Preliminary Assessment of Sea Star (Echinodermata, Asteroidea) Diversity in the Coastal Magellanic Region (South Chile) and Their Geographical Distribution
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Morphological Identification
2.2. COI Amplification
2.3. DNA Barcoding and Database Compilation
2.4. Bioinformatics
3. Results
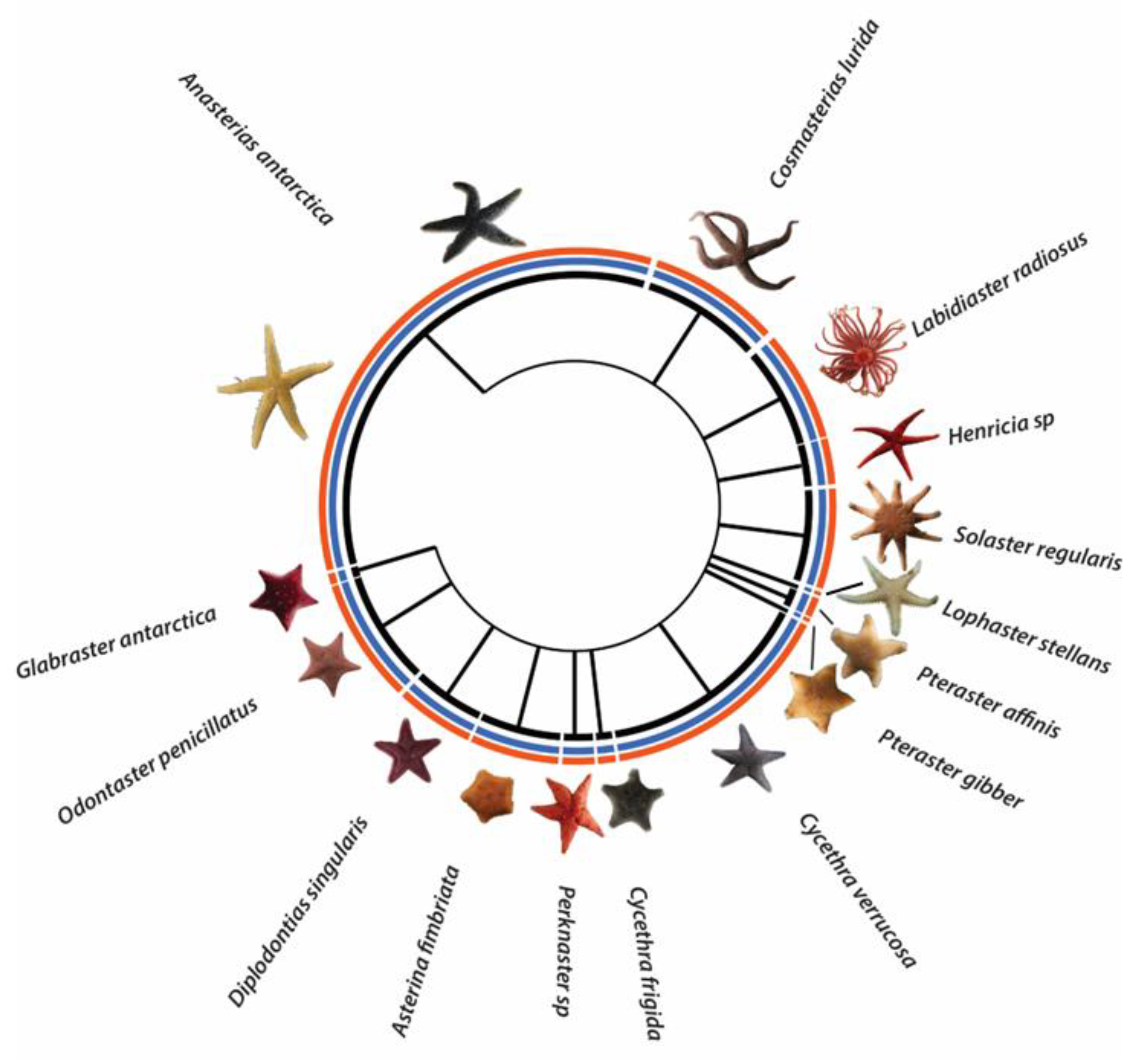
3.1. Sea Star Diversity in the Magellanic Region
| Order | Family | Genus | Species | N New Sequences | N Sequences BOLD | N Total | π | N Haplotypes | Hd | Genetic Variability (%) | BOLD BIN | Developmental Mode |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Forcipulatida | Asteriidae | Anasterias | Anasterias antarctica Lütken, 1857 [61] | 93 | 19 | 112 | 0.0039 | 14 | 0.622 | 0.38 | BOLD:AAA8344 | Brooder 1,2 |
| Heliasteridae | Labidiaster | Labidiaster radiosus Lütken, 1871 [62] | 17 | 2 | 19 | 0.0042 | 9 | 0.813 | 0.43 | BOLD:ACB6572 | Unknown | |
| Stichasteridae | Cosmasterias | Cosmasterias lurida Philippi, 1858 [63] | 21 | 0 | 21 | 0.0055 | 12 | 0.929 | 0.57 | Private | Broadcaster 1 | |
| Spinulosida | Echinasteridae | Henricia | Henricia sp. | 7 | 0 | 7 | 0.0051 | 5 | 0.905 | 0.51 | No match | Unknown |
| Valvatida | Asterinidae | Asterina | Asterina fimbriata Perrier, 1875 [64] | 13 | 5 | 18 | 0.0023 | 6 | 0.699 | 0.23 | BOLD:ACI1273 | Brooder 1 |
| Ganeriidae | Cycethra | Cycethra verrucosa Philippi, 1857 [65] | 33 | 0 | 33 | 0.0106 | 14 | 0.884 | 0.95 | BOLD:AAR5363 | Unknown | |
| Cycethra frigida Koehler, 1917 [66] | 3 | 12 | 15 | 0.0017 | 5 | 0.695 | 0.17 | BOLD:ADG2622 | Broadcaster 2 | |||
| Peknaster | Perknaster sp. | 5 | 0 | 5 | 0.0021 | 4 | 0.900 | 0.31 | No match | Broadcaster 1,3 | ||
| Odontasteridae | Diplodontias | Diplodontias singularis Müller and Troschel, 1843 [67] | 11 | 0 | 11 | 0.0023 | 6 | 0.855 | 0.23 | BOLD:AEH4090 | Unknown | |
| Odontaster | Odontaster penicillatus Philippi, 1870 [68] | 19 | 45 | 64 | 0.0049 | 27 | 0.936 | 0.58 | BOLD:ABW1983 | Broadcaster 1,3 | ||
| Poraniidae | Glabraster | Glabraster antarctica Smith, 1876 [69] | 20 | 357 | 377 | 0.0171 | 146 | 0.980 | 1.71 | BOLD:AAB6633 | Broadcaster 1,3 | |
| Solasteridae | Solaster | Solaster regularis Sladen, 1889 [70] | 15 | 5 | 20 | 0.0027 | 9 | 0.842 | 0.27 | BOLD:AAM2777 | Unknown | |
| Lophaster | Lophaster stellans Sladen, 1889 [70] | 1 | 1 | 2 | NA | 2 | NA | NA | BOLD:AAE4820 | Unknown | ||
| Velatida | Pterasteridae | Pteraster | Pteraster affinis * Smith, 1876 [69] | 3 | 20 | 23 | 0.0018 | 10 | 0.707 | 0.18 | BOLD:AAC7424 | Brooder and broadcaster 1,4 |
| Pteraster gibber Sladen, 1882 [71] | 1 | 9 | 10 | 0.0038 | 4 | 0.533 | 0.37 | BOLD:ABW2318 | Unknown | |||
| TOTAL | 262 | 475 | 737 |
3.2. Biogeography and Faunal Affinities
3.2.1. Occurrences vs. Barcoded Occurrences
3.2.2. Estimating Species Distribution
- South America-only
- 2.
- Sub-Antarctic
- 3.
- Sub-Antarctic and Antarctic Peninsula
- 4.
- True circum-Antarctic
4. Discussion
4.1. Sea Star Diversity in the Magellanic Region
4.1.1. Species Richness
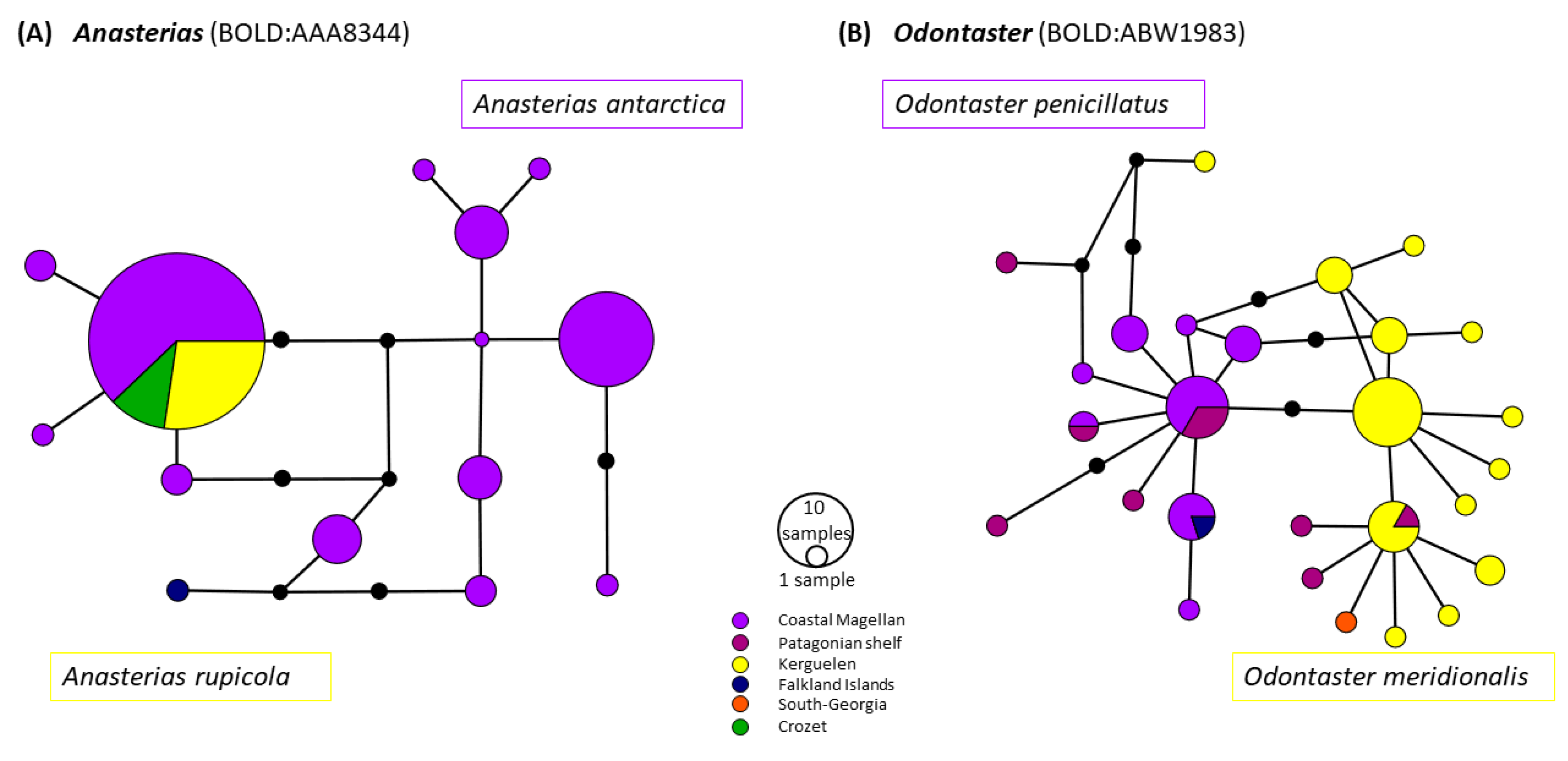
4.1.2. Taxonomic Discrepancies Highlighting the Importance of an Integrative Approach
4.2. Geographical Patterns of Magellanic Sea Stars in the Southern Ocean
Factors Influencing Species Distribution
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Griffiths, H.J.; Waller, C.L. The first comprehensive description of the biodiversity and biogeography of Antarctic and Sub-Antarctic intertidal communities. J. Biogeogr. 2016, 43, 1143–1155. [Google Scholar] [CrossRef]
- Moreau, C.; Saucede, T.; Jossart, Q.; Agüera, A.; Brayard, A.; Danis, B. Reproductive strategy as a piece of the biogeographic puzzle: A case study using Antarctic sea stars (Echinodermata, Asteroidea). J. Biogeogr. 2017, 44, 848–860. [Google Scholar] [CrossRef]
- Pérez-Ruzafa, A.; Alvarado, J.J.; Solís-Marín, F.A.; Hernández, J.C.; Morata, A.; Marcos, C.; Abreu-Pérez, M.; Aguilera, O.; Alió, J.; Bacallado-Aránega, J.J.; et al. Latin America Echinoderm Biodiversity and Biogeography: Patterns and Affinities; Echinoderm research and diversity in Latin America: Berlin/Heidelberg, Germany, 2013; pp. 511–542. [Google Scholar]
- Moreau, C.; Jossart, Q.; Danis, B.; Eléaume, M.; Christiansen, H.; Guillaumot, C.; Downey, R.; Saucède, T. The high diversity of Southern Ocean sea stars (Asteroidea) reveals original evolutionary pathways. Prog. Oceanogr. 2021, 190, 102472. [Google Scholar] [CrossRef]
- Fraysse, C.; Calcagno, J.; Pérez, A.F. Asteroidea of the southern tip of South America, including Namuncurá Marine Protected Area at Burdwood Bank and Tierra del Fuego Province, Argentina. Polar Biol. 2018, 41, 2423–2433. [Google Scholar] [CrossRef]
- Rahman, M.A.; Molla, M.H.R.; Megwalu, F.O.; Asare, O.E.; Tchoundi, A.; Shaikh, M.M. The sea stars (Echinodermata: Asteroidea): Their biology, ecology, evolution and utilization. SF J. Biotechnol. Biomed. Eng. 2018, 1, 1007. [Google Scholar]
- Le Bourg, B. Trophic Ecology of Southern Ocean Sea Stars: Influence of Environmental Drivers on Trophic Diversity. Ph.D. Thesis, Université de Liège, Liège, Belgium, 2020. [Google Scholar]
- Moreau, C. Diversity and Phylogeography of Southern Ocean Sea Stars (Asteroidea). Ph.D. Thesis, Université Bourgogne Franche-Comté; Université Libre de Bruxelles, Bruxelles, Belgium, 2019. [Google Scholar]
- Janosik, A.M.; Mahon, A.R.; Halanych, K.M. Evolutionary history of Southern Ocean Odontaster sea star species (Odontasteridae; Asteroidea). Polar Biol. 2011, 34, 575–586. [Google Scholar] [CrossRef]
- Mutschke, E.; Gerdes, D.; Ríos, C. Distribution and abundance patterns of echinoderms in the fjord and channel complex from a subantarctic north Patagonian Ice field, Magellan region. Rev. Biol. Trop. 2017, 65, S60–S72. [Google Scholar] [CrossRef][Green Version]
- Jossart, Q.; Kochzius, M.; Danis, B.; Saucède, T.; Moreau, C.V. Diversity of the Pterasteridae (Asteroidea) in the Southern Ocean: A molecular and morphological approach. Zool. J. Linn. Soc. 2021, 192, 105–116. [Google Scholar] [CrossRef]
- Guzzi, A.; Alvaro, M.C.; Danis, B.; Moreau, C.; Schiaparelli, S. Not all that glitters is gold: Barcoding effort reveals taxonomic incongruences in iconic Ross Sea sea stars. Diversity 2022, 14, 457. [Google Scholar] [CrossRef]
- Madsen, F.J. Asteroidea: With a Survey of the Asteroidea of the Chilean Shelf; CWK Gleerup: 1956. Available online: https://www.marinespecies.org/aphia.php?p=sourcedetails&id=125933 (accessed on 3 August 2023).
- Moles, J.; Figuerola, B.; Campanya-Llovet, N.; Monleon-Getino, T.; Taboada, S.; Avila, C. Distribution patterns in Antarctic and Subantarctic echinoderms. Polar Biol. 2015, 38, 799–813. [Google Scholar] [CrossRef]
- Moore, J.M.; Carvajal, J.I.; Rouse, G.W.; Wilson, N.G. The Antarctic Circumpolar Current isolates and connects: Structured circumpolarity in the sea star Glabraster antarctica. Ecol. Evol. 2018, 8, 10621–10633. [Google Scholar] [CrossRef] [PubMed]
- Mah, C.L.; Foltz, D.W. New taxa and taxonomic revisions to the Poraniidae (Valvatacea; Asteroidea) with comments on feeding biology. Zootaxa 2014, 3795, 327–372. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.G.; Pérez-Portela, R.; Griffiths, C.L. Determining the correct identity of South African Marthasterias (Echinodermata: Asteroidea). Afr. J. Mar. Sci. 2016, 38, 443–455. [Google Scholar] [CrossRef]
- Layton, K.K.; Corstorphine, E.A.; Hebert, P.D. Exploring Canadian echinoderm diversity through DNA barcodes. PLoS ONE 2016, 11, e0166118. [Google Scholar] [CrossRef]
- Knott, K.E.; Ringvold, H.; Blicher, M.E. Morphological and molecular analysis of Henricia Gray, 1840 (Asteroidea: Echinodermata) from the Northern Atlantic Ocean. Zool. J. Linn. Soc. 2018, 182, 791–807. [Google Scholar] [CrossRef]
- Uthicke, S.; Byrne, M.; Conand, C. Genetic barcoding of commercial Bêche-de-mer species (Echinodermata: Holothuroidea). Mol. Ecol. Resour. 2010, 10, 634–646. [Google Scholar] [CrossRef]
- Christiansen, H.; Dettai, A.; Heindler, F.M.; Collins, M.A.; Duhamel, G.; Hautecoeur, M.; Steinke, D.; Volckaert, F.A.M.; Van de Putte, A.P. Diversity of mesopelagic fishes in the Southern Ocean—A phylogeographic perspective using DNA barcoding. Front. Ecol. Evol. 2018, 6, 120. [Google Scholar] [CrossRef]
- Peck, L.S.; Clark, M.S.; Dunn, N.I. Morphological variation in taxonomic characters of the Antarctic starfish Odontaster validus. Polar Biol. 2018, 41, 2159–2165. [Google Scholar] [CrossRef]
- Ringvold, H.; Moum, T. On the genus Crossaster (Echinodermata: Asteroidea) and its distribution. PLoS ONE 2020, 15, e0227223. [Google Scholar]
- Jossart, Q.; Sands, C.J.; Sewell, M.A. Dwarf brooder versus giant broadcaster: Combining genetic and reproductive data to unravel cryptic diversity in an Antarctic brittle star. Heredity 2019, 123, 622–633. [Google Scholar] [CrossRef]
- Ward, R.D.; Holmes, B.H.; O’hara, T.D. DNA barcoding discriminates echinoderm species. Mol. Ecol. Resour. 2008, 8, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Petrov, N.B.; Vladychenskaya, I.P.; Drozdov, A.L.; Kedrova, O.S. Molecular genetic markers of intra-and interspecific divergence within starfish and sea urchins (Echinodermata). Biochemistry 2016, 81, 972–980. [Google Scholar] [CrossRef] [PubMed]
- BOLD. Barcode of Life Data Systems Home Page. 2022. Available online: https://www.boldsystems.org (accessed on 15 June 2023).
- Gong, S.; Ding, Y.; Wang, Y.; Jiang, G.; Zhu, C. Advances in DNA barcoding of toxic marine organisms. Int. J. Mol. Sci. 2018, 19, 2931. [Google Scholar] [CrossRef] [PubMed]
- Mah, C.L. World Asteroidea Database. 2022. Available online: https://www.marinespecies.org/asteroidea (accessed on 3 August 2023).
- Hajibabaei, M.; Singer, G.A.; Hebert, P.D.; Hickey, D.A. DNA barcoding: How it complements taxonomy, molecular phylogenetics and population genetics. Trends Genet. 2007, 23, 167–172. [Google Scholar] [CrossRef]
- Gostel, M.R.; Kress, W.J. The Expanding Role of DNA Barcodes: Indispensable Tools for Ecology, Evolution, and Conservation. Diversity 2022, 14, 213. [Google Scholar] [CrossRef]
- Hedgpeth, J.W. Introduction to Antarctic Zoogeography. In Distribution of Selected Groups of Marine Invertebrates in Waters South of 35° S Latitude; Bushnell, V.C., Hedgpeth, J.W., Eds.; Antarctic Map Folio Series, Folio 11; American Geographical Society: New York, NY, USA, 1969; pp. 1–9. [Google Scholar]
- de Moura Barboza, C.A.; de Moura, R.B.; Lanna, A.M.; Oackes, T.; Campos, L.S. Echinoderms as clues to Antarctic—South American connectivity. Oecologia Aust. 2011, 15, 86–110. [Google Scholar] [CrossRef]
- Poulin, E.; González-Wevar, C.; Díaz, A.; Gérard, K.; Hüne, M. Divergence between Antarctic and South American marine invertebrates: What molecular biology tells us about Scotia Arc geodynamics and the intensification of the Antarctic Circumpolar Current. Glob. Planet. Change 2014, 123, 392–399. [Google Scholar] [CrossRef]
- Casares, B.M.; Àngel, J.J.S.; Cantero, Á.L.P. Towards a better understanding of Southern Ocean biogeography: New evidence from benthic hydroids. Polar Biol. 2017, 40, 1975–1988. [Google Scholar] [CrossRef]
- Díaz, A.; Féral, J.P.; David, B.; Saucède, T.; Poulin, E. Evolutionary pathways among shallow and deep-sea echinoids of the genus Sterechinus in the Southern Ocean. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2011, 58, 205–211. [Google Scholar] [CrossRef]
- Cumming, R.A.; Nikula, R.; Spencer, H.G.; Waters, J.M. Transoceanic genetic similarities of kelp-associated sea slug populations: Long-distance dispersal via rafting? J. Biogeogr. 2014, 41, 2357–2370. [Google Scholar] [CrossRef]
- González-Wevar, C.A.; Hüne, M.; Segovia, N.I.; Nakano, T.; Spencer, H.G.; Chown, S.L.; Saucède, T.; Johnstone, G.; Mansilla, A.; Poulin, E. Following the Antarctic Circumpolar Current: Patterns and processes in the biogeography of the limpet Nacella (Mollusca: Patellogastropoda) across the Southern Ocean. J. Biogeogr. 2017, 44, 861–874. [Google Scholar] [CrossRef]
- Güller, M.; Puccinelli, E.; Zelaya, D.G. The Antarctic Circumpolar Current as a dispersive agent in the Southern Ocean: Evidence from bivalves. Mar. Biol. 2020, 167, 1–13. [Google Scholar] [CrossRef]
- Moon, K.L.; Chown, S.L.; Fraser, C.I. Reconsidering connectivity in the sub-Antarctic. Biol. Rev. 2017, 92, 2164–2181. [Google Scholar] [CrossRef]
- Helmuth, B.S.; Veit, R.R.; Holberton, R. Dispersal of benthic invertebrates in the Scotia Arc by kelp rafting. Antarct. J.-Rev. 1994, 29, 145–147. [Google Scholar]
- González-Wevar, C.A.; Segovia, N.I.; Rosenfeld, S.; Noll, D.; Maturana, C.S.; Hüne, M.; Naretto, J.; Gérard, K.; Díaz, A.; Spencer, H.G.; et al. Contrasting biogeographical patterns in Margarella (Gastropoda: Calliostomatidae: Margarellinae) across the Antarctic Polar Front. Mol. Phylogenet Evol. 2021, 156, 107039. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.M. Asteroidea. Antarctic Research Expedition 1929–1931. B9: 1-104. Available online: https://www.vliz.be/nl/catalogus?module=ref&refid=253385 (accessed on 3 August 2023).
- Clark, A.M.; Downey, M.E. Starfishes of the Atlantic; Chapman & Hall: London, UK, 1992. [Google Scholar]
- O’Hara, T.D. Systematics and biology of Macquarie Island echinoderms. Mem. Mus. Vic. 1998, 57, 167–223. [Google Scholar] [CrossRef]
- Mutschke, E.; Mah, C. Asteroidea–Starfish. Marine Benthic Fauna of Chilean Patagonia; Nature in Focus: Santiago, Chile, 2009; pp. 802–830. [Google Scholar]
- McKnight, D.G. Echinodermata: Asteroidea (Seastars). 3. Orders Velatida, Spinulosida, Forcipulatida, Brisingida with adenda to Paxillosida, Valvatida; National Institute of Water and Atmospheric Research: Auckland, New Zealand, 2006. [Google Scholar]
- Janosik, A.M.; Halanych, K.M. Seeing stars: A molecular and morphological investigation into the evolutionary history of Odontasteridae (Asteroidea) with description of a new species from the Galapagos Islands. Mar. Biol. 2013, 160, 821–841. [Google Scholar] [CrossRef]
- Geller, J.; Meyer, C.; Parker, M.; Hawk, H. Redesign of PCR primers for mitochondrial cytochrome c oxidase subunit I for marine invertebrates and application in all-taxa biotic surveys. Mol. Ecol. Res. 2013, 13, 851–861. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sánchez-Gracia, A. DnaSP 6, DNA sequence polymorphism analysis of large data sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Goodall-Copestake, W.P.; Tarling, G.A.; Murphy, E. On the comparison of population-level estimates of haplotype and nucleotide diversity: A case study using the gene cox1 in animals. Heredity 2012, 109, 50–56. [Google Scholar] [CrossRef]
- GBIF.org. GBIF Home Page. 2022. Available online: https://www.gbif.org (accessed on 15 June 2023).
- QGIS.org. QGIS Geographic Information System. QGIS Association. 2022. Available online: http://www.qgis.org (accessed on 15 June 2023).
- Clement, M.; Snell, Q.; Walker, P.; Posada, D.; Crandall, K. TCS: Estimating gene genealogies. Parallel Distrib. Process. Symp. Int. Proc. 2002, 2, 184. [Google Scholar]
- Madsen, F.J. The Henricia sanguinolenta complex (Echinodermata, Asteroidea) of the Norwegian Sea and adjacent waters. A re-evaluation, with notes on related species. Steenstruia 1987, 13, 201–268. [Google Scholar]
- Bratova, O.A.; Paskerova, G.G. Henricia spp. (Echinodermata: Asteroidea: Echinasteridae) of the White Sea: Morphology, morphometry, and synonymy. Can. J. Zool. 2018, 96, 341–355. [Google Scholar] [CrossRef]
- Bosch, I.; Pearse, J.S. Developmental types of shallow-water asteroids of McMurdo Sound, Antarctica. Mar. Biol. 1990, 104, 41–46. [Google Scholar] [CrossRef]
- McClary, D.J.; Mladenov, P.V. Reproductive pattern in the brooding and broadcasting sea star Pteraster militaris. Mar. Biol. 1989, 103, 531–540. [Google Scholar] [CrossRef]
- Lütken, C. De ved Danmarks kyster levende Pighude. Videns Kabel. Meddelelser Fra Den Naturhist 1857, 1856, 88–110. [Google Scholar]
- Lütken, C. Fortasatte kritiske og beskrivende Bidrag til Kundskab om Sostjernerne (Asteriderne). Vidensk. Meddelelser Fra Dan. Naturhistorisk Foren. 1871, 23, 227–304. [Google Scholar]
- Philippi, A. Beschreibungen einiger neuer Seesterne aus dem Meere von Chiloe. Archiv Für Naturgeschichte 1858, 24, 264–268. [Google Scholar]
- Perrier, E. Révision de la Collection de Stellérides du Museum d’Histoire Naturelle de Paris; Reinwald: Paris, France, 1875; 384p. [Google Scholar]
- Philippi, R.A. Vier neue Echinodermen des Chilenischen Meeres. Arch. Für Naturgeschichte 1857, 23, 130–148. [Google Scholar]
- Koehler, R. Échinodermes (astéries, ophiures et échinides) Recueillis par M. Rallier du Baty, aux îles Kerguelen, en 1913–1914. Ann. Inst. Océanogr. 1917, 7, 46–48. [Google Scholar]
- Müller, J.; Troschel, F.H. Neue Beiträge zur Kenntnis der Asteriden. Arch. Für Naturgeschichte 1843, 9, 113–131. [Google Scholar]
- Philippi, R.A. Neue Seesterne aus Chile. Arch. Für Naturgeschichte 1870, 36, 268–275. [Google Scholar]
- Smith, E.A. Descriptions of species of Asteridae and Ophiuridae from Kerguelen Islands. Ann. Mag. Natural Hist. 1876, 4, 105–113. [Google Scholar] [CrossRef]
- Sladen, W.P. Report on the Asteroidea. Report on the Scientific Results of the Voyage of H.M.S. Challenger during the years 1873–1876. Zoology 1889, 30, 454–455. [Google Scholar]
- Sladen, W.P. The Asteroidea of H.M.S. Challenger Expedition, pt. 1. Pterasteridae. J. Linn. Soc. 1882, 16, 189–246. [Google Scholar] [CrossRef]
- Hurtado-García, J.; Manjón-Cabeza, E. Species composition of sea stars (Echinodermata: Asteroidea) in the Patagonian Argentinian deep sea, including seven new records: Connectivity with sub-Antarctic and Antarctic fauna. Pol. Biol. 2022, 45, 1211–1228. [Google Scholar] [CrossRef]
- Meudec, L. Analyse de la Diversité des Astéries du Plateau des Kerguelen par Approches Génétique et Morphologique, et Modélisation des Habitats. Master’s Thesis, Université Libre de Bruxelles, Bruxelles, Belgium, 2021. [Google Scholar]
- Perrier, E.; Echinoderma, I. Stellérides. Mission Scientifique du Cap Horn, 1882–1883. 6. Zoologie 1891, 3, 104–105. [Google Scholar]
- Maldonado, C.; Molina, C.I.; Zizka, A.; Persson, C.; Taylor, C.M.; Albán, J.; Chilquillo, E.; Rønsted, N.; Antonelli, A. Estimating species diversity and distribution in the era of Big Data: To what extent can we trust public databases? Glob. Ecol. Biogeogr. 2015, 24, 973–984. [Google Scholar] [CrossRef] [PubMed]
- Moudrý, V.; Devillers, R. Quality and usability challenges of global marine biodiversity databases: An example for marine mammal data. Ecol. Inform. 2020, 56, 101051. [Google Scholar] [CrossRef]
- Fraser, C.I.; Nikula, R.; Waters, J.M. Oceanic rafting by a coastal community. Proc. R. Soc. B Biol. Sci. 2011, 278, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Waters, J.M.; King, T.M.; Fraser, C.I.; Garden, C. Rafting dispersal in a brooding southern sea star (Asteroidea: Anasterias). Invertebr. Syst. 2018, 32, 253–258. [Google Scholar] [CrossRef]
- Waters, J.M.; King, T.M.; Fraser, C.I.; Craw, D. An integrated ecological, genetic and geological assessment of long-distance dispersal by invertebrates on kelp rafts. Front. Biogeogr. 2018, 10, 1–12. [Google Scholar] [CrossRef]
- Gibson, R.N.; Atkinson, R.J.A.; Gordon, J.D.M. The ecology of rafting in the marine environment. II. The rafting organisms and community. Oceanogr. Mar. Biol. An Annu. Rev. 2005, 43, 279–418. [Google Scholar]
- Fraser, C.I.; Morrison, A.K.; Hogg, A.M.; Macaya, E.C.; van Sebille, E.; Ryan, P.G.; Padovan, A.; Jack, C.; Valdivia, N.; Waters, J.M. Antarctica’s ecological isolation will be broken by storm-driven dispersal and warming. Nat. Clim. Change 2018, 8, 704–708. [Google Scholar] [CrossRef]
- Smith, S.D. Kelp rafts in the Southern Ocean. Glob. Ecol. Biogeogr. 2002, 11, 67–69. [Google Scholar] [CrossRef]
- Stuart-Smith, R.D.; Edgar, G.J.; Bates, A.E. Thermal limits to the geographic distributions of shallow-water marine species. Nat. Ecol. Evol. 2017, 1, 1846–1852. [Google Scholar] [CrossRef]
- Clarke, A.; Barnes, D.K.; Hodgson, D.A. How isolated is Antarctica? Tree 2005, 20, 1–3. [Google Scholar] [CrossRef]
- Molina, A.N.; Pulgar, J.M.; Rezende, E.L.; Carter, M.J. Heat tolerance of marine ectotherms in a warming Antarctica. Glob. Change Biol. 2023, 29, 179–188. [Google Scholar] [CrossRef]
- Silva, N.; Calvete, C. Características oceanográficas físicas y químicas de canales australes chilenos entre el golfo de Penas y el estrecho de Magallanes (Crucero Cimar-Fiordo 2). Rev. Cienc. Tecnol. Mar. 2002, 25, 23–88. [Google Scholar]
- Valdenegro, M. Caracterización Oceanográfica Física y Química de la Zona de Canales y Fiordos Australes de Chile Entre el Estrecho de Magallanes y el Cabo de Hornos, Cimar 3, Fiordo. Ph.D. Thesis, Universidad Católica de Valparaíso, Valparaíso, Chile, 2002. [Google Scholar]
- Kashenko, S.D. The reaction of the starfish Asterias amurensis and Patiria pectinifera (Asteroidea) from Vostok Bay (Sea of Japan) to a salinity decrease. Russ J. Mar. Biol. 2003, 29, 110–114. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vantomme, L.; Jossart, Q.; Gérard, K.; Danis, B.; Moreau, C. Preliminary Assessment of Sea Star (Echinodermata, Asteroidea) Diversity in the Coastal Magellanic Region (South Chile) and Their Geographical Distribution. Diversity 2023, 15, 1129. https://doi.org/10.3390/d15111129
Vantomme L, Jossart Q, Gérard K, Danis B, Moreau C. Preliminary Assessment of Sea Star (Echinodermata, Asteroidea) Diversity in the Coastal Magellanic Region (South Chile) and Their Geographical Distribution. Diversity. 2023; 15(11):1129. https://doi.org/10.3390/d15111129
Chicago/Turabian StyleVantomme, Luka, Quentin Jossart, Karin Gérard, Bruno Danis, and Camille Moreau. 2023. "Preliminary Assessment of Sea Star (Echinodermata, Asteroidea) Diversity in the Coastal Magellanic Region (South Chile) and Their Geographical Distribution" Diversity 15, no. 11: 1129. https://doi.org/10.3390/d15111129
APA StyleVantomme, L., Jossart, Q., Gérard, K., Danis, B., & Moreau, C. (2023). Preliminary Assessment of Sea Star (Echinodermata, Asteroidea) Diversity in the Coastal Magellanic Region (South Chile) and Their Geographical Distribution. Diversity, 15(11), 1129. https://doi.org/10.3390/d15111129







