Seasonal Variation in the Organization of Dung Beetle Communities in the Moroccan Middle Atlas (Coleoptera: Scarabaeoidea)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Sampling
2.3. Identification of Dung Beetles
2.4. Statistical Analysis
2.5. Environmental Data
3. Results
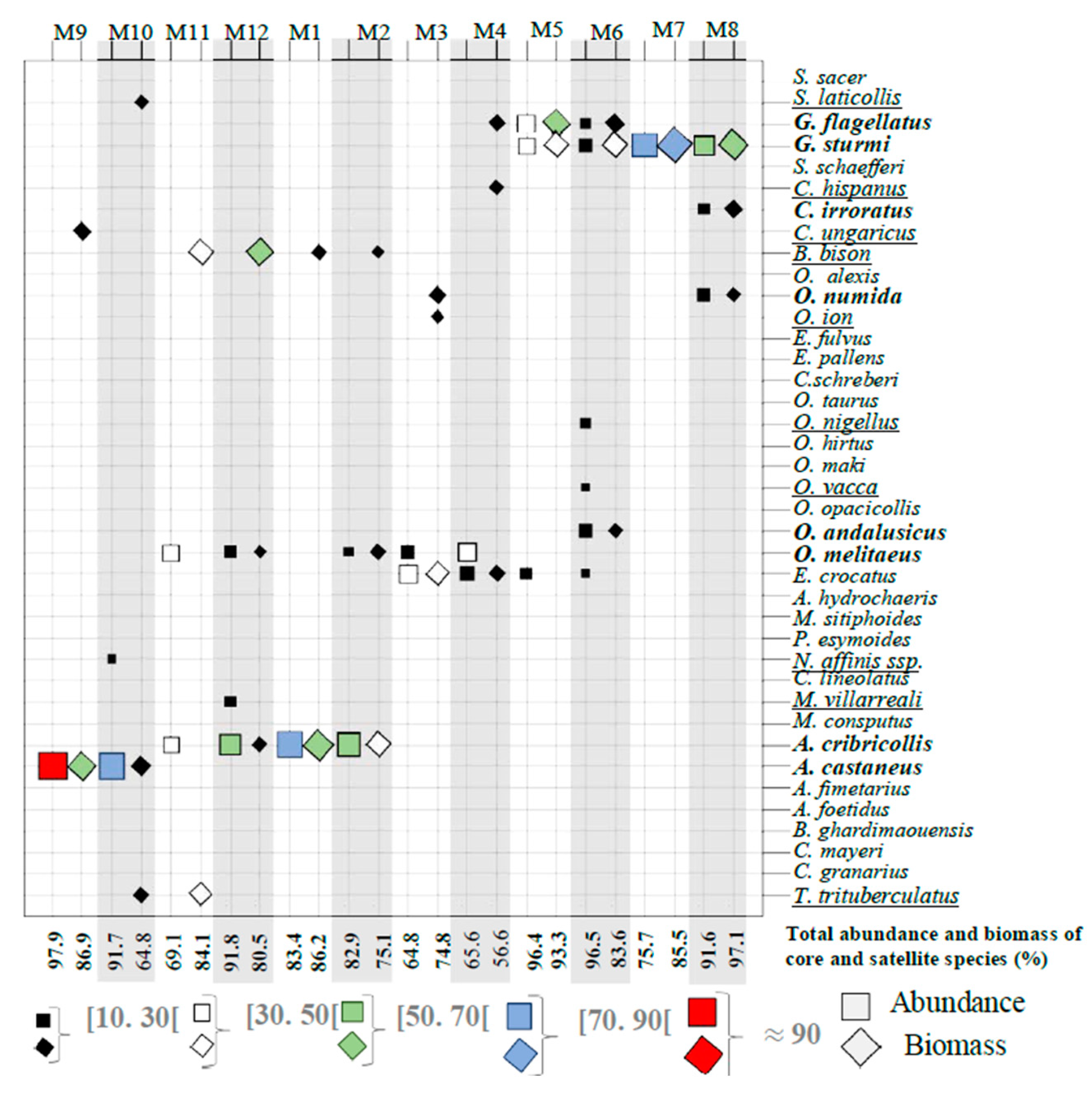
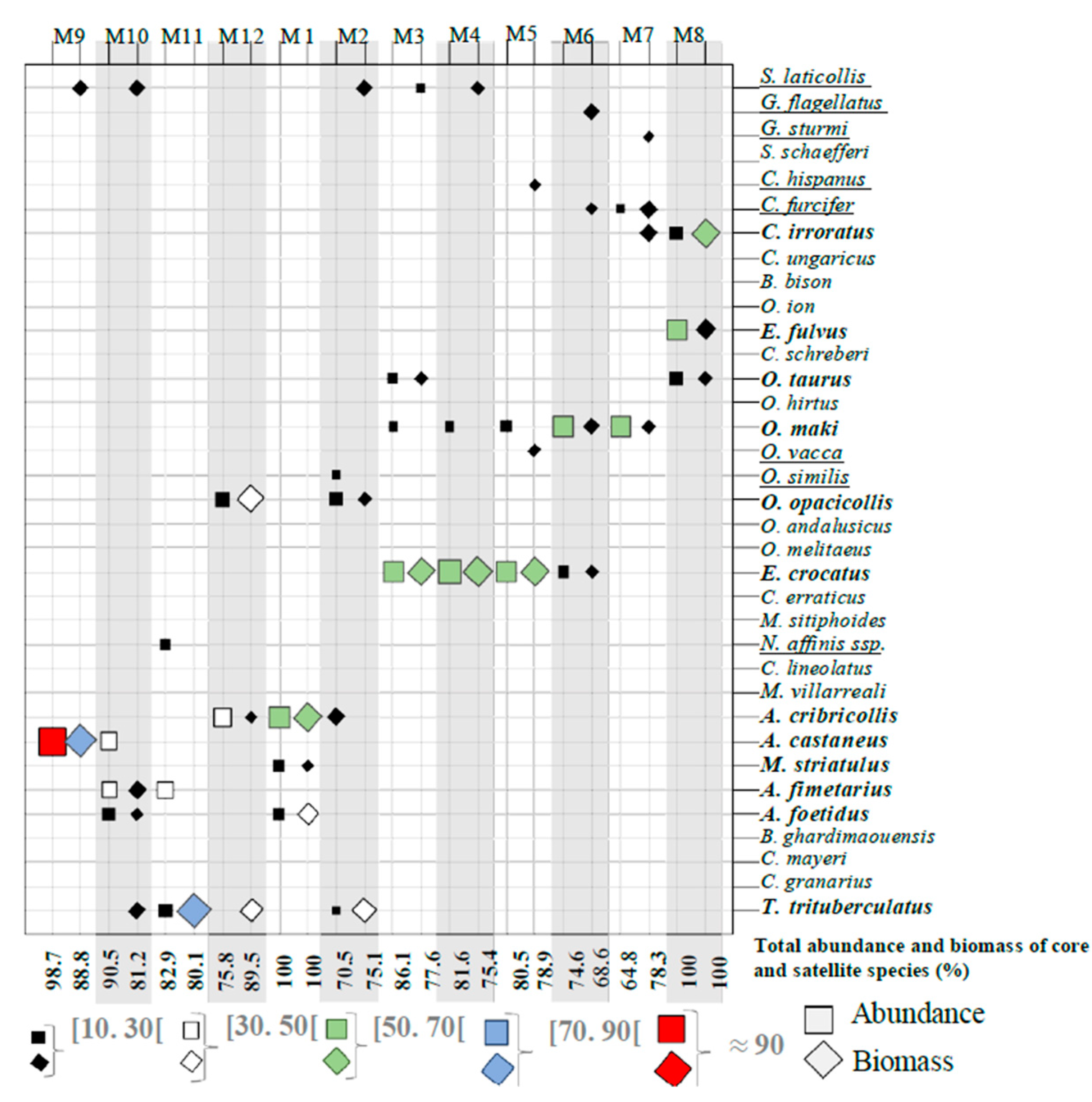
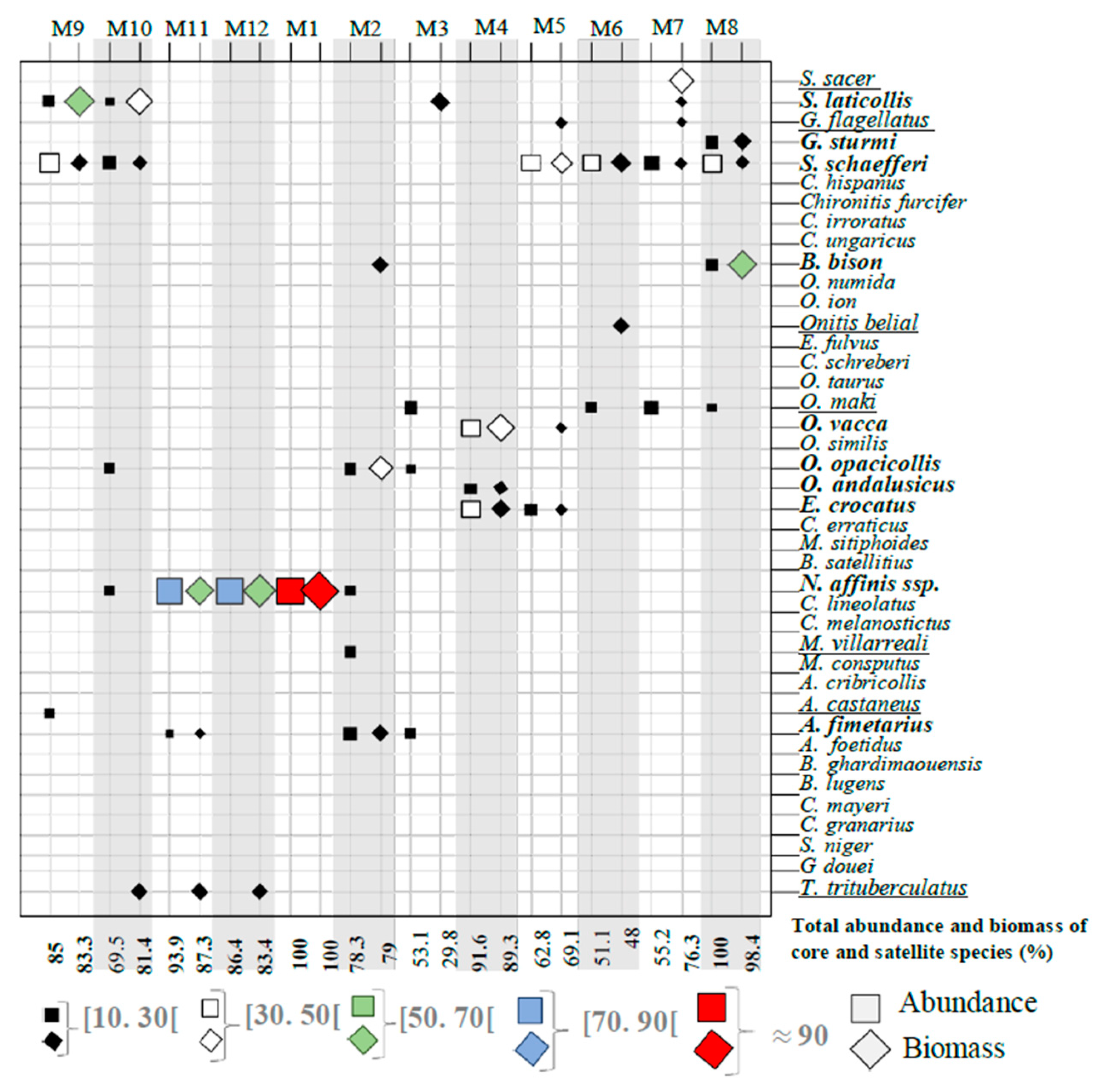
3.1. Faunal Composition of Assemblages and Organization of the Dung Beetle Communities
3.2. Core Species and Satellite Species
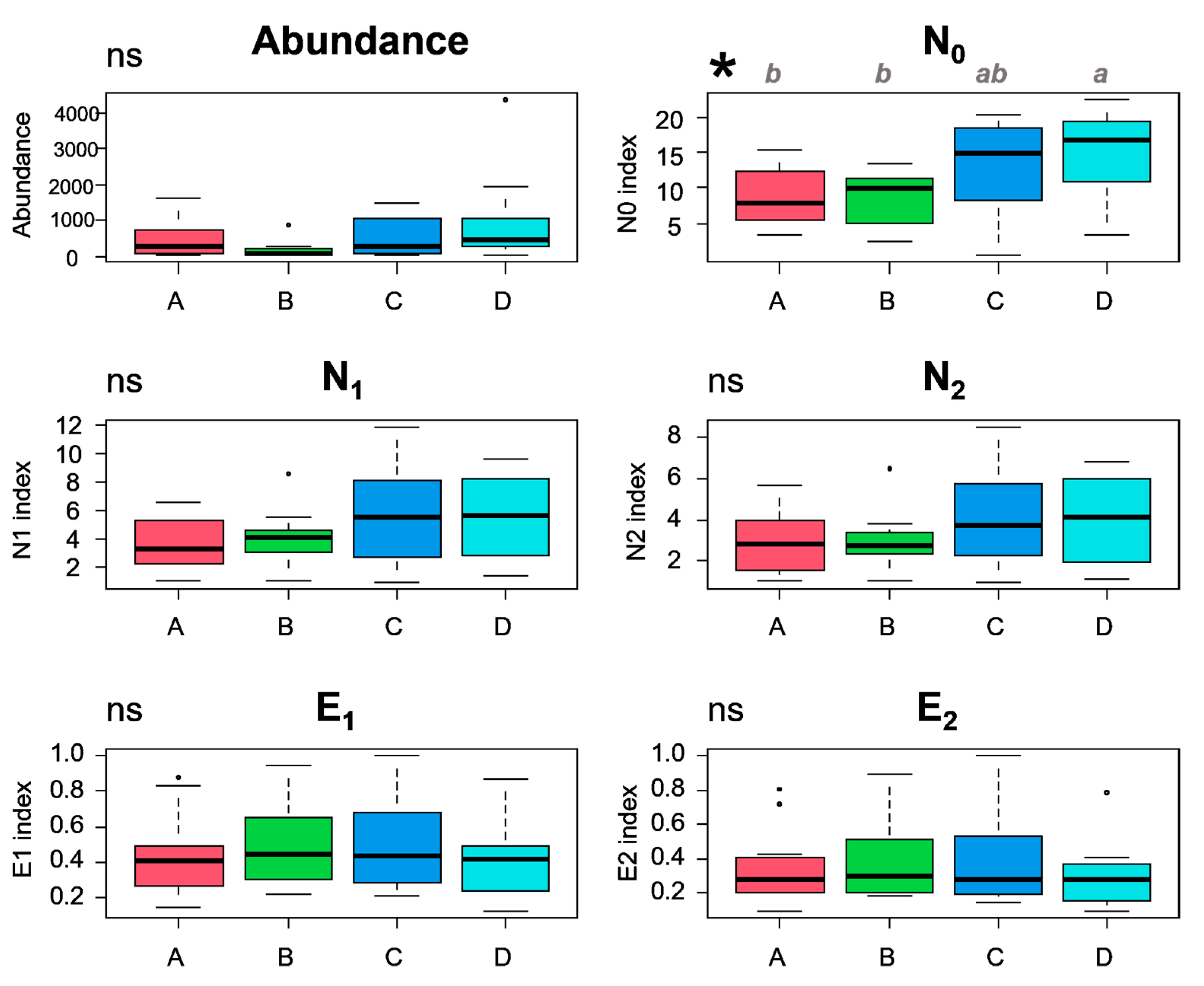
3.3. Diversity in the Communities and Environmental Variables
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Braga, R.F.; Korasaki, V.; Andresen, E.; Louzada, J. Dung beetle community and functions along a habitat-disturbance gradient in the Amazon: A rapid assessment of ecological functions associated to biodiversity. PLoS ONE 2013, 8, e57786. [Google Scholar] [CrossRef] [PubMed]
- Louzada, J.N.C.; Silva, P.R.C.E. Utilisation of introduced Brazilian pastures ecosystems by native dung beetles: Diversity patterns and resource use. Insect Conserv. Divers. 2009, 2, 45–52. [Google Scholar] [CrossRef]
- Nichols, E.; Spector, S.; Louzada, J.; Larsen, T.; Amezquita, S.; Favila, M.E. Ecological functions and ecosystem services provided by Scarabaeinae dung beetles. Biol. Conserv. 2008, 141, 1461–1474. [Google Scholar] [CrossRef]
- Stanbrook, R.; King, J.R. Dung beetle community composition affects dung turnover in subtropical US grasslands. Ecol. Evol. 2022, 12, e8660. [Google Scholar] [CrossRef]
- Bang, H.S.; Lee, J.-H.; Kwon, O.S.; Na, Y.E.; Jang, Y.S.; Kim, W.H. Effects of paracoprid dung beetles (Coleoptera: Scarabaeidae) on the growth of pasture herbage and on the underlying soil. Appl. Soil Ecol. 2005, 29, 165–171. [Google Scholar] [CrossRef]
- Vulinec, K. Dung beetles (Coleoptera: Scarabaeidae), monkeys, and conservation in Amazonia. Fla. Entomol. 2000, 83, 229–241. [Google Scholar] [CrossRef]
- Nichols, E.; Gómez, A. Dung beetles and fecal helminth transmission: Patterns, mechanisms, and questions. Parasitology 2014, 141, 614–623. [Google Scholar] [CrossRef]
- Fowler, F.; Denning, S.; Hu, S.; Watson, W. Carbon neutral: The failure of dung beetles (Coleoptera: Scarabaeidae) to affect dung-generated greenhouse gases in the pasture. Environ. Entomol. 2000, 49, 1105–1116. [Google Scholar] [CrossRef]
- Penttilä, A.; Slade, E.M.; Simojoki, A.; Riutta, T.; Minkkinen, K.; Roslin, T. Quantifying beetle-mediated effects on gas fluxes from dung pats. PLoS ONE 2013, 8, e71454. [Google Scholar] [CrossRef]
- Verdú, J.R.; Sánchez-Piñero, F.; Lobo, J.M.; Cortez, V. Evaluating long-term ivermectin use and the role of dung beetles in reducing short-term CH4 and CO2 emissions from livestock faeces: A mesocosm design under Mediterranean conditions. Ecol. Entomol. 2019, 45, 109–120. [Google Scholar] [CrossRef]
- Doube, B.M. A functional classification for the analysis of dung beetle assemblages. Ecol. Entomol. 1990, 15, 371–383. [Google Scholar] [CrossRef]
- Tonelli, M. Some considerations on the terminology applied to dung beetle functional groups. Ecol. Entomol. 2021, 46, 772–776. [Google Scholar] [CrossRef]
- Favila, M.; Halffter, G. The use of indicator groups for measuring biodiversity as related to community structure and function. Acta Zool. Mex. 1997, 72, 1–25. [Google Scholar] [CrossRef]
- Bicknell, J.; Phelps, S.; Davies, R.; Mann, D.; Struebig, M.; Davies, Z.; Davies, G. Dung beetles as indicators for rapid impact assessments: Evaluating best practice forestry in the neotropics. Ecol. Indic. 2014, 43, 154–161. [Google Scholar] [CrossRef]
- Carvalho, R.L.; Andresen, E.; Barônio, G.J.; Oliveirad, V.H.F.; Louzada, J.; Rodrigo, F.; Braga, R.F. Is dung removal a good proxy for other dung beetle functions when monitoring for conservation? A case study from the Brazilian Amazon. Ecol. Indic. 2020, 109, 105840. [Google Scholar] [CrossRef]
- Gómez-Cifuentes, A.; Munevar, A.; Gimenez, V.C.; Gatti, M.G.; Zurita, G. Influence of land use on the taxonomic and functional diversity of dung beetles (Coleoptera: Scarabaeinae) in the southern Atlantic Forest of Argentina. J. Insect Conserv. 2017, 21, 147–156. [Google Scholar] [CrossRef]
- Nunes, C.A.; Braga, R.F.; de Moura Resende, F.; Neves, F.; Figueira, J.; Fernandes, G. Linking biodiversity, the environment and ecosystem functioning: Ecological functions of dung beetles along a tropical elevational gradient. Ecosystems 2018, 21, 1244–1254. [Google Scholar] [CrossRef]
- Verdú, J.R.; Lobo, J.M.; Sánchez-Piñero, F.; Gallego, B.; Numa, C.; Lumaret, J.-P.; Cortez, V.; Ortiz, A.J.; Tonelli, M.; García-Teba, J.P.; et al. Ivermectin residues disrupt dung beetle diversity, soil properties and ecosystem functioning: An interdisciplinary field study. Sci. Total Environ. 2018, 618, 219–228. [Google Scholar] [CrossRef]
- Lumaret, J.-P.; Kadiri, N.; Martínez, M.I. The Global Decline of Dung Beetles. In Imperiled: The Encyclopedia of Conservation; DellaSala, D.A., Goldstein, M.I., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; Volume 3, pp. 553–562. [Google Scholar]
- Löbl, I.; Löbl, D. Catalogue of Palaearctic Coleoptera. Volume 3, Scarabaeoidea, Scirtoidea, Dascilloidea, Buprestoidea, Byrrhoidea; Revised and updated edition; Brill: Leiden, The Netherlands; Boston, MA, USA, 2016. [Google Scholar]
- Lumaret, J.-P.; Lobo, J.M. Geographic distribution of endemic dung beetles (Coleoptera, Scarabaeoidea) in the Western Palaearctic Region. Divers. Distrib. 1996, 3, 192–199. [Google Scholar] [CrossRef]
- Numa, C.; Tonelli, M.; Lobo, J.M.; Verdú, J.R.; Lumaret, J.-P.; Sánchez-Piñero, F.; Ruiz, J.L.; Dellacasa, M.; Ziani, S.; Arriaga, A.; et al. The Conservation Status and Distribution of Mediterranean Dung Beetles; IUCN: Gland, Switzerland; Málaga, Spain, 2020. [Google Scholar]
- Errouissi, F.; Jay-Robert, P.; Lumaret, J.-P.; Piau, O. Composition and structure of dung beetle (Coleoptera: Aphodiidae, Geotrupidae, Scarabaeidae) assemblages in mountain grasslands of the Southern Alps. Ann. Entomol. Soc. Am. 2004, 97, 701–709. [Google Scholar] [CrossRef]
- Haloti, S.; Janati-Idrissi, A.; Chergui, H.; Lumaret, J.-P. Structure des communautés de Scarabéides coprophages du Maroc nord-occidental (Coleoptera, Scarabaeoidea). Bull. Inst. Sci. Rabat Sci. Vie 2006, 28, 25–34. [Google Scholar]
- Lobo, J.M.; Lumaret, J.-P.; Jay-Robert, P. Diversity, distinctiveness, and conservation status of the Mediterranean coastal dung beetle assemblages in the Regional Natural Park of the Camargue (France). Divers. Distrib. 2001, 7, 257–270. [Google Scholar] [CrossRef]
- Horgan, F.G. Burial of bovine dung by coprophagous beetles (Coleoptera: Scarabaeidae) from horse and cow grazing sites in El Salvador. Eur. J. Soil Biol. 2001, 37, 103–111. [Google Scholar] [CrossRef]
- Lobo, J.M. Estimation of dung beetle biomass (Coleoptera: Scarabaeoidea). Eur. J. Entomol. 1993, 90, 235–238. [Google Scholar]
- Lumaret, J.-P.; Kadiri, N.; Bertrand, M. Changes in resources: Consequences for the dynamics of dung beetle communities. J. Appl. Ecol. 1992, 29, 349–356. [Google Scholar] [CrossRef]
- Kadiri, N. Effets des Activités Sylvo-Pastorales sur la Structure et la Dynamique des Communautés de Scarabéidés Coprophages en Région Méditerranéenne. Ph.D. Dissertation, Faculté des Sciences et Techniques de Saint-Jérôme, Université Aix-Marseille 3, Aix-en-Provence, France, 1993. [Google Scholar]
- Stiernet, N.; Lumaret, J.-P. Organisation des peuplements de Scarabéidés coprophages de Vanoise (Insectes Coléoptères). In Sciences Naturelles et Montagnes; Deloince, R., Ed.; Actes 116ème Congrès national des Sociétés historiques et scientifiques, Chambéry-Annecy 1991; Les Editions du CTHS (Publ.): Paris, France, 1993; pp. 225–239. [Google Scholar]
- Hanski, I. Dynamics of regional distribution: The core and satellite species hypothesis. Oikos 1982, 38, 210–221. [Google Scholar] [CrossRef]
- Kadiri, N.; Lumaret, J.-P.; Floate, K.D. Functional diversity and seasonal activity of dung beetles (Coleoptera: Scarabaeoidea) on native grasslands in southern Alberta, Canada. Can. Entomol. 2014, 146, 291–305. [Google Scholar] [CrossRef]
- Hanski, I.; Cambefort, Y. Dung Beetle Ecology; Princeton University Press: Princeton, NJ, USA, 1991. [Google Scholar]
- Janati-Idrissi, A. Les Scarabéides Coprophages des Pelouses Sèches du Maroc Occidental: Structure des Communautés et Rôle Ecologique. Ph.D. Dissertation, Faculté des Sciences Dhar el Mahraz, Univ. Sidi Mohamed Ben Abdellah, Fez, Morocco, 2000. [Google Scholar]
- Lobo, J.M.; Martin-Piera, F.; Veiga, C.M. Las trampas con cebo, sus posibilidades en el estudio de la communidades coprófagas de Scarabaeoidea (Col.). I: Características determinantes de su capacidad de captura. Rev. Ecol. Biol. Sol. 1988, 25, 77–100. [Google Scholar]
- Baraud, J. Coléoptères Scarabaeoidea: Faune du Nord de l’Afrique, du Maroc au Sinaï; Lechevalier Publ.: Paris, France, 1985. [Google Scholar]
- Dray, S.; Dufour, A.-B. The ade4 Package: Implementing the Duality Diagram for Ecologists. J. Stat. Softw. 2007, 22, 1–20. [Google Scholar] [CrossRef]
- Borcard, D.; Gillet, F.; Legendre, P. Numerical Ecology with R, 2nd ed.; Springer International Publ.: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Alatalo, R.; Alatalo, R. Components of diversity: Multivariate analysis with interaction. Ecology 1977, 58, 900–906. [Google Scholar] [CrossRef]
- Legendre, P.; Legendre, L. Numerical Ecology, 3rd ed.; Elsevier Publ.: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Hill, M.O. Diversity and evenness: A unifying notation and its consequences. Ecology 1973, 54, 427–432. [Google Scholar] [CrossRef]
- Peet, R.K. The measurement of species diversity. Annu. Rev. Ecol. Evol. Syst. 1974, 5, 285–307. [Google Scholar] [CrossRef]
- Alatalo, R.V. Problems in the measurement of evenness in ecology. Oikos 1981, 37, 199–204. [Google Scholar] [CrossRef]
- Brignon, C.; Sauvage, C.H. Carte des étages bioclimatiques au 1/2,000,000. Comité national Géogr. du Maroc: Atlas du Maroc, sect. II. Bull. Inst. Sci. de Rabat, 1962. [Google Scholar]
- Nealis, V.G. Habitat associations and community analysis of South Texas dung beetles (Coleoptera: Scarabaeinae). Can. J. Zool. 1977, 55, 138–147. [Google Scholar] [CrossRef]
- Lumaret, J.-P. Structure des peuplements de coprophages Scarabaeidae en région méditerranéenne française: Relations entre les conditions écologiques et quelques paramètres biologiques des espèces. Bull. Soc. Entomol. Fr. 1983, 88, 481–495. [Google Scholar] [CrossRef]
- Lumaret, J.P.; Kirk, A. Ecology of dung beetles in the French Mediterranean region (Coleoptera: Scarabaeidae). Acta Zool. Mex. (Nueva Ser.) 1987, 24, 1–55. [Google Scholar]
- Lumaret, J.-P.; Stiernet, N. Biogeography of dung beetle communities in the western and central Alps (Coleoptera, Scarabaeoidea). Biogeographia 1992, 16, 425–436. [Google Scholar]
- Floate, K.D.; Kadiri, N. Dung beetles (Coleoptera: Scarabaeidae) associated with cattle dung on native grasslands of southern Alberta, Canada. Can. Entomol. 2013, 145, 647–654. [Google Scholar] [CrossRef]
- Halffter, G.; Matthews, E.G. The natural history of dung beetles of the subfamily Scarabaeinae (Coleoptera: Scarabaeidae). Folia Entomol. Mex. 1966, 12–14, 1–312. [Google Scholar]
- Jay-Robert, P.; Lumaret, J.-P.; Lebreton, J.D. Spatial and temporal variation of mountain dung beetle assemblages and their relationships with environmental factors (Aphodiinae: Geotrupinae: Scarabaeinae). Ann. Entomol. Soc. Am. 2008, 101, 58–69. [Google Scholar] [CrossRef]
- Lobo, J.M.; Guéorguiev, B.; Chehlarov, E. Convergences and divergences between two European mountain dung beetle assemblages (Coleoptera, Scarabaeoidea). Anim. Biodivers. Conserv. 2007, 30, 83–96. [Google Scholar] [CrossRef]
- Menéndez, R.; Gutiérrez, D. Altitudinal effects on habitat selection of dung beetles (Scarabaeoidea Aphodiidae) in the northern Iberian Peninsula. Ecography 1996, 19, 313–317. [Google Scholar] [CrossRef]
- Janati-Idrissi, A.; Kadiri, N.; Lumaret, J.-P. Le partage du temps et de l’espace entre les guildes de Coléoptères coprophages dans le Moyen-Atlas (Maroc). Ann. Soc. Entomol. Fr. 1999, 35, 213–221. [Google Scholar]
- Mantoni, C.; Tsafack, N.; Palusci, E.; Di Pietro, S.; Fattorini, S. Diversity patterns of dung beetles along a Mediterranean elevational gradient. Insects 2021, 12, 781. [Google Scholar] [CrossRef]
- Lobo, J.M. Species diversity and composition of dung beetle (Coleoptera: Scarabaeoidea) assemblages in North America. Can. Entomol. 2000, 132, 307–321. [Google Scholar] [CrossRef]
- Martínez, I.M.; Lumaret, J.-P. Escarabajos Estercoleros: Biología Reproductiva y su Regulación (Coleoptera: Scarabaeidae y Geotrupidae); Asociación Española de Entomología Publ.: Madrid, Spain, 2022; 406p, Available online: https://www.entomologica.es/cont/publicaciones/docs/Docpubli1_26.pdf (accessed on 1 February 2023).
- Lumaret, J.-P. Desiccation rate of excrements: A selective pressure on dung beetles. In Time Scales of Biological Responses to Water Constraints. The Case of Mediterranean Biota; Roy, J., Aronson, J., Di Castri, F., Eds.; SPB Academic Publishing: Amsterdam, The Netherlands, 1995; pp. 105–118. [Google Scholar]
- Sanders, N.J.; Rahbek, C. The patterns and causes of elevational diversity gradients. Ecography 2012, 35, 1–3. [Google Scholar] [CrossRef]
- Wolda, H. Insect seasonality: Why? Annu. Rev. Ecol. Evol. Syst. 1988, 19, 1–18. [Google Scholar] [CrossRef]
- Tshikae, B.P.; Davis, A.L.; Scholtz, C.H. Species richness—Energy relationships and dung beetle diversity across an aridity and trophic resource gradient. Acta Oecol. Oecol. Gen. 2013, 49, 71–82. [Google Scholar] [CrossRef]
- Daoudi, L.; Chavanon, G.; Taybi, A.F.; Mabrouki, Y. Composition and phenology of the beetle community (Coleoptera: Scarabaeoidea, Staphylinidae, Histeridae, Hydrophilidae) associated to dung of equines in an arid environment. Ann. Soc. Entomol. Fr. 2022, 58, 155–164. [Google Scholar] [CrossRef]




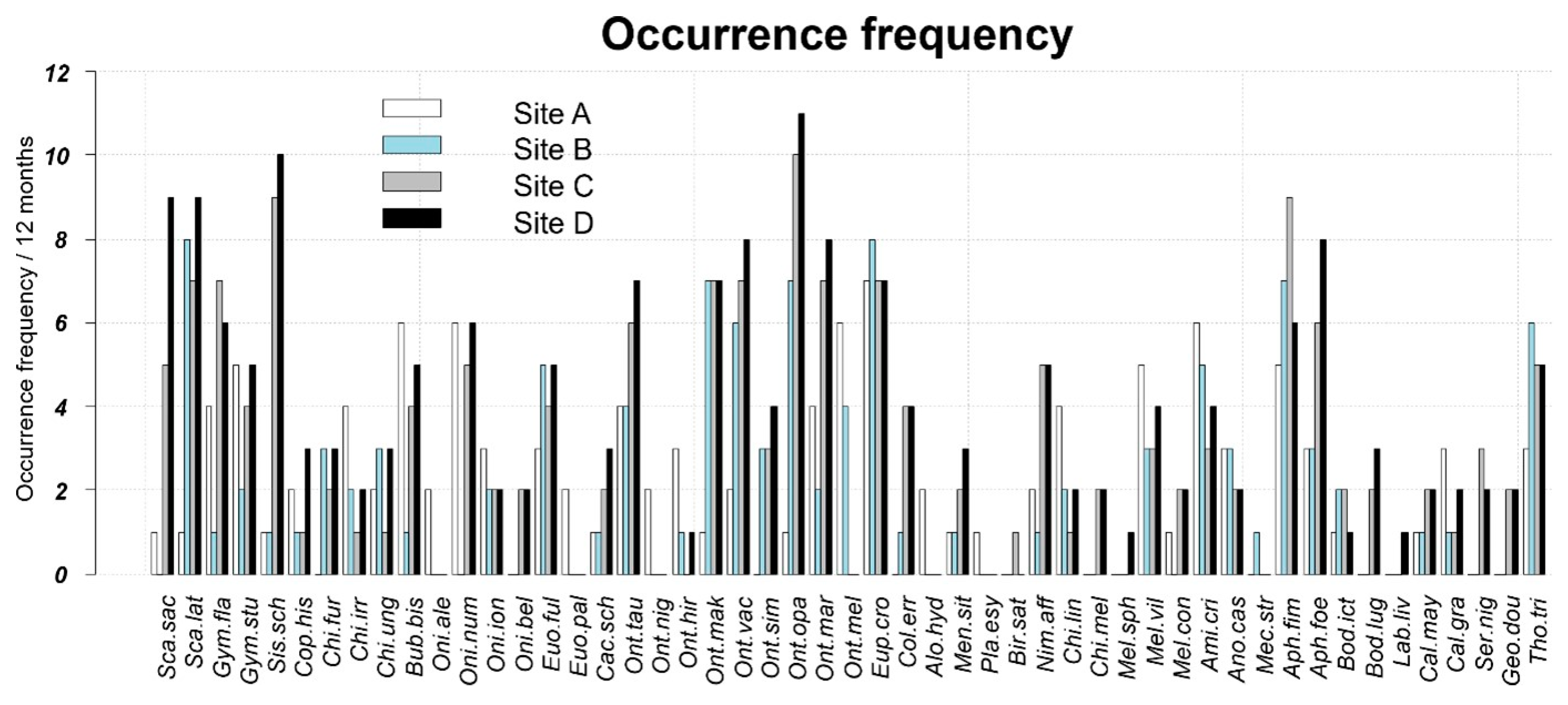


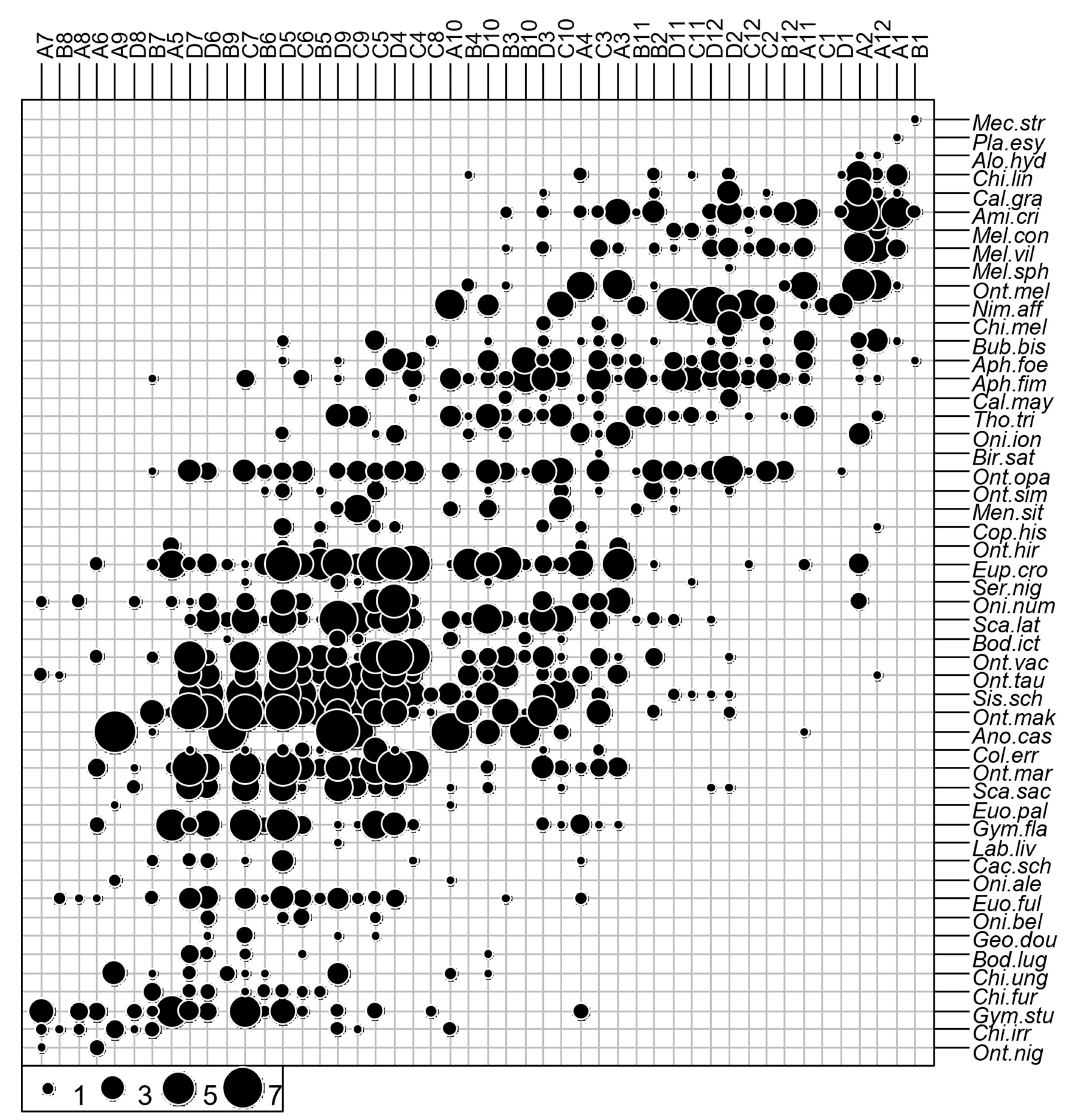
| Family/Subfamily | Species List | Individual Biomass (mg) | Guild | Presence or Absence in Stations | |||
|---|---|---|---|---|---|---|---|
| Fez-Sais | Immouzzer | Ifrane I | Ifrane II | ||||
| Scarabaeinae | Scarabaeus sacer Linnaeus 1758 | 650.3 | T | X | 0 | X | X |
| Scarabaeus laticollis Linnaeus 1767 | 173.0 | T | X | X | X | X | |
| Gymnopleurus flagellatus (Fabricius 1787) | 98.9 | T | X | X | X | X | |
| Gymnopleurus sturmi (McLeay 1821) | 85.0 | T | X | X | X | X | |
| Sisyphus schaefferi (Linnaeus 1758) | 29.0 | T | X | X | X | X | |
| Copris hispanus (Linnaeus 1764) | 427.6 | P | X | X | X | X | |
| Cheironitis furcifer (Rossi 1792) | 68.5 | P | 0 | X | X | X | |
| Cheironitis irroratus (Rossi 1790) | 93.7 | P | X | X | X | X | |
| Cheironitis ungaricus (Rossi 1790) | 92.8 | P | X | X | X | X | |
| Bubas bison (Linnaeus 1767) | 161.6 | P | X | X | X | X | |
| Onitis alexis Klug 1835 | 128.5 | P | X | 0 | X | 0 | |
| Onitis numida (Laporte de Castelnau 1840) | 51.3 | P | X | 0 | X | X | |
| Onitis ion (Olivier 1789) | 44.7 | P | X | X | X | X | |
| Onitis belial (Fabricius 1798) | 433.3 | P | 0 | 0 | X | X | |
| Euoniticellus fulvus (Goeze 1777) | 25.0 | P | X | X | X | X | |
| Euoniticellus pallens (Olivier 1789) | 19.5 | P | X | 0 | 0 | 0 | |
| Caccobius schreberi (Linnaeus 1758) | 7.0 | P | X | X | X | X | |
| Onthophagus (s.str.) taurus (Schreber 1759) | 21.6 | P | X | X | X | X | |
| Onthophagus (Parentius) nigellus (Illiger 1803) | 5.5 | P | X | 0 | 0 | 0 | |
| Onthophagus (Trichonthophagus) hirtus (Illiger 1803) | 11.5 | P | X | X | 0 | X | |
| Onthophagus (Trichonthophagus) maki (Illiger 1803) | 10.0 | P | X | X | X | X | |
| Onthophagus (Palaeonthophagus) vacca (Linnaeus 1767) | 41.4 | P | X | X | X | X | |
| Onthophagus (Palaeonthophagus) similis (Scriba 1790) | 7.6 | P | 0 | X | X | X | |
| Onthophagus (Palaeonthophagus) opacicollis Reitter 1893 | 22.0 | P | X | X | X | X | |
| Onthophagus (Palaeonthophagus) marginalis spp. andalusicus Waltl 1835 | 31.7 | P | X | X | X | X | |
| Onthophagus (Amphionthophagus) melitaeus (Fabricius 1798) | 7.6 | P | X | X | 0 | 0 | |
| Euonthophagus crocatus (Mulsant & Godart 1873) | 22.1 | P | X | X | X | X | |
| Aphodiinae | Colobopterus erraticus (Linnaeus 1758) | 22.1 | P | 0 | X | X | X |
| Alocoderus hydrochaeris (Fabricius 1798) | 11.1 | D | X | 0 | 0 | 0 | |
| Mendidaphodius sitiphoides (D’Orbigny 1896) | 3.4 | D | X | X | X | X | |
| Plagiogonus esymoides (Reitter 1892) | 2.7 | D | X | 0 | 0 | 0 | |
| Biralus satellitius (Herbst 1789) | 23.0 | D | 0 | 0 | X | 0 | |
| Nimbus affinis ssp. orbignyi (Clouët des Pesruches 1896) | 5.8 | D | X | X | X | X | |
| Chilothorax lineolatus (Illiger 1803) | 4.7 | D | X | X | X | X | |
| Chilothorax melanostictus (W. Schmidt 1840) | 3.5 | D | 0 | 0 | X | X | |
| Melinopterus sphacelatus (Panzer 1798) | 3.8 | D | 0 | 0 | 0 | X | |
| Melinopterus villarreali Baraud 1975 | 4.3 | D | X | X | X | X | |
| Melinopterus consputus (Creutzer 1799) | 3.6 | D | X | 0 | X | X | |
| Amidorus cribricollis (Lucas 1846) | 4.1 | D | X | X | X | X | |
| Anomius castaneus (Illiger 1803) | 3.0 | D | X | X | X | X | |
| Mecynodes striatulus (Waltl 1835) | 3.1 | D | 0 | X | 0 | 0 | |
| Aphodius (s.str.) fimetarius (Linnaeus 1758) | 10.0 | D | X | X | X | X | |
| Aphodius (s.str.) foetidus (Herbst 1783) | 7.0 | D | X | X | X | X | |
| Bodiloides ictericus ghardimaouensis (Balthasar 1929) | 4.0 | D | X | X | X | X | |
| Bodilus lugens (Creutzer 1799) | 5.1 | D | 0 | 0 | X | X | |
| Labarrus lividus (Olivier 1789) | 4.7 | D | 0 | 0 | 0 | X | |
| Calamosternus mayeri (Pilleri 1953) | 3.6 | D | X | X | X | X | |
| Calamosternus granarius (Linnaeus 1767) | 3.3 | D | X | X | X | X | |
| Geotrupidae | Sericotrupes niger (Marsham 1802) | 210.3 | P | 0 | 0 | X | X |
| Geotrupes (Stereopyge) douei Gory 1841 | 523.7 | P | 0 | 0 | X | X | |
| Thorectes trituberculatus (Reitter 1893) | 129.0 | P | X | X | X | X | |
| Total Species | 51 | 39 | 35 | 42 | 43 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hajji, H.; Janati-Idrissi, A.; Taybi, A.F.; Caron, V.; Lumaret, J.-P.; Mabrouki, Y. Seasonal Variation in the Organization of Dung Beetle Communities in the Moroccan Middle Atlas (Coleoptera: Scarabaeoidea). Diversity 2023, 15, 1138. https://doi.org/10.3390/d15111138
Hajji H, Janati-Idrissi A, Taybi AF, Caron V, Lumaret J-P, Mabrouki Y. Seasonal Variation in the Organization of Dung Beetle Communities in the Moroccan Middle Atlas (Coleoptera: Scarabaeoidea). Diversity. 2023; 15(11):1138. https://doi.org/10.3390/d15111138
Chicago/Turabian StyleHajji, Hasnae, Abdellatif Janati-Idrissi, Abdelkhaleq Fouzi Taybi, Valérie Caron, Jean-Pierre Lumaret, and Youness Mabrouki. 2023. "Seasonal Variation in the Organization of Dung Beetle Communities in the Moroccan Middle Atlas (Coleoptera: Scarabaeoidea)" Diversity 15, no. 11: 1138. https://doi.org/10.3390/d15111138
APA StyleHajji, H., Janati-Idrissi, A., Taybi, A. F., Caron, V., Lumaret, J.-P., & Mabrouki, Y. (2023). Seasonal Variation in the Organization of Dung Beetle Communities in the Moroccan Middle Atlas (Coleoptera: Scarabaeoidea). Diversity, 15(11), 1138. https://doi.org/10.3390/d15111138









