. Tethys 1973, 4, 899–934. [Google Scholar]
- Bellan-Santini, D.; Ruffo, S. Biogeography of benthic marine amphipods in Mediterranean Sea. Biogeographia 2003, 24, 273–292. [Google Scholar] [CrossRef]
- Coll, M.; Parodic, C.; Steenbeek, J.; Kaschner, K.; Ben Rais Lasram, F.; Aguzzi, J.; Ballesteros, E.; Nike-Bianchi, C.; Corbera, J.; Dailianis, T.; et al. The biodiversity of the Mediterranean Sea: Estimates, patterns, and threats. PLoS ONE 2010, 5, e11842. [Google Scholar] [CrossRef]
- Coleman, C.O. Taxonomy in times of the taxonomic impediment–examples from the community of experts on amphipod crustaceans. J. Crustac. Biol. 2015, 35, 729–740. [Google Scholar] [CrossRef]
- Zenetos, A.; Albano, P.G.; López Garcia, E.; Stern, N.; Tsiamis, K.; Galanidi, M. Established non-indigenous species increased by 40% in 11 years in the Mediterranean Sea. Mediterr. Mar. Sci. 2022, 23, 196–212. [Google Scholar] [CrossRef]
- Marchini, A.; Cardeccia, A. Alien amphipods in a sea of troubles: Cryptogenic species, unresolved taxonomy and overlooked introductions. Mar. Biol. 2017, 164, 69. [Google Scholar] [CrossRef]
- Beermann, J.; Hall-Mullen, A.K.; Havermans, C.; Coolen, J.W.; Crooijmans, R.P.; Dibbits, B.; Held, C.; Desiderato, A. Ancient globetrotters—Connectivity and putative native ranges of two cosmopolitan biofouling amphipods. PeerJ 2020, 8, e9613. [Google Scholar] [CrossRef]
- Conlan, K.E.; Desiderato, A.; Beermann, J. Jassa (Crustacea: Amphipoda): A new morphological and molecular assessment of the genus. Zootaxa 2021, 4939, 1–191. [Google Scholar] [CrossRef] [PubMed]
- Gerovasileiou, V.; Bancila, R.I.; Katsanevakis, S.; Zenetos, A. Introduced species in Mediterranean marine caves: An increasing but neglected threat. Mediterr. Mar. Sci. 2022, 23, 995–1005. [Google Scholar] [CrossRef]
- Ruffo, S. Contributo alla conoscenza dei Crostacei Anfipodi delle acque sotterranee della Sardegna e delle Baleari. Atti I R. Ist. Veneto Sci. Lett. 1960, 118, 180. [Google Scholar]
- Pavesi, L.; De Matthaeis, E. Life history of the talitrid amphipod Macarorchestia remyi (Schellenberg, 1950) on a Tyrrhenian sandy beach, Italy. Hydrobiologia 2009, 635, 171–180. [Google Scholar] [CrossRef]
- Pavesi, L.; De Matthaeis, E.; Tiedemann, R.; Ketmaier, V. Temporal Population Genetics and COI Phylogeography of the Sandhopper Macarorchestia remyi (Amphipoda: Talitridae). Zool. Stud. 2011, 50, 220–229. [Google Scholar]
- Lowry, J.K.; Myers, A.A. New genera of Talitridae in the revised Superfamily Talitroidea Bulycheva 1957 (Crustacea, Amphipoda, Senticaudata). Zootaxa 2019, 4553, 1–100. [Google Scholar] [CrossRef]
- Takahashi, T.; Morino, H.; Tomikawa, K.; Lai, Y.T.; Nakano, T. Molecular phylogenetic position of Minamitalitrus zoltani elucidates a further troglobisation pattern in cave-dwelling terrestrial amphipods (Crustacea: Talitridae). Mol. Phylogenet Evol. 2021, 154, 106984. [Google Scholar] [CrossRef]
- González, A.R.; Guerra-García, J.M.; Maestre, M.J.; Ruiz-Tabares, A.; Espinosa, F.; Gordillo, I.; Sánchez-Moyano, E.; García-Gómez, J.C. Community structure of caprellids (Crustacea: Amphipoda: Caprellidae) on seagrasses from southern Spain. Helgoland Mar. Res. 2008, 62, 189–199. [Google Scholar] [CrossRef]
- Guerra-García, J.M.; Navarro-Barranco, C.; Corzo, J.; Cobos-Munoz, V.; García-Adiego, E.M.; Giménez, F.S.; García-Gómez, J.C. An illustrated key to the soft-bottom caprellids (Crustacea: Amphipoda) of the Iberian Peninsula and remarks to their ecological distribution along the Andalusian coast. Helgoland Mar. Res. 2013, 67, 321–336. [Google Scholar] [CrossRef]
- Piazzi, L.; Bonaviri, C.; Castelli, A.; Ceccherelli, G.; Costa, G.; Curini-Galletti, M.; Langeneck, J.; Manconi, R.; Montefalcone, M.; Pipitone, C.; et al. Biodiversity in canopy-forming algae: Structure and spatial variability of the Mediterranean Cystoseira assemblages. Estuar. Coast. Shelf Sci. 2018, 207, 132–141. [Google Scholar] [CrossRef]
- Bellisario, B.; Camisa, F.; Abbattista, C.; Cimmaruta, R. A network approach to identify bioregions in the distribution of Mediterranean amphipods associated with Posidonia oceanica meadows. PeerJ 2019, 7, e6786. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Barranco, C.; Moreira, J.; Espinosa, F.; Ros, M.; Rallis, I.; Sempere-Valverde, J.; Ostalé-Valriberas, E.; Altamirano, M.; García-Gómez, J.C.; Guerra-García, J.M. Evaluating the vulnerability of coralligenous epifauna to macroalgal invasions. Aquat. Conserv. 2021, 31, 2305–2319. [Google Scholar] [CrossRef]
- Gerovasileiou, V.; Chintiroglou, C.C.; Konstantinou, D.; Voultsiadou, E. Sponges as “living hotels” in Mediterranean marine caves. Sci. Mar. 2016, 80, 279–289. [Google Scholar] [CrossRef]
- Koukouras, A.; Russo, E.; Voultsiadou-Koukoura, C.; Dounas, H.; Chintiroglou, C. Relationships of sponge macrofauna with the morphology of their hosts in the North Aegean Sea. Int. Rev. ges Hydrobiol. Hydrogr. 1992, 77, 609–619. [Google Scholar] [CrossRef]
- Pavloudi, C.; Christodoulou, M.; Mavidis, M. Macrofaunal assemblages associated with the sponge Sarcotragus foetidus Schmidt, 1862 (Porifera: Demospongiae) at the coasts of Cyprus and Greece. Biodivers. Data J. 2016, 4, e8210. [Google Scholar] [CrossRef]
- Navarro-Barranco, C.; Guerra-García, J.M.; Sánchez-Tocino, L.; Ros, M.; Florido, M.; García-Gómez, J.C. Colonization and successional patterns of the mobile epifaunal community along an environmental gradient in a marine cave. Mar. Ecol. Prog. Ser. 2015, 521, 105–115. [Google Scholar] [CrossRef]
- Cattaneo, R.; Pastorino, M.V. Popolamenti algali e fauna bentonica nelle cavità naturali della regione litorale mediterranea. Rass. Speleol. Ital. 1974, 12, 272–281. [Google Scholar]
- Bellan-Santini, D. Mediterranean deep-sea amphipods: Composition, structure and affinities of the fauna. Prog. Oceanogr. 1990, 24, 275–287. [Google Scholar] [CrossRef]
- Vacelet, J.; Boury-Esnault, N.; Harmelin, J.G. Hexactinellid cave, a unique deep-sea habitat in the scuba zone. Deep. Sea Res. I Oceanogr. Res. Pap. 1994, 41, 965–973. [Google Scholar] [CrossRef]
- Rosso, A.; Sanfilippo, R.; Taddei Ruggiero, E.; Di Martino, E. Faunas and ecological groups of Serpuloidea, Bryozoa and Brachiopoda from submarine caves in Sicily (Mediterranean Sea). Boll. Soc. Paleontol. Ital. 2013, 52, 167–176. [Google Scholar]
- Fernandez-Gonzalez, V.; Sanchez-Jerez, P. Fouling assemblages associated with off-coast aquaculture facilities: An overall assessment of the Mediterranean Sea. Mediterr. Mar. Sci. 2017, 18, 87–96. [Google Scholar] [CrossRef]
- Saenz-Arias, P.; Navarro-Barranco, C.; Guerra-García, J.M. Influence of environmental factors and sessile biota on vagile epibionts: The case of amphipods in marinas across a regional scale. Mediterr. Mar. Sci. 2022, 23, 1–13. [Google Scholar] [CrossRef]
- Conradi, M.; López-González, P.J.; García-Gómez, J.C. The amphipod community as a bioindicator in Algeciras Bay (southern Iberian Peninsula) based on a spatio-temporal distribution. Mar. Ecol. 1997, 18, 97–111. [Google Scholar] [CrossRef]
- De-la-Ossa-Carretero, J.A.; Del-Pilar-Ruso, Y.; Loya-Fernández, A.; Ferrero-Vicente, L.M.; Marco-Méndez, C.; Martinez-Garcia, E.; Sánchez-Lizaso, J.L. Response of amphipod assemblages to desalination brine discharge: Impact and recovery. Estuar. Coast. Shelf Sci. 2016, 172, 13–23. [Google Scholar] [CrossRef]
- Bianchi, C.N. Flora e fauna: Lineamenti generali e prospettive. In Grotte Marine: Cinquant’Anni di Ricerca in Italia; Cicogna, F., Ed.; Ministero dell’Ambiente e della Tutela del Territorio: Rome, Italy, 2003; pp. 137–146. [Google Scholar]
- Rastorgueff, P.A.; Harmelin-Vivien, M.; Richard, P.; Chevaldonné, P. Feeding strategies and resource partitioning mitigate the effects of oligotrophy for marine cave mysids. Mar. Ecol. Prog. Ser. 2011, 440, 163–176. [Google Scholar] [CrossRef]
- Bussotti, S.; Di Franco, A.; Bianchi, C.N.; Chevaldonné, P.; Egea, L.; Fanelli, E.; Guidetti, P. Fish mitigate trophic depletion in marine cave ecosystems. Sci. Rep. 2018, 8, 9193. [Google Scholar] [CrossRef]
- Stefanidou, D.; Voultsiadou-Koukoura, E. An account of our knowledge of the amphipod fauna of the Aegean Sea. Crustaceana 1995, 68, 597–615. [Google Scholar] [CrossRef]
- Fishelson, L. Marine animal assemblages along the littoral of the Israeli Mediterranean seashore: The Red-Mediterranean Seas communities of species. Ital. J. Zool. 2000, 67, 393–415. [Google Scholar] [CrossRef]
- Antoniadou, C.; Chintiroglou, C. Biodiversity of zoobenthic hard-substrate sublittoral communities in the Eastern Mediterranean (North Aegean Sea). Estuar. Coast. Shelf Sci. 2005, 62, 637–653. [Google Scholar] [CrossRef]
- Bakir, A.K.; Katağan, T. Distribution of littoral benthic amphipods off the Levantine coast of Turkey with new records. Turk. J. Zool. 2014, 38, 23–34. [Google Scholar] [CrossRef]
- Ballesteros, E. Mediterranean coralligenous assemblages: A synthesis of present knowledge. Oceanogr. Mar. Biol. 2006, 44, 123–195. [Google Scholar]
- Rosso, A.; Di Martino, E. Bryozoan diversity in the Mediterranean Sea: An update. Mediterr. Mar. Sci. 2016, 17, 567–607. [Google Scholar] [CrossRef]
- Bauer, D.M.; Bell, K.P.; Nelson, E.J.; Calhoun, A.J. Managing small natural features: A synthesis of economic issues and emergent opportunities. Biol. Conserv. 2017, 211, 80–87. [Google Scholar] [CrossRef]
- Jaume, D.; Garcia, L. A new Psammogammarus (Amphipoda: Melitidae) from Cabrera (Balearic Islands). Stygologia 1992, 7, 107–115. [Google Scholar]
- Bou, C.; Ruffo, S. Contributo alla conoscenza delle Bogidiella di Grecia. Natura Soc. Ital. Sei. Nat. Mus. Civ. Stor. Nat. Acquario Civ. 1979, 70, 1979. [Google Scholar]
- Gràcia, F.; Jaume, D. La fauna aquàtica dels hàbitats anquihalins i dolçaquícoles de les cavitats balears. Endins Publicació D’espeleologia 2011, 17, 257–268. [Google Scholar]
- Ruffo, S. Lo stato attuale delle conoscenze sulla distribuzione geografica degli anfipodi delle acqua sotteranee europee e dei paesi mediterranei. In Premier Congres International de Spéléologie Paris; AbeBooks: Paris, France, 1953; pp. 13–37. [Google Scholar]
- Jaume, D.; Christenson, K. Amphi-Atlantic distribution of the subterranean amphipod family Metacrangonyctidae (Crustacea, Gammaridea). Contrib. Zool. 2001, 70, 99–125. [Google Scholar] [CrossRef]
- Sket, B. The ecology of anchihaline caves. Trends Ecol. Evol. 1996, 11, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Gottstein, S.; Kršinić, F.; Ternjej, I.; Cukrov, N.; Kutleša, P.; Jalžić, B. Shedding light on crustacean species diversity in the anchihaline caves of Croatia. Nat. Croatica 2012, 21 (Suppl. 1), 54–58. [Google Scholar]
- Ozimec, R.; Bedek, J.; Gottstein, S.; Jalžić, B.; Slapnik, R.; Bilandžija, H. Crvena Knjiga Špiljske Faune Hrvatske; Ministarstvo Kulture Republike Hrvatske, Državni Zavod za Zaštitu Prirode: Zagreb, Croatia, 2009. [Google Scholar]
- Fišher, C.; Trontelj, P.; Luštrik, R.; Sket, B. Toward a unified taxonomy of Niphargus (Crustacea: Amphipoda): A review of morphological variability. Zootaxa 2009, 2061, 1–22. [Google Scholar] [CrossRef]
- Delić, T.; Švara, V.; Coleman, C.O.; Trontelj, P.; Fišer, C. The giant cryptic amphipod species of the subterranean genus Niphargus (Crustacea, Amphipoda). Zool. Scr. 2017, 46, 740–752. [Google Scholar] [CrossRef]
- Hupalo, K.; Stoch, F.; Karaouzas, I.; Wysocka, A.; Rewicz, T.; Mamos, T.; Grabowski, M. Freshwater malacostraca of the Mediterranean islands-diversity, origin, and conservation perspectives. In Recent Advances in Freshwater Crustacean Biodiversity and Conservation; Kawai, T., Rogers, D.C., Eds.; CRC Press: Boca Raton, FL, USA, 2021; Volume 22. [Google Scholar]
- Pesce, G.L. A new subterranean crustacean from southern Italy, Metahadzia adriatica n. sp.; with notes on Hadzia minuta Ruffo (Amphipoda, Gammaridae). Bijd Dierkunde 1979, 49, 102–108. [Google Scholar] [CrossRef]
- Gottstein Matočec, S.; Bakran-Petricioli, T.; Bedek, J.; Bukovec, D.; Buzjak, S.; Franičević, M.; Jalžic, B.; Kerovec, M.; Kletečkii, E.; Kralj, J.; et al. An overview of the cave and interstitial biota of Croatia. Nat. Croatica 2002, 11 (Suppl. 1), 1–112. [Google Scholar]
- Ruffo, S. Rinvenimento di Gammarus (Neogammarus) rhipidiophorus Catta nelle acque sotterranee della Liguria. Doriana 1951, 1, 1–4. [Google Scholar]
- Stock, J.H. A revision of the Sarathrogammarus-group (Crustacea, Amphipoda). Bijd Dierkunde 1971, 41, 94–129. [Google Scholar] [CrossRef]
- Jaume, D.; Vonk, R. A new species of Salentinella Ruffo, 1947 from a thermo-mineral cave in southern Spain, with comments on the systematic position of the family Salentinellidae (Amphipoda). J. Crustac. Biol. 2021, 41, 1–11. [Google Scholar] [CrossRef]
- Karaman, G.S. Contributions to the knowledge of the Amphipoda. 109. The problem of Salentinella angelieri Del. Deb. and Ruffo, 1952 and its subspecies. Poljopr. I Summ. Titogr. 1979, 25, 24–44. [Google Scholar]
- Pesce, G.L. New records for Salentinella Ruffo (Crustacea Amphipoda) from phreatic waters of Italy and Greece. Int. J. Speleol. 1984, 14, 3. [Google Scholar]
- Pérez-Moreno, J.L.; Iliffe, T.M.; Bracken-Grissom, H.D. Life in the Underworld: Anchialine cave biology in the era of speleogenomics. Int. J. Speleol. 2016, 45, 149–170. [Google Scholar] [CrossRef]
- Bauzà-Ribot, M.M.; Jaume, D.; Fornós, J.J.; Juan, C.; Pons, J. Islands beneath islands: Phylogeography of a groundwater amphipod crustacean in the Balearic archipelago. BMC Evol. Biol. 2011, 11, 221. [Google Scholar] [CrossRef] [PubMed]
- Stokkan, M.; Jurado-Rivera, J.A.; Oromí, P.; Juan, C.; Jaume, D.; Pons, J. Species delimitation and mitogenome phylogenetics in the subterranean genus Pseudoniphargus (Crustacea: Amphipoda). Mol. Phylogenet. Evol. 2018, 127, 988–999. [Google Scholar] [CrossRef]
- Notenboom, J. Marine regressions and the evolution of groundwater dwelling amphipods (Crustacea). J. Biogeogr. 1991, 18, 437–454. [Google Scholar] [CrossRef]
- Borko, Š.; Trontelj, P.; Seehausen, O.; Moškrič, A.; Fišer, C. A subterranean adaptive radiation of amphipods in Europe. Nat. Commun. 2021, 12, 3688. [Google Scholar] [CrossRef]
- Kokalj, A.J.; Fišer, Ž.; Dolar, A.; Novak, S.; Drobne, D.; Bračko, G.; Fišer, C. Screening of NaCl salinity sensitivity across eight species of subterranean amphipod genus Niphargus. Ecotoxicol. Environ. Saf. 2022, 236, 113456. [Google Scholar] [CrossRef]
- Hervant, F.; Malard, F. Adaptations: Low oxygen. In Encyclopedia of Caves, 3rd ed.; White, W.B., Culver, D.C., Pipan, T., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 8–15. [Google Scholar]
- Holsinger, J.R.; Culver, D.C. The invertebrate cave fauna of Virginia and a part of eastern Tennessee—Zoogeography and ecology. Brimleyana 1988, 14, 1–162. [Google Scholar]
- Bishop, R.E.; Kakuk, B.; Torres, J.J. Life in the hypoxic and anoxic zones: Metabolism and proximate composition of Caribbean troglobitic crustaceans with observations on the water chemistry of two anchialine caves. J. Crustac. Biol. 2004, 24, 379–392. [Google Scholar] [CrossRef]
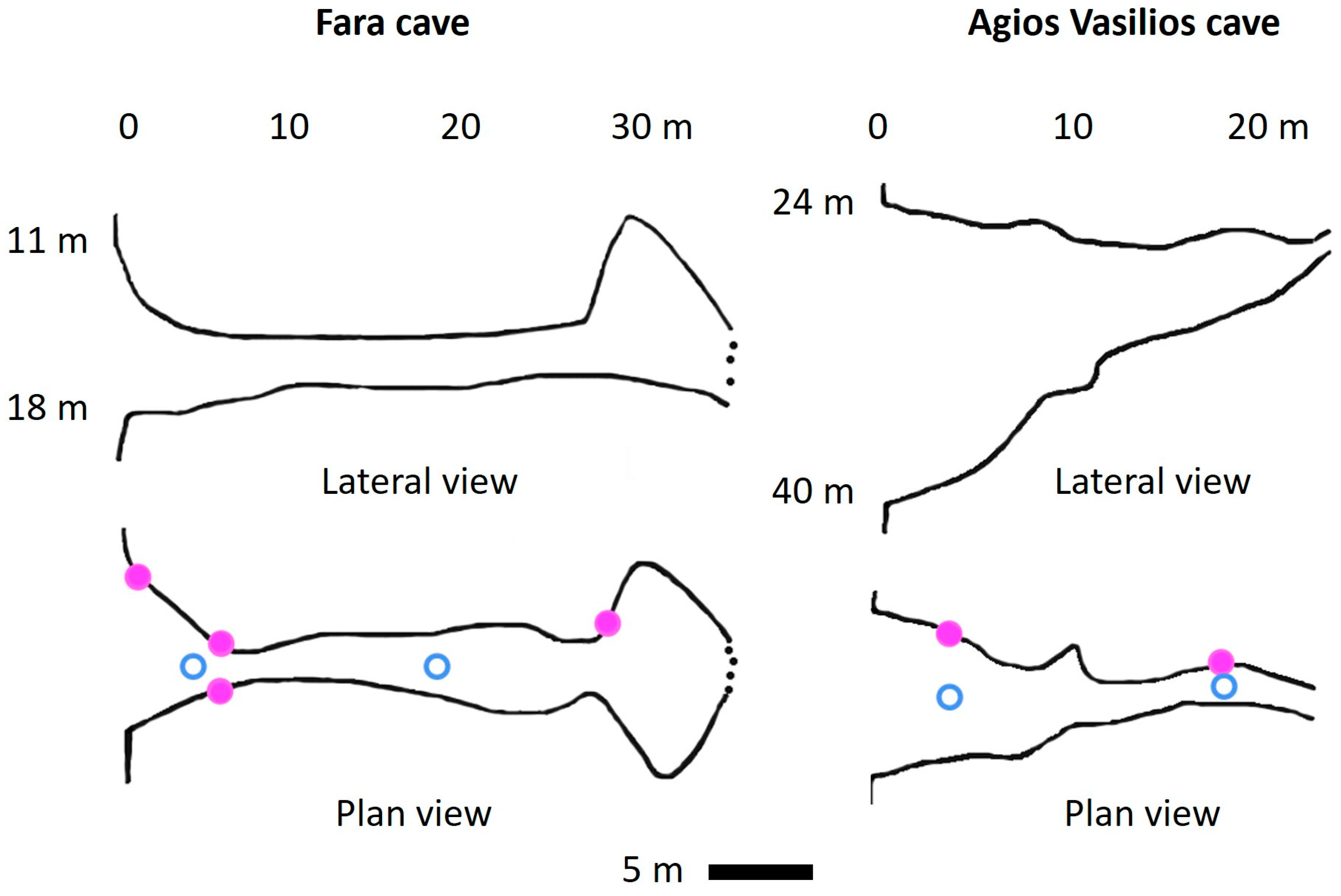

| Fara Cave | Agios Vasilios Cave | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample location | L w | C | R w | L w | C | Walls | C | Walls | C | Walls |
| Distance from entrance (m) | Entr | 5 | 5-10 | 5-10 | 15-20 | 20-30 | 5-10 | 5-10 | 15-20 | 15-20 |
| Biocoenosis | Cor | SD | SD | SD | Trans | Dark | SD | SD | Dark | Dark |
| Dominant encrusters | Rh | Sc-Sp | Sc-Sp | Sp | Sr-Br | Sr-Sp-Br | Sc-Sp | Sp | Sc-Sp-Sr | Sp-Sr |
| Species Sampling stations | F1 | FC1 | F2 | F3 | FC2 | F4 | VC1 | V1 | VC2 | V2 |
| Apherusa sp. | 0.3 | |||||||||
| Colomastix pusilla | 0.3 | 0.3 | 0.7 | 0.3 | 3.0 | |||||
| Gammarus subtypicus | 0.7 | |||||||||
| Iphimedia carinata | 0.3 | 0.3 | ||||||||
| Iphimedia sp. | 0.3 | |||||||||
| Leptocheirus bispinosus | 10.0 | 21.7 | 5.3 | 2.3 | 0.3 | |||||
| Leptocheirus pectinatus | 3.0 | 2.3 | 0.3 | 0.3 | ||||||
| Leucothoe spinicarpa | 1.3 | 0.3 | ||||||||
| Liljeborgia dellavallei | 0.3 | 3.7 | 2.3 | 2.7 | 0.3 | |||||
| Lysianassina longicornis | 0.3 | |||||||||
| Perrierella audouiniana | 2.0 | 10.0 | 0.7 | 3.0 | 1.0 | |||||
| Stenothoe antennulariae | 0.7 | |||||||||
| Amphipoda unid. | 1.0 | 0.3 | ||||||||
| Total number of individuals | 8 | 73 | 74 | 12 | 16 | 0 | 37 | 22 | 3 | 2 |
| Mean abundance | 1 | 8 | 8.3 | 1.3 | 1.7 | 0 | 2.3 | 4.0 | 0.3 | 0.3 |
| Species richness | 3 | 5 | 3 | 3 | 1 | 0 | 6 | 9 | 2 | 3 |
| Species Richness | Abundance | |||||||
|---|---|---|---|---|---|---|---|---|
| Cave | Source | df | MS | Pseudo-F | P (Perm) | MS | Pseudo-F | P (Perm) |
| Agios Vasilios | Station | 3 | 12.56 | 10.76 | 0.005 | 93.56 | 13.37 | 0.006 |
| Res | 8 | 1.17 | 7.00 | |||||
| Fara | Station | 5 | 2.89 | 5.78 | 0.015 | 379.17 | 5.95 | 0.010 |
| Res | 12 | 0.50 | 63.72 | |||||
| CS | Blue Cave | FC | Shrimp Cave | SC | BC | Korakonisi | PL | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Substrate | AO | AO | CO | AO | AO | Hy | AO | AO | AO | Br | AO |
| Species | |||||||||||
| Apocorophium acutum | 1 | 1 | |||||||||
| Apolochus picadurus | 1 | ||||||||||
| Colomastix pusilla | 1 | 17 | 1 | 6 | 1 | ||||||
| Coxischyrocerus inexpectatus | 1 | ||||||||||
| Gitana sarsi | 5 | 9 | 1 | ||||||||
| Leptocheirus bispinosus | 3 | 1 | |||||||||
| Leptocheirus guttatus | 1 | ||||||||||
| Leptocheirus pectinatus | 1 | 2 | 5 | ||||||||
| Leucothoe spinicarpa | 1 | ||||||||||
| Phtisica marina | 1 | 1 | |||||||||
| Pseudoprotella phasma | 1 | 1 | |||||||||
| Stenothoe monoculoides | 1 | ||||||||||
| Stenothoe sp. | 1 | ||||||||||
| Family | Species | Biogeographical Region | ||||||
|---|---|---|---|---|---|---|---|---|
| Alboran Sea | Algero-Provençal Basin | Tyrrhenian Sea | Tunisian Plateau | Adriatic Sea | Ionian Sea | Aegean Sea | ||
| Ampeliscidae | Ampelisca rubella A. Costa, 1864 | - | - | 1 | - | - | - | - |
| Ampelisca serraticaudata Chevreux, 1888 | - | - | - | - | - | - | - | |
| Ampelisca truncata Bellan-Santini & Kaim-Malja, 1977 | - | - | - | 1 | - | - | - | |
| Ampelisca typica (Spence-Bate, 1856) | 1 | - | - | - | - | - | - | |
| Amphilochidae | Amphilochus manudens Spence Bate, 1862 | - | 1 | - | - | - | - | - |
| Apolochus neapolitanus (Della Valle, 1893) | - | - | 1 | - | - | - | - | |
| Apolochus picadurus (J.L. Barnard, 1962) * | - | - | - | - | - | 1 | - | |
| Gitana cf. abyssicola G.O. Sars, 1892 | 1 | - | - | - | - | - | - | |
| Gitana sarsi Boeck, 1871 * | - | 1 | - | - | - | 4 | - | |
| Ampithoidae | Ampithoe ramondi Audouin, 1826 | - | 2 | 1 | - | - | - | - |
| Pleonexes helleri (Karaman, 1975) | - | - | 1 | - | - | - | - | |
| Aoridae | Aora spinicornis Afonso, 1976 | - | 1 | 1 | - | - | - | - |
| Autonoe rubromaculatus (Ledoyer, 1973) | - | - | 1 | - | - | - | - | |
| Lembos websteri Spence Bate, 1857 | 4 | 3 | - | - | - | - | - | |
| Microdeutopus algicola Della Valle, 1893 | 1 | - | - | - | - | - | - | |
| Aristiidae | Aristias neglectus Hansen, 1988 | - | 5 | - | - | - | - | - |
| Perrierella audouiniana (Spence Bate, 1857) * | - | 1 | 1 | - | - | - | 2 | |
| Atylidae | Nototropis swammerdamei (H. Milne-Edwards, 1830) | - | - | 1 | - | - | - | - |
| Nototropis vedlomensis (Spence Bate & Westwood, 1862) | - | 1 | - | - | - | - | - | |
| Bogidiellidae | Marinobogidiella tyrrhenica (Schiecke, 1979) | - | - | 1 | - | - | - | - |
| Calliopiidae | Apherusa bispinosa (Spence Bate, 1857) | - | 2 | 1 | - | - | - | - |
| Caprellidae | Caprella hirsuta Mayer, 1890 | - | - | 1 | - | - | - | - |
| Caprella liparotensis Haller, 1879 | - | 1 | 1 | - | - | - | - | |
| Liropus minimus Mayer, 1890 | - | - | 1 | - | - | - | - | |
| Phtisica marina Slabber, 1796 | 3 | 5 | - | - | - | 3 | 2 | |
| Pseudolirius kroyeri (Haller, 1879) | 4 | - | - | - | - | - | - | |
| Pseudoprotella phasma (Montagu, 1804) * | 4 | 2 | 2 | - | - | 3 | 1 | |
| Colomastigidae | Colomastix pusilla Grube, 1861 | - | 5 | 3 | - | - | 6 | 2 |
| Corophiidae | Apocorophium acutum (Chevreux, 1908) * | 1 | 1 | 1 | - | - | 1 | - |
| Leptocheirus bispinosus Norman, 1908 | 1 | 1 | - | - | - | 2 | 2 | |
| Leptocheirus guttatus (Grube, 1864) * | - | - | - | - | - | 1 | - | |
| Leptocheirus longimanus Ledoyer, 1973 | 1 | - | - | - | - | - | - | |
| Leptocheirus pectinatus (Norman, 1869) * | 2 | 3 | - | - | - | 2 | 1 | |
| Monocorophium sextonae (Crawford, 1937) | - | 1 | - | - | - | - | - | |
| Cressidae | Cressa cristata Myers, 1969 | - | - | - | 2 | - | - | - |
| Cressa mediterranea Ruffo, 1979 | - | 2 | - | 1 | - | - | - | |
| Cyproideidae | Peltocoxa marioni Catta, 1875 | - | - | 1 | - | - | - | - |
| Dexaminidae | Dexamine spiniventris (Costa, 1853) | 1 | 5 | 1 | - | - | - | - |
| Dexamine spinosa (Montagu, 1813) | 1 | 3 | 1 | - | - | - | - | |
| Tritaeta gibbosa (Spence Bate, 1862) | 1 | - | 3 | - | - | - | - | |
| Gammaridae | Gammarus subtypicus Stock, 1966 | - | - | - | - | - | - | 1 |
| Hyalidae | Apohyale crassipes (Heller, 1866) | - | 1 | - | - | - | - | - |
| Protohyale (Protohyale) schmidtii (Heller, 1866) | - | 1 | 1 | - | - | - | - | |
| Iphimediidae | Iphimedia carinata Heller, 1866 * | - | - | - | - | - | - | 2 |
| Iphimedia eblanae Spence Bate, 1857 | - | 2 | - | - | - | - | - | |
| Iphimedia minuta G.O. Sars, 1883 | - | 4 | - | - | - | - | - | |
| Ischyroceridae | Centraloecetes dellavallei (Stebbing, 1899) | - | 1 | - | - | - | - | - |
| Coxischyrocerus inexpectatus (Ruffo, 1959) * | 3 | - | 1 | - | - | 1 | - | |
| Ericthonius brasiliensis (Dana, 1853) | - | 1 | 1 | - | - | - | - | |
| Ericthonius punctatus (Spence Bate, 1857) | 1 | - | - | - | - | - | - | |
| Jassa marmorata Holmes, 1905 | - | 1 | 1 | - | - | - | - | |
| Jassa slatteryi Conlan, 1990 | 1 | - | - | - | - | - | - | |
| Microjassa cumbrensis (Stebbing & Robertson, 1891) | 2 | - | - | - | - | - | - | |
| Plumulojassa ocia (Spence Bate, 1862) * | - | - | - | - | - | - | 1 | |
| Kamakidae | Cerapopsis longipes Della-Valle, 1893 | 4 | - | - | - | - | - | - |
| Leucothoidae | Leucothoe oboa Karaman, 1971 | 1 | - | - | - | - | - | - |
| Leucothoe spinicarpa (Abildgaard, 1789) | 1 | 5 | 1 | - | - | 1 | 2 | |
| Liljeborgiidae | Idunella nana (Schiecke, 1973) | - | - | 1 | - | - | - | - |
| Liljeborgia dellavallei Stebbing, 1906 | 1 | 3 | 2 | - | - | - | 1 | |
| Lysianassidae | Lysianassa caesarea Ruffo, 1987 | - | - | - | - | - | - | 1 |
| Lysianassa costae H. Milne Edwards, 1830 | - | 5 | - | - | - | - | - | |
| Lysianassa pilicornis Heller, 1866 | - | 2 | - | - | - | - | - | |
| Lysianassina longicornis (Lucas, 1846) * | - | 3 | - | - | 1 | - | 1 | |
| Maeridae | Elasmopus rapax Costa, 1853 | - | 1 | - | - | - | - | - |
| Elasmopus pectenicrus (Spence Bate, 1862) † | 1 | - | - | - | - | - | - | |
| Elasmopus pocillimanus (Spence Bate, 1862) | - | 1 | - | - | - | - | - | |
| Maera grossimana (Montagu, 1808) | - | - | 1 | - | - | - | - | |
| Maeropsis revelata (Krapp-Shcickel, Martì & Ruffo, 1996) | - | - | - | 1 | - | - | - | |
| Othomaera othonis (H. Milne Edwards, 1830) | - | 1 | - | - | - | - | - | |
| Quadrimaera inaequipes (A. Costa in Hope, 1851) | 1 | 4 | 1 | 1 | - | - | - | |
| Melitidae | Melita palmata (Montagu, 1804) | - | 1 | - | - | - | - | - |
| Nuuanuidae | Gammarella fucicola (Leach, 1814) | 3 | 2 | - | - | - | - | - |
| Oedicerotidae | Deflexilodes acutipes (Ledoyer, 1893) | - | - | - | - | - | - | - |
| Deflexilodes griseus (Della Valle, 1893) | 3 | 1 | - | - | - | - | - | |
| Monoculodes packardi Boeck, 1871 | 1 | - | - | - | - | - | - | |
| Perioculodes longimanus (Spence-Bate & Westwood, 1868) | 5 | - | - | - | - | - | - | |
| Pontocrates arenarius (Spence Bate, 1858) | 1 | - | - | - | - | - | - | |
| Synchelidium cf. longidigitatum Ruffo, 1947 | 1 | - | - | - | - | - | - | |
| Phliantidae | Pereionotus testudo (Montagu, 1808) | - | 1 | 1 | - | - | - | - |
| Photidae | Gammaropsis crenulata Krapp-Schickel & Myers, 1979 | - | 1 | - | - | - | - | - |
| Gammaropsis dentata Chevreux, 1900 | - | - | - | - | 1 | - | - | |
| Gammaropsis maculata (Johnston, 1828) | 4 | 2 | - | - | - | - | - | |
| Phoxocephalidae | Harpinia ala Karaman, 1987 | 2 | - | - | - | - | - | - |
| Harpinia antennaria Meinert, 1890 | 2 | - | - | - | - | - | - | |
| Harpinia crenulata Boeck, 1871 | 4 | - | 1 | - | - | - | - | |
| Harpinia pectinata Sars, 1891 | 4 | - | - | - | - | - | - | |
| Hippomedon massiliensis Bellan-Santini, 1965 | 2 | - | - | - | - | - | - | |
| Metaphoxus fultoni (Scott, 1890) | 2 | - | - | - | - | - | - | |
| Metaphoxus gruneri Karaman, 1986 | - | - | - | 1 | - | - | - | |
| Pleustidae | Stenopleustes nodifer (G.O. Sars, 1883) | - | 1 | - | - | - | - | - |
| Podoceridae | Parunciola seurati Chevreux, 1911 | - | 2 | - | - | - | - | - |
| Podocerus variegatus Leach, 1814 * | - | - | - | - | - | - | 1 | |
| Pontogeneiidae | Eusiroides dellavallei Chevreux, 1899 | 1 | 3 | 1 | - | - | - | - |
| Stenothoidae | Stenothoe antennulariae Della Valle, 1893 * | - | - | - | - | - | - | 1 |
| Stenothoe cavimana Chevreux, 1908 | 1 | 1 | - | - | - | - | - | |
| Stenothoe dollfusi Chevreux, 1887 | 1 | 1 | - | - | - | - | - | |
| Stenothoe monoculoides (Montagu, 1813) * | - | - | 2 | - | - | 1 | - | |
| Stenothoe pieropan Krapp-Schickel, 1996 | - | - | 1 | - | - | - | - | |
| Stenothoe tergestina (Nebeski, 1881) | 1 | 4 | - | - | - | - | - | |
| Stenothoe sp. | - | - | - | - | - | 1 | 1 | |
| Talitridae | Macarorchestia remyi (Schellenberg, 1950) | - | - | 1 | - | - | - | - |
| Tryphosidae | Lepidepecreum crypticum Ruffo & Schiecke, 1977 | - | - | 1 | - | - | - | - |
| Orchomene humilis (Costa, 1853) | - | 1 | 1 | - | - | - | - | |
| Tryphosella minima (Chevreux, 1911) | - | 1 | - | - | - | - | - | |
| Uristidae | Tmetonyx nardonis (Heller, 1867) | - | 1 | - | - | - | - | - |
| Urothoidae | Urothoe elegans Spence Bate, 1857 | 1 | - | - | - | - | - | - |
| Family | Species | Biogeographical Region | ||
|---|---|---|---|---|
| Al-Pr Basin | Adriatic Sea | Ionian Sea | ||
| Bogidiellidae | Bogidiella balearica Dancau, 1973 | 5 | - | - |
| Bogidiella cerberus Bou & Ruffo, 1979 | - | - | 1 | |
| Bogidiellidae | Racovella birramea Jaume, Gràcia & Boxshall, 2007 | 1 | - | - |
| Cheluridae | Chelura terebrans Philippi, 1839 | 1 | - | - |
| Eriopisidae | Psammogammarus burri Jaume & García, 1992 | 1 | - | - |
| Hadziidae | Hadzia fragilis S. Karaman, 1932 | - | 6 | - |
| Metahadzia minuta (Ruffo, 1947) | - | 2 | 3 | |
| Metacrangonyctidae | Metacrangonyx longipes Chevreux, 1909 | 13 | - | - |
| Niphargidae | Niphargus angelieri Ruffo, 1954 | 1 | - | - |
| Niphargus delamarei Ruffo, 1954 | 2 | - | - | |
| Niphargus hebereri Schellenberg, 1933 | - | 18 | - | |
| Niphargus pectencoronatae Sket & G. Karaman, 1990 | - | 3 | - | |
| Niphargus salonitanus S. Karaman, 195- | - | 1 | - | |
| Pseudoniphargidae | Pseudoniphargus leucatensis Bréhier & Jaum, 2009 | 1 | - | - |
| Pseudoniphargus mercadali Pretus, 1988 | 1 | - | - | |
| Salentinellidae | Salentinella angelieri Delamare-Deboutteville & Ruffo, 1952 | 17 | 2 | 2 |
| Salentinella gracillima Ruffo, 1947 | - | - | 3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro-Barranco, C.; Martínez, A.; Sempere-Valverde, J.; Chebaane, S.; Digenis, M.; Plaitis, W.; Voultsiadou, E.; Gerovasileiou, V. Amphipods in Mediterranean Marine and Anchialine Caves: New Data and Overview of Existing Knowledge. Diversity 2023, 15, 1180. https://doi.org/10.3390/d15121180
Navarro-Barranco C, Martínez A, Sempere-Valverde J, Chebaane S, Digenis M, Plaitis W, Voultsiadou E, Gerovasileiou V. Amphipods in Mediterranean Marine and Anchialine Caves: New Data and Overview of Existing Knowledge. Diversity. 2023; 15(12):1180. https://doi.org/10.3390/d15121180
Chicago/Turabian StyleNavarro-Barranco, Carlos, Alejandro Martínez, Juan Sempere-Valverde, Sahar Chebaane, Markos Digenis, Wanda Plaitis, Eleni Voultsiadou, and Vasilis Gerovasileiou. 2023. "Amphipods in Mediterranean Marine and Anchialine Caves: New Data and Overview of Existing Knowledge" Diversity 15, no. 12: 1180. https://doi.org/10.3390/d15121180
APA StyleNavarro-Barranco, C., Martínez, A., Sempere-Valverde, J., Chebaane, S., Digenis, M., Plaitis, W., Voultsiadou, E., & Gerovasileiou, V. (2023). Amphipods in Mediterranean Marine and Anchialine Caves: New Data and Overview of Existing Knowledge. Diversity, 15(12), 1180. https://doi.org/10.3390/d15121180









