A New Iason Species from Crete (Coleoptera, Carabidae, Anillini) with Notes about Anillini of Greece and Anatolia
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Iason assingi n. sp. (Figure 1 and Figure 2)
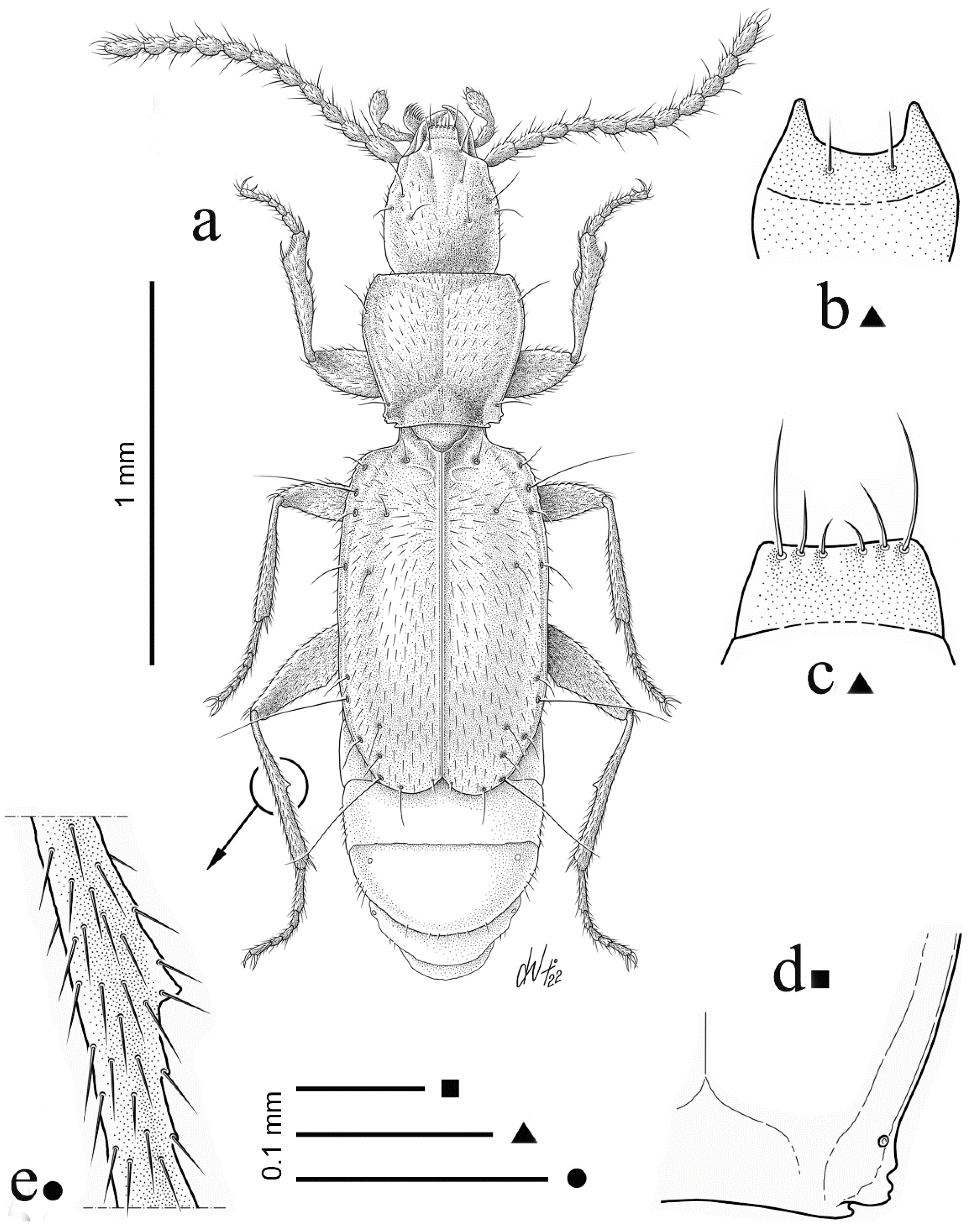
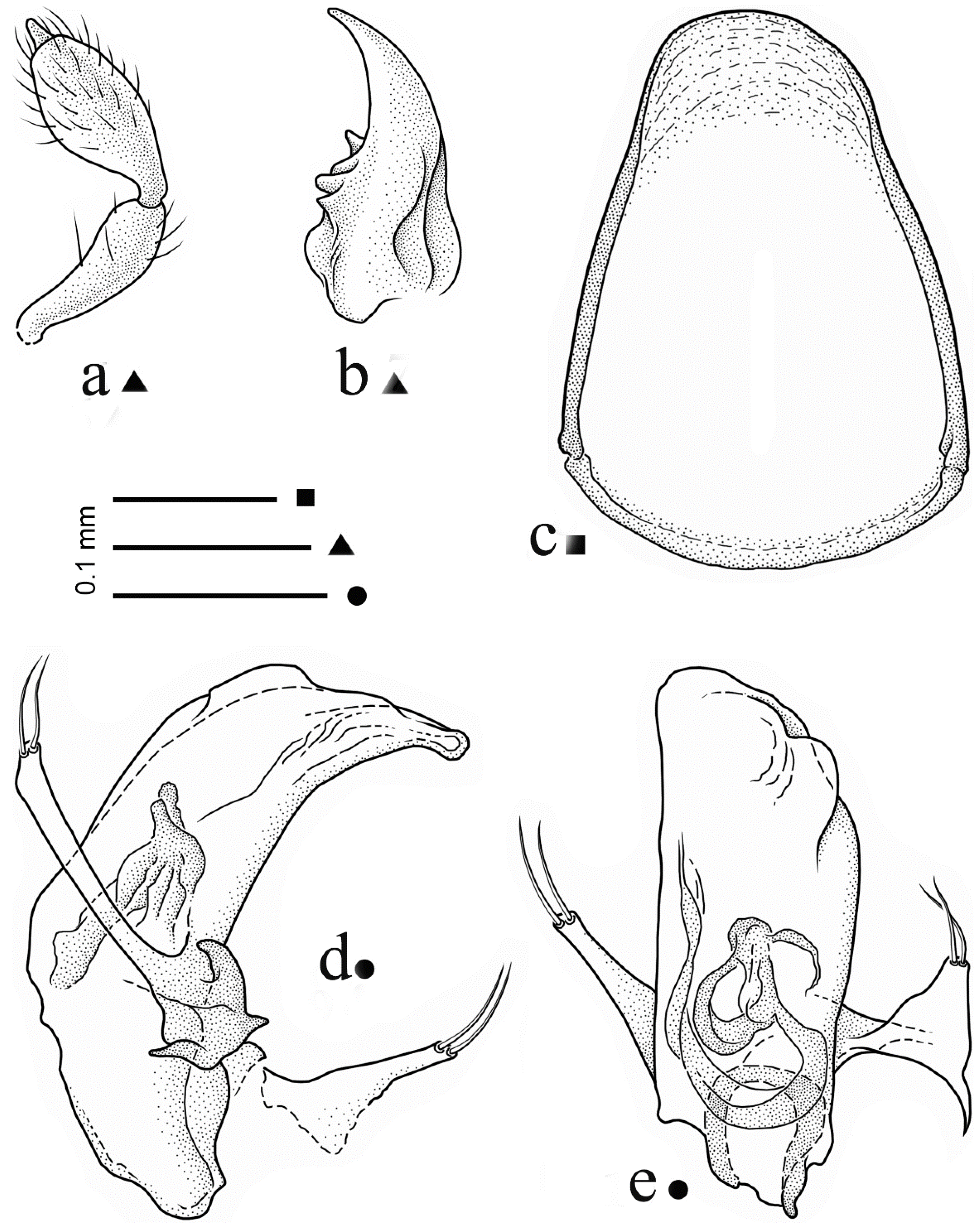
3.1.1. Loc. Typ.
3.1.2. Type Series
3.1.3. Diagnosis
3.1.4. Description
3.1.5. Etymology
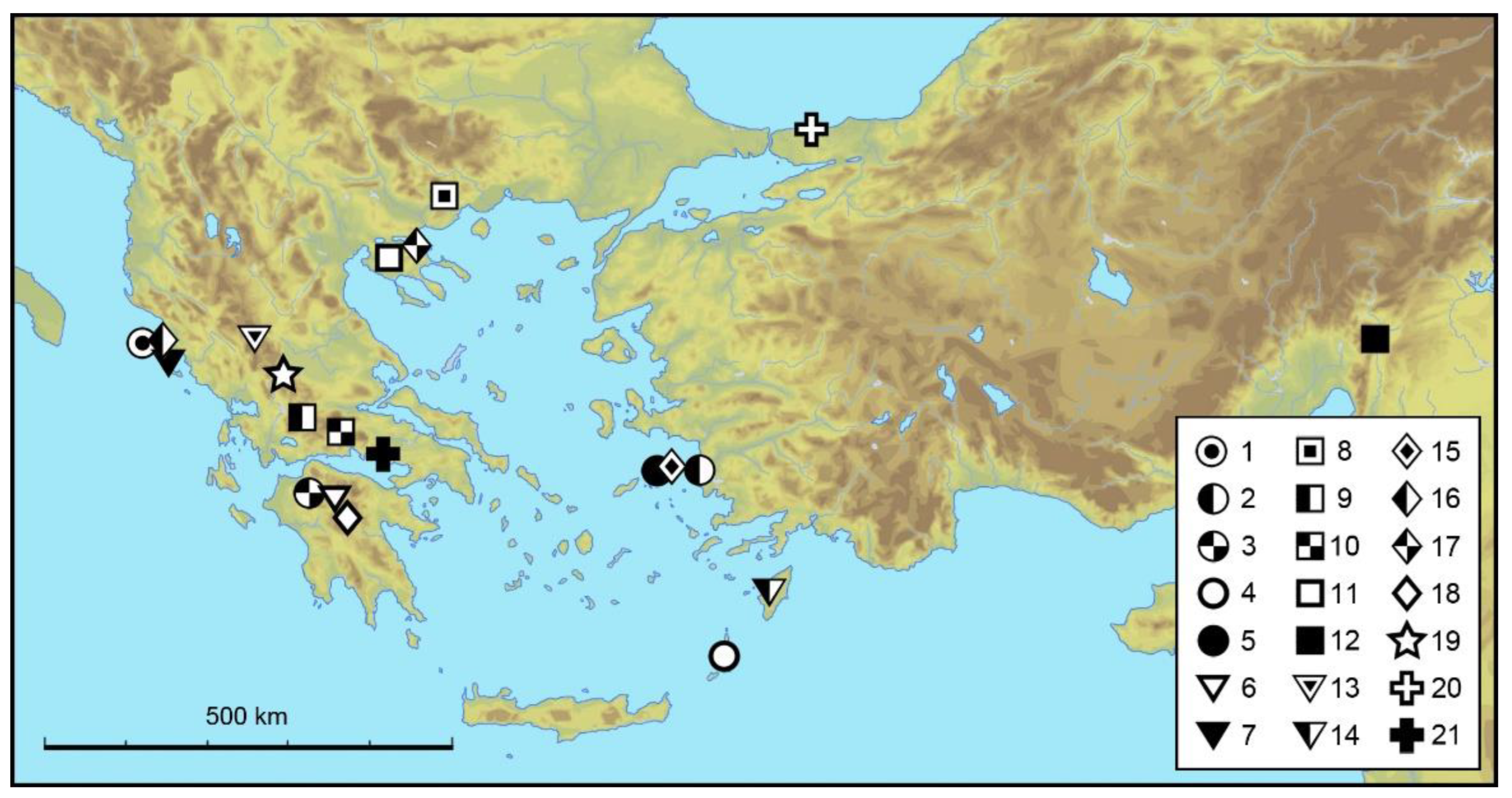
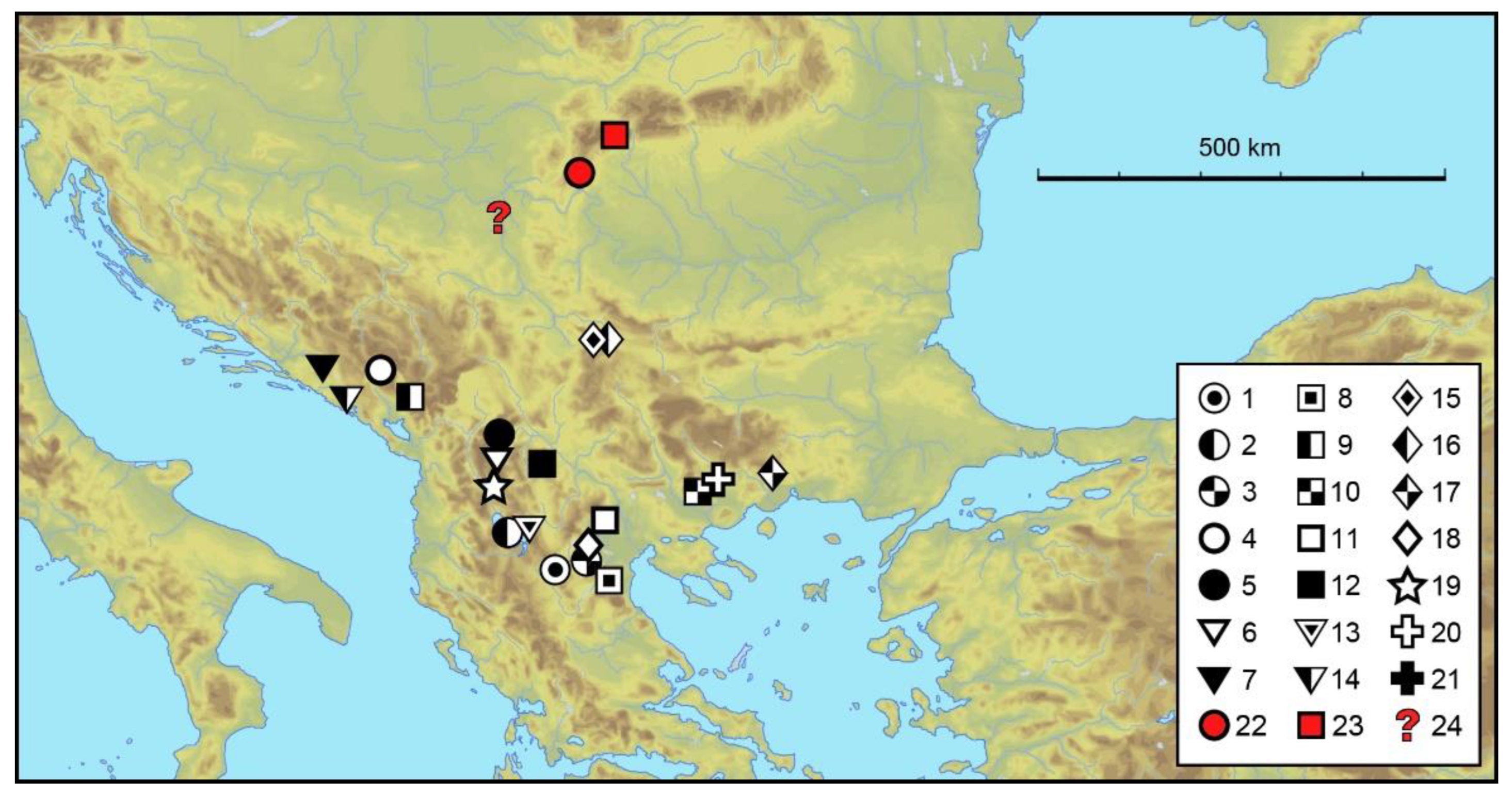
3.1.6. Distribution and Ecology
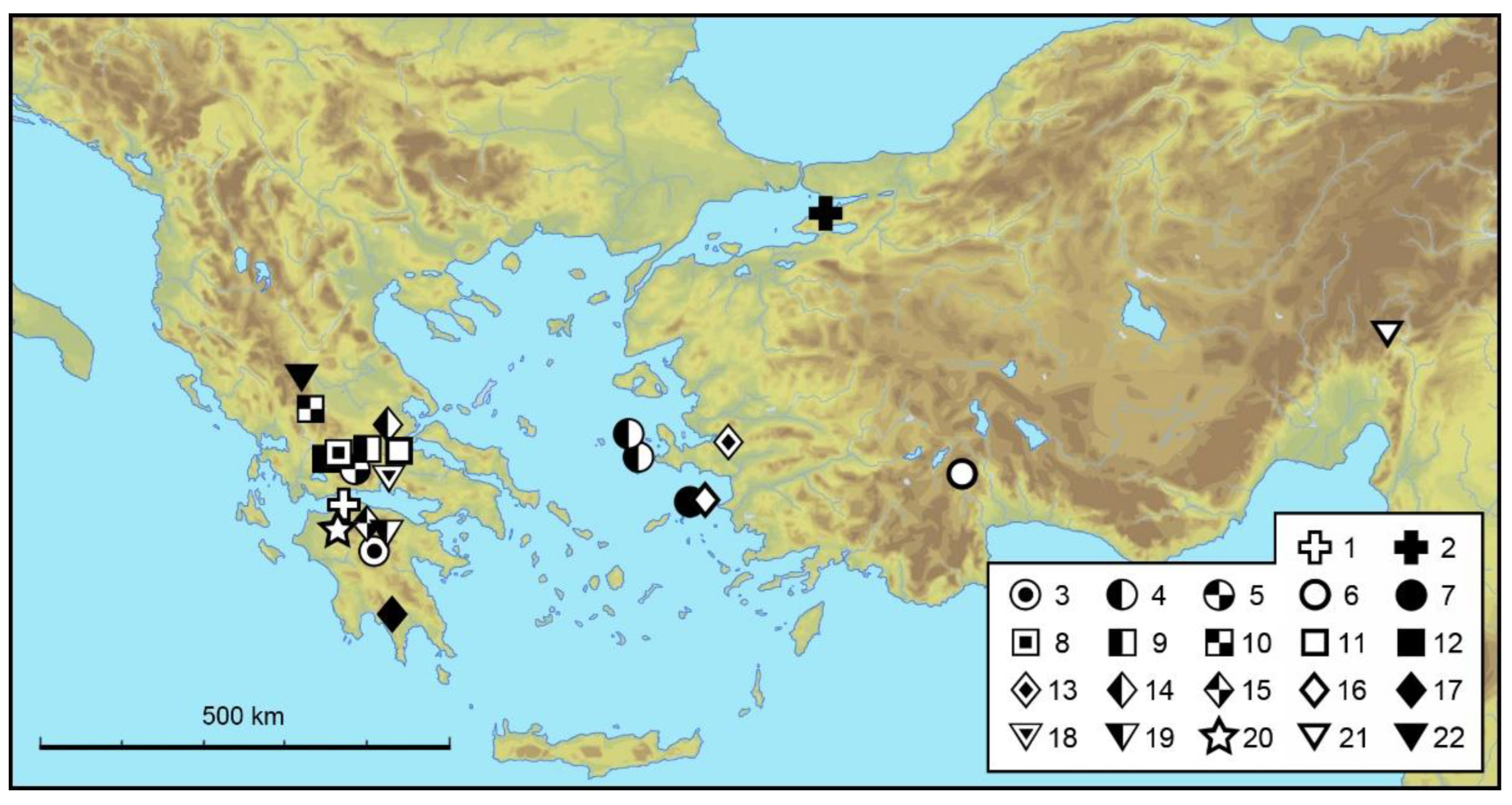
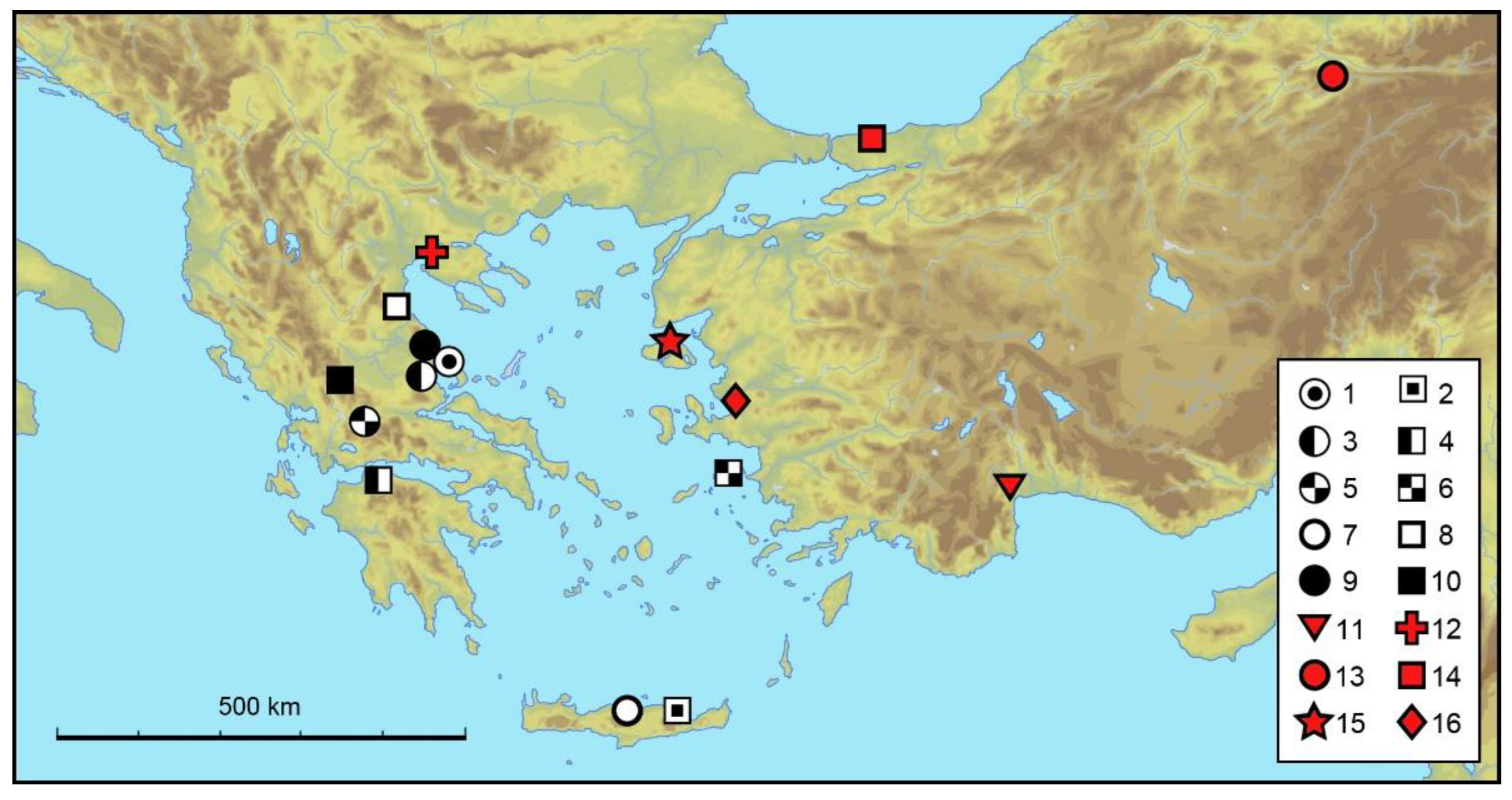
4. Discussion
Zoogeographic Remarks
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Materials examined are deposited in the following collections: | |
| BMNH | The Natural History Museum, London (United Kingdom) |
| CCa | Achille Casale Collection, Torino (Italia) |
| CGi | Pier Mauro Giachino Collection, San Martino Canavese (TO) (Italia) |
| CVa | Dante Vailati Collection, Brescia (Italia) |
| For type material, the following acronyms were used: | |
| HT | Holotype |
| PT(T) | Paratype (s) |
| The following acronyms were used for measurements: | |
| L | Overall length from apex of mandibles to apex of elytra |
| TL | Total length from apex of mandibles to apex of last urotergite |
| PL | Pronotum length |
| PW | Pronotum width |
| EL | Length of elytra |
| EW | Width of elytra |
References
- Zaballos, J.P.; Pérez-Gonzáles, S.; Andújar Fernández, C.; Giachino, P.M. Family Carabidae, subfamily Trechinae, tribe Anillini Jeannel, 1937. In Catalogue of Palaearctic Coleoptera. Revised and Updated Edition, Vol. 1. Archostemata-Myxophaga-Adephaga; Löbl, I., Löbl, D., Eds.; Brill: Leiden, The Netherlands; Boston, MA, USA, 2017; pp. 286–294, 1443. [Google Scholar]
- Giachino, P.M.; Vailati, D. Review of the Anillina of Greece (Coleoptera, Carabidae, Bembidiini). Biodivers. J. Monogr. 2011, 1, 1–112. [Google Scholar]
- Giachino, P.M.; Vailati, D. Anillina. In Ground Beetles (Carabidae) of Greece; Arndt, E., Schnitter, P., Sfenthourakis, S., Wrase, D., Eds.; Pensoft Publishers: Sofia, Bulgaria, 2011; pp. 91–102, 394. [Google Scholar]
- Arndt, E.; Schnitter, P.; Sfenthourakis, S.; Wrase, D.W. (Eds.) Ground beetles (Carabidae) of Greece; Series Faunistica; Pensoft: Sofia, Bulgaria, 2011; Volume 100, p. 393. [Google Scholar]
- Zaballos, J.P. Family Carabidae, subfamily Trechinae, tribe Anillini Jeannel, 1937. In Catalogue of Palaearctic Coleoptera. Volume 1. Archostemata–Mixophaga–Adephaga; Löbl, I., Smetana, A., Eds.; Apollo Books: Stenstrup, Denmark, 2003; pp. 237–241, 819. [Google Scholar]
- Lorenz, W. Systematic list of extant Ground Beetles of the World (Insecta Coleoptera “Geadephaga”: Trachypachidae and Carabidae incl. Paussinae, Cicindelinae, Rhysodinae); W. Lorenz: Tutzing, Germany, 2005; p. 530. [Google Scholar]
- Casale, A. Un nuovo Winklerites di Grecia (Col., Carabidae, Bembidiinae). Boll. del Mus. di Zool. dell’universita di Torino 1977, 6, 77–83. [Google Scholar]
- Casale, A.; Giachino, P.M.; Etonti, M. Nuovi Coleotteri endogei e cavernicoli (Carabidae Trechinae e Bembidiinae, Cholevidae Bathysciinae) della Grecia nord-orientale e dei Rodopi Bulgari, e loro significato zoogeografico. Boll. del Mus. Reg. di Sci. Nat. Di Torino 1990, 8, 545–580. [Google Scholar]
- Jeannel, R. Les Bembidiides endogés (Col. Carabidae). Monographie d’une lignee gondwanienne. Rev. Fr. d’Entomologie 1937, 3, 241–396. [Google Scholar]
- Jeannel, R. Monographie des “Anillini”, Bembidiides endogés (Coleoptera Trechidae). In Memoires du Museum National d’Histoire Naturelle, Paris (A); 1963; Volume 28, pp. 33–204. [Google Scholar]
- Giachino, P.M.; Vailati, D. Review of Anillina of Macedonia and description of two new species of Prioniomus from Greece (Coleoptera, Carabidae). Fragm. Entomol. 2012, 44, 33–64. [Google Scholar] [CrossRef]
- Fancello, L.; Magrini, P. Due nuove specie di Prioniomus Jeannel, 1937 della Penisola Calcidica (Grecia nord-orientale) (Coleoptera, Carabidae, Bembidiini). G. Ital. Di Entomol. 2015, 14, 1–16. [Google Scholar]
- Giachino, P.M.; Vailati, D. Nuovi Anillini (Coleoptera Carabidae) di Grecia e Anatolia. Mem. della Soc. Entomol. Ital. 2019, 95, 3–44. [Google Scholar] [CrossRef]
- Jeanne, C. Sur la classification des Bembidiides endogés de la région euro-méditerranéenne (Col. Carabidae, Bembidiinae, Anillini). Nouv. Rev. Française d’Entomologie 1973, 3, 83–102. [Google Scholar]
- Vigna Taglianti, A. Considerazioni sui Carabidi cavernicoli ed endogei dell’Asia Minore (Coleoptera, Carabidae). Int. J. Speleol. 1973, 5, 349–360. [Google Scholar] [CrossRef]
- Coiffait, H. Notes sur les Anillini. Faune de Turquie et de la France. Rev. Française d’Entomologie 1956, 23, 77–83. [Google Scholar]
- Jedlička, A. Neue Carabiden aus der palaearktischen Region (Coleoptera, Carabidae). Reichenbachia 1968, 8, 285–296. [Google Scholar]
- Vigna Taglianti, A. Un nuovo Anillino dell’Asia Minore (Coleoptera, Carabidae). Rev. Suisse de Zool. 1976, 83, 373–379. [Google Scholar]
- Casale, A.; Vigna Taglianti, A. Caraboid Beetles (excl. Cicindelidae) of Anatolia and their biogeographical significance. (Coleoptera, Caraboidea). Biogeogr. Biogeogr. dell’Anatolia 1999, 20, 277–406. [Google Scholar] [CrossRef]
- Pavesi, M. Ridefinizione del genere Prioniomus Jeannel, 1937 e descrizione di Prioniomus cassiopaeus n. sp. dell’Isola di Kerkyra (Greece, Isole Ionie). Fragm. Entomol. Roma 2010, 42, 415–448. [Google Scholar] [CrossRef]
- Giachino, P.M.; Vailati, D. The Subterranean Environment. Hypogean life, concepts and collecting techniques. In WBA Handbooks; WBA Project: Verona, Italy, 2010; Volume 3, p. 130. [Google Scholar]
- Giachino, P.M. Revision of the Australian Anillina (Coleoptera, Carabidae, Bembidiini). In Results of the Zoological Missions to Australia of the Regional Museum of Natural Sciences of Turin. II. Monografie del Museo Regionale di Scienze Naturali, Torino; Daccordi, M., Giachino, P.M., Eds.; Museo Regionale di Scienze Naturali: Torino, Italy, 2005; Volume 42, pp. 137–238. [Google Scholar]
- Popov, S.V.; Rögl, F.; Rozanov, A.Y.; Steininger, F.F.; Shcherba, I.G.; Kovac, M. Lithological-Paleogeographic maps of Paratethys. 10 Maps late Eocene to Pliocene. Cour. Forsch. Senckenberg 2004, 250, 1–46, 10 maps. [Google Scholar]
- Casale, A.; Giachino, P.M.; Vailati, D. Il genere Duvalius nell’isola di Creta, con descrizione di Duvalius (Duvalius) augusti specie nuova. Mem. della Soc. Entomol. Ital. 2021, 97, 191–201. [Google Scholar] [CrossRef]
- Jeannel, R. Le sillon transégéen et description de Coléoptères cavernicoles nouveaux de la Grèce. Bull. de la Société des Sci. de Cluj 1929, 4, 59–84. [Google Scholar]
- Jeannel, R. La genèse des Faunes Terrestres. Eléments de Biogéographie; Presses Universitaires de France: Paris, France, 1942; p. 514. [Google Scholar]
- Furon, R. La Paléogéographie. Essai sur l’évolution des Continents et des Océans; Payot: Paris, Italy, 1941; p. 530. [Google Scholar]
- Giachino, P.M.; Vailati, D. Revisione degli Anemadinae. In Monografie di Natura Bresciana; Museo Civico di Scienze Naturali: Brescia, Italy, 1993; Volume 18, p. 314. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giachino, P.M.; Vailati, D. A New Iason Species from Crete (Coleoptera, Carabidae, Anillini) with Notes about Anillini of Greece and Anatolia. Diversity 2023, 15, 75. https://doi.org/10.3390/d15010075
Giachino PM, Vailati D. A New Iason Species from Crete (Coleoptera, Carabidae, Anillini) with Notes about Anillini of Greece and Anatolia. Diversity. 2023; 15(1):75. https://doi.org/10.3390/d15010075
Chicago/Turabian StyleGiachino, Pier Mauro, and Dante Vailati. 2023. "A New Iason Species from Crete (Coleoptera, Carabidae, Anillini) with Notes about Anillini of Greece and Anatolia" Diversity 15, no. 1: 75. https://doi.org/10.3390/d15010075
APA StyleGiachino, P. M., & Vailati, D. (2023). A New Iason Species from Crete (Coleoptera, Carabidae, Anillini) with Notes about Anillini of Greece and Anatolia. Diversity, 15(1), 75. https://doi.org/10.3390/d15010075







