Transformations of Vascular Flora of a Medieval Settlement Site: A Case Study of a Fortified Settlement in Giecz (Wielkopolska Region, Western Poland)
Abstract
1. Introduction
2. Materials and Methods
2.1. Wielkopolska as a Physiographic and Geobotanical Region
2.2. Study Site
2.3. Nomenclature and Classifications

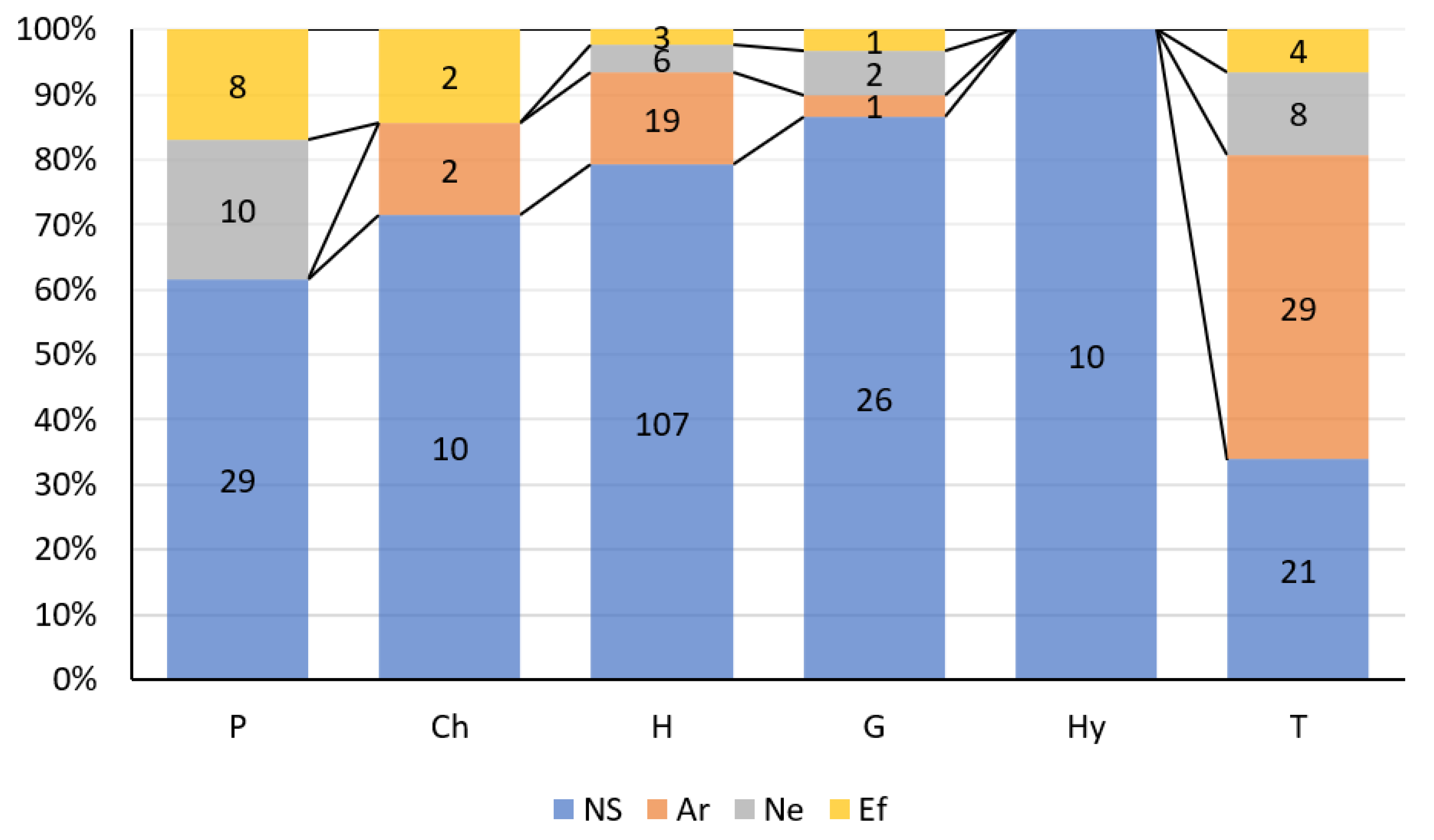
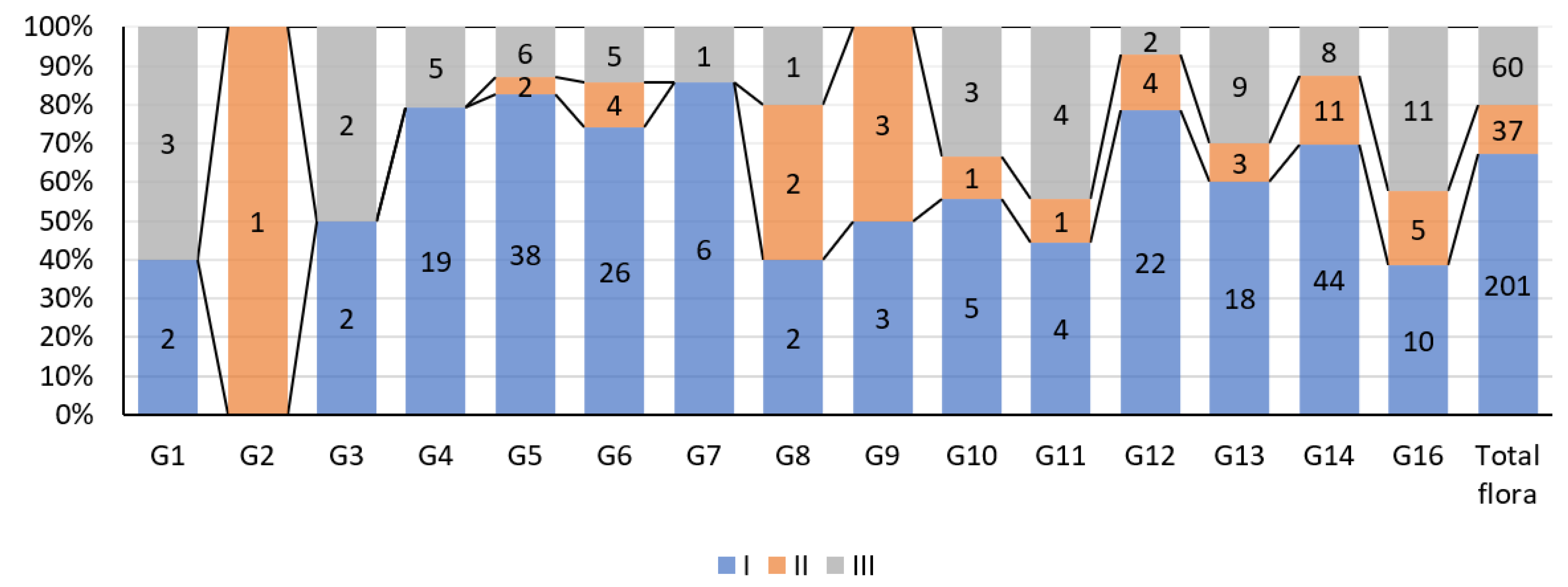
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Behm, H. Das sichtbare und das verbogene historische Kulturlandschaften—Analyse, Schutz und zukunftsorientierte Entwicklung. Traditio Innov. 1997, 2, 29–34. [Google Scholar]
- Kobyliński, Z. Ochrona krajobrazu archeologicznego. Kraj. Dziedzictwa Nar. 2000, 2, 45–53. [Google Scholar]
- Myczkowski, Z. Cultural landscape—Phenomenon of integrating cultural and natural heritage protection. J. Herit. Conserv. 2018, 56, 70–87. [Google Scholar] [CrossRef]
- Godłowski, K.; Kozłowski, J.K. Historia Starożytna Ziem Polskich; Państwowe Wydawnictwo Naukowe: Warszawa, Poland, 1985; p. 200. [Google Scholar]
- Bogdanowski, J. Architektura obronna w krajobrazie Polski. Od Biskupina do Westerplatte; Wydawnictwo Naukowe PWN: Warszawa, Poland; Kraków, Poland, 2002; p. 612. [Google Scholar]
- Sudnik-Wójcikowska, B.; Moysiyenko, I.I.; Dembicz, I.; Galera, H.; Rowińska, A.; Zachwatowicz, M. Kurhany na „Dzikich Polach”—Dziedzictwo Kultury i Ostoja Ukraińskiego Stepu; Wydawnictwa Uniwersytetu Warszawskiego: Warszawa, Poland, 2012; p. 195. [Google Scholar]
- Leciejewicz, L. Słowianie zachodni. Z dziejów tworzenia się średniowiecznej Europy; Zakład Narodowy im. Ossolińskich—Wydawnictwo: Wrocław, Poland, 1989; p. 424. [Google Scholar]
- Serafini, L. (Ed.) Historia Powszechna; Instituto Geografico de Agostini S.p. A.: Novara, Italy, 2007; Volume 7, p. 719. [Google Scholar]
- Dehnen-Schmutz, K. Nichteinheimische Pflanzen in der Flora mittelalterlicher Burgen. Diss. Bot. 2000, 334, 1–119. [Google Scholar]
- Bednarek, R.; Kamiński, D.; Markiewicz, M.; Chrzanowski, W.; Zbyszewska, K. Transformations of soils and forest communities in the areas of early-medieval strongholds (examples from Chełmno Land). Pol. J. Soil Sci. 2010, 53, 93–101. [Google Scholar]
- Celka, Z. Relics of cultivation in the vascular flora of medieval West Slavic settlements and castles. Biodiv. Res. Conserv. 2011, 22, 1–110. [Google Scholar] [CrossRef]
- Hejcman, M.; Karlík, P.; Ondráček, J.; Klír, T. Short-Term Medieval Settlement Activities Irreversibly Changed Forest Soils and Vegetation in Central Europe. Ecosystems 2013, 16, 652–663. [Google Scholar] [CrossRef]
- Kamiński, D. Szata Roślinna grodzisk Wczesnośredniowiecznych Ziemi Chełmińskiej; Wydawnictwo Naukowe Uniwersytetu Mikołaja Kopernika: Toruń, Poland, 2014; p. 244. [Google Scholar]
- Lityńska-Zając, M.; Wasylikowa, K. Przewodnik do Badań Archeobotaniczych; Wydawnictwo Sorus: Poznań, Poland, 2005; p. 566. [Google Scholar]
- Sádlo, J.; Matoušek, V. Aktuální vegetace jako předmět historických a archeologických interpretací. In Bioarcheologie v České republice; Beneš, J., Pokorný, P., Eds.; Jihočeská universita, Přírodiovědecká fakulta et Archeologický ústav AVČR: Praha, Czech Republic, 2008; pp. 489–514. [Google Scholar]
- Dayneko, P.; Moysiyenko, I.; Dembicz, I.; Zachwatowicz, M.; Sudnik-Wójcikowska, B. Ancient settlements in Southern Ukraine: How do local and landscape factors shape vascular plant diversity patterns in the last remnants of grass steppe vegetation? Tuexenia 2020, 40, 459–478. [Google Scholar] [CrossRef]
- Karschon, R.; Weinstein, A. Wall flora and vegetation at Qal’at nimrud, the castle of Banyas. Isr. J. Bot. 1985, 34, 59–64. [Google Scholar] [CrossRef]
- Duvigneaud, J. Un haut lieu pour les plantes castrales: Le site de Saint-Berthauld à Chaumont-Porcien (Départment des Ardennes, France). Nat. Mosana 1991, 44, 49–54. [Google Scholar]
- Siegl, A. Flora und Vegetation mittelalterlicher Burgruinen. In Naturschutz und Denkmalpflege: Wege zu einem Dialog im Garten; Kowarik, I., Schmidt, E., Siegl, B., Eds.; Hochschulverlag ETH: Zürich, Switzerland, 1998; pp. 193–202. [Google Scholar]
- Pavlova, D.; Tonkov, S. The wall flora of the Nebet Tepe Architectural Reserve in the city of Plovdiv (Bulgaria). Acta Bot. Croat. 2005, 64, 357–368. [Google Scholar]
- Iatrou, G.; Trigas, P.; Pettas, N. The vascular flora of Akrokorinthos Castle and its surrounding area (NE Peloponnese, Greece). Phytol. Balc. 2007, 13, 83–93. [Google Scholar]
- Hübl, E.; Scharfetter, E. Zur Gefäßpflanzenflora von Burgruinen in Niederösterreich. Braunschw. Geobot. Arb. 2008, 9, 249–310. [Google Scholar]
- Nedelcheva, A.; Vasileva, A. Vascular Plants from the Old Walls in Kystendil (Southwestern Bulgaria). Biotechnol. Biotechnol. Equip. 2009, 23 (Suppl. 1), 154–157. [Google Scholar] [CrossRef]
- Nedelcheva, A. Observations on the wall flora of Kyustendil (Bulgaria). EurAsian J. Biosci. 2011, 5, 80–90. [Google Scholar] [CrossRef]
- Sudnik-Wójcikowska, B.; Moysiyenko, I.I.; Zachwatowicz, M.; Jabłońska, E. The value and need for protection of kurgan flora in the anthropogenic landscape of steppe zone in Ukraine. Plant Biosyst. 2011, 145, 638–653. [Google Scholar] [CrossRef]
- Scharfetter, E.; Hübl, E. Gefäßpflanzenflora niederösterreichischer Ruinen (Abhandlungen der Zoologisch-Botanischen Gesellschaft in Österreich). Abh. Der Zool.-Bot. Ges. Österreich 2013, 39, 1–187. [Google Scholar]
- Moysiyenko, I.I.; Zachwatowicz, M.; Sudnik-Wójcikowska, B.; Jabłońska, E. Kurgans help to protect endangered steppe species in the Pontic grass steppe zone, Ukraine. Wulfenia 2014, 21, 83–94. [Google Scholar]
- Moysiyenko, I.I.; Dayneko, P.M.; Sudnik-Wójcikowska, B.; Dembicz, I.; Zachwatowicz, M.; Zakharova, M.Y. Conspectus of old settlements flora of the Lower Dnipro. Chornomors’k. Bot. Z. 2020, 16, 6–39. [Google Scholar] [CrossRef]
- Türkmen, N.; Düzenli, A.; Karakuş, H.; Uma, M.U. Anthropogenic characteristics and conservation status of the vascular flora of Kozan castle and its surrounding area (Turkey). Fresenius Environ. Bull. 2015, 24, 1189–1194. [Google Scholar]
- Dayneko, P. Systematic structure of the ancient settlements flora in the Lower Dnipro. Chornomors’k. Bot. Z. 2020, 16, 230–239. [Google Scholar] [CrossRef]
- Panitsa, M.; Trigas, P.; Kontakos, D.; Valli, A.-T.; Iatrou, G. Natural and cultural heritage interaction: Aspects of plant diversity in three East Peloponnesian castles (Greece) and conservation evaluation. Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2022, 156, 538–552. [Google Scholar] [CrossRef]
- Helwing, J.A. Flora Quasimodogenita Sive Enumeratio Aliguot plantarum indigenarum in Prussia; Imprimebat Joannes Daniel Stollius: Gdańsk, Poland, 1712; p. 74. Available online: https://books.google.pl/books?id=2QgZAAAAYAAJ&printsec=frontcover&hl=pl&source=gbs_ge_summary_r&cad=0#v=onepage&q&f=false (accessed on 20 December 2022).
- Helwing, J.A. Litographia Angerburgica sive Lapidum et fossilium In Districtu Angerburgensi & ejus vicinia; Literis Johannis Stelteri: Königsberg, Germany, 1717; p. 132. Available online: https://books.google.pl/books?id=3c8Gk8mf7PoC&printsec=frontcover&hl=pl&source=gbs_ge_summary_r&cad=0#v=onepage&q&f=false (accessed on 20 December 2022).
- Zabłocki, J. Wyniki badań nad współczesną roślinnością grodziska i jego otoczenia w Jeziorku, pow. Giżycko. Mater. Starożytne 1958, 3, 79–84. [Google Scholar]
- Duchoslav, M. Flora and vegetation of stony walls in East Bohemia (Czech Republic). Preslia 2002, 74, 1–25. [Google Scholar]
- Simonová, D. Alien flora on walls in southern and western Moravia. In Plant Invasions: Human Perception, Ecological Impacts and Management; Tokarska-Guzik, B., Brock, J.H., Brundu, G., Child, L., Daehler, C.C., Pyšek, P., Eds.; Backhuys Publishers: Leiden, The Netherlands, 2008; pp. 317–332. [Google Scholar]
- Bauch, R. Vorzeitliche und frühzeitliche Kulturrelikte in der Pflanzenwelt Mecklenburgs. Beih. Zum Bot. Cent. 1937, B 57, 77–138. [Google Scholar]
- Brandes, D. Zur Flora der Burgen im nördlichen Harzvorland. Braunschw. Naturk. Sehr. 1987, 2, 797–801. [Google Scholar]
- Dehnen-Schmutz, K. Medieval castles as centers of spread of non-native plant species. In Plant Invasions: Ecological Mechanisms and Human Responses; Starfinger, U., Edwards, K., Kowarik, I., Williamson, M., Eds.; Blackhuys Publishers: Leiden, The Netherlands, 1998; pp. 307–312. [Google Scholar]
- Dehnen-Schmutz, K. Alien species reflecting history: Medieval castles in Germany. Divers. Distrib. 2004, 10, 147–151. [Google Scholar] [CrossRef]
- Russow, B.; Schulz, A. Die Vegetationsveränderung auf Inseln im Strelitzer Land. Labus 2002, 16, 14–19. [Google Scholar]
- Buliński, M. Flora roślin naczyniowych doliny Wierzycy w warunkach antropogenicznych przemian środowiska przyrodniczego. Acta Biol. 1993, 8, 7–52. [Google Scholar]
- Buliński, M. Systematyczny przegląd flory roślin naczyniowych doliny Wierzycy i dolin jej trzech dopływów. Acta Biol. 1994, 9, 1–174. [Google Scholar]
- Celka, Z. Rośliny naczyniowe grodzisk Wielkopolski. Pr. Zakładu Taksonomii Roślin UAM W Pozn. 1999, 9, 1–159. [Google Scholar]
- Celka, Z. Distribution Atlas of Vascular Plants on the Earthworks of Wielkopolska. Publ. Dep. Plant Taxon. Adam Mickiewicz Univ. Poznań 2004, 13, 1–447. [Google Scholar]
- Kamiński, D. Floristic diversity on the early medieval earthworks of Chełmno Land (Ziemia Chełmińska) in NW Poland. Biodiv. Res. Conserv. 2006, 3–4, 344–347. [Google Scholar]
- Suder, D. Participation of thermophilous species in plant communities of earthworks and castle ruins in the Western Carpathians. Annales Universitatis Mariae Curie-Skłodowska Lublin–Polonia, Sect. C 2011, 46, 21–31. [Google Scholar] [CrossRef][Green Version]
- Suder, D. Trawy (Poaceae) we florze wybranych grodzisk i zamczysk w Karpatach Zachodnich. Fragm. Flor. Geobot. Pol. 2011, 18, 331–340. [Google Scholar]
- Hollnagel, A. Kulturreliktpflanzen auf slawischen Inselsiedlungen im Kreis Neustrelitz. Bodenkmalpflege Mecklenbg. 1953, 1953, 151–164. [Google Scholar]
- Hollnagel, A. Pflanzen als Kulturrelikte auf slawischen Inselsiedlungen. Heim. des Kreises Neustrelitz 1953, 1953, 96–99. [Google Scholar]
- Russow, B. Slawische Kulturreliktpflanzen im naturschutzfachlichen Gutachten—Ein Beispiel. Labus 2000, 11, 57–60. [Google Scholar]
- Russow, B. Pflanzen auf ur- und frühgeschichtlichen Siedlungsplatzen—Ein Diskussionsbeitrag zur Problematik der Kulturreliktpflanzen. Pulsatilla 2002, 5, 37–49. [Google Scholar]
- Russow, B.; Schulz, A. Die Schutzproblematik slawischer Kulturreliktpflanzen am Beispiel der Bestandssituation auf den Inselsiedlungen des Altkreises Neustrelitz—Ein Vergleich 1950–2000. Nat. Mecklenbg.-Vorpommern 2001, 44, 75–80. [Google Scholar]
- Derwich, M.; Żurek, A. (Eds.) U źródeł Polski. Do roku 1038. Polska, Dzieje cywilizacji i narodu; Horyzont, Grupa Wyd. Bertelsmann Media, Warszawa, Wyd. Dolnośląskie: Wrocław, Poland, 2002; p. 237. [Google Scholar]
- Kurnatowska, Z. Próba odtworzenia organizacji zarządu terytorialnego państwa pierwszych Piastów w Wielkopolsce. Pr. Kom. Archeol. 1984, 1, 81–91. [Google Scholar]
- Kurnatowska, Z.; Łosińska, A. Weryfikacja grodzisk wielkopolskich na półmetku. Fontes Archeol. Posnan. 1983, 32, 25–62. [Google Scholar]
- Kurnatowska, Z. Wczesnopiastowskie grody centralne. Podobieństwa i różnice, In Gniezno i Poznań w państwie pierwszych Piastów; Wójtowicz, A., Ed.; Ośrodek Wydawnictw Naukowych: Poznań, Poland, 2000; pp. 9–31. [Google Scholar]
- Buko, A. Ośrodki centralne a problem najstarszego "patrymonium" dynastii Piastów. Archeol. Pol. 2012, 57, 133–159. [Google Scholar]
- Richling, A.; Solon, J.; Macias, A.; Balon, J.; Borzyszkowski, J.; Kistowski, M. (Eds.) Regionalna Geografia Fizyczna Polski; Bogucki Wyd. Nauk: Poznań, Poland, 2021; p. 608. [Google Scholar]
- Szafer, W. Szata roślinna Polski Niżp. owej. In Szata Roślinna Polski; Szafer, W., Zarzycki, K., Eds.; PWN: Warszawa, Poland, 1977; Volume 2, pp. 17–188. [Google Scholar]
- Matuszkiewicz, J.M. Geobotanical Regionalization of Poland. IGiPZ PAN, Warszawa. 2008. Available online: http://www.igipz.pan.pl/Regiony-geobotaniczne-zgik.html (accessed on 14 October 2022).
- Kurnatowska, Z.; Łosińska, A. Stan i potrzeby badań nad wczesnym średniowieczem w Wielkopolsce. PTPN Pr. Kom. Archeol. 1990, 11, 105–153. [Google Scholar]
- Grygorowicz, A.; Milecka, K.; Tobolski, K. Architektoniczno-Przestrzenne i Przyrodnicze Podstawy Rekonstrukcji WczesnośrednioWiecznych Założeń Obronnych Giecza; Instytut Architektury o Planowania Przestrzennego Wydziału Architektury Politechniki Poznańskiej: Poznań, Poland, 2007; p. 204. [Google Scholar]
- Krysztofiak, T. Gród Piastowski w Gieczu; Wyd. Muzeum Pierwszych: Piastów na Lednicy, Poland, 1998. [Google Scholar]
- Krysztofiak, T. Rezerwat archeologiczny Gród Wczesnopiastowski w Gieczu. Available online: https://lednicamuzeum.pl/strona,rezerwat-archeologiczny-grod-w-gieczu.html (accessed on 14 October 2022).
- Wyrwa, A.M. Gdecz-Giecz. Scire est reminisci. Outlines of the Piast Residence as Being Taken Out of Oblivion; Musaeum Primorum Principum ex Stirpe Piastea in Lednica, Dziekanowice: Lednica, Poland, 2014; p. 154. [Google Scholar]
- Rutkowski, L. Klucz do Oznaczania Roślin Naczyniowych Polski Niżowej; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2004; p. 814. [Google Scholar]
- Celka, Z. Zróżnicowanie flory naczyniowej grodziska w Gieczu (pow. średzki). Studia Lednickie 2000, 6, 351–372. [Google Scholar]
- Mirek, Z.; Piękoś-Mirkowa, H.; Zając, A.; Zając, M. Vascular Plants of Poland: An Annotated Checklist; W. Szafer Institute of Botany, Polish Academy of Sciences: Kraków, Poland, 2020; p. 526. [Google Scholar]
- Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. Available online: http://www.plantsoftheworldonline.org/ (accessed on 20 December 2022).
- Zarzycki, K.; Trzcińska-Tacik, H.; Różański, W.; Szeląg, Z.; Wołek, J.; Korzeniak, U. Ecological Indicator Values of Vascular Plants of Poland; W. Szafer Institute of Botany, Polish Academy of Sciences: Kraków, Poland, 2002; p. 183. [Google Scholar]
- Jackowiak, B. Atlas of distribution of vascular plants in Poznań. Publ. Dep. Plant Taxon. Adam Mickiewicz Univ. Poznań 1993, 3, 1–409. [Google Scholar]
- Zając, A.; Zając, M. Methodical problems in distinguishing the group of archaeophytes. Acta Bot. Sil. 2011, 6, 55–62. [Google Scholar]
- Jackowiak, B.; Celka, Z.; Chmiel, J.; Latowski, K.; Żukowski, W. Checklist of the vascular flora of Wielkopolska (Poland): Native species and naturalized alien species. Biodiv. Res. Conserv. 2013, 31, 9–96. [Google Scholar] [CrossRef]
- Jackowiak, B.; Celka, Z.; Chmiel, J.; Latowski, K.; Żukowski, W. Checklist of the vascular flora of Wielkopolska (Poland): Casual alien species. Biodiv. Res. Conserv. 2017, 46, 35–55. [Google Scholar] [CrossRef]
- Żukowski, W.; Latowski, K.; Jackowiak, B.; Chmiel, J. Rośliny naczyniowe Wielkopolskiego Parku Narodowego. Pr. Zakładu Taksonomii Roślin UAM w Pozn. 1995, 4, 1–229. [Google Scholar]
- Maarel, E.M. van der, Florastatistieken als bijdrage tot de evalutie van natuurgebieden. Gorteria 1971, 5, 176–188. [Google Scholar]
- Kunick, W. Veränderungen von Flora und Vegetation einer Großstadt, dargestellt am Beispiel von Berlin (West). Diss. Tech. Univ. Berl. 1974, 83, 1–472. [Google Scholar]
- Jackowiak, B. Antropogeniczne przemiany flory roślin naczyniowych Poznania. Wyd. Nauk. UAM Poznań Ser. Biol. 1990, 42, 232. [Google Scholar]
- Jackowiak, B. Struktura przestrzenna flory dużego miasta. Studium metodyczno-problemowe. Pr. Zakładu Taksonomii Roślin UAM w Pozn. 1998, 8, 1–227. [Google Scholar]
- Chmiel, J. Flora roślin naczyniowych wschodniej części Pojezierza Gnieźnieńskiego i jej antropogeniczne przeobrażenia w wieku XIX i XX, cz. 1 i 2. Pr. Zakładu Taksonomii Roślin UAM w Pozn. 1993, 1, 1–202, 2, 1–212. [Google Scholar]
- Chmiel, J. Zróżnicowanie przestrzenne flory jako podstawa ochrony przyrody w krajobrazie rolniczym. Pr. Zakładu Taksonomii Roślin UAM w Pozn. 2006, 14, 1–250. [Google Scholar]
- Jäger, E.J.; Werner, K. (Eds.) Rothmaler. Exkursionsflora von Deutschland. Band 4, Gefäßpflanzen: Kritischer Band, 10th ed.; Spektrum Akademischer Verlag, Elsevier Gmbh: München, Germany, 2005; p. 980. [Google Scholar]
- Matuszkiewicz, W. Przewodnik do oznaczania zbiorowisk roślinnych Polski; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2008; p. 540. [Google Scholar]
- Ratyńska, H.; Wojterska, M.; Brzeg, A.; Kołacz, M. Multimedialna encyklopedia zbiorowisk roślinnych Polski, ver. 1.1. (CD); Narodowy Fundusz Ochrony Środowiska i Gospodarki Wodnej, Instytut Edukacyjnych Technologii Informatycznych, Uniwesytet Kazimierza Wielkiego: Bydgoszcz, Poland, 2010. [Google Scholar]
- Jackowiak, B.; Celka, Z.; Chmiel, J.; Latowski, K.; Żukowski, W. Red list of vascular flora of Wielkopolska (Poland). Biodiv. Res. Conserv. 2007, 5–8, 95–127. [Google Scholar]
- Kaźmierczakowa, R.; Bloch-Orłowska, J.; Celka, Z.; Cwener, A.; Dajdok, Z.; Michalska-Hejduk, D.; Pawlikowski, P.; Szczęśniak, E.; Ziarnek, K. Polish Red List of Pteridophytes and Flowering Plants; Instytut Ochrony Przyrody Polskiej Akademii Nauk: Kraków, Poland, 2016; p. 44. [Google Scholar]
- Bilz, M.; Kell, S.P.; Maxted, N.; Lansdown, R.V. European Red List of Vascular Plants; Publications Office of the European Union: Luxembourg, 2011; Available online: https://op.europa.eu/en/publication-detail/-/publication/ad44df42-f7d2-4297-a4c2-932859effccd/language-en (accessed on 20 December 2022).
- Hahs, A.K.; McDonnell, M.J.; McCarthy, M.C.; Vesk, P.A.; Corlett, R.T.; Norton, B.A.; Clemants, S.E.; Duncan, R.P.; Thompson, K.; Schwartz, M.W.; et al. A global synthesis of plant extinction rates in urban areas. Ecol. Lett. 2009, 12, 1165–1173. [Google Scholar] [CrossRef]
- Gao, J.G.; Liu, H.; Wang, N.; Yang, J.; Zhang, X.-L. Plant extinction excels plant speciation in the Anthropocene. BMC Plant Biol. 2020, 20, 430. [Google Scholar] [CrossRef] [PubMed]
- Lughadha, E.N.; Bachman, S.P.; Leão, T.C.C.; Forest, F.; Halley, J.M.; Moat, J.; Acedo, C.; Bacon, K.L.; Brewer, R.F.A.; Gâteblé, G.; et al. Extinction risk and threats to plants and fungi. Plants People Planet 2020, 2, 389–408. [Google Scholar] [CrossRef]
- Pyšek, P.; Richardson, D.M.; Pergl, J.; Jarošík, W.; Sixtová, Z.; Weber, E. Geographical and taxonomic biases in invasion ecology. Trends Ecol. Evol. 2008, 23, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, B.; Levine, J.M. Plant invasions and extinction debts. PNAS 2013, 110, 1744–1749. [Google Scholar] [CrossRef] [PubMed]
- Essl, F.; Dullinger, S.; Genovesi, P.; Hulme, P.E.; Jeschke, J.M.; Katsanevakis, S.; Kühn, I.; Lenzner, B.; Pauchard, A.; Pyšek, P.; et al. A Conceptual Framework for Range-Expanding Species that Track Human-Induced Environmental Change. BioScience 2019, 69, 908–919. [Google Scholar] [CrossRef]
- Steinbauer, M.S.; Gohlke, A.; Mahler, C.; Schmiedinger, A.; Beierkuhnlein, C. Quantification of wall surface heterogeneity and its influence on species diversity at medieval castles—Implications for the environmentally friendly preservation of cultural heritage. J. Cult. Herit. 2013, 14, 219–228. [Google Scholar] [CrossRef]
- Liccari, F.; Sigura, M.; Tordoni, E.; Boscutti, F.; Bacaro, G. Determining Plant Diversity within Interconnected Natural Habitat Remnants (Ecological Network) in an Agricultural Landscape: A Matter of Sampling Design? Diversity 2022, 14, 12. [Google Scholar] [CrossRef]
- Brandes, D. Naturschutzaspekte bei der Denkmalpflege unter besonderer Berücksichtigung der Mauervegetation. Ber. Der ANL 1996, 20, 145–149. [Google Scholar]
- Celka, Z. Sites of medieval settlements as refuges for vascular plants. Ecol. Quest. 2004, 4, 99–104. [Google Scholar]
- Kamiński, D. Early medieval fortified settlements at Kałdus and Płutowo (Chełmno land, northern Poland)—Places of plant invasion and refuges. Ecol. Quest. 2004, 4, 105–114. [Google Scholar]
- Korczyński, M. Flora grodzisk Wyszogrodu i Zamczyska na terenie miasta Bydgoszczy. In Ciepłolubne Murawy w Polsce—Stan Zachowania i Perspektywy Ochrony; Ratyńska, H., Waldon, B., Eds.; Wyd. Uniw. K. Wielkiego: Bydgoszcz, Poland, 2010; pp. 201–207. [Google Scholar]
- Sudnik-Wójcikowska, B.; Moysiyenko, I.I. Ukrainian kurgans as refugia of steppe flora and their role in steppe restoration. In Steppenlebensräume Europas—Gefährdung, Erhaltungsmaßnahmen und Schutz; Pfützenreuter, H.S., Ed.; Thüringer Ministerium für Landwirtschaft, Forsten, Umwelt und Naturschutz (TMLFUN)—Stabsstelle Presse, Öffentlichkeitsarbeit: Reden, Germany, 2013; pp. 201–210. [Google Scholar]
- Wójcik, T.; Ziaja, M. Zbiorowiska roślinne wzgórza Kamieniec na Pogórzu Dynowskim (Karpaty Zachodnie). Park. Nar. I Rezerw. Przyr. 2015, 34, 57–74. [Google Scholar]
- Łaguna, W.; Rayss, J. Zmiany w krajobrazie kulturowym wywołane rekonstrukcją grodziska średniowiecznego w Owidzu (gmina Starogard Gd.)—jako przykład świadomego kształtowania krajobrazu historycznego. Teka Kom. Arch. Urb. Stud. Krajobr.-OL PAN 2013, 9, 55–69. [Google Scholar]
- Kołos, A.; Banaszuk, P. Mowing may bring about vegetation change, but its effect is strongly modified by hydrological factors. Wetl. Ecol Manag. 2018, 26, 879–892. [Google Scholar] [CrossRef]
- Rysiak, A.; Chabuz, W.; Sawicka-Zugaj, W.; Zdulski, J.; Grzywaczewski, G.; Kulik, M. Comparative impacts of grazing and mowing on the floristics of grasslands in the buffer zone of Polesie National Park, eastern Poland. Glob. Ecol. Conserv. 2021, 27, e01612. [Google Scholar] [CrossRef]
- Latowski, K.; Chmiel, J.; Jackowiak, B.; Żukowski, Ż. Udział antropofitów we Florze segetalnej Wielkopolski. Fragm. Agron. 2010, 27, 103–111. [Google Scholar]
- Zając, M.; Zając, A.; Tokarska-Guzik, B. Extinct and endangered archaeophytes and the dynamics of their diversity in Poland. Biodiv. Res. Conserv. 2009, 13, 17–24. [Google Scholar] [CrossRef]
- Pándi, I.; Penksza, K.; Botta-Dukát, Z.; Kröel-Dulay, G. People move but cultivated plants stay: Abandoned farmsteads support the persistence and spread of alien plants. Biodivers. Conserv. 2014, 23, 1289–1302. [Google Scholar] [CrossRef]
- Kaźmierczakowa, R.; Zarzycki, K.; Mirek, Z. (Eds.) Polish Red Data Book of Plants, 3rd ed.; Instytut Ochrony Przyrody PAN: Kraków, Poland, 2014; p. 895. [Google Scholar]
- Pokorná, A.; Kočár, P.; Novák, J.; Šálková, T.; Žáčková, P.; Komárková, V.; Vaněček, Z.; Sádlo, J. Ancient and Early Medieval man-made habitats in the Czech Republic: Colonization history and vegetation changes. Preslia 2018, 90, 171–193. [Google Scholar] [CrossRef]
- Zając, M.; Zając, A. Survival problems of archaeophytes in the Polish flora. Biodiv. Res. Conserv. 2014, 35, 47–56. [Google Scholar] [CrossRef]
- Zając, A.; Zając, M. (Eds.) Distribution Atlas of Vascular Plants in Poland; Laboratory of Computer Chorology, Institute of Botany, Jagiellonian University: Cracow, Poland, 2001; pp. xii+714. [Google Scholar]
- Tokarska-Guzik, B. The establishment and Spread of Alien Plant Species (kenophytes) in the Flora of Poland; Wydawnictwo Uniwersytetu Śląskiego: Katowice, Poland, 2015; p. 192. [Google Scholar]
- Tokarska-Guzik, B.; Dajdok, Z.; Zając, M.; Zając, A.; Urbisz, A.; Danielewicz, W.; Hołdyński, C. Rośliny obcego pochodzenia w Polsce ze szczególnym uwzględnieniem gatunków inwazyjnych; Generalna Dyrekcja Ochrony Środowiska: Warszawa, Poland, 2012; p. 197. [Google Scholar]
- Denisov, B.; Wrzesień, M.; Mamchur, Z.; Chuba, M. Invasive flora within urban railway areas: A case study from Lublin (Poland) and Lviv (Ukraine). Acta Agrobot. 2017, 70, 1727. [Google Scholar] [CrossRef]
- Jamil, M.D.; Waheed, M.; Akhtar, S.; Bangash, N.; Chaudhari, S.K.; Majeed, M.; Hussain, M.; Ali, K.; Jones, D.A. Invasive Plants Diversity, Ecological Status, and Distribution Pattern in Relation to Edaphic Factors in Different Habitat Types of District Mandi Bahauddin, Punjab, Pakistan. Sustainability 2022, 14, 13312. [Google Scholar] [CrossRef]
- Brandes, D. The flora of old town centres in Europe. In Urban Ecology as the Basis of Urban Planning; Sukopp, H., Numata, M., Huber, A., Eds.; Academic Publishing bv: Amsterdam, The Netherlands, 1995; pp. 49–58. [Google Scholar]
- Korniak, T.; Urbisz, A. Trawy synantropijne. In Księga Polskich Traw; Frey, L., Ed.; Instytut Botaniki im. W. Szafera, Polska Akademia Nauk: Kraków, Poland, 2007; pp. 317–342. [Google Scholar]
- Tokarska-Guzik, B. Trawy inwazyjne. In Księga Polskich Traw; Frey, L., Ed.; Instytut Botaniki im. W. Szafera, Polska Akademia Nauk: Kraków, Poland, 2007; pp. 361–387. [Google Scholar]
- Szczęśniak, E. Ekspansja Eragrostis minor (Poaceae) we Wrocławiu. Fragm. Flor. Geobot. Pol. 2010, 17, 305–314. [Google Scholar]
- Guzik, J. Dynamika rozprzestrzeniania się w Krakowie i warunki występowania gatunków z rodzaju Eragrostis (Poaceae). Fragm. Flor. Gebot. Pol. 2011, 18, 231–247. [Google Scholar]
- Rostański, K.; Sowa, R. Alfabetyczny wykaz efemerofitów Polski. Fragm. Flor. Geobot. 1986/1987, 31–32, 151–205. [Google Scholar]
- Urbisz, A. Occurrence of temporarily-introduced alien plant species (ephemerophytes) in Poland—scale and assessment of the phenomenon. Pr. Nauk. Uniw. Śląskiego W Katowicach 2012, 2897, 1–200. [Google Scholar]
- Brandes, D. Burgruinen als Habitatinseln. Ihre Flora und Vegetation sowie die Bedeutung für Sukzessionsforschung und Naturschutz dargestellt unter besonderer Berücksichtigung der Burgruinen des Harzgebietes. Braunschw. Nat. Schr. 1996, 5, 125–163. [Google Scholar]
- Pivarci, R.; Behm, H. Kulturreliktpflanzen—Ein wenig beachtetes kulturelles Erbe, dargestellt am Beispiel Nordostdeutschalands. In Kulturelles Erbe—Landschaften im Spannungsfeld zwischen Zerstörung und Bewahrung; Behm, H., Ed.; Beitrage zur Tagung vom 26.-28. 1998 in Rostock; Inter Media GmbH: Wittenburg, Germany, 2001; pp. 147–150. [Google Scholar]
- Blay, D. Tropical secondary forest management in humid Africa: Reality and perspectives. In Proceedings of the Workshop on Tropical Secondary Forest Management in Africa: Reality and Perspectives, Nairobi, Kenya, 9–13 December 2002; Available online: http://www.fao.org/docrep/006/j0628e/J0628E12.htm (accessed on 20 December 2022).
- Parker, K.C.; Hamrick, J.L.; Hodgson, W.C.; Trapnell, D.W.; Parker, A.J.; Kuzoff, R.K. Genetic consequences of pre-Columbian cultivation for Agave murpheyi and A. delamateri (Agavaceae). Am. J. Bot. 2007, 94, 1479–1490. [Google Scholar] [CrossRef]
- Hohla, M. Lebendige Spuren aus der Vergangenheit—Pflanzen unserer Burgen, Schlösser und Klöster. Öko·L 2009, 31, 13–24. [Google Scholar]
- Powling, A. The Palms of Buton, Indonesia, an Island in Wallacea. Palms 2009, 53, 84–91. [Google Scholar]
- Möller, S. Populationsgenetik und Phylogeographie des Archäophyten und Kulturreliktzeigers Vinca minor L. (Apocynaceae). Doktors der Naturwissenschaften (Dr. rer. nat) im Fachbereich 10 Mathematik und Naturwissenschaften der Universität Kassel. 2015, p. 216. Available online: https://kobra.uni-kassel.de/bitstream/handle/123456789/2015041748149/DissertationSinaMoeller.pdf?sequence=6&isAllowed=y (accessed on 20 December 2022).
- Hlásná Čepková, P.; Karlík, P.; Viehmannova, I.; Müllerova, V.; Smejda, L.; Hejcman, M. Genetic and leaf-trait variability of Vinca minor at ancient and recent localities in Central Europe. Biochem. Syst. Ecol. 2016, 64, 22–30. [Google Scholar] [CrossRef]
- Dempsey, K. ‘Sowing Seeds of Interdisciplinary Work’: Relict Plants at Medieval Castles; Castle Studies Trust: UK, 2021; p. 47. Available online: http://castlestudiestrust.org/blog/2020/05/05/sowing-seeds-of-interdisciplinary-work-relict-plants-at-medieval-castles/ (accessed on 20 December 2022).
- Behm, H.; Pivarci, R. Slawische Kulturreliktpflanzen in Mecklenburg-Vorpommern. In Bodenkmal und Kulturlandschaft—Planungs- und Landnutzungsorientierte Grundlagen Nachhaltiger Raumententwicklung unter Besonderer Beachtung Mecklenburg-Vorpommerns. Veröffentlichte Habilschrift; Behm, H., Ed.; Universität Rostock: Rostock, Germany, 1998; Volume 2, pp. 7–12. [Google Scholar]
- Celka, Z. Problems of Cultivation Relicts. Publ. Dep. Plant Taxon. Adam Mickiewicz Univ. Poznań 2000, 10, 185–191. [Google Scholar]
- Celka, Z. Relikty dawnych upraw we współczesnej florze Polski. Botanical Guidebooks 2005, 28, 281–296. [Google Scholar]
- Celka, Z.; Drapikowska, M. Relics of cultivation in Central Europe: Malva alcea L. as an example. Veget. Hist. Archaeobot. 2008, 17 (Suppl. 1), 251–255. [Google Scholar] [CrossRef]
- Koch, O. Flora von Teterow. Arch. Des Ver. Der Freunde Der Nat. Mecklenbg. 1897, 50, 246–270. [Google Scholar]
- Krause, L. Bericht über die 50. Generalversammlung des Vereins der Freunde der Naturgeschichte in Mecklenburg am 26. Mai 1896 zu Teterow. Arch. Des Ver. Der Freunde Der Nat. Mecklenbg. 1897, 50, 344–354. [Google Scholar]



| Raunkiaer Plant Life-Form | Number of Species | Contribution (%) |
|---|---|---|
| Phanerophytes | 47 | 15.8 |
| Chamaephytes | 14 | 4.7 |
| Hemicryptophytes | 135 | 45.3 |
| Geophytes | 30 | 10.1 |
| Hydrophytes | 10 | 3.4 |
| Therophytes | 62 | 20.8 |
| Indices of Anthropogenic Changes | Total Flora |
|---|---|
| Indices of flora synanthropization total (WSc) | 83.9 |
| permanent (WSt) | 82.8 |
| Indices of apophytization | |
| total (WApc) | 52.0 |
| permanent (Wapt) | 55.6 |
| Spontaneophyte apophytization index (Wap) | 76.4 |
| Indices of flora anthropophytization | |
| total (Wanc) | 31.9 |
| permanent (Want) | 27.2 |
| Indices of flora archaeophytization | |
| total (Warc) | 17.1 |
| permanent (Wart) | 18.3 |
| Indices of flora kenophytization | |
| total (WKnc) | 8.4 |
| permanent (WKnt) | 9.0 |
| Flora modernization index (WM) | 33.3 |
| Index of floristic fluctuations (WF) | 6.4 |
| Flora naturalness index (WN) | 16.1 |
| Indices of flora permanence | |
| of anthropophytes (WTA) | 80.0 |
| total (WTC) | 93.6 |
| Species | Cultivation Period | Major Use | Found in Archaeological Deposits | Frequency at Archaeological Sites of Central Europe | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pr | Md | Me | M | F | P | D | O | |||
| Artemisia absinthium | + | + | + | + | + | + | + | + | + | ** |
| Leonurus cardiaca | - | + | + | + | - | + | + | + | + | ** |
| Lycium barbarum | - | - | + | + | - | - | + | + | - | * |
| Malva alcea | + | + | + | + | + | + | + | + | + | *** |
| Pastinaca sativa | + | + | + | + | + | - | - | + | + | ** |
| Saponaria officinalis | +? | + | + | + | - | + | + | + | + | * |
| Viola odorata | + | + | + | + | - | - | + | + | + | ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Celka, Z.; Brzeg, A.; Sobczyński, A. Transformations of Vascular Flora of a Medieval Settlement Site: A Case Study of a Fortified Settlement in Giecz (Wielkopolska Region, Western Poland). Diversity 2023, 15, 35. https://doi.org/10.3390/d15010035
Celka Z, Brzeg A, Sobczyński A. Transformations of Vascular Flora of a Medieval Settlement Site: A Case Study of a Fortified Settlement in Giecz (Wielkopolska Region, Western Poland). Diversity. 2023; 15(1):35. https://doi.org/10.3390/d15010035
Chicago/Turabian StyleCelka, Zbigniew, Andrzej Brzeg, and Adam Sobczyński. 2023. "Transformations of Vascular Flora of a Medieval Settlement Site: A Case Study of a Fortified Settlement in Giecz (Wielkopolska Region, Western Poland)" Diversity 15, no. 1: 35. https://doi.org/10.3390/d15010035
APA StyleCelka, Z., Brzeg, A., & Sobczyński, A. (2023). Transformations of Vascular Flora of a Medieval Settlement Site: A Case Study of a Fortified Settlement in Giecz (Wielkopolska Region, Western Poland). Diversity, 15(1), 35. https://doi.org/10.3390/d15010035






