New Data on the Distribution of Southern Forests for the West Siberian Plain during the Late Pleistocene: A Paleoentomological Approach
Abstract
1. Introduction
2. Material and Methods
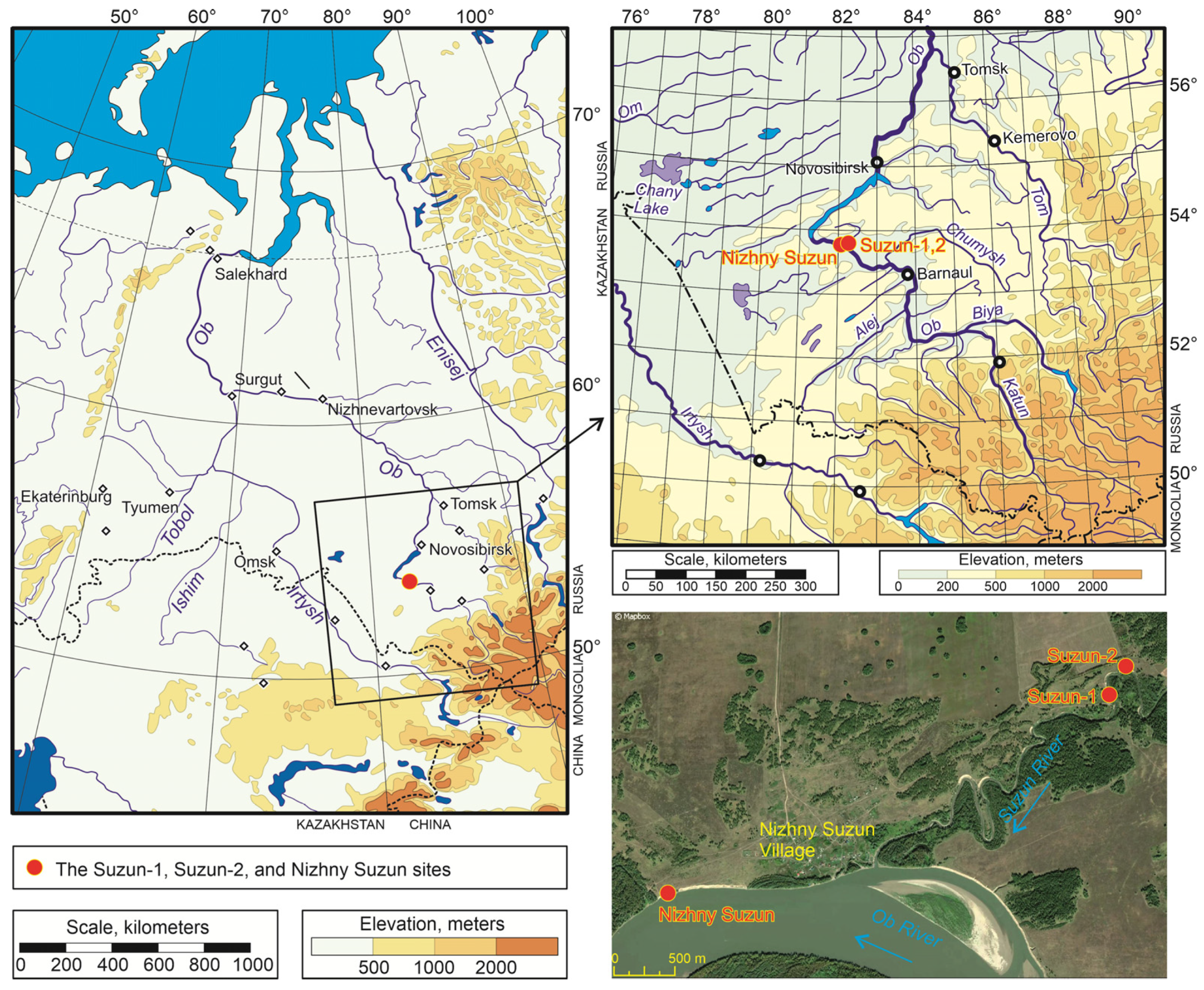
2.1. Study Area
2.2. Methods and Geological Settings
| Layer No. | Depth of Bedding, m | Thickness, m | Description |
|---|---|---|---|
| 1 | 0.0–0.3 | 0.3 | Modern soil. |
| 2 | 0.3–2.3 | 2.0 | Light brown sandy loam. Dense, dry, porous, with spots of ferrugination. |
| 3 | 2.3–2.4 | 0.1 | Gray loam. Dense. |
| 4 | 2.4–3.2 | 0.8 | Brown sandy loam. Dense, with ferrugination along the roots of trees. |
| 5 | 3.2–4.5 | 1.3 | Gray-brown loam with spots of ferrugination. |
| 6 | 4.5–6.1 | 1.6 | Horizontal interbedding of medium-grained light brown sands and loams with ferruginous inclusions. |
| 7 | 6.1–6.9 | 0.8 | Alternation of dark brown sandy loams and loams with inclusions of plant detritus. |
| 8 | 6.9–7.85 | 0.95 | Blue-gray clays with lenses and layers of alluvial plant detritus. Five samples were taken for entomological analysis (Table 4). |
| 9 | 7.85–8.25 | 0.4 | Gray sand. Medium-grained, moisture-saturated, goes under the water level. |
| Layer No. | Depth of Bedding, m | Thickness, m | Description |
|---|---|---|---|
| 1 | 0.0–0.5 | 0.5 | Modern soil. |
| 2 | 0.5–5.5 | 5.0 | Light brown sandy loam. Dense, dry, porous, with spots of ferrugination. |
| 3 | 5.5–12.0 | 6.5 | Gray loam. Dense, interbedded with brownish-gray sandy loam. |
| 4 | 12.0–13.3 | 1.3 | Brown sandy loam. Dense, with sparse lenses and sublayers of alluvial detritus. |
| 5 | 13.3–16.0 | 2.7 | Bluish-gray sandy loam. Dense, with lenses and sublayers of alluvial detritus. Four samples were taken for entomological analysis (Table 4). |
| 6 | 16.0–16.5 | 0.5 | Gray sand. Medium-grained, moisture-saturated, goes under the water level. |
| Layer No. | Depth of Bedding, m | Thickness, m | Description |
|---|---|---|---|
| 1 | 0.0–0.6 | 0.6 | Modern soil. |
| 2 | 0.6–1.6 | 1.0 | Sandy loam. Dense, dry, porous, with spots of ferrugination. |
| 3 | 1.6–3.1 | 1.5 | Light gray sands. Cross-bedded, medium- and coarse-grained. |
| 4 | 3.1–4.5 | 1.4 | Light gray sands. Horizontal-bedded, medium- and coarse-grained, with sublayers of cross-bedded sands. |
| 5 | 4.5–4.6 | 0.1 | Sublayer of buried soil. |
| 6 | 4.6–5.1 | 0.6 | Brownish sands. Horizontal-bedded, medium- and coarse-grained, with spots and sublayers of ferrugination. |
| 7 | 5.1–10.0 | 4.9 | Light-gray sands. Horizontal-bedded, medium- and coarse-grained, with spots and sublayers of ferrugination. |
| 8 | 10.0–11.5 | 1.5 | Dark brown sandy loam. Wet. |
| 9 | 11.5–12.0 | 0.5 | Bluish-gray clay with sublayers of alluvial plant detritus. Two samples were taken for entomological analysis (Table 4). |
| Section | Coordinates | Samples: Depth, m | Radiocarbon Date, BP | Laboratory Code | Calibrated Age, cal BP |
|---|---|---|---|---|---|
| Suzun-1 | N53.73169°; E82.18172° | S1: 6.9–7.1 S2: 7.1–7.25 S3: 7.25–7.45 S4: 7.45–7.65 S5: 7.65–7.85 | S1: 21,190 ± 500 | SPb_3011 | 24,893–25,966 |
| Suzun-2 | N53.73334°; E82.18352° | S1: 13.3–13.6 S2: 13.6–13.8 S3: 14.1–14.3 S4: 14.5–14.7 | S2: 16,984 ± 120 | SPb_3125 | 20,379–20,699 |
| Nizhny Suzun | N53.71668°; E82.12691° | S1: 11.5 –11.7 S2: 11.7–11.95 | S2: 23,737 ± 200 | SPb_3126 | 27,693–28,126 |
2.3. Material
3. Results
3.1. Taxonomic Composition
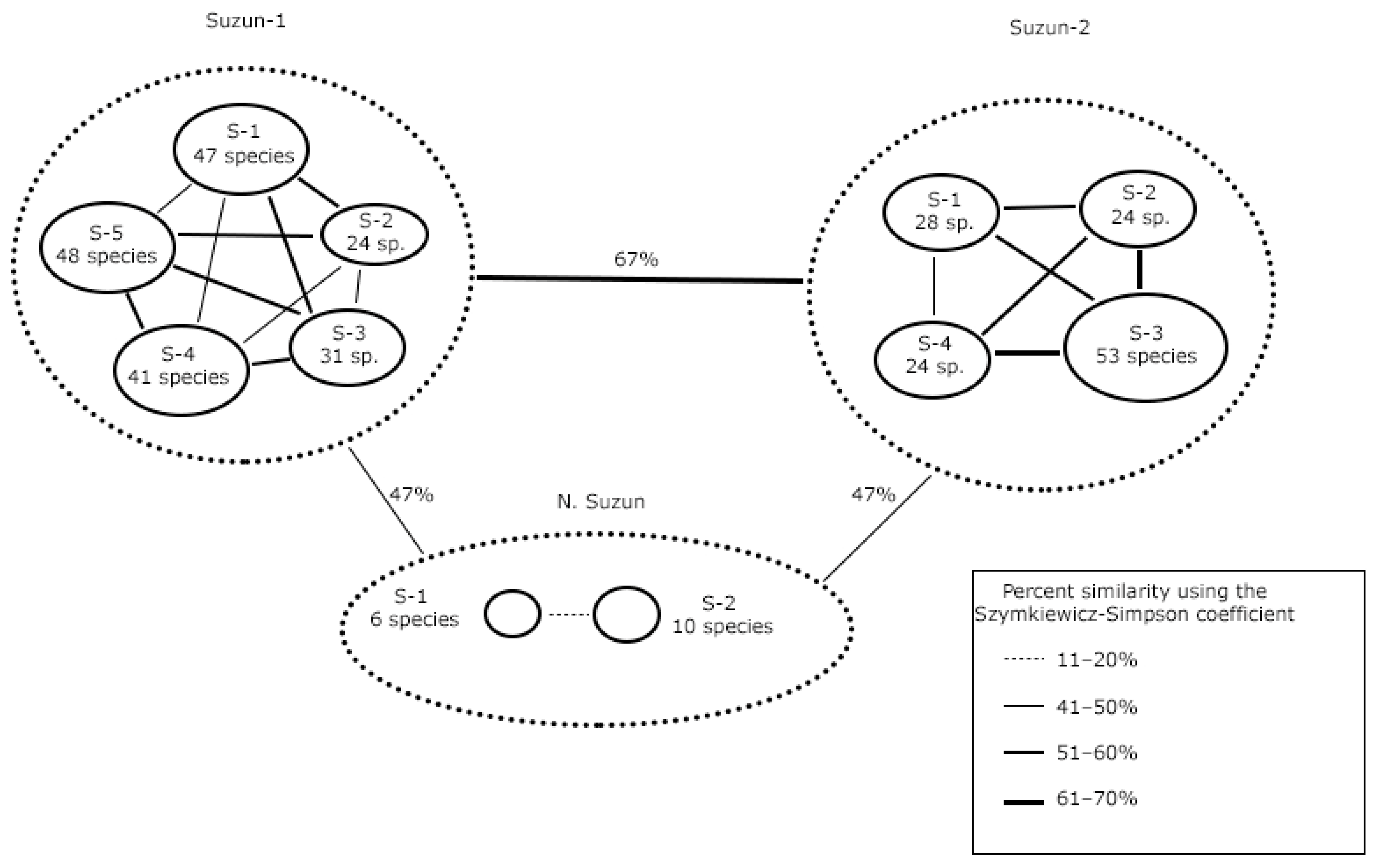
3.2. Comparison of the Species Composition
4. Discussion
4.1. Modern Distribution of Species
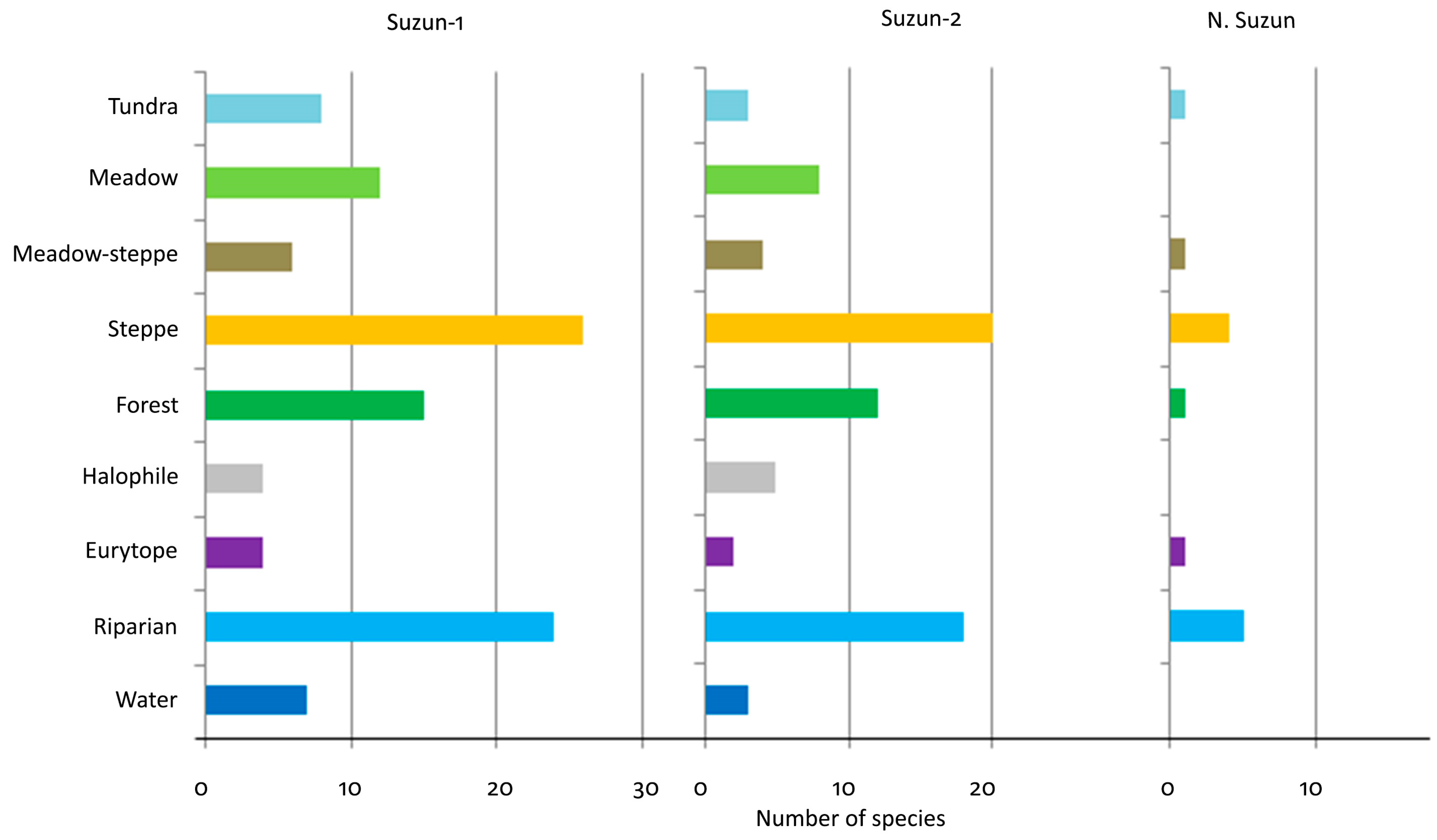
4.2. Ecology
4.3. Comparison with the Region Entomocomplexes of a Similar Age
4.4. Paleobotanical and Theriological Landscape Reconstructions for the Region in MIS 3 and MIS 2
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| No | Species | N | Nmin | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Suzun-1 | Suzun-2 | Nizhny Suzun | Ʃ | ||||||||||||||
| H | P | E | O | S1 | S2 | S3 | S4 | S5 | S1 | S2 | S3 | S4 | S1 | S2 | |||
| DYTISCIDAE | |||||||||||||||||
| 1 | * Agabus ?adpressus Aube, 1837 | – | – | 1 | – | – | – | – | 1 | – | – | – | – | – | – | – | 1 |
| 2 | * Agabus congener (Thunberg, 1794) | – | 2 | – | – | – | – | 1 | – | 1 | – | – | – | – | – | – | 2 |
| 3 | * Agabus coxalis Sharp, 1882 | – | 1 | – | – | 1 | – | – | – | – | – | – | – | – | – | – | 1 |
| 4 | * Agabus labiatus (Brahm, 1791) | – | 1 | – | – | – | – | – | – | – | 1 | – | – | – | – | – | 1 |
| 5 | * Agabus pallens Poppius, 1905 | – | 5 | – | – | 2 | – | – | – | 1 | 1 | – | 1 | – | – | – | 5 |
| 6 | * Ilybius subaeneus Erichson, 1837 | – | 1 | – | 1 | – | – | – | 1 | 1 | – | – | – | – | – | – | 2 |
| 7 | Ilybius sp.1 | – | – | 1 | – | – | – | – | – | – | – | 1 | – | – | – | – | 1 |
| 8 | * Nebrioporus ?depressus (Fabricius, 1775) | – | 1 | – | – | 1 | – | – | – | – | – | – | – | – | – | – | 1 |
| 9 | * Porhydrus lineatus (Fabricius, 1775) | – | 1 | – | – | – | – | – | – | 1 | – | – | – | – | – | – | 1 |
| – | Dytiscidae indet. | – | 1 | – | – | – | – | – | – | – | – | – | 1 | – | – | – | 1 |
| CARABIDAE | |||||||||||||||||
| 10 | Pelophila borealis (Paykull, 1790) | – | 1 | 2 | – | 1 | – | – | 1 | – | – | – | 1 | – | – | – | 3 |
| 11 | Notiophilus aquaticus/N. cf. aquaticus (Linnaeus, 1758) | – | – | 4 | – | – | 1 | – | – | 1 | – | – | 1 | – | – | – | 3 |
| 12 | Nebria gyllenhali (Schönherr, 1806) | – | 2 | – | – | – | – | – | – | 1 | – | – | 1 | – | – | – | 2 |
| 13 | * Carabus henningi Fischer von Waldheim, 1817 | – | – | 1 | – | – | – | 1 | – | – | – | – | – | – | – | – | 1 |
| 14 | * Carabus regalis Fischer von Waldheim, 1820 | – | – | 1 | – | – | – | – | – | – | 1 | – | – | – | – | – | 1 |
| 15 | * Carabus arvensis Herbst, 1784 | – | – | 1 | – | – | – | – | – | – | – | – | – | – | 1 | – | 1 |
| 16 | Diacheila polita (Faldermann, 1835) | – | – | 1 | – | 1 | – | – | – | – | – | – | – | – | – | – | 1 |
| 17 | Blethisa multipunctata (Linnaeus, 1758) | – | – | 2 | – | 1 | 1 | – | – | – | – | – | – | – | – | – | 2 |
| 18 | Clivina fossor (Linnaeus, 1758) | – | 1 | 5 | – | – | – | 1 | 1 | 1 | – | 1 | 2 | – | – | – | 6 |
| 19 | * Dyschiriodes cf. rufipes (Dejean, 1825) | – | – | 1 | – | 1 | – | – | – | – | – | – | – | – | – | – | 1 |
| 20 | Dyschiriodes tristis/D. cf. tristis (Stephens, 1827) | – | 1 | 1 | – | – | – | – | 1 | – | – | – | – | – | – | – | 1 |
| – | Dyschiriodes sp. | – | – | 1 | – | – | – | – | 1 | – | – | – | – | – | – | – | 1 |
| 21 | * Bembidion (Bracteon) lapponicum Zetterstedt, 1828 | – | – | 1 | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 |
| 22 | Bembidion (Chlorodium) almum almum J.Sahlberg, 1900 | – | 4 | 9 | – | 1 | 1 | 1 | 1 | 1 | – | – | 2 | 2 | – | 1 | 10 |
| 23 | * Bembidion (Notaphus) obliquum/B. cf. obliquum Sturm, 1825 | – | 2 | 6 | – | 1 | – | – | 1 | – | – | – | 2 | – | – | – | 4 |
| 24 | Bembidion (Eupetedromus) sp. | – | – | 1 | – | 1 | – | – | – | – | – | – | – | – | – | – | 1 |
| 25 | Bembidion (Semicampa) sp.1 | – | – | 2 | – | – | – | – | – | – | 1 | – | 1 | – | – | – | 2 |
| 26 | Bembidion (Philochtus) cf. aeneum Germar, 1823 | – | – | 2 | – | – | – | – | – | – | – | – | 1 | 1 | – | – | 2 |
| 27 | * Bembidion (Bembidion) cf. paediscum Bates, 1883 | – | 1 | 3 | – | 1 | – | 1 | – | – | – | – | 1 | – | – | – | 3 |
| 28 | Bembidion (Plataphus) difficile (Motschulsky, 1844) | – | – | 4 | – | 1 | 1 | 1 | – | – | – | – | 1 | – | – | – | 4 |
| 29 | Bembidion (Ocydromus) cf. scopulinum (Kirby 1837) | – | 1 | 2 | – | 1 | – | – | – | 1 | – | – | – | – | – | – | 2 |
| 30 | Bembidion (Asioperyphus) cf. infuscatum Dejean, 1831 | – | 2 | 5 | – | – | – | 1 | 1 | 1 | 1 | 1 | 1 | 1 | – | – | 7 |
| 31 | Bembidion (Asioperyphus) sp.1 | – | – | 7 | – | – | – | – | – | – | 1 | 1 | 2 | – | 1 | – | 5 |
| 32 | Bembidion (Peryphus) cf. dauricum (Motschulsky, 1844) | – | – | 3 | – | – | – | – | – | 1 | – | – | 1 | 1 | – | – | 3 |
| 33 | * Bembidion (Peryphus) cf. jedlickai Fassati, 1945 | – | – | 2 | – | – | 1 | – | – | – | – | – | – | – | – | – | 1 |
| 34 | Bembidion (Peryphus) obscurellum (Motschulsky, 1845) | – | – | 8 | – | – | – | – | 1 | – | 1 | 2 | 3 | 1 | – | – | 8 |
| 35 | Bembidion (Testediolum) kokandicum Solsky, 1874 | – | – | 5 | – | – | – | – | 1 | – | – | 1 | 1 | – | 1 | – | 4 |
| 36 | * Bembidion (Pamirium) cf. roborovskii Mikhailov, 1988 | – | 4 | 19 | – | 3 | – | 2 | 1 | 1 | 3 | – | 6 | 2 | – | – | 18 |
| – | Bembidion (Ocydromus s.l.) spp. | – | 3 | – | – | 1 | – | – | 2 | – | – | – | – | – | – | – | 3 |
| – | Bembidion spp. | 4 | 8 | 5 | – | 3 | – | 1 | 1 | 2 | 1 | 1 | 3 | – | – | 1 | 13 |
| 37 | Pogonus punctulatus Dejean, 1828 | – | 2 | 4 | – | – | 1 | – | 1 | 1 | – | – | 1 | – | – | – | 4 |
| 38 | Patrobus cf. septentrionis Dejean, 1828 | 1 | 2 | 2 | – | – | – | – | – | 1 | 1 | 1 | 1 | – | 1 | – | 5 |
| 39 | Poecilus cf. ravus (Lutschnik, 1922) | – | 6 | 19 | – | 1 | 2 | 4 | 1 | 2 | – | 1 | 1 | 2 | 1 | – | 15 |
| 40 | Pterostichus (Pseudomaseus) nigrita (Paykull, 1790) | – | – | 1 | – | – | – | – | – | 1 | – | – | – | – | – | – | 1 |
| 41 | Pterostichus (Phonias) sp. | – | 2 | 4 | – | 1 | – | 1 | 1 | – | – | – | 2 | – | – | – | 5 |
| 42 | Pterostichus (Cryobius) sp.1 | – | – | 3 | – | 1 | – | – | 1 | – | – | – | – | – | – | – | 2 |
| 43 | Pterostichus (Cryobius) sp.2 | – | – | 1 | – | – | – | – | – | – | – | – | 1 | – | – | – | 1 |
| – | Pterostichus (Cryobius) spp. | – | 2 | 1 | – | – | – | 1 | – | 1 | – | – | 1 | – | – | – | 3 |
| 44 | Pterostichus (Eosteropus) cf. maurusiacus (Mannerheim, 1825) | – | – | 1 | – | – | – | 1 | – | – | – | – | – | – | – | – | 1 |
| 45 | * Pterostichus (Petrophilus) cf. altainus Jedlička, 1958 | – | – | 1 | – | – | – | 1 | – | – | – | – | – | – | – | – | 1 |
| – | Pterostichus spp. | – | – | 5 | – | – | 1 | 1 | 1 | 1 | – | – | 1 | – | – | – | 5 |
| 46 | * Amara (Amara) cf. depressangula Poppius, 1908 | – | – | 2 | – | 1 | – | – | – | – | 1 | – | – | – | – | – | 2 |
| 47 | * Amara (Celia) cf. infima (Duftschmid, 1812) | – | 1 | – | – | – | – | – | – | – | – | – | 1 | – | – | – | 1 |
| 48 | * Amara (Celia) rupicola/A. cf. rupicola Zimmermann, 1832 | – | 2 | 1 | – | – | – | 1 | 1 | – | – | – | – | – | – | – | 2 |
| 49 | * Amara (Celia) cf. saginata Ménétriés, 1847 | – | – | 1 | – | – | – | – | – | – | – | – | – | – | 1 | – | 1 |
| 50 | Amara (Paracelia) quenseli (Schönherr 1806) | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | 1 | 1 |
| 51 | * Amara (Amathitis) cf. microdera (Chaudoir, 1844) | – | 1 | – | – | – | – | – | – | 1 | – | – | – | – | – | – | 1 |
| 52 | Curtonotus cf. alpinus (Paykull, 1790) | – | 1 | – | – | – | – | – | – | 1 | – | – | – | – | – | – | 1 |
| 53 | * Curtonotus cf. fodinae (Mannerheim, 1825) | – | 1 | 1 | – | 1 | – | – | – | – | – | – | 1 | – | – | – | 2 |
| – | Curtonotus sp. | – | – | 1 | – | – | – | – | – | – | – | – | – | – | 1 | – | 1 |
| 54 | * Agonum carbonarium/A. cf. carbonarium Dejean, 1828 | – | 1 | 2 | – | – | 1 | – | – | – | – | 1 | – | – | – | – | 2 |
| – | Agonum sp. | – | – | 3 | – | – | – | – | 1 | 1 | 1 | – | – | – | – | – | 3 |
| 55 | Dicheirotrichus (Trichocellus) sp. | – | – | 4 | – | – | – | 1 | – | – | 1 | 1 | 1 | – | – | – | 4 |
| 56 | Harpalus amputatus Say, 1830 | – | 8 | 9 | – | 1 | 1 | – | 1 | 2 | 1 | 2 | – | 2 | – | – | 10 |
| 57 | * Harpalus anxius-group (Duftschmid, 1812) | – | – | 3 | – | – | 1 | – | – | – | – | – | – | – | – | – | 1 |
| 58 | * Harpalus pusillus-group (Motschulsky, 1850) | – | 1 | – | – | – | – | – | – | 1 | – | – | – | – | – | – | 1 |
| 59 | * Harpalus salinus Dejean, 1829 | – | – | 2 | – | 1 | – | – | – | – | – | – | – | 1 | – | – | 2 |
| 60 | Harpalus sp.1 | – | – | 3 | – | – | – | – | 1 | 1 | – | – | – | – | – | – | 2 |
| – | Harpalus spp. | – | – | 4 | – | 2 | – | – | – | – | – | – | – | 1 | – | – | 3 |
| 61 | Cymindis binotata/C. cf. binotata Fischer von Waldheim, 1820 | – | – | 4 | – | 2 | – | 1 | – | – | – | – | 1 | – | – | – | 4 |
| 62 | Cymindis cf. kasakh Kryzhanovskij et Emetz, 1973 | – | 1 | 1 | – | – | – | – | – | 1 | – | – | 1 | – | – | – | 2 |
| 63 | Syntomus sp. | – | – | 2 | – | – | 1 | – | – | – | – | – | 1 | – | – | – | 2 |
| 64 | Lebia punctata Gebler, 1843 | – | – | 1 | – | – | 1 | – | – | – | – | – | – | – | – | – | 1 |
| – | Carabidae indet. | 3 | 3 | 8 | – | 1 | – | – | 1 | 2 | – | – | – | 1 | – | – | 5 |
| HELOPHORIDAE | |||||||||||||||||
| 65 | Helophorus obscurellus Poppius, 1907 | – | 2 | 3 | – | 1 | – | 1 | 1 | 1 | – | – | 1 | – | – | – | 5 |
| 66 | * Helophorus orientalis Motschulsky, 1860 | – | 1 | 1 | – | 1 | – | – | – | – | – | – | – | – | – | – | 1 |
| 67 | * Helophorus pallidus Gebler, 1830 | – | 1 | 1 | – | – | – | – | – | – | – | – | 1 | – | – | – | 1 |
| 68 | * Helophorus ?parajacutus Angus, 1970 | – | – | 1 | – | 1 | – | – | – | – | – | – | – | – | – | – | 1 |
| – | Helophorus sp. | – | – | 2 | – | – | – | – | – | 1 | – | – | 2 | – | – | – | 3 |
| HYDROPHILIDAE | |||||||||||||||||
| 69 | Enochrus quadripunctatus (Herbst, 1797) | – | – | – | 1 | – | – | – | – | – | 1 | – | – | – | – | – | 1 |
| 70 | Hydrobius sp. | – | – | 1 | – | – | – | – | – | – | 1 | – | – | – | – | – | 1 |
| – | Hydrophilidae indet. | – | – | 2 | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 |
| HYDRAENIDAE | |||||||||||||||||
| 71 | Ochthebius (?Asiobates) sp.1 | – | – | 3 | – | 1 | – | – | – | – | – | – | 2 | – | – | – | 3 |
| 72 | Ochthebius sp.2 | – | – | 1 | – | – | – | – | 1 | – | – | – | – | – | – | – | 1 |
| LEIODIDAE | |||||||||||||||||
| 73 | Leiodidae indet. | – | – | 6 | – | 2 | – | – | – | 1 | – | – | 2 | – | – | – | 5 |
| SILPHIDAE | |||||||||||||||||
| 74 | Aclypea bicarinata (Gebler, 1830) | – | – | 1 | – | – | – | – | – | 1 | – | – | – | – | – | – | 1 |
| 75 | Aclypea calva (Reitter, 1890) | – | 1 | – | – | – | – | – | – | 1 | – | – | – | – | – | – | 1 |
| 76 | Aclypea opaca (Linnaeus, 1758) | – | 4 | 8 | – | 2 | 1 | 1 | – | 1 | – | – | 1 | 2 | – | 1 | 9 |
| 77 | Aclypea sericea (Zoubkoff, 1833) | – | – | 7 | – | 1 | – | 1 | – | – | 1 | – | 1 | 1 | – | – | 5 |
| – | Aclypaea sp. | – | 1 | – | – | – | – | 1 | – | – | – | – | – | – | – | – | 1 |
| 78 | * Silpha carinata Herbst, 1783 | – | 1 | – | – | – | 1 | – | – | – | – | – | – | – | – | – | 1 |
| 79 | Thanatophilus trituberculatus (Kirby, 1837) | – | 1 | – | – | – | – | – | – | – | 1 | – | – | – | – | – | 1 |
| – | Silphidae indet. | – | 17 | 2 | 1 | 1 | 2 | 1 | 1 | 2 | 1 | – | 2 | 4 | – | – | 14 |
| STAPHYLINIDAE | |||||||||||||||||
| 80 | Aleocharinae indet. sp.1 | – | 1 | – | – | 1 | – | – | – | – | – | – | – | – | – | – | 1 |
| 81 | Aleocharinae indet. sp.2 | – | 5 | – | – | 3 | – | 1 | – | – | – | – | 1 | – | – | – | 5 |
| 82 | Aleocharinae indet. sp.3 | – | 2 | – | – | – | – | 1 | – | – | – | – | 1 | – | – | – | 2 |
| 83 | Aleocharinae indet. sp.4 | – | 5 | – | – | 3 | – | – | 2 | – | – | – | – | – | – | – | 5 |
| 84 | Aleocharinae indet. sp.5 | – | 1 | – | – | – | – | – | 1 | – | – | – | – | – | – | – | 1 |
| 85 | Aleocharinae indet. sp.6 | – | 1 | – | – | – | – | – | – | 1 | – | – | – | – | – | – | 1 |
| 86 | Aleocharinae indet. sp.7 | – | 1 | – | – | – | – | – | – | – | 1 | – | – | – | – | – | 1 |
| 87 | Aleocharinae indet. sp.8 | – | 1 | – | – | – | – | – | – | – | – | – | 1 | – | – | – | 1 |
| 88 | Aleocharinae indet. sp.9 | – | 1 | – | – | – | – | 1 | – | – | – | – | – | – | – | – | 1 |
| 89 | Aleocharinae indet. sp.10 | – | 1 | – | – | – | – | 1 | – | – | – | – | – | – | – | – | 1 |
| – | Aleocharinae indet. spp. | – | – | 1 | – | 1 | – | – | – | – | – | – | – | – | – | – | 1 |
| 90 | * Olophrum cf. fuscum(Gravenhorst, 1806) | – | 2 | 10 | – | 2 | – | – | 1 | 1 | 2 | – | 2 | – | – | – | 8 |
| 91 | Olophrum sp.1 | – | 1 | 6 | – | 1 | – | 1 | – | 1 | 1 | – | 1 | – | – | – | 5 |
| 92 | * Phloeostiba lapponica (Zetterstedt, 1838) | – | 1 | – | – | – | – | – | 1 | – | – | – | – | – | – | – | 1 |
| – | Omaliinae sp. | – | 1 | 1 | – | 1 | – | – | – | – | – | – | – | – | – | – | 1 |
| 93 | Bledius sp. | – | 6 | 7 | 1 | 2 | – | 2 | 3 | 1 | – | – | – | – | – | – | 8 |
| 94 | * Platystethus cf. cornutus (Gravenhorst, 1802) | 7 | 13 | 7 | – | 3 | – | 4 | 2 | 5 | 2 | 1 | 1 | – | – | – | 18 |
| 95 | Lathrobium sp. | – | 2 | – | – | 1 | – | – | 1 | – | – | – | – | – | – | – | 2 |
| 96 | Ochthephilum sp. | – | – | 2 | – | – | – | – | – | – | 1 | – | – | – | – | – | 1 |
| 97 | Philonthus sp.1 | – | – | 1 | – | 1 | – | – | – | – | – | – | – | – | – | – | 1 |
| 98 | Philonthus sp.2 | – | – | 2 | – | – | – | – | – | – | 1 | – | 1 | – | – | – | 2 |
| 99 | Philonthus sp.3 | – | – | 1 | – | – | – | – | – | 1 | – | – | – | – | – | – | 1 |
| 100 | Philonthus sp.4 | – | 1 | – | – | – | – | – | 1 | – | – | – | – | – | – | – | 1 |
| 101 | Stenus sp. | – | – | 1 | – | – | – | 1 | – | – | – | – | – | – | – | – | 1 |
| 102 | Lordithon sp. | – | – | 2 | – | 1 | – | – | 1 | – | – | – | – | – | – | – | 2 |
| 103 | Mycetoporus sp. | – | 1 | – | – | – | – | – | – | – | – | – | 1 | – | – | – | 1 |
| 104 | * Tachinus cf. rufipes Linnaeus,1758 | – | – | 1 | – | – | – | – | – | – | 1 | – | – | – | – | – | 1 |
| 105 | Tachyporus spp. | – | 1 | 1 | – | 1 | – | – | – | – | – | – | 1 | – | – | – | 2 |
| 106 | Staphylinidae indet. sp.1 | – | – | 1 | – | – | – | 1 | – | – | – | – | – | – | – | – | 1 |
| – | Staphylinidae indet. | – | 2 | – | 1 | 1 | – | 1 | – | – | – | – | 1 | – | – | – | 3 |
| SCARABAEIDAE | |||||||||||||||||
| 107 | * Aphodius (Acanthobodilus) cf. immundus Creutzer, 1799 | – | – | 1 | – | – | – | – | – | – | – | – | 1 | – | – | – | 1 |
| 108 | * Aphodius (Bodilus) cf. lugens (Creutzer, 1799) | – | – | 2 | – | 1 | – | – | – | – | 1 | – | – | – | – | – | 2 |
| 109 | Aphodius (Chilothorax) melanostictus W.Schmidt, 1840 | – | – | 1 | – | – | – | – | – | – | – | 1 | – | – | – | – | 1 |
| 110 | * Aphodius (Colobopterus) erraticus (Linnaeus, 1758) | – | – | 1 | – | – | – | – | 1 | – | – | – | – | – | – | – | 1 |
| 111 | Aphodius (Liothorax) plagiatus/A. cf. plagiatus (Linnaeus, 1767) | – | – | 7 | – | – | 1 | – | 2 | 2 | – | – | – | 1 | – | – | 6 |
| 112 | * Aphodius (Loraspis) frater Mulsant et Rey, 1870 | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | 1 | 1 |
| 113 | Aphodius (Nobius) cf. serotinus (Panzer, 1799) | – | – | 13 | – | 2 | 1 | 2 | – | – | – | 2 | 3 | 1 | – | – | 11 |
| 114 | Aphodius (Phaeaphodius) rectus Motschulsky, 1866 | – | – | 1 | – | – | – | – | – | 1 | – | – | – | – | – | – | 1 |
| 115 | * Aphodius (Subrinus) sturmi (Harold, 1870) | – | – | 1 | – | 1 | – | – | – | – | – | – | – | – | – | – | 1 |
| – | Aphodius spp. | 3 | 2 | 8 | – | 1 | – | 2 | 1 | 1 | – | – | 1 | 1 | 1 | – | 8 |
| 116 | Aegialia sp. | – | – | 2 | – | – | – | – | 1 | – | – | – | – | – | 1 | – | 2 |
| BYRRHIDAE | |||||||||||||||||
| 117 | Porcinolus murinus (Fabricius, 1794) | – | 1 | 4 | – | – | – | 1 | 1 | 2 | – | – | – | – | – | – | 4 |
| 118 | Morychus ostasiaticus Tshernyshev, 1997 | – | 5 | 12 | – | 1 | 1 | 1 | 2 | 3 | – | – | 1 | 1 | – | – | 10 |
| – | Byrrhidae indet. | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | 1 | 1 |
| HETEROCERIDAE | |||||||||||||||||
| 119 | Augyles sp. | – | – | – | 1 | – | – | – | – | – | – | 1 | – | – | – | – | 1 |
| 120 | * Heterocerus marginatus (Fabricius, 1787) | – | – | 1 | – | – | – | 1 | – | – | – | – | – | – | – | – | 1 |
| ELATERIDAE | |||||||||||||||||
| 121 | Berninelsonius hyperboreus (Gyllenhal, 1827) | – | – | 1 | – | 1 | – | – | – | – | – | – | – | – | – | – | 1 |
| 122 | Hypnoidus cf. rivularius (Gyllenhal, 1808) | – | – | 3 | – | – | – | – | 1 | – | 1 | – | 1 | – | – | – | 3 |
| 123 | * Hypoganomorphus laevicollis (Mannerheim, 1852) | – | – | 2 | – | – | – | – | – | – | – | – | 1 | – | – | – | 1 |
| 124 | * Pristilophus punctatissimus (Ménétriés, 1851) | – | – | – | 1 | 1 | – | – | – | – | – | – | – | – | – | – | 1 |
| BUPRESTIDAE | |||||||||||||||||
| 125 | Buprestidae? indet. | – | 1 | – | – | – | – | – | – | – | – | – | 1 | – | – | – | 1 |
| MALACHIIDAE | |||||||||||||||||
| 126 | Malachiidae? indet. | – | – | 2 | – | 1 | – | – | – | – | – | – | 1 | – | – | – | 2 |
| MELOIDAE | |||||||||||||||||
| 127 | * Mylabris ledebouri Gebler, 1829 | – | – | 1 | – | – | – | – | – | – | – | – | 1 | – | – | – | 1 |
| – | Mylabris sp. | – | – | 1 | – | – | – | – | – | – | – | – | 1 | – | – | – | 1 |
| TENEBRIONIDAE | |||||||||||||||||
| 128 | Centorus rufipes (Gebler, 1833) | – | – | 13 | – | 2 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | – | – | 11 |
| 129 | * Centorus ?crassipes borealis (Fischer von Waldheim, 1844) | – | – | 1 | – | 1 | – | – | – | – | – | – | – | – | – | – | 1 |
| – | Centorus spp. | 1 | 3 | 11 | 1 | 2 | 1 | 1 | 1 | 2 | 1 | – | 1 | 1 | – | – | 10 |
| 130 | Platyscelis sp. | 1 | – | – | 1 | – | – | 1 | 1 | – | – | – | – | – | – | – | 2 |
| 131 | Scythis sp. | 2 | 2 | – | – | – | – | – | 1 | 1 | – | – | – | – | – | – | 2 |
| 132 | Tenebrionidae indet. sp.1 | 1 | – | 2 | – | 1 | – | – | – | – | – | – | – | – | – | 1 | 2 |
| CERAMBYCIDAE | |||||||||||||||||
| 133 | * Eodorcadion carinatum (Fabricius, 1781) | – | 1 | – | – | – | 1 | – | – | – | – | – | – | – | – | – | 1 |
| CHRYSOMELIDAE | |||||||||||||||||
| 134 | * Donacia dentata Hoppe, 1795 | – | – | 2 | – | – | 1 | 1 | – | – | – | – | – | – | – | – | 2 |
| 135 | * Plateumaris sericea (Linnaeus, 1761) | – | – | 1 | – | – | – | 1 | – | – | – | – | – | – | – | – | 1 |
| 136 | * Chrysolina cf. gebleri L.Medvedev, 1979 | – | 1 | – | – | 1 | – | – | – | – | – | – | – | – | – | – | 1 |
| 137 | * Chrysolina graminis artemisiae (Motschulsky, 1860) | – | 1 | – | – | – | – | – | – | – | – | 1 | – | – | – | – | 1 |
| 138 | * Crosita altaica Gebler, 1823 | – | – | 1 | – | – | – | – | – | – | – | 1 | – | – | – | – | 1 |
| 139 | * Colaphellus alpicola (Warchałowski, 2004) | – | 3 | – | – | 1 | 1 | – | – | – | – | – | 1 | – | – | – | 3 |
| 140 | * Charaea minutum (Joannis, 1865) | – | – | 2 | – | – | – | – | – | – | – | 1 | 1 | – | – | – | 2 |
| 141 | * Galeruca ?daurica Joannis, 1866 | – | – | 1 | – | – | – | – | – | – | 1 | – | – | – | – | – | 1 |
| 142 | * Luperus cf. longicornis (Fabricius, 1781) | – | – | 2 | – | – | – | – | – | – | – | 1 | 1 | – | – | – | 2 |
| 143 | * Hippuriphila modeeri (Linnaeus, 1761) | – | – | 1 | – | – | – | – | – | 1 | – | – | – | – | – | – | 1 |
| 144 | * Phyllotreta ?tetrastigma (Comolli, 1837) | – | – | 1 | – | – | – | – | – | – | – | – | 1 | – | – | – | 1 |
| 145 | * Phyllotreta nemorum (Linnaeus, 1758) | – | – | 6 | – | – | 1 | 1 | – | – | – | 1 | 1 | 1 | – | – | 5 |
| – | Alticinae indet. | – | 2 | 1 | – | 1 | – | – | – | – | – | – | 1 | – | – | – | 2 |
| 146 | * Pachybrachis scriptidorsum Marseul, 1875 | – | – | 1 | – | – | – | – | – | – | – | – | 1 | – | – | – | 1 |
| – | Chrysomelidae indet. | – | 4 | 36 | – | 1 | 3 | 1 | 2 | 2 | 3 | 1 | 3 | 1 | 1 | 2 | 20 |
| BRENTIDAE | |||||||||||||||||
| 147 | * Taphrotopium ircutense (Faust, 1888) | – | 1 | – | – | – | – | – | – | – | – | – | 1 | – | – | – | 1 |
| 148 | Taphrotopium steveni (Gyllenhal, 1839) | – | – | 1 | – | – | – | – | 1 | – | – | – | – | – | – | – | 1 |
| 149 | Eutrichapion facetum (Gyllenhal, 1839) | – | – | 1 | – | – | – | – | – | – | – | – | 1 | – | – | – | 1 |
| 150 | * Ceratapion onopordi (Kirby, 1808) | – | – | 1 | – | – | – | – | – | – | – | – | 1 | – | – | – | 1 |
| 151 | Cyanapion sp. | – | – | 1 | – | – | – | – | – | 1 | – | – | – | – | – | – | 1 |
| 152 | * Ompholapion hookerorum (Kirby, 1808) | – | – | 2 | – | – | – | – | – | 2 | – | – | – | – | – | – | 2 |
| 153 | Trichapion simile (Kirby, 1811) | – | – | 1 | – | – | – | – | 1 | – | – | – | – | – | – | – | 1 |
| – | Apioninae indet. | 1 | 1 | 1 | – | 1 | – | – | 1 | – | – | – | – | – | – | – | 2 |
| CURCULIONIDAE | |||||||||||||||||
| 154 | Tournotaris bimaculata (Fabricius, 1787) | 23 | 14 | 160 | – | 9 | 4 | 11 | 13 | 18 | 4 | 1 | 7 | 4 | – | 1 | 72 |
| 155 | * Notaris scirpi (Fabricius, 1792) | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | 1 | 1 |
| 156 | Bagous sp.1 | – | – | 2 | – | – | – | – | 1 | 1 | – | – | – | – | – | – | 2 |
| 157 | Bagous sp.2 | 1 | 1 | 2 | – | 1 | – | 1 | – | – | – | – | – | 1 | – | – | 3 |
| 158 | Stephanocleonus foveifrons Chevrolat, 1873 | 1 | – | 6 | 2 | 1 | 1 | – | – | 1 | 1 | 1 | – | 1 | – | – | 6 |
| 159 | Stephanocleonus suvorovi Legalov, 1999 | 1 | – | – | – | 1 | – | – | – | – | – | – | – | – | – | – | 1 |
| 160 | * Baris artemisiae (Herbst, 1795) | – | 1 | – | – | 1 | – | – | – | – | – | – | – | – | – | – | 1 |
| 161 | Aulacobaris lepidii (Germar, 1823) | 1 | 1 | 6 | – | 1 | – | 1 | – | 3 | – | – | 1 | – | – | – | 6 |
| 162 | * Phytobius leucogaster (Marsham, 1802) | – | 1 | 1 | – | – | – | – | – | 1 | – | – | 1 | – | – | – | 2 |
| 163 | Pelenomus sp. | – | – | 1 | – | – | – | – | 1 | – | – | – | – | – | – | – | 1 |
| 164 | Ceutorhynchus sp.1 | 1 | 1 | – | – | – | – | 1 | – | – | – | – | – | – | – | – | 1 |
| 165 | Ceutorhynchus sp.2 | – | – | 1 | – | – | – | – | – | – | – | – | 1 | – | – | – | 1 |
| 166 | * Anoplus plantaris (Næzén, 1794) | – | – | 1 | – | – | – | – | 1 | – | – | – | – | – | – | – | 1 |
| 167 | * Tychius quinquepunctatus (Linnaeus, 1758) | – | – | 1 | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 |
| 168 | Tychius sp.1 | – | – | 1 | – | – | – | – | – | 1 | – | – | – | – | – | – | 1 |
| 169 | * Eremochorus steppensis (Motschulsky, 1860) | 2 | – | – | – | – | – | – | 2 | – | – | – | – | – | – | – | 2 |
| 170 | Trichalophus biguttatus Gebler, 1832 | 2 | – | – | – | – | – | – | – | 2 | – | – | – | – | – | – | 2 |
| 171 | * Sitona obscuratus Faust, 1882 | 1 | – | – | – | – | – | – | – | 1 | – | – | – | – | – | – | 1 |
| 172 | Sitona ovipennis Hochhuth, 1851 | – | – | 1 | – | – | – | – | 1 | – | – | – | – | – | – | – | 1 |
| 173 | Sitona sp.1 | – | 1 | – | – | – | – | – | 1 | – | – | – | – | – | – | – | 1 |
| 174 | Sitona sp.2 | – | – | 1 | – | 1 | – | – | – | – | – | – | – | – | – | – | 1 |
| 175 | Sitona sp. 3 | 1 | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 |
| 176 | Chlorophanus sibiricus Gyllenhal, 1834 | – | – | 5 | – | 1 | – | – | – | 1 | 1 | – | – | – | – | – | 3 |
| 177 | Eusomus ovulum Germar, 1823 | – | – | 1 | – | – | – | – | – | – | – | – | 1 | – | – | – | 1 |
| 178 | Paophilus albilaterus (Faust, 1882) | – | – | 6 | – | – | – | – | 1 | – | 1 | 1 | 1 | – | – | – | 4 |
| 179 | * Phyllobius crassipes Motschulsky, 1860 | – | – | 1 | – | – | – | – | – | 1 | – | – | – | – | – | – | 1 |
| 180 | Phyllobius pomaceus Gyllenhal, 1834 | – | – | 1 | – | – | – | – | – | – | 1 | – | – | – | – | – | 1 |
| 181 | Phyllobius virideaeris (Laicharting, 1781) | 1 | 2 | 9 | – | 2 | – | – | 1 | – | 1 | 1 | 3 | – | – | – | 8 |
| 182 | Phyllobius sp.1 | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | 1 | 1 |
| 183 | Phyllobius sp.2 | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | 1 | 1 |
| 184 | Polydrusus amoenus (Germar, 1823) | – | – | 2 | – | – | – | 1 | – | – | – | – | 1 | – | – | – | 2 |
| 185 | * Polydrusus corruscus Germar, 1823 | – | – | 1 | – | – | – | – | 1 | – | – | – | – | – | – | – | 1 |
| 186 | * Eudipnus mollis (Stroem, 1768) | – | – | 1 | – | – | – | – | – | 1 | – | – | – | – | – | – | 1 |
| 187 | Otiorhynchus politus Gyllenhal, 1834 | 4 | 5 | – | – | 2 | – | 1 | 1 | 1 | 1 | – | – | 2 | – | – | 8 |
| 188 | Otiorhynchus pullus Gyllenhal, 1834 | 3 | 3 | 1 | – | – | – | 1 | 1 | 1 | – | 1 | – | 1 | – | 1 | 6 |
| 189 | Otiorhynchus subocularis L. Arnoldi, 1975 | 9 | 12 | 19 | – | 3 | 4 | – | 2 | 4 | 2 | 1 | 1 | 2 | – | – | 19 |
| 190 | Otiorhynchus af. ursus Gebler, 1844 | 17 | 28 | 44 | – | 4 | 3 | 3 | 6 | 6 | 2 | 1 | 4 | 4 | – | – | 33 |
| – | Otiorhynchus af. ursus/O. bardus Boheman, 1842 | 2 | – | – | – | – | – | – | – | – | – | – | – | – | – | 2 | 2 |
| 191 | Otiorhynchus unctuosus Germar, 1823 | – | – | 3 | – | – | – | – | – | – | – | – | – | – | – | 2 | 2 |
| 192 | Otiorhynchus obscurus Gyllenhal, 1834 | 1 | 2 | 4 | – | – | – | – | – | – | – | – | – | – | 1 | 2 | 3 |
| – | Curculionidae indet. | – | 11 | 17 | – | 3 | 1 | 1 | 3 | 2 | 2 | – | 3 | – | – | 1 | 16 |
| SCOLYTIDAE | |||||||||||||||||
| 193 | Phloeotribus spinulosus (Rey, 1883) | – | – | 26 | – | 3 | – | – | 5 | 5 | 1 | – | 2 | – | – | – | 18 |
| 194 | * Polygraphus subopacus Thomson, 1871 | – | – | 1 | – | – | – | – | 1 | – | – | – | – | – | – | – | 1 |
| – | COLEOPTERA indet. | 5 | 9 | 28 | 5 | 4 | – | 2 | 3 | 5 | 1 | – | 2 | – | – | – | 17 |
| Coleoptera in total | 100 | 277 | 795 | 16 | 123 | 43 | 78 | 105 | 123 | 57 | 33 | 125 | 49 | 11 | 21 | 770 | |
| Number of Coleoptera species | 25 | 83 | 144 | 10 | 67 | 27 | 45 | 59 | 60 | 39 | 28 | 74 | 27 | 11 | 16 | 194 | |
| 194 | 145 | 101 | 23 | ||||||||||||||
| HEMIPTERA indet. | – | 3 | 1 | – | 1 | – | – | 1 | – | 1 | – | 1 | – | – | – | 4 | |
| HYMENOPTERA indet. | 4 | 1 | – | 2 | 2 | 1 | 1 | 1 | 1 | – | – | – | – | – | – | 6 | |
| DIPTERA indet. | – | – | – | 1 | – | – | – | 1 | – | – | – | – | – | – | – | 1 | |
| INSECTA indet. | 2 | – | 1 | 7 | 1 | – | – | 1 | 1 | 1 | – | – | – | – | – | 4 | |
| ARANEI indet. | – | – | – | 2 | – | – | 1 | 1 | – | – | – | – | – | – | – | 2 | |
References
- Arkhipov, S.A.; Volkova, V.S. Geologicheskaya Istoria, Landshafty I Klimaty Pleistotsena Zapadnoi Sibiri [Geological History, Landscapes and Climates of the Pleistocene of Western Siberia]; Institute of Geology and Geophysics SB RAS: Novosibirsk, Russia, 1994; pp. 1–106. [Google Scholar]
- Volkova, V.S.; Arkhipov, S.A.; Babushkin, A.E.; Kulkova, I.A.; Guskov, S.A.; Kuzmina, O.B.; Levchuk, L.K.; Mikhailova, I.V.; Sukhorukova, S.S. Stratigrafia Neftegazonosnykh Basseinov Sibiri. Kainozoi Zapadnoi Sibiri [Stratigraphy of Oil and Gas Bearing Basins of Siberia. Cenozoic of Western Siberia]; SB RAS: Novosibirsk, Russia, 2002; pp. 1–246. [Google Scholar]
- Zinovyev, E.V. Sub-fossil beetle assemblages associated with the «mammoth fauna» in the late Pleistocene localities of the Ural Mountains and West Siberia. ZooKeys 2011, 100, 149–169. [Google Scholar] [CrossRef] [PubMed]
- Coope, G.R. Changes in the thermal climate in Northwestern Europe during Marine Oxygen Isotope Stage 3, estimated from fossil insect assemblages. Quat. Res. 2002, 57, 401–408. [Google Scholar] [CrossRef]
- Lemdahl, G. Lateglacial and early Holocene insect assemblages from sites at different altitudes in the Swiss Alps—Implications on climate and environment. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2000, 159, 293–312. [Google Scholar] [CrossRef]
- Kuzmina, S.A. Macroentomology analysis: Methods, opportunities, and examples of reconstructions of Paleoclimatic and Paleoenvironmental conditions in the Quaternary of the Northeastern Siberia. Contemp. Probl. Ecol. 2017, 10, 336–349. [Google Scholar] [CrossRef]
- Grushevskii, I.I.; Medvedev, L.N. Preliminary data on the application of coleopterological analysis to study the continental deposits of Northern Yakutia. In Sbornik Statei Po Paleontologii I Biostratigrafii; NIIGA: Leningrad, USSR, 1962; pp. 38–42. [Google Scholar]
- Kiselev, S.V. Pozdnekainozoiskie Zhestkokrylye Severo-Vostoka Sibiri [Late Cenozoic Coleoptera of the North-East of Siberia]; Nauka: Moscow, Russia, 1981; pp. 1–116. [Google Scholar]
- Kuzmina, S.A.; Korotyaev, B.A. New species of the pill beetle genus Morychus Er. (Coleoptera, Byrrhidae) from the North-East of the USSR. Entomol. Obozr. 1987, 66, 342–344. [Google Scholar]
- Kuzmina, S.A. Quaternary Insects of the Coastal Lowlands of Yakutia. Master’s Thesis, Paleontological Institute, Russian Academy of Sciences, Moscow, Russia, 2001. [Google Scholar]
- Kuzmina, S.A.; Kolosnikov, S.F. Insects of the Upper Pleistocene and Holocene deposits of Medvezhyi Islands (East Siberian Sea). Byulleten MOIP. Otd. Geol. 2000, 2, 68–71. [Google Scholar]
- Sher, A.V.; Kuzmina, S.A.; Kuznetsova, T.V.; Sulerzhitsky, L.D. New insights into the Weichselian environment and climate of the East Siberian Arctic, derived from fossil insects, plants, and mammals. Quat. Sci. Rev. 2005, 24, 533–569. [Google Scholar] [CrossRef]
- Kuzmina, S.A.; Matthews, J.V. Late Cenozoic insects of Beringia. Euroasian Entomol. J. 2012, 11 (Suppl. S1), 59–97. [Google Scholar]
- Kuzmina, S.A. Quaternary Insects and Environment of the Northeastern Asia. Paleontol. J. 2015, 49, 679–867. [Google Scholar] [CrossRef]
- Kiselev, S.V. Late Pleistocene Coleoptera of Transurals. Paleontol. J. 1973, 4, 70–73. [Google Scholar]
- Kiselev, S.V. Pleistocene and Holocene Coleoptera of Western Siberia. In Sovremennoe Sostoyanie I Istoria Zhivotnogo Mira Zapadno-Sibirskoi Nizmennosti; UrO AN SSSR: Sverdlovsk, Russia, 1988; pp. 97–118. [Google Scholar]
- Zinovyev, E.V. Problems of ecological interpretation of quaternary insect faunas from the central part of Northern Eurasia. Quat. Sci. Rev. 2006, 25, 1821–1840. [Google Scholar] [CrossRef]
- Sheinkman, V.; Sedov, S.; Shumilovskikh, L.; Korkina, E.; Korkin, S.; Zinovyev, E.; Golyeva, A. First results from the Late Pleistocene paleosols in northern Western Siberia: Implications for pedogenesis and landscape evolution at the end of MIS3. Quat. Int. 2016, 418, 132–146. [Google Scholar] [CrossRef]
- Zinovyev, E.V. Coleoptera of the Mega duct site. In Sovremennoe Sostoyanie I Istoria Zhivotnogo Mira Zapadno-Sibirskoi Nizmennosti; UrO AN SSSR: Sverdlovsk, Russia, 1988; pp. 119–122. [Google Scholar]
- Zinovyev, E.V. Late Karginian entomocomplexes of low reaches of Irtysh River as exemplified by sites at Skorodum-95 and Kazakovka-95. Euroasian Entomol. J. 2003, 2, 83–93. [Google Scholar]
- Zinovyev, E.V. Early holocene entomocomplexes from the middle reaches of the Ob’ River in West Siberia. Euroasian Entomol. J. 2005, 4, 283–292. [Google Scholar]
- Zinovyev, E.V.; Gilev, A.V.; Khantemirov, R.M. Changes in the insect fauna of Southern Yamal Peninsula in connection with shifts of the northern timberline in the Holocene. Entomol. Obozr. 2001, 80, 843–852. [Google Scholar]
- Zinovyev, E.V.; Borodin, A.V.; Kotov, A.A.; Korkin, S.E. Palaeoenvironment of MIS5 in the North of Western Siberia, reconstructed on the sub-fossil insect, crustacean and plant macrofossil data. Quat. Int. 2019, 534, 171–182. [Google Scholar] [CrossRef]
- Legalov, A.A.; Dudko, R.Y.; Zinovyev, E.V. Sub-fossil weevils (Coleoptera, Curculionoidea) from the central part of West Siberia provide evidence of range expansion during the last glaciations. Quat. Int. 2016, 420, 233–241. [Google Scholar] [CrossRef]
- Zinovyev, E.V.; Dudko, R.Y.; Gurina, A.A.; Prokin, A.A.; Mikhailov, Y.E.; Tsepelev, K.A.; Tshernyshev, S.E.; Kireev, M.S.; Kostyunin, A.E.; Legalov, A.A. First records of sub-fossil insects from Quaternary deposits in the southeastern part of West Siberia, Russia. Quat. Int. 2016, 420, 221–232. [Google Scholar] [CrossRef]
- Gurina, A.A.; Dudko, R.Y.; Zinovyev, E.V.; Borodin, A.V.; Tshernyshev, S.E.; Legalov, A.A. Late Pleistocene taphocoenosis of insects and small mammals from the upper reaches of the Ob River. Paleontol. J. 2018, 53, 1610–1622. [Google Scholar] [CrossRef]
- Gurina, A.A.; Dudko, R.Y.; Tshernyshev, S.E.; Zinovyev, E.V.; Legalov, A.A. Late Pleistocene insects from the Dubrovino site at Ob River (West Siberia, Russia) and their paleoenvironmental significance. Palaeontol. Electron. 2019, 22.1.3, 1–18. [Google Scholar] [CrossRef]
- Gurina, A.A.; Dudko, R.Y.; Prosvirov, A.S.; Tshernyshev, S.E.; Legalov, A.A.; Zinovyev, E.V. Coleoptera assemblages from the Quaternary deposits of Kizikha river, the southernmost late Pleistocene insects of the West Siberian Plain. Invertebr. Zool. 2019, 16, 165–182. [Google Scholar] [CrossRef]
- Smirnov, N.N. Istoricheskaya Ekologiya Presnovodnykh Zootsenozov [Historical Ecology of Freshwater Zoocenoses]; KMK: Moscow, Russia, 2010; pp. 1–225. [Google Scholar]
- Neretina, A.N.; Gololobova, M.A.; Neplyukhina, A.A.; Zharov, A.A.; Rogers, C.D.; Horne, D.J.; Protopopov, A.V.; Kotov, A.A. Crustacean remains from the Yuka mammoth raise questions about non-analogue freshwater communities in the Beringian region during the Pleistocene. Sci. Rep. 2020, 10, 859. [Google Scholar] [CrossRef] [PubMed]
- Zharov, A.A.; Neretina, A.N.; Rogers, C.D.; Reshetova, S.A.; Sinitsa, S.M.; Kotov, A.A. Pleistocene Branchiopods (Cladocera, Anostraca) from Transbaikalian Siberia demonstrate morphological and ecological stasis. Water 2020, 12, 3063. [Google Scholar] [CrossRef]
- Rogers, D.C.; Zharov, A.A.; Neretina, A.A.; Kuzmina, S.A.; Kotov, A.A. A review of recently discovered remains of the Pleistocene branchiopods (Anostraca, Notostraca) from NE Siberia and Arctic Canada. Water 2021, 13, 280. [Google Scholar] [CrossRef]
- Lyubimova, N.K. (Ed.) Atlas Novosibirskoi Oblasti [Atlas of the Novosibirsk Region], 2nd ed.; Roskartografia: Moscow, Russia, 2002; pp. 1–56. [Google Scholar]
- Alisov, B.P. Klimat SSSR [Climate of the USSR]; MGU Publ.: Moscow, Russia, 1956; pp. 1–126. [Google Scholar]
- Makunina, N.I. Vegetation of the Forest-Steppe of the West Siberian Plain and the Altai-Sayan Mountain Region: Classification, Structure and Botanical and Geographical Patterns. Doctoral Dissertation, Central Siberian Botanical Garden, SB RAS: Novosibirsk, Russia, 2015. [Google Scholar]
- Coope, G.R. A Late Pleistocene insect fauna from Chelford, Cheshire. Proc. Royal Soc. B 1959, 151, 70–86. [Google Scholar] [CrossRef]
- Shotton, F.W.; Osborne, P.J. The fauna of the Hoxnian interglacial deposits of Nechells, Birmingham. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1965, 248, 357–378. [Google Scholar] [CrossRef]
- Gurina, A.A.; Dudko, R.Y.; Zinoviev, E.V.; Zinchenko, V.K.; Tshernyshev, S.E.; Legalov, A.A. Sub-fossil insects from Late Holocene alluvial deposits in the bank of river Alei, Altaiskii Krai, Russia. Euroasian Entomol. J. 2016, 15, 555–562. [Google Scholar]
- Pesenko, Y.A. Printsipy I Metody Kolichestvennogo Analiza v Faunisticheskikh Issledovaniyakh [Principle and Methods of the Quantitative Analysis in Faunistic Researches]; Nauka: Moscow, Russia, 1982; pp. 1–287. [Google Scholar]
- Magurran, A.E. Measuring Biological Diversity; Blackwell Publ.: Oxford, UK, 2004; pp. 1–256. [Google Scholar]
- Chernov, Y.I.; Makarov, K.V.; Eremin, P.K. Ground beetle families (Coleoptera, Carabidae) in the arctic fauna. Report 2. Zool. Zhurnal 2001, 80, 285–293. [Google Scholar]
- Legalov, A.A. Revised checklist of weevils (Coleoptera: Curculionoidea excluding Scolytidae and Platypodidae) from Siberia and the Russian Far East. Acta biol. Sib. 2020, 6, 437–549. [Google Scholar] [CrossRef]
- Kataev, B.M. Ground beetles of Harpalus Latr. “affinis” group (Coleoptera, Carabidae). In Novye I Maloizvestnye Zhestkokrylye Nasekomye; ZIN: Leningrad, Russia, 1987; pp. 3–41. [Google Scholar]
- Nikolajev, G.V. Additional data about fauna and biology of phytophagous carrion beetles of the genus Aclypea Reitter, 1885 (Coleoptera: Silphidae) in Asia. Kavk. Entomol. Byull. 2010, 6, 143–147. [Google Scholar] [CrossRef]
- Dudko, R.Y.; Danukalova, G.A.; Gurina, A.A.; Ivanov, A.V.; Mikhailov, Y.E.; Osipova, E.M.; Prosvirov, A.S.; Solodovnikov, A.Y.; Legalov, A.A.; Zinovyev, E.V. Insects and molluscs of the Late Pleistocene at the Gornovo site (Southern Ural foreland, Russia): New data on palaeoenvironment reconstructions. Quat. Int. 2022, 632, 154–177. [Google Scholar] [CrossRef]
- Hieke, F. Revision einiger Gruppen und neue Arten der Gattung Amara Bonelli, 1810 (Coleoptera: Carabidae). Ann. Hist. Nat. Mus. Nat. Hung. 2000, 92, 41–143. [Google Scholar]
- Krupa, E.G. Zooplankton Limnicheskikh I Loticheskikh Ekosistem Kazakhstana. Struktura, Zakonomernosti Formirivaniya [Zooplankton of Limnic and Lotic Ecosystems of Kazakhstan. Structure, Peculiarities of Formation]; Palmarium Academic Publ.: Kishinev, Moldova, 2012; pp. 1–392. [Google Scholar]
- Vekhov, N.V. Fauna of fairy shrimps (Anostraca) and shield shrimps (Notostraca) in water bodies of the forest-steppe and steppe zones of Eastern Europe, Northern Kazakhstan and Siberia. Sib. Biol. Zhurnal 1993, 3, 43–50. [Google Scholar]
- Lindroth, C.H. Ground-Beetles (Carabidae) Of Fennoscandia. A Zoogeographic Study. Part Iii. General Analysis with a Discussion on Biogeographic Principles. Amerind Publ.: New Delhi, India, 1992; pp. 1–814. [Google Scholar]
- Hieke, F. Die Paracelia-Gruppe der Gattung Amara Bonelli, 1810 (Insecta: Coleoptera: Carabidae). In Biodiversität UND Naturausstattung Im Himalaya II; Hartmann, M., Weipert, J., Eds.; Verein der Freunde und Förderer des Naturkundemuseums Erfurt e. V.: Erfurt, Germany, 2006; pp. 245–314. [Google Scholar]
- Bobkovskaya, N.E. Large mammals of the Pleistocene of the Lower Irtysh region. In Pleistotsenovye I Golotsenovye Fauny Urala; Universitet Publ.: Ekaterinburg, Russia, 2002; pp. 56–61. [Google Scholar]
- Vasiliev, S.K. Large Mammals of the Kazantsevian and Karginian Time of the Novosibirsk Ob Region: Based on Materials from the Krasny Yar Site. Master’s Thesis, Institute of Systematics and Ecology of Animals SB RAS, Novosibirsk, Russia, 2005. [Google Scholar]
- Borodin, A.V. Quaternary small mammal faunas from the West Siberian Plain. Acta Zool. Cracoviensia 1996, 39, 75–81. [Google Scholar]
- Panychev, V.A. Radiouglerodnaya Khronologia Allyuvialnykh Otlozhenii Predaltaiskoi Ravniny [Radiocarbon Chronology of Alluvial Deposits of the Prealtai Plain]; Nauka: Novosibirsk, Russia, 1979; pp. 1–103. [Google Scholar]
- Volkova, V.S.; Volkov, I.A. Deposits and landscapes of the Sartan stage of glaciation in the lower reaches of the Chulym River. In Paleopalinologia Sibiri; Nauka: Moscow, Russia, 1980; pp. 93–96. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gurina, A.A.; Dudko, R.Y.; Ivanov, A.V.; Kotov, A.A.; Mikhailov, Y.E.; Prokin, A.A.; Prosvirov, A.S.; Solodovnikov, A.Y.; Zinovyev, E.V.; Legalov, A.A. New Data on the Distribution of Southern Forests for the West Siberian Plain during the Late Pleistocene: A Paleoentomological Approach. Diversity 2023, 15, 56. https://doi.org/10.3390/d15010056
Gurina AA, Dudko RY, Ivanov AV, Kotov AA, Mikhailov YE, Prokin AA, Prosvirov AS, Solodovnikov AY, Zinovyev EV, Legalov AA. New Data on the Distribution of Southern Forests for the West Siberian Plain during the Late Pleistocene: A Paleoentomological Approach. Diversity. 2023; 15(1):56. https://doi.org/10.3390/d15010056
Chicago/Turabian StyleGurina, Anna A., Roman Y. Dudko, Alexander V. Ivanov, Alexey A. Kotov, Yuri E. Mikhailov, Alexander A. Prokin, Alexander S. Prosvirov, Alexey Y. Solodovnikov, Evgenii V. Zinovyev, and Andrei A. Legalov. 2023. "New Data on the Distribution of Southern Forests for the West Siberian Plain during the Late Pleistocene: A Paleoentomological Approach" Diversity 15, no. 1: 56. https://doi.org/10.3390/d15010056
APA StyleGurina, A. A., Dudko, R. Y., Ivanov, A. V., Kotov, A. A., Mikhailov, Y. E., Prokin, A. A., Prosvirov, A. S., Solodovnikov, A. Y., Zinovyev, E. V., & Legalov, A. A. (2023). New Data on the Distribution of Southern Forests for the West Siberian Plain during the Late Pleistocene: A Paleoentomological Approach. Diversity, 15(1), 56. https://doi.org/10.3390/d15010056











