Fossil History of Ambrosia Beetles (Coleoptera; Platypodidae) with Description of a New Genus from Dominican Amber
Abstract
1. Introduction
2. Materials and Methods
3. Results
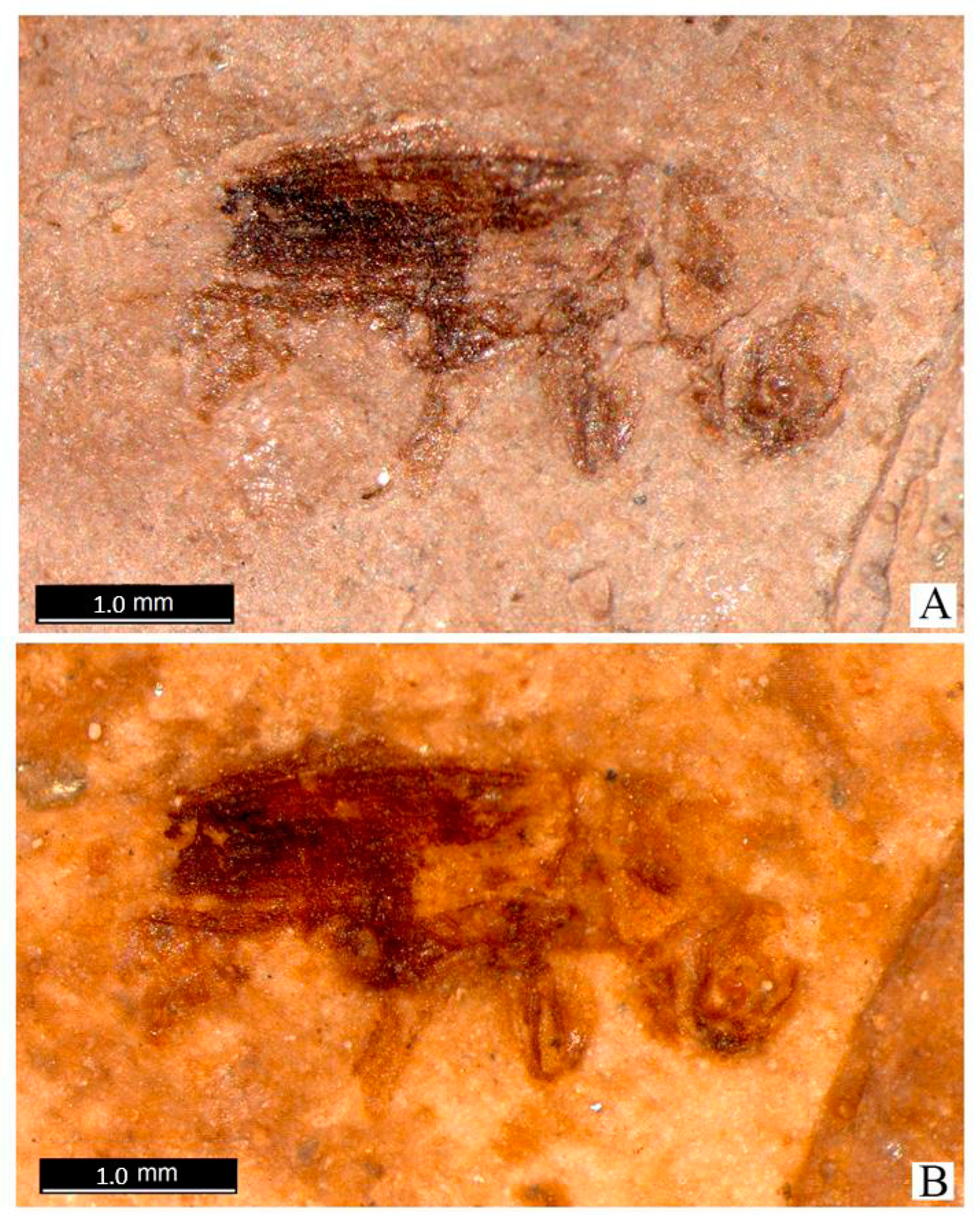
Description of New Fossil Taxa of Platypodidae
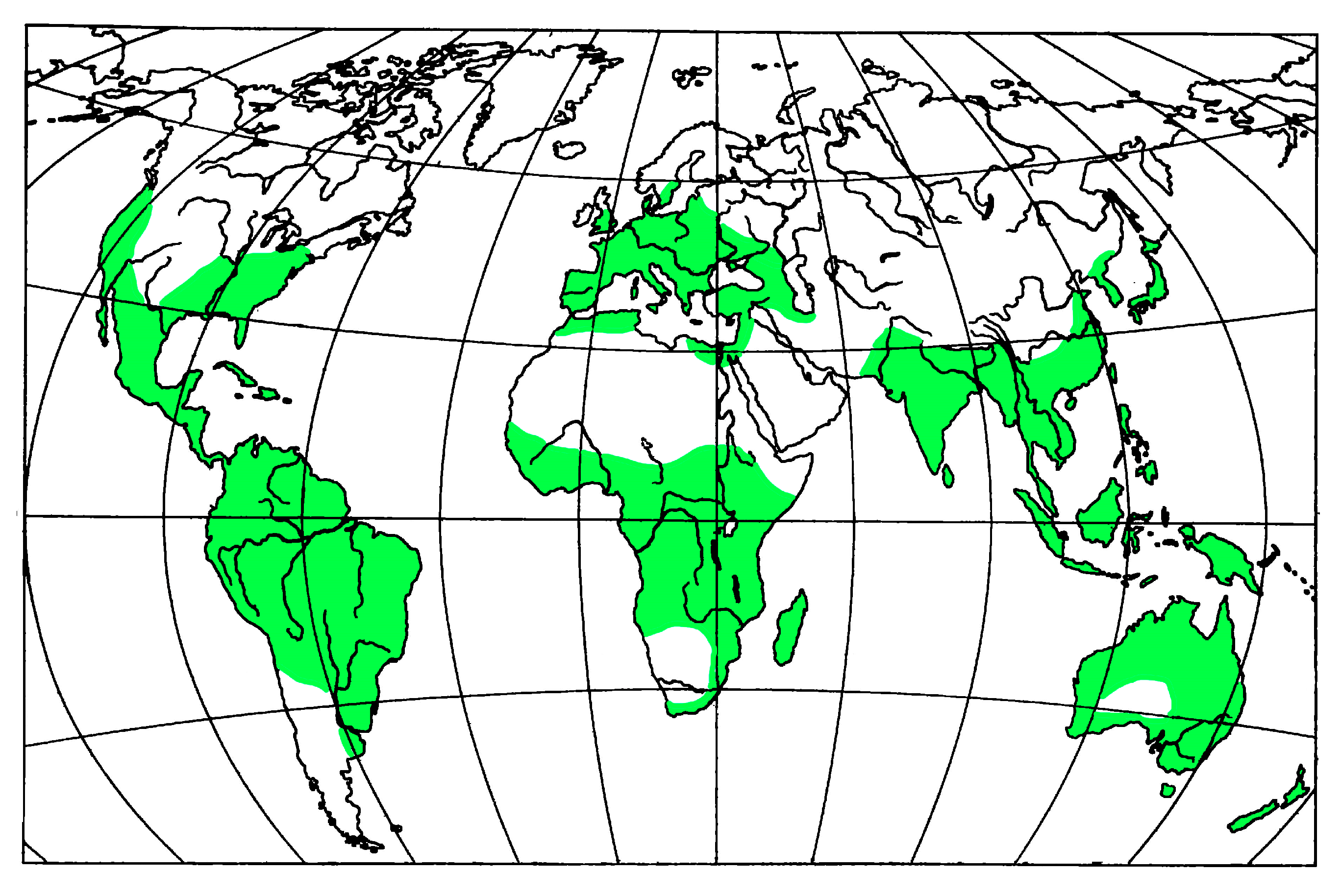
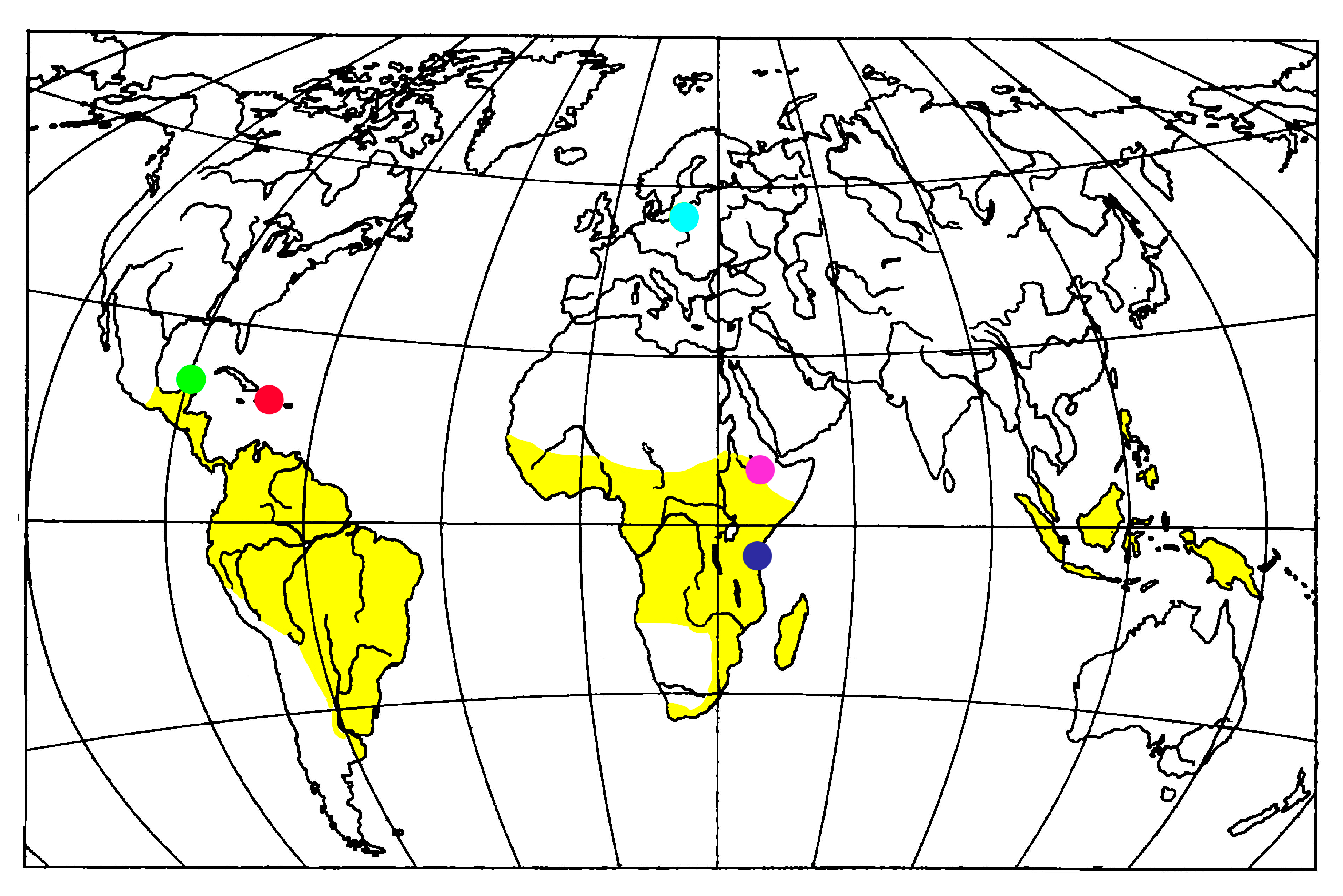
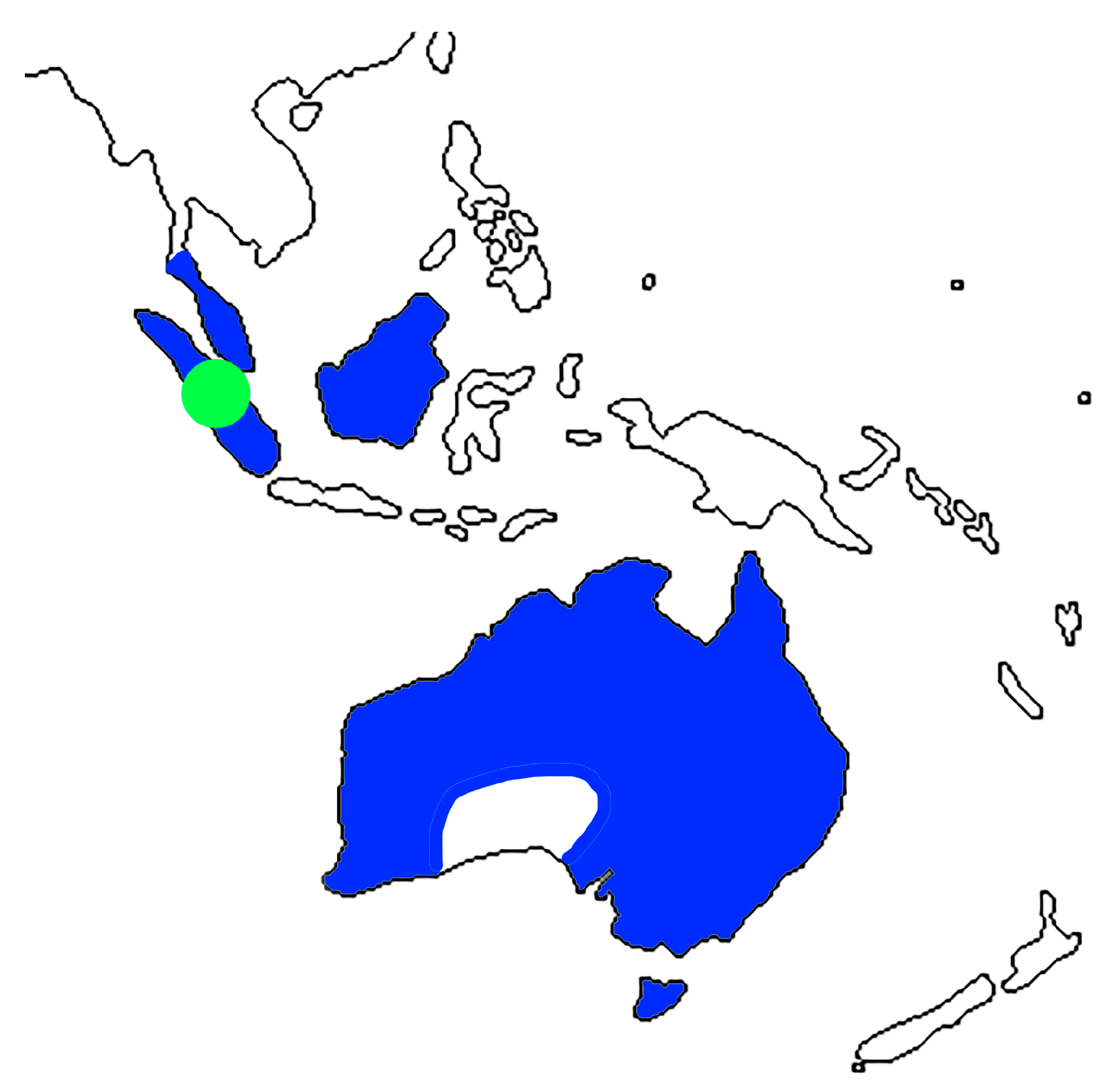
4. Fossil Ambrosia Beetles Review
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jordal, B.H. 3.7.13. Platypodinae Shuckard, 1840. In Handbook of Zoology. Arthropoda: Insecta. Coleoptera, Beetles. Volume 3: Morphology and Systematics (Phytophaga); Kristensen, N.P., Beutel, R.G., Eds.; Walter de Gruyter: Berlin, Germany; New York, NY, USA, 2014; pp. 642–648. [Google Scholar]
- Wood, S.L.; Bright, D.E., Jr. A catalog of Scolytidae and Platypodidae (Coleoptera), part 2: Taxonomic index. Great Bas. Nat. Mem. 1992, 13, 835–1553. [Google Scholar]
- Wood, S.L. Revision of the genera of Platypodidae (Coleoptera). Great Bas. Nat. 1993, 53, 259–281. [Google Scholar]
- Bright, D.E. A catalog of Scolytidae and Platypodidae (Coleoptera): Supplement 3 (2000–2010), with notes on subfamily and tribal reclassifications. Insecta Mundi 2014, 356, 1–336. [Google Scholar]
- Thompson, R.T. Observations on the morphology and classification of weevils (Coleoptera, Curculionoidea) with a key to major groups. J. Nat. Hist. 1992, 26, 835–891. [Google Scholar] [CrossRef]
- Legalov, A.A. Fossil Mesozoic and Cenozoic weevils (Coleoptera, Obrienioidea, Curculionoidea). Paleontol. J. 2015, 49, 1442–1513. [Google Scholar] [CrossRef]
- Legalov, A.A. A review of the Curculionoidea (Coleoptera) from European Eocene ambers. Geosciences 2020, 10, 16. [Google Scholar] [CrossRef]
- Legalov, A.A. Fossil history of Curculionoidea (Coleoptera) from the Paleogene. Geosciences 2020, 10, 358. [Google Scholar] [CrossRef]
- Schedl, K.E. New Platypodidae from Mexican amber. J. Paleontol. 1962, 36, 1035–1038. [Google Scholar]
- Schawaller, W. Pseudoskorpione (Cheliferidae) phoretisch auf Käfern (Platypodidae) in Dominikanischem Bernstein (Stuttgarter Bernsteinsammlung: Pseudoscorpionidea und Coleoptera). Stuttg. Beitr. Natur. 1981, 71, 1–17. [Google Scholar]
- Peris, D.; Solórzano-Kraemer, M.M.; Smith, S.M.; Cognato, A.I. Eoplatypus jordali gen.n. et sp.n., the first described Platypodinae (Coleoptera: Curculionidae) from Baltic amber. Arthropod Syst. Phyl. 2017, 75, 85–194. [Google Scholar]
- Bright, D.E.; Poinar, G.O., Jr. Scolytidae and Platypodidae (Coleoptera) from Dominican Republic amber. Ann. Entomol. Soc. Am. 1994, 87, 170–194. [Google Scholar] [CrossRef]
- Davis, S.R.; Engel, M.S. A new ambrosia beetle in Miocene amber of the Dominican Republic (Coleoptera: Curculionidae: Platypodinae). Alavesia 2007, 1, 121–124. [Google Scholar]
- Peris, D.; Solórzano-Kraemer, M.M.; Peñalver, E.; Delclos, X. New ambrosia beetles (Coleoptera: Curculionidae: Platypodinae) from Miocene Mexican and Dominican ambers and their paleobiogeographical implications. Org. Divers. Evol. 2015, 15, 527–542. [Google Scholar] [CrossRef]
- Solórzano-Kraemer, M.M.; Hammel, J.U.; Kunz, R.; Xu, C.-P.; Cognato, A.I. Miocene pinhole borer ambrosia beetles: New species of Diapus (Coleoptera: Curculionidae: Platypodinae). Palaeoworld, 2021, in press. [CrossRef]
- Legalov, A.A.; Pankowski, M.G. Two new species of the tribe Tesserocerini (Coleoptera: Platypodidae) from Miocene Ethiopian amber. Paleontol. J. 2023, in press.
- Lawrence, J.F.; Beutel, R.G.; Leschen, R.A.B.; Slipinsky, S.A. Chapter 2. Glossary of morphological terms. In Handbook of Zoology. Arthropoda: Insecta. Coleoptera, Beetles. Volume 2: Morphology and Systematics (Elateroidea, Bostrichiformia, Cucujiformia partim); Kristensen, N.P., Beutel, R.G., Eds.; Walter de Gruyter: Berlin, Germany; New York, NY, USA, 2010; pp. 9–20. [Google Scholar]
- Wickham, H.F. Fossil Coleoptera from the Wilson Ranch near Florissant, Colorado. Bull. Lab. Nat. Hist. State. Univ. Iowa 1913, 6, 3–29. [Google Scholar]
- Poinar, G.O., Jr.; Vega, F.E.; Legalov, A.A. New subfamily of ambrosia beetles (Coleoptera: Platypodidae) from mid-Cretaceous Burmese amber. Hist. Biol. 2020, 32, 137–142. [Google Scholar] [CrossRef]
- Kohring, R.; Schlueter, T. Historische und paläontologische des simetits, eines fossilen harzes mutmaßlich mio/pliozänen alters aus Sizilien. Doc. Natur. 1989, 56, 33–58. [Google Scholar]
- Guerin-Meneville, F.E. Lettre de Maravigna et note sur la lettre de Maravigna sur des insectes trouvés dans l’ambre de Sicile. Rev. Zool. Soc. Cuvierienne 1838, 1, 168–170. [Google Scholar]
- Schedl, K.E. Monographie der Familie Platypodidae. Coleoptera; Verlag, W., Junk, N., Den Haag, V., Eds.; Springer: Berlin/Heidelberg, Germany, 1972; p. 322. [Google Scholar]
- Sanborne, M. Biology of Ithycerus noveboracensis (Forster) (Coleoptera) and weevil phylogeny. Evol. Monogr. 1981, 4, 1–80. [Google Scholar]
- Wood, S.L. A reclasification of the genera of Scolytidae (Coleoptera). Great Basin Nat. Mem. 1986, 10, 1–126. [Google Scholar]
- Zherikhin, V.V.; Egorov, A.B. Weevils (Coleoptera, Curculionidae) from Russian Far East; Institute Biology Soil Science: Vladivostok, Russia, 1991. [Google Scholar]
- Legalov, A.A. Annotated key to weevils of the world. Part 1. Families Nemonychidae, Anthribidae, Belidae, Ithyceridae, Rhynchitidae, Brachyceridae and Brentidae. Ukr. J. Ecol. 2018, 8, 780–831. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Zarazaga, M.A.; Lyal, C.H.C. A World Catalogue of Families and Genera Curculionoidea (Insecta: Coleoptera) (Excepting Scolytidae and Platypodidae); Entomopraxis: Barcelona, Spain, 1999; p. 315. [Google Scholar]
- Morimoto, K.; Kojima, H. Systematic position of the tribe Phylloplatypodini, with remarks on the definitions of the families Scolytidae, Platypodidae, Dryophthoridae and Curculionidae (Coleoptera: Curculionoidea). Esakia 2004, 44, 153–168. [Google Scholar] [CrossRef]
- Gratshev, V.G.; Legalov, A.A. The Mesozoic stage of evolution of the family Nemonychidae (Coleoptera, Curculionoidea). Paleontol. J. 2014, 48, 851–944. [Google Scholar] [CrossRef]
- Kuschel, G. A phylogenetic classification of Curculionoidea to families and subfamilies. Mem. Entomol. Soc. Wash. 1995, 14, 5–33. [Google Scholar]
- Kuschel, G.; Leschen, R.A.B.; Zimmerman, E.C. Platypodidae under scrutiny. Inverteb. Tax. 2000, 14, 771–805. [Google Scholar] [CrossRef]
- Marvaldi, A.E.; Sequeira, A.S.; O’Brien, C.W.; Farrell, B.D. Molecular and morphological phylogenetics of weevils (Coleoptera: Curculionoidea): Do niche shifts accompany diversification? Syst. Biol. 2002, 51, 761–785. [Google Scholar] [CrossRef]
- Oberprieler, R.G.; Marvaldi, A.E.; Anderson, R.S. Weevils, weevils, weevils everywhere. Zootaxa 2007, 1668, 491–520. [Google Scholar] [CrossRef]
- Gillett, C.P.D.T.; Crampton-Platt, A.; Timmermans, M.J.T.N.; Jordal, B.H.; Emerson, B.C.; Vogler, A.P. Bulk de novo mitogenome assembly from pooled total DNA elucidates the phylogeny of weevils (Coleoptera: Curculionoidea). Mol. Biol. Evol. 2014, 31, 2223–2237. [Google Scholar] [CrossRef]
- Shin, S.; Clarke, D.J.; Lemmon, A.R.; Moriarty-Lemmon, E.; Aitken, A.L.; Haddad, S.; Farrell, B.D.; Marvaldi, A.E.; Oberprieler, R.G.; McKenna, D.D. Phylogenomic data yield new and robust insights into the phylogeny and evolution of weevils. Mol. Biol. Evol. 2017, 35, 823–836. [Google Scholar] [CrossRef]
- Mugu, S.; Pistone, D.; Jordal, B.H. New molecular markers resolve the phylogenetic position of the enigmatic wood-boring weevils Platypodinae (Coleoptera: Curculionidae). Arth. Syst. Phyl. 2018, 76, 45–58. [Google Scholar]
- Clarke, D.; Limaye, A.; McKenna, D.; Oberprieler, R. The weevil fauna preserved in Burmese amber—Snapshot of a unique, extinct lineage (Coleoptera: Curculionoidea). Diversity 2019, 11, 1. [Google Scholar] [CrossRef]
- Deng, C.; Ślipiński, A.; Ren, D.; Pang, H. The first Mesozoic colydiid beetles (Coleoptera: Zopheridae: Colydiinae) from the upper Cretaceous amber of Myanmar. Cret. Res. 2017, 78, 71–77. [Google Scholar] [CrossRef]
- Háva, J. A new species of Paleoendeitoma Deng, Ślipiński, & Pang 2017 (Coleoptera: Zopheridae: Colydiinae) from Upper Cretaceous Burmese amber. Folia Heyrovskyana 2019, 27, 9–12. [Google Scholar]
- Bullis, D.A. A new species of Paleoendeitoma (Coleoptera: Zopheridae: Colydiinae) from mid-Cretaceous Burmese amber. Palaeoentomology 2020, 3, 46–49. [Google Scholar] [CrossRef]
- Cheng, G.; Tihelka, E.; Shi, H.; Tian, L.; Huang, D.; Cai, C. Specialised subcortical cylindrical bark beetles from mid-Cretaceous Burmese amber (Coleoptera: Zopheridae: Colydiinae). Hist. Biol. 2020, 33, 2584–2590. [Google Scholar] [CrossRef]
- Poinar, G., Jr.; Vega, F.E. A new genus of cylindrical bark beetle (Coleoptera: Zopheridae: Colydiinae) in mid-Cretaceous Burmese amber. Biosis Biol. Syst. 2020, 1, 134–140. [Google Scholar] [CrossRef]
- Li, Y.-D.; Huang, D.-Y.; Cai, C.-Y. An aberrant colydiine-like tenebrionoid beetle from mid-Cretaceous Burmese amber (Coleoptera: Tenebrionoidea: Zopheridae). Palaeoentomology 2021, 4, 614–619. [Google Scholar] [CrossRef]
- Legalov, A.A. Fossil history of Mesozoic weevils (Coleoptera: Curculionoidea). Insect Sci. 2012, 19, 683–698. [Google Scholar] [CrossRef]
- Alonso-Zarazaga, M.A.; Lyal, C.H.C. A catalogue of family and genus group names in Scolytinae and Platypodinae with nomenclatural remarks (Coleoptera: Curculionidae). Zootaxa 2009, 2258, 1–134. [Google Scholar] [CrossRef]
- Alonso-Zarazaga, M.A.; Barrios, H.; Borovec, R.; Bouchard, P.; Caldara, R.; Colonnelli, E.; Gültekin, L.; Hlavá, P.; Korotyaev, B.; Lyal, C.H.C.; et al. Cooperative catalogue of Palaearctic Coleoptera Curculionoidea. Monogr. Electrón. 2017, 8, 1–729. [Google Scholar]
- Ngo-Muller, V.; Garrouste, R.; Carbuccia, B.; Pouillon, J.-M.; Nel, A. First terrestrial arthropod records from the Miocene amber of Sumatra. In Proceedings of the 8th International Conference on Fossil Insects, Arthropods and Amber Saint Domingo, Colonial City, Dominican Republic, 7–13 April 2019; pp. 116–117. [Google Scholar]
- Ngô-Muller, V.; Garrouste, R.; Pouillon, J.-M.; Christophersen, V.; Christophersen, A.; Nel, A. The first representative of the fly genus Trentepohlia subgenus Mongoma in amber from the Miocene of Sumatra (Diptera: Limoniidae). Hist. Biol. 2021, 33, 254–257. [Google Scholar] [CrossRef]
- Perez-Gelabert, D.E. Checklist, bibliography and quantitative data of the arthropods of Hispaniola. Zootaxa 2020, 4749, 1–668. [Google Scholar] [CrossRef] [PubMed]
- Nunberg, M. Eine fossile Kernkäfer-Art aus der Gattung Periommatus Chap. (Platypodidae). Ann. Zool. 1959, 18, 127–138. [Google Scholar]
- Schlüter, T.; von Gnielinski, F. The East African Copal—Its Geologic, Stratigraphic, Palaeontologic Significance and Comparison with Other Fossil Resins of Similar Age; National Museums of Tanzania: Dar es Salaam, Tanzania, 1987; Volume 8, pp. 1–32. [Google Scholar]
- Solórzano-Kraemer, M.M.; Kunz, R.; Hammel, J.U.; Peñalver, E.; Delclòs, X.; Engel, M.S. Stingless bees (Hymenoptera: Apidae) in Holocene copal and Defaunation resin from Eastern Africa indicate Recent biodiversity change. Holocene 2022, 32, 414–432. [Google Scholar] [CrossRef]
- MacGinitie, H.D. An Early Middle Eocene Flora from the Yellowstone-Absaroka Volcanic Province, Northwestern Wind River Basin, Wyoming, 1st ed.; University of California Press: Berkeley, CA, USA, 1974. [Google Scholar]
- Allen, S.E. The Uppermost Lower Eocene Blue Rim flora from the Bridger Formation of Southwestern Wyoming: Floristic Composition, Paleoclimate, and Paleoecology. Ph.D. Thesis, University of Florida, Gainesville, FL, USA, 2017. [Google Scholar]
- MacGinitie, H.D. The Eocene Green River flora of northwestern Colorado and Northeastern Utah; University of California Press: Berkeley, CA, USA, 1969. [Google Scholar]
- Wolfe, J.A.; Wehr, W. Middle Eocene dicotyledonous plants from Republic, northeastern Washington. US Geol. Surv. Bull. 1987, 1597, 1–25. [Google Scholar]
- McClain, A.M.; Manchester, S.R. Dipteronia (Sapindaceae) from the Tertiary of North America and implications for the phytogeographic history of the Aceroideae. Am. J. Bot. 2001, 88, 1316–1325. [Google Scholar] [CrossRef]
- Jud, N.A.; Allen, S.E.; Nelson, C.W.; Bastos, C.L.; Chery, J.G. Climbing since the early Miocene: The fossil record of Paullinieae (Sapindaceae). PLoS ONE 2021, 16, e0248369. [Google Scholar] [CrossRef]
- Graham, A. Studies in neotropical paleobotany. 1V. The Eocene communities of Panama. Ann. Mo. Bot. Gard. 1985, 72, 504–534. [Google Scholar] [CrossRef]
- Graham, A. Tropical American Tertiary floras and paleoenvironments: Mexico, Costa Rica, and Panama. Am. J. Bot. 1987, 74, 1519–1531. [Google Scholar] [CrossRef]
- MacGinitie, H.D. Fossil Plants of the Florissant Beds, Colorado; Carnegie Institution of Washington Publication: Washington, DC, USA, 1953; 599p. [Google Scholar]
- Zherikhin, V.V. History of the tropical rain-forest biome. Zh. Obsh. Biol. 1993, 54, 659–666. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Legalov, A.A.; Poinar, G.O., Jr. Fossil History of Ambrosia Beetles (Coleoptera; Platypodidae) with Description of a New Genus from Dominican Amber. Diversity 2023, 15, 45. https://doi.org/10.3390/d15010045
Legalov AA, Poinar GO Jr. Fossil History of Ambrosia Beetles (Coleoptera; Platypodidae) with Description of a New Genus from Dominican Amber. Diversity. 2023; 15(1):45. https://doi.org/10.3390/d15010045
Chicago/Turabian StyleLegalov, Andrei A., and George O. Poinar, Jr. 2023. "Fossil History of Ambrosia Beetles (Coleoptera; Platypodidae) with Description of a New Genus from Dominican Amber" Diversity 15, no. 1: 45. https://doi.org/10.3390/d15010045
APA StyleLegalov, A. A., & Poinar, G. O., Jr. (2023). Fossil History of Ambrosia Beetles (Coleoptera; Platypodidae) with Description of a New Genus from Dominican Amber. Diversity, 15(1), 45. https://doi.org/10.3390/d15010045






