A New Miniature Species of Arthroleptis (Anura: Arthroleptidae) from Nyungwe National Park, Rwanda †
Abstract
1. Introduction
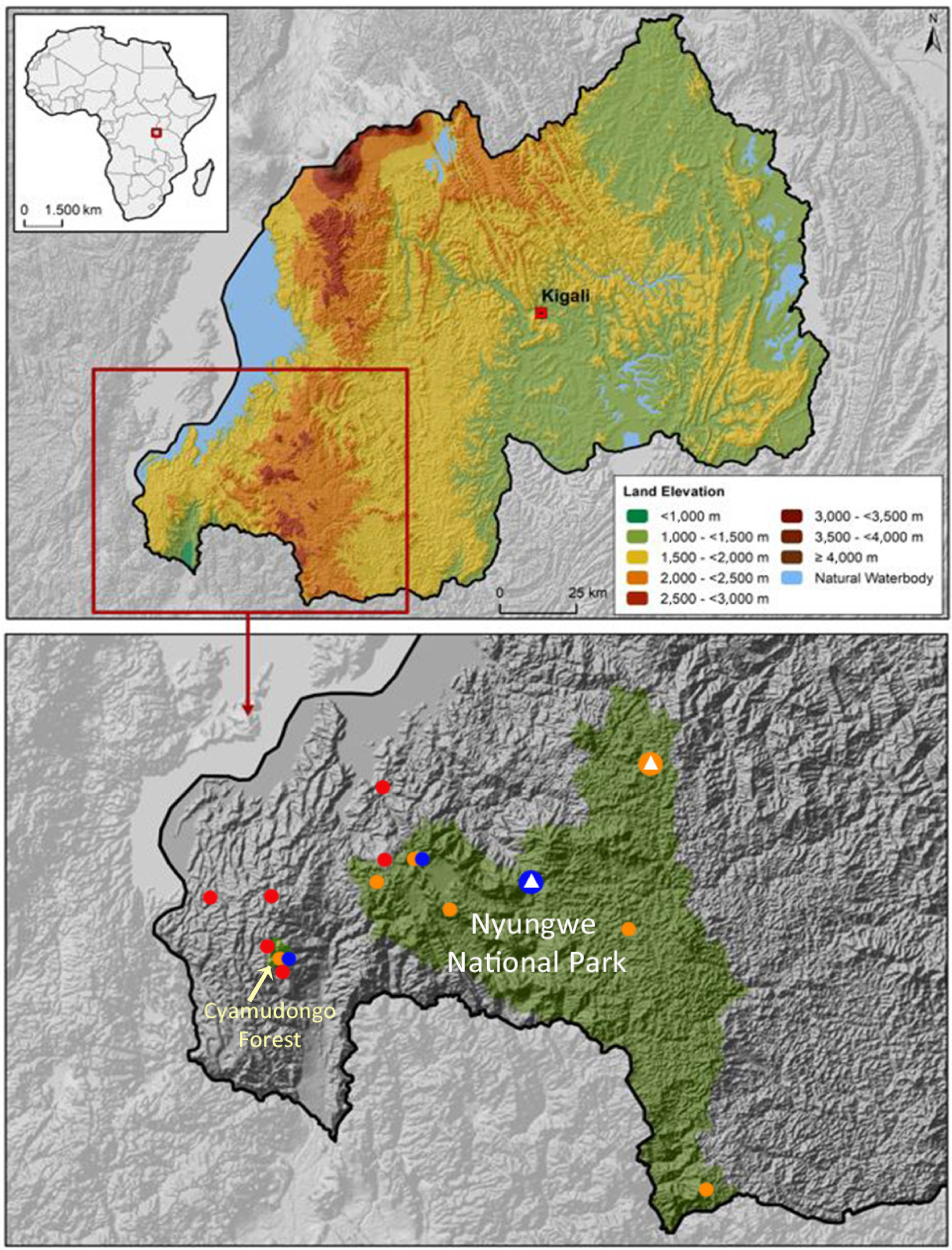
2. Materials and Methods
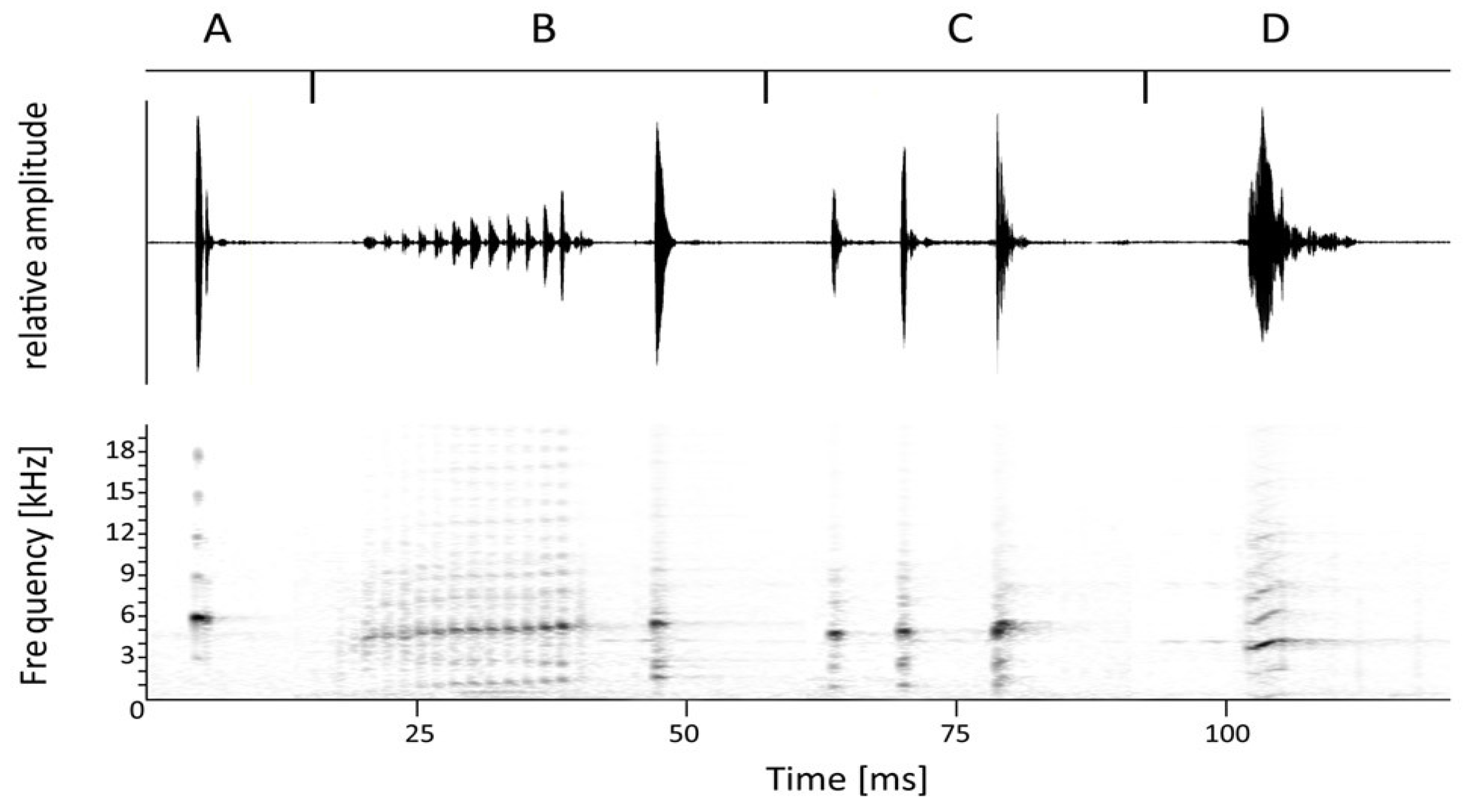
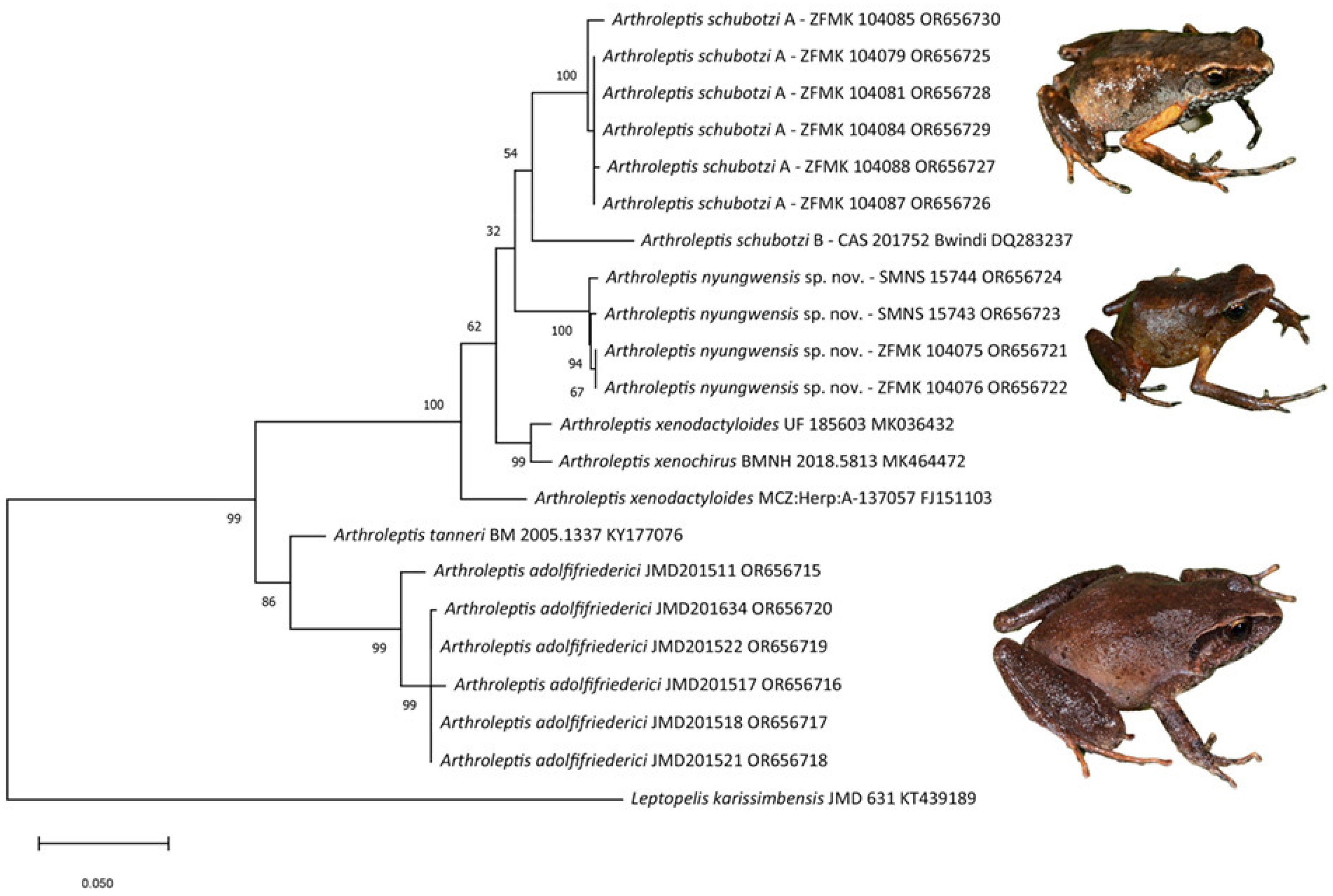
3. Results
- Arthroleptis nyungwensis sp. nov.
- Nyungwe Squeaker
- urn:lsid:zoobank.org:act:E8F25561-AD7C-46D8-9107-73F6ED10799C
- Arthroleptis sp.—Dehling & Sinsch 2023: 13 [17].
4. Discussion
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Species | Status | Voucher # | Country of Origin | Locality |
|---|---|---|---|---|
| A. adolfifriederici | syntype | ZMB 21787 | Rwanda | Rugegewald [=northeastern Nyungwe Forest] |
| A. adolfifriederici | topotype | 201511 | Rwanda | Rugegewald [=northeastern Nyungwe Forest] |
| A. adolfifriederici | - | JMD 597, 202103 | Rwanda | Nyungwe Forest |
| A. adolfifriederici | - | JMD 201517–23 | Rwanda | Gishwati Forest |
| A. adolfifriederici | - | JMD 201634 | Rwanda | Cyamudongo Forest |
| A. hematogaster | possible types | RMCA “Caja 40, Lote 60” (2 specs.) | DRC | May ya Moto |
| A. loveridgei | syntypes | RMCA 2624–25 | DRC | Arebi |
| A. nyungwensis | holotype | ZFMK 104075 | Rwanda | Rukuzi (2.4635 S, 29.2293 E; 2200 m), Nyungwe National Park |
| A. nyungwensis | paratype | ZFMK 104076 | Rwanda | Rukuzi (2.4635 S, 29.2293 E; 2200 m), Nyungwe National Park |
| A. nyungwensis | paratype | ZFMK 104077 | Rwanda | Cyamudongo Forest (2.546 S, 28.989 E, about 2000 m) |
| A. nyungwensis | paratype | SMNS 15743–44 | Rwanda | Cyamudongo Forest (2.5378 S, 29.9942 E; 1830 m) |
| A. phrynoides | holotype | RMCA 75-43-B-23 | DRC | Lomami, Terr. de Lomela, Sankuru |
| A. pyrrhoscelis | possible types | RMCA “Caja 9, Lote 98” (5 females, 2 males) | DRC | Haute Lubitshako, Terr. de Fizi (Kabobo) |
| A. pyrrhoscelis | possible types | RMCA “Caja 39, Lote 64” (many specs.) | DRC | Haute Lubitshako, Terr. de Fizi (Kabobo) |
| A. schubotzi | holotype | ZMB 21774 | Burundi | Usumbura [=Bujumbura] |
| A. schubotzi | - | ZMB 22860 | DRC | Insel Kwidschwi |
| A. schubotzi | - | ZFMK 104078–104092, JMD 201909–10 | Rwanda | Cyamudongo Forest |
| A. schubotzi | - | ZFMK 104081,-84,-85 | Rwanda | Buhinga |
| A. schubotzi | syntypes of Schoutedenella kivuensis | RMCA 48.783–784 | DRC | N’Zulu (Lac Kivu)—Passe de Sake (alt. 1500 m.) |
| A. schubotzi | possible types of Schoutedenella discodactyla | RMCA “Caja 31, Lote 81” (2 specs.) | DRC | Lutunguru |
| A. spinalis | holotype | RMCA 455 | DRC | Plaine St. Louis (Baudounville) |
| A. spinalis | holotype of A. boulengeri | RMCA 534 | DRC | Plaine St. Louis (Baudounville) |
| A. vercammeni | - | RMCA “Caja 4, Lote 3” | DRC | Mwana |
| A. xenochirus | syntypes of Arthroleptis globosa | RMCA 586, B 585/A–F (7 specs.) | DRC | Lofoi |
| A. xenochirus | syntypes of Arthroleptis lameeri | RMCA 180–180D | DRC | Lofoi |
| A. xenochirus | syntypes of Schoutedenella muta | RMCA 16219– 16248 | DRC | Kando près Tenke |
| Species | Location | Voucher | GenBank# | Original Source |
|---|---|---|---|---|
| Arthroleptis adolfifriederici | Nyungwe Forest, Rwanda | JMD 201511 | OR656715 | this study |
| A. adolfifriederici | Gishwati Forest, Rwanda | JMD 201517 | OR656716 | this study |
| JMD 201518 | OR656717 | |||
| JMD 201521 | OR656718 | |||
| JMD 201522 | OR656719 | |||
| A. adolfifriederici | Cyamudongo Forest, Rwanda | JMD 201634 | OR656720 | this study |
| A. nyungwensis | Rukuzi, Nyungwe National Park, Western Province, Rwanda | ZFMK 104075 | OR656721 | this study |
| ZFMK 104076 | OR656722 | |||
| A. nyungwensis | Cyamudongo Forest (2.5378 S, 29.9942 E; 1830 m), Western Province, Rwanda | SMNS 15743 | OR656723 | this study |
| SMNS 15744 | OR656724 | |||
| A. schubotzi | Cyamudongo Forest, Rwanda | ZFMK 104079 | OR656725 | this study |
| ZFMK 104087 | OR656726 | |||
| ZFMK 104088 | OR656727 | |||
| A. schubotzi | Buhinga, Rwanda | ZFMK 104081 | OR656728 | this study |
| ZFMK 104084 | OR656729 | |||
| ZFMK 104085 | OR656730 | |||
| A. schubotzi | Bwindi Impenetrable National Park, Uganda | CAS 201752 | DQ283237 | [12] |
| A. xenochirus | Lukwakwa, Zambia | BMNH 2018.5813 | MK464472 | [39] |
| A. xenodactyloides | Angola | UF185602 | MK036432 | [40] |
| A. xenodactyloides | Misuku Mountains, Mughese Forest Reserve, Malawi | MCZ:Herp:A-137057 | FJ151103 | [6] |
| Leptopelis karissimbensis | Rwasenkoko swamp, Nyungwe National Park, Rwanda | JMD 631 | KT439189 | [41] |
References
- Channing, A.; Rödel, M.-O. Field Guide to the Frogs & Other Amphibians of Africa; Penguin Random House: Cape Town, South Africa, 2019. [Google Scholar]
- Frost, D.R. Amphibian Species of the World: An Online Reference. Version 6.2. Electronic Database; American Museum of Natural History: New York, NY, USA, 2023; Available online: https://amphibiansoftheworld.amnh.org/index.php (accessed on 20 September 2023).
- Laurent, R.F. Remarques sur le genre Schoutedenella Witte. Ann. Musée R. Congo Belg. Sci. Zool. 1954, 1, 34–40. [Google Scholar]
- Laurent, R.F. Amphibiens. In Exploration du Parc National des Virunga; Deuxième Series; Fondation pour Favoriser les Recherches Scientifique en Afrique: Bruxelles, Belgium, 1972; Volume 22, pp. 1–125. [Google Scholar]
- Schmidt, K.; Inger, R. Amphibians exclusive of the genera Afrixalus and Hyperolius. In Exploration du Parc National de l’Upemba; Institut des Parcs Nationaux du Congo Belge: Bruxelles, Belgium, 1959; Volume 56, pp. 1–294. [Google Scholar]
- Blackburn, D.C. Biogeography and evolution of body size and life history of African frogs: Phylogeny of squeakers (Arthroleptis) and long-fingered frogs (Cardioglossa) estimated from mitochondrial data. Mol. Phylogenetics Evol. 2008, 49, 806–826. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, D.C. Description and phylogenetic relationships of two new species of miniature Arthroleptis (Anura: Arthroleptidae) from the Eastern Arc Mountains of Tanzania. Breviora 2009, 517, 1–17. [Google Scholar] [CrossRef]
- de Witte, G.-F. Description de batraciens nouveaux du Congo Belge. Rev. Zool. Afr. 1921, 9, 1–27. [Google Scholar]
- Laurent, R.F. The natural classification of the Arthroleptinae (Amphibia, Hyperoliidae). Rev. Zool. Bot. Afr. 1973, 87, 666–678. [Google Scholar]
- Loveridge, A. Check list of the reptiles and amphibians of East Africa (Uganda; Kenya; Tanganyika; Zanzibar). Bull. Mus. Comp. Zool. Harv. Coll. 1957, 117, 151–362. [Google Scholar]
- Poynton, J.C.; Broadley, D.G. Amphibia Zambesiaca 1. Scolecomorphidae, Pipidae, Microhylidae, Hemisidae, Arthroleptidae. Ann. Natal Mus. 1985, 26, 503–553. [Google Scholar]
- Frost, D.R.; Grant, T.; Faivovich, J.; Bain, R.H.; Haas, A.; Haddad, C.F.B.; de Sá, R.O.; Channing, A.; Wilkinson, M.; Donnellan, S.C.; et al. The amphibian tree of life. Bull. Am. Mus. Nat. Hist. 2006, 297, 1–370. [Google Scholar] [CrossRef]
- Nieden, F. Neue ostafrikanische Frösche aus dem Kgl. Zool. Museum in Berlin. Sitzungsberichte Der Ges. Naturforschender Freunde Zu Berl. 1911, 1910, 436–441. [Google Scholar]
- Boulenger, G.A. Descriptions d’un ophidien et d’un batracien nouveaux du Congo. Rev. Zool. Afr. 1919, 7, 186–187. [Google Scholar]
- de Witte, G.F. Batraciens et reptiles. In Exploration du Parc National Albert, Mission G. F. de Witte (1933–1935), Fascicle 33; Fondation pour Favoriser les Recherches Scientifiques en Afrique: Bruxelles, Belgium, 1941; 261p. [Google Scholar]
- Laurent, R. Reptiles et batraciens nouveaux du massif du mont Kabobo et du plateau des Marungu. Rev. De Zool. Et De Bot. Afr. 1952, 46, 18–34. [Google Scholar]
- Dehling, J.M.; Sinsch, U. Amphibians of Rwanda: Diversity, community features, and conservation status. Diversity 2023, 15, 512. [Google Scholar] [CrossRef]
- Nieden, F. Neues Verzeichnis der Kriechtiere (außer den Schlangen) von Deutsch-Ostafrika. II. Teil: Amphibia. Mitteilungen Aus Dem Zool. Mus. Berl. 1915, 7, 345–390. [Google Scholar]
- Hinkel, H.; Fischer, E. Checklist of amphibians and reptiles of Nyungwe Forest, Rwanda. Tauraco Res. Rep. 1990, 3, 135–138. [Google Scholar]
- Nieden, F. Amphibia. In Wissenschaftliche Ergebnisse der Deutschen Zentral-Afrika-Expedition 1907–1908 unter Führung Adolf Friedrichs, Herzogs zu Mecklenburg; Band, I.V., Zoologie, I.I., Schubotz, H., Eds.; Klinkhardt & Biermann: Leipzig, Germany, 1912; Volume IV, pp. 165–196. [Google Scholar]
- de Witte, G.-F. Batraciens nouveaux du Congo Belge. Rev. Zool. Bot. Afr. 1933, 24, 97–103. [Google Scholar] [CrossRef]
- Channing, A.; Howell, K.M. Amphibians of East Africa; Edition Chimaira: Frankfurt, Germany, 2006. [Google Scholar]
- Palumbi, S. Nucleic acids II: The polymerase chain reaction. In Molecular Systematics; Hillis, D.M., Moritz, C., Mable, B.K., Eds.; Sinauer Associates: Sunderland, MA, USA, 1996; pp. 205–247. [Google Scholar]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and high-performance computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Köhler, J.; Jansen, M.; Rodríguez, A.; Kok, P.J.R.; Toledo, L.F.; Emmrich, M.; Glaw, F.; Haddad, C.F.B.; Rödel, M.-O.; Vences, M. The use of bioacoustics in anuran taxonomy: Theory, terminology, methods and recommendations for best practice. Zootaxa 2017, 4251, 1–124. [Google Scholar] [CrossRef]
- Zimkus, B.M.; Blackburn, D.C. Distinguishing features of the sub-Saharan frog genera Arthroleptis and Phrynobatrachus: A short guide for field and museum researchers. Breviora 2008, 513, 1–12. [Google Scholar] [CrossRef]
- Dehling, D.M.; Dehling, J.M. Elevated alpha diversity in disturbed sites obscures regional decline and homogenization of amphibian taxonomic, functional and phylogenetic diversity. Sci. Rep. 2023, 13, 1710. [Google Scholar] [CrossRef] [PubMed]
- Mercurio, V. Amphibians of Malawi. In An Analysis of Their Richness and Community Diversity in a Changing Landscape; Edition Chimaira: Frankfurt am Main, Germany, 2011. [Google Scholar]
- Pickersgill, M. Frog Search. Results of Expeditions to Southern and Eastern Africa; Edition Chimaira: Frankfurt am Main, Germany, 2007. [Google Scholar]
- Maiditsch, I.; Liedtke, H.C.; Ng’wava, J.M.; Hödl, W. Advertisement and close-range encounter call of Arthroleptis schubotzi Nieden, 1911, with notes on phonotaxis and sexual dimorphism in the third manual digit (Anura: Arthroleptidae). Herpetozoa 2011, 24, 23–31. [Google Scholar]
- Wells, K.D. The Ecology and Behavior of Amphibians; The University of Chicago Press: Chicago, IL, USA; London, UK, 2007. [Google Scholar]
- Dehling, J.M. An African glass frog: A new Hyperolius species (Anura: Hyperoliidae) from Nyungwe National Park, southern Rwanda. Zootaxa 2012, 3391, 52–64. [Google Scholar] [CrossRef]
- Channing, A.; Hillers, A.; Lötters, S.; Rödel, M.O.; Schick, S.; Conradie, W.; Rödder, D.; Mercurio, V.; Wagner, P.; Dehling, J.M.; et al. Taxonomy of the super-cryptic Hyperolius nasutus group of long reed frogs of Africa (Anura: Hyperoliidae), with descriptions of six new species. Zootaxa 2013, 3620, 301–350. [Google Scholar] [CrossRef]
- Hughes, D.F.; Tolley, K.A.; Behangana, M.; Lukwago, W.; Menegon, M.; Dehling, J.M.; Stipala, J.; Tilbury, C.R.; Khan, A.M.; Kusamba, C.; et al. Cryptic diversity in Rhampholeon boulengeri (Sauria: Chamaeleonidae), a pygmy chameleon from the Albertine Rift biodiversity hotspot. Mol. Phylogenetics Evol. 2018, 122, 125–141. [Google Scholar] [CrossRef] [PubMed]
- Dehling, J.M.; Sinsch, U. Partitioning of morphospace in larval and adult reed frogs (Anura: Hyperoliidae: Hyperolius) of the Central African Albertine Rift. Zool. Anz. 2019, 280, 65–77. [Google Scholar] [CrossRef]
- Greenbaum, E.; Portik, D.M.; Allen, K.E.; Vaughan, E.R.; Badjedjea, G.; Barej, M.F.; Behangana, M.; Conkey, N.; Dumbo, B.; Gonwouo, L.N.; et al. Systematics of the Central African spiny reed frog Afrixalus laevis (Anura: Hyperoliidae), with the description of two new species from the Albertine Rift. Zootaxa 2022, 5174, 201–232. [Google Scholar] [CrossRef]
- Bittencourt-Silva, G.B. A herpetological survey of western Zambia. Amphib. Reptile Conserv. 2019, 13, 1–28. [Google Scholar]
- Hayes, D.T.; Ceriaco, L.M.P.; Jongsma, G.F.M.; Bandeira, S.; Hedley, R.W.; Stanley, E.L.; Bauer, A.M.; Blackburn, D.C. Amphibian Surveys at Two Important Type Localities in Malanje Province, Angola. Available online: https://www.ncbi.nlm.nih.gov/nuccore/MK036432 (accessed on 20 September 2023).
- Lehr, E.; Dehling, J.M.; Greenbaum, E.; Sinsch, U. Embryogenesis and tadpole description of Hyperolius castaneus Ahl, 1931 and H. jackie Dehling, 2012 (Anura, Hyperoliidae) from montane bog pools. ZooKeys 2015, 546, 125–152. [Google Scholar] [CrossRef][Green Version]


| specimen | status | sex | SVL | HL | HW | EYE | TD | SL | EN | NS |
|---|---|---|---|---|---|---|---|---|---|---|
| ZFMK 104075 | holotype | male | 16.5 | 6.1 | 5.4 | 2.1 | 1.0 | 2.5 | 1.3 | 1.4 |
| ZFMK 104076 | paratype | male | 16.0 | 5.6 | 5.4 | 1.9 | 0.9 | 2.2 | 1.1 | 1.0 |
| ZFMK 104077 | paratype | male | 16.1 | 5.6 | 5.1 | 2.0 | 0.9 | 2.5 | 1.2 | 1.1 |
| specimen | status | NN | EE | EW | IO | HND | 3FL | FoL | TFL | THL |
| ZFMK 104075 | holotype | 2.2 | 3.2 | 1.2 | 1.9 | 6.9 | 5.0 | 7.1 | 6.9 | 7.3 |
| ZFMK 104076 | paratype | 2.0 | 3.1 | 1.0 | 2.0 | 6.1 | 5.5 | 7.1 | 7.2 | 7.4 |
| ZFMK 104077 | paratype | 1.9 | 3.0 | 1.0 | 2.0 | 6.8 | 4.7 | 7.1 | 7.3 | 7.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dehling, J.M. A New Miniature Species of Arthroleptis (Anura: Arthroleptidae) from Nyungwe National Park, Rwanda. Diversity 2023, 15, 1104. https://doi.org/10.3390/d15101104
Dehling JM. A New Miniature Species of Arthroleptis (Anura: Arthroleptidae) from Nyungwe National Park, Rwanda. Diversity. 2023; 15(10):1104. https://doi.org/10.3390/d15101104
Chicago/Turabian StyleDehling, J. Maximilian. 2023. "A New Miniature Species of Arthroleptis (Anura: Arthroleptidae) from Nyungwe National Park, Rwanda" Diversity 15, no. 10: 1104. https://doi.org/10.3390/d15101104
APA StyleDehling, J. M. (2023). A New Miniature Species of Arthroleptis (Anura: Arthroleptidae) from Nyungwe National Park, Rwanda. Diversity, 15(10), 1104. https://doi.org/10.3390/d15101104





