Abstract
Insect communities in tropical forests tend to be structured vertically and with respect to tree fall gaps and edges. Furthermore, insect communities vary over time. Insight into such habitat specificity and temporal variation is needed to design and interpret biodiversity surveys and to compare conservation value among habitats. Some aspects of tropical insect community structure, such as the proportion of canopy specialists and temporal variation, vary among biogeographical regions and climatic zones. To date, few regions have been sampled systematically, so generalization remains difficult. We compared fruit-feeding butterfly communities among understory, canopy, natural treefalls, and forest edge, in a tropical forest of the Western Ghats, a strip of rainforest that is isolated from Sundaland, the large rainforest block of South-East Asia. During a yearlong study, we captured 3018 individuals belonging to 32 species and representing 14 genera. While some butterflies were captured in the canopy, no species was significantly more abundant in the canopy than in the understory. This observation was contrary to studies elsewhere in the tropics where 14–55% of the species could be classified as canopy specialists. Even though the largest number of species was captured at forest edges, species diversity was highest in the gaps. The communities at the forest edge differed importantly from those in treefall gaps: at the forest edge, we caught grassland species in addition to the forest species. Larger treefall gaps had higher butterfly abundance than smaller gaps. Both abundance and diversity peaked during the late monsoon season, and all common species in our sample also peaked during this period. The spatiotemporal community structure appears to depend on biogeography (less vertical stratification further from large forest blocks) and climate (more synchrony among species in seasonal abundance when there is a more severe dry season).
1. Introduction
Since insect species have habitat preferences and their abundances vary through time, insect communities in tropical forests tend to be structured in space and in time [1]. Insight into habitat preferences can be used to predict responses of insect communities to habitat change and management, whereas data on temporal variation of communities is needed to interpret biodiversity surveys and to identify drivers of population fluctuations [2]. Studies on insect community structure attributes of tropical forests have revealed similarities among distant communities in species-abundance-distributions, e.g., [3,4] and prevalence of inter-annual variation over seasonality in community structure [5]. However, there are important differences among regions [6,7] that may be related to differences in climate or (paleo-) biogeography [8,9]. For example, communities on islands tend to have fewer species than mainland communities [10] and may also show differences in the proportion of particular habitat specialists [11]. Hence, the spatial and temporal structure of insect communities needs to be described for the various regions, including sites that are part of large forest blocks, as well as those situated in (habitat) islands.
Forests typically have a canopy fauna of species that are rarely observed at the understory level [12]. For example, a large percent of fruit-feeding butterfly species can mainly be observed in the canopy [13]. Nevertheless, many biodiversity studies are carried out only at ground level, thus missing an important portion of the biodiversity. When the canopy is broken due to treefalls (see below) or selective logging, such canopy species may be more frequently caught in understory traps [14,15]. Consequently, omitting canopy sampling may not only underestimate the total number of species but also give wrong estimates of relative species richness between closed-canopy and more disturbed sites. Notably, the proportion of canopy species within particular insect guilds appears to vary among biogeographical regions [12], but vertical stratification has been studied in too few regions to test for global patterns in the evolutionary ecology of canopy specialization in insects.
Treefall gaps play an important role in structuring insect communities [16]. In undisturbed forests, large branches or whole trees occasionally fall down, creating a more or less random distribution of treefall gaps. Such treefall gaps play a fundamental role in tropical forest ecology [17,18]. The increased light penetration in forest gaps results in more vigorous plant growth (saplings, understory shrubs, vines, and regenerating trees crushed by the falling tree, or the fallen tree itself), and warmer microclimates [19,20,21]. The increased plant growth in treefall gaps can benefit herbivorous insects and their natural enemies, and warmer microhabitats can be used for thermoregulation by insects [22]. In some insects, males defend territories in treefall gaps as part of their mate acquisition strategy [23]. Moreover, treefall gaps may form isolated habitat islands for some animals, so that animals that do reach treefall gaps may experience reduced competition or natural enemy pressure compared to those in larger continuous habitats, such as forest edges. In short, treefall gaps may feature distinct insect faunas, probably depending on their size.
The microclimate of forest edges may seem similar to that of treefall gaps in terms of structure and irradiation, but they experience more wind, do not form habitat islands, and are more stable in time [24,25]. Moreover, animals specialized on habitats on both sides of the edge may be found in edge habitats [26,27]. Notably, individuals may use these habitats for different activities. For example, an individual may spend the night in the understory, warm up in the canopy, forage in the understory, court at the forest edge, and lay eggs in treefall gaps [28].
Herbivorous insects in tropical forests tend to become more abundant during wet seasons, probably because it usually coincides with a peak in plant growth [29]. However, three longer-term studies of tropical insect community dynamics show that seasonality in overall abundance is weak compared to inter-annual variation in abundance [5,29,30]. Moreover, individual species in these communities rarely showed seasonal abundance patterns and species were rarely synchronous in abundance [5]. However, these studies were from humid environments, and when there is a more pronounced dry season, insect seasonality is probably stronger and species will show more synchrony in abundance [8]. Since even one-year-long studies on tropical insect communities are rare, much remains to be learned about the geography of tropical insect temporal abundance patterns [8].
The conservation value of a habitat is not simply the number of species it harbours but should incorporate the conservation value of these species [31]. For example, endemic species have more conservation value than widespread species. Thus, while forest disturbance may not reduce the number of species, it tends to reduce the conservation value of forests because forest specialists are often species of limited distribution, and these are replaced by widespread species when the forest is disturbed [32]. Similarly, some studies have found differences in spatial species turnover (beta diversity) among understory and canopy habitats [7,9].
We compared fruit-feeding butterfly communities among understory, canopy, natural treefall gaps, and forest edge, in a sub-tropical forest of the Western Ghats to test the hypotheses that (1) fruit-feeding butterfly communities in the Western Ghats are vertically stratified and (2) fruit-feeding butterfly communities differ between treefall gaps and forest edge. We also investigated the effect of size of treefall gaps on fruit-feeding butterfly abundance, and describe temporal abundance patterns. Finally, we used estimates of the conservation value of butterfly species to compare the conservation value of these four habitats. Baited traps have been deployed widely for describing community structure of fruit-feeding butterfly communities in tropical forests [33,34], but previous studies in the Western Ghats did not include the canopy [35,36].
2. Methods
2.1. Study Area
The Western Ghats, a rainforest-clad mountain chain ranging from south to north-west India, has a moderately rich flora and fauna, with high levels of endemism [31,37]. They are situated more than a thousand kilometres from the nearest rain forest block in East Asia (North East India), which makes the Western Ghats an isolated rain forest biome. We sampled in Silent Valley National Park (SVNP; 11°03′–11°13′ N and 76°21′–76°35′ E), Palakkad District of Kerala, India. The core zone of SVNP stretches over 237.52 square kilometres and it is part of the Nilgiri Biosphere Reserve, covering over 5000 square kilometres. Most of the park lies within the altitude range of 880 m to 1200 m with highest at 2383 m. The forests of SVNP are categorised as Malabar rain forests, and vegetation comprises mainly species of the west-coast tropical evergreen and semi-evergreen forests and montane shola forest [38,39]. The forest is interspersed with high-altitude natural shola grasslands. At our study site, the annual mean temperature ranges between 18 °C to 23 °C and mean accumulated precipitation ranges between 3200 mm to 5000 mm per year.
2.2. Fruit-Feeding Butterflies
Based on the feeding habits of adults, butterflies can be classified into two main feeding guilds; nectar-feeding, and fruit-feeding [13,40]. Fruit-feeding butterflies are attracted to fermented fruit baits, but may also feed on sap oozing from trees, honey-dew, some puddle on mud, excrements, or carrion, and some may also visit flowers [40,41,42].
2.3. Habitat Definitions
Understory habitats were located under closed canopy forest, at least 20 m from treefall gaps and forest edge. Understory habitats were paired with canopy habitats directly above the selected understory habitats at heights of 25–40 m. We identified treefall gaps using the protocol developed by Runkle [43]. Furthermore, we measured the surface area of treefall gaps. Edge habitats were at clear edges between forest and natural grassland.
2.4. Sampling Regime
We placed six live traps for butterflies [44] in each of the four habitats, using trap dimensions of DeVries [13]. Traps were baited with a mixture of mashed banana with squashed pineapple fermented for at least 48 h, and rum was added before baiting. New bait was added to each trap every second day. The traps were placed >100 m from each other (apart from paired understory and canopy traps). This is more than the 20 m recommended [34] based on the area sampled by a trap. Traps were scored daily between 14:00 and 17:00 h for 12 days per month, and all trapped butterflies were identified in the field using field guides [45,46,47]. Before release, butterflies were marked with a felt-tipped pen, and recaptures were excluded from our analyses. Sampling was carried out during eleven months (August 2006–June 2007), covering all the major seasons. Sampling in July was not possible because of heavy rain. All traps were run simultaneously, but some trap days had to be excluded because bait was disturbed by monkeys, birds, or elephants. In some cases, captured butterflies were eaten by a Giant Asian Mantis (Hierodula membranacea), or other (unidentified) predators, and rainstorms toppled some of the branches on which canopy traps were hung. As a result, the number of trapping days was not equal among habitats (Table 1).

Table 1.
Trapping effort, abundances, and diversity indices for butterflies trapped in fruit-baited traps in the Silent Valley National Park of the Western Ghats, India.
2.5. Data Analysis
We calculated diversity in terms of species richness and evenness, and calculated the Shannon–Wiener index [48]. The species richness among habitats were compared using rarefaction [49], and community evenness using Simpson’s index. Bootstrap methods were used to calculate 95% confidence intervals for Simpson and Shannon–Wiener indices. To compare butterfly community composition across the four habitats, we constructed a Bray–Curtis similarity matrix and then summarized these similarities in a dendrogram by using the BioDiversity Pro Software (https://www.sams.ac.uk/science/outputs/, accessed on 1 December 2021). To investigate butterfly abundance in treefall gaps further, we performed a regression analysis on butterfly abundance with the surface of treefall gaps as predictor. We used conservation value estimates provided by Kunte [31], which are based on global distribution, local distribution, habitat preference and status of each species. We visualized temporal variation of total butterfly abundance by plotting monthly abundance (individuals/trap/day) separately for each habitat. One year of data is not sufficient for statistical time-series analyses.
3. Results
Abundance and Diversity among Habitats
Our 2173 trap-days yielded 3018 individuals, representing 32 species in 13 genera and 6 subfamilies (Table 1 and Table 2). The average number of butterflies per trap varied between months from 0 to 32, with an overall average of 1.4 individuals per trap per day (n = 24 sampling unit/day). Butterfly abundance was highest in forest edge and gap habitats and lowest in the canopy (Table 1). The Chao 1 predicts a total number of fruit-feeding butterfly species in SVNP of 41 species, the second order Jack-knife 47.7 species and bootstrap 39.3 species. This is in agreement with natural history records [31] and indicates that our study captured the vast majority of the fruit-feeding butterfly species that are present at the site.

Table 2.
Butterflies recorded from fruit-baited traps in the four principal habitats in the Silent Valley National Park of the Western Ghats, India.
The largest number of species was caught in the forest edge traps, followed by those in treefall gaps, understory, and canopy (Table 1). However, the Shannon–Wiener estimate of diversity and the Simpson’s index (species evenness) were highest in treefall gaps. The catches in understory traps were dominated (52%) by common evening browns (Melanitis leda L. 1758), which accounted for 46% of individuals caught overall (Table 2). The similarity in the fruit-feeding butterfly community between the understory and forest edge traps was 68%. Similarity was greatest between the sets of traps in understory and treefall gaps (75.5%). Communities in canopy traps showed low similarity with those in the forest edge (Figure 1).
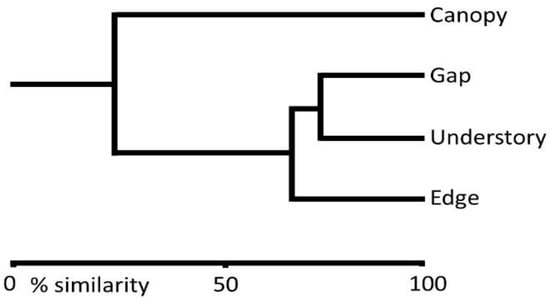
Figure 1.
Bray–Curtis Cluster diagram on the similarity of fruit-feeding butterfly communities among habitats for butterflies trapped in fruit-baited traps in the Silent Valley National Park of the Western Ghats, India.
Only one fruit-feeding species could be regarded as a possible canopy species: the Red-spot Duke Dophla evelina Stoll, 1790 (N = 2 out of 5; Nymphalidae, fruit-feeding). Barons (Euthalia aconthea Cramer, 1777) were represented with 18% of their total abundance in canopy traps, Black Prince (Rohana parisatis Westwood, 1885) and Bamboo Treebrown (Lethe europa Fabricius, 1775) with 7%, and Common Evening Brown (M. leda) with 5%. This was always a minority when compared to understory traps alone (Table 2). Therefore, no species was significantly more abundant in the canopy, and thus no species could be classified as a canopy species. In larger treefall gaps, we found a higher abundance of butterflies in traps (Figure 2).
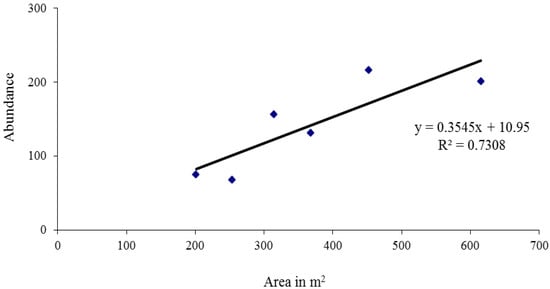
Figure 2.
The relationships between the abundance of the fruit-feeding butterflies and the area of the gaps in Silent Valley National Park, Western Ghats, India.
Temporal variation in abundance was modest, with a peak near the end of the wet season. This was consistent among the four habitats (Figure 3). All common species followed this pattern (all discernible peaks fell in the period October–December), hence there was a high degree of synchrony among species in seasonal abundance during the study year.
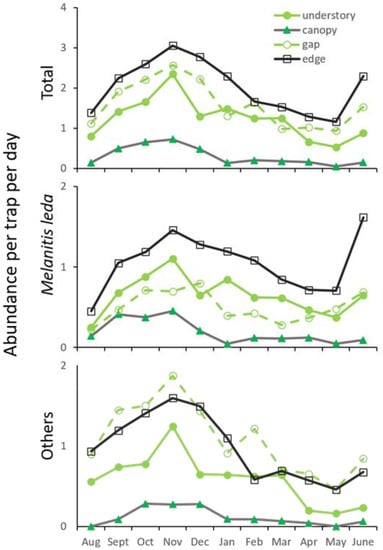
Figure 3.
Temporal variation in fruit-feeding butterfly abundance (all species combined, the most common species, the remaining species combined) in the four principal habitats in Silent Valley National Park, Western Ghats, India.
4. Discussion
We described the partitioning of an insect community into the four main forest habitats (understory, canopy, treefall gaps, and forest edge), and sampled during all seasons of a monsoon climate. Information on such insect community structure parameters for tropical forest regions that are isolated from major rain forest blocks are scarce [11]. By trapping fruit-feeding butterflies using standard methodology, we facilitated comparison with results of similar studies carried out in other tropical forests. In particular, we found a fair abundance but a low number of fruit-feeding butterfly species, and no canopy specialists. Temporal dynamics suggested continuous breeding with synchronous abundance fluctuations, peaking near the end of the wet season. The present study is also in conformity with the findings of other studies in tropical forests, where greater canopy openness and light intensity resulted in higher butterfly diversity [16,32].
We recorded 32 species of fruit-feeding butterfly in this study and estimated that there are approximately 40 species, which is a bit less than found in sites that are part of the Sundaland forest block (54, 53, and 43 species respectively found; [15,16,50]) but more than the 28 estimated species diversity on Siberut Island near Sumatra, Indonesia [11]. The lower diversity of this isolated rain forest biome compared to the larger Sundaland forest block is consistent with lower diversity of island biota compared to mainland [10].
In contrast to all other studies of fruit-feeding butterflies in the tropics that included canopy traps, a canopy fauna could hardly be identified in this forest. The canopy fauna was the most distinct of the four habitats sampled because species that are abundant in the understory differed in their propensity to be occasionally captured in the canopy. We assume that abundance and diversity of butterflies in canopy traps is lower than in understory in part because traps are less effective in the canopy. For example, canopy traps may be affected more by wind, which may disturb butterflies and desiccate bait, and butterflies may escape while lowering canopy traps for inspection. However, we believe that any difference in trap-efficiency only explains a small portion of the observed difference in butterfly abundance between canopy and understory because the same trapping issues would also occur in other regions. The Redspot Duke (D. evelina) is the only true fruit-feeding canopy butterfly in this system, with two individuals out of five caught in the canopy (note that sampling effort was ¼ in canopy). Barons (E. aconthea) may often be caught in the canopy because larvae feed on leaves of certain trees [47], and the presence of Bamboo Treebrowns (L. europa) in the canopy indicates that this species breeds on the higher tips of bamboo. Dolpho evelina is known to breed in the lower canopy, and satyrines on grasses. Nevertheless, these were captured occasionally in the canopy, as is also the case for some grass-feeding satyrines in Africa [3]. We assume these species are using the canopy for other activities, such as sleeping at night, or sun basking. At this point, we may only conclude on the particular forest sampled. However, we predict that canopy specialists are rare throughout the Western Ghats since such proportions of canopy specialists appear to vary little within regions (Table 3; [51,52]).

Table 3.
Vertical stratification of fruit-feeding butterflies in (relatively undisturbed) tropical forests as extracted from the literature [3,7,9,11,15,50,51,52,53,54,55,56,57,58,59]. Note that the % canopy species that we calculated from the reported data will depend on sampling effort and butterfly abundance, and studies vary in their use of trap heights. Within each continent, we distinguish biomes that are large forest blocks (highlighted in gray) and outlying biomes. There appears to be a lower proportion of fruit-feeding butterflies that is specialized in the canopy in more outlying rainforests.
The near-absence of canopy specialists in this forest may be consistent with the possible trend that the proportion of canopy specialist decreases as you move further away from large tropical rainforest blocks (Table 3). For a comparison with Western Ghats (at most one canopy specialist), we can look at Sulawesi, where as many as 40% of fruit-feeding butterfly species in a natural forest were classified as canopy specialists [15]. Our hypothesis that the proportion of canopy specialists increases with size or age of the rain forest biome needs to be tested with further data from rain forest blocks and outlying rain forest biomes.
That traps at the forest edge caught the highest number of species appears mainly due to the effect of adding open habitat species to the nearly complete set of forest species. This corroborates results from other sites and animal groups [27,60]. Future studies should also include traps in the matrix of the grassland to more safely identify grassland species that come to forest edges.
In larger treefall gaps, we found a higher abundance of butterflies in traps (Figure 2). Probably in larger gaps, the microclimate becomes warmer, which may make them suitable for basking butterflies in this quite cool sub-montane forest. In addition, there will be more of the rapid vegetation growth in larger treefall gaps that butterflies may breed on [19]. Our results emphasize the importance of treefall gaps for forest insects.
The temporal variation in fruit-feeding butterfly abundance during this study year was modest, with a consistent peak in the late monsoon, corroborating other butterfly studies from the region [35,36,61,62]. This indicates that most species are actively breeding throughout the year but more successfully so when rains facilitate increased vegetation growth. While the timing of the overall abundance peak is consistent with those found in other tropical forests [5,29,30], the synchrony in timing of abundance peaks among species is usually much lower in humid tropical forests [63,64]. Probably, such synchrony among species in seasonal temporal abundance is typical for tropical forests with a severe dry season [8].
Regarding methodological implications of our results, the canopy fauna in this forest appears so sparse that biodiversity studies in this region may be limited to understory sampling. However, our results do show the importance of a careful consideration of the trap micro-environment: whether a trap is in a treefall gap, how large that treefall gap is, or whether the trap is near the forest edge, greatly affects results.
5. Conclusions
We found a fair abundance of fruit-feeding butterflies, but a low number of species, and we could not identify any canopy species. Species richness was higher at the forest edge where open-landscape species were added to the forest species. In treefall gaps, we found mainly forest-understory species and these were more abundant in larger gaps. During the study year, most species were found year-round, with a synchronous abundance peak near the end of the monsoon season. The degree of vertical stratification appears to depend on biogeography (less vertical stratification further from large forest blocks) and temporal abundance variation varies with climate (more synchrony among species in seasonal abundance when there is a more severe dry season). A concurrent study with the microclimatic parameters in similar micro habitats is envisaged to have a better understanding of the habitat preferences of butterfly fauna of the region.
Author Contributions
The study was designed by K.S.A.D. K.S.A.D. and D.R. collected field data. K.S.A.D. analysed the data with advice from F.M. and wrote the first draft of the MS. F.M. contributed to the writing of the manuscript and interpretation of the results. All authors have read and agreed to the published version of the manuscript.
Funding
Funding was provided by the Wildlife Trust, USA and University Grants Commission, New Delhi. F.M. was funded by grant 2021/43/B/NZ8/00966 from the National Science Centre (NCN, Poland). K.S.A.D. thanks DST-FIST, SERB-CRG (Government of India) for ongoing support.
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not Applicable.
Data Availability Statement
The data presented in this study not included in the appendices are available on request from the corresponding author.
Acknowledgments
The authors acknowledge L. Vijayan, for their valuable guidance and extend gratitude to V.S. Vijayan, R. Sankaran, P.A. Azeez, T.V. Sajeev, J.K. Hill, P. Balakrishnan, and P.R. Arun for their inspiring discussions during the project. Thanks to O.P. Abdurahiman, K.K. Abida, P.P. Manzur Ali, E. Anas, L.K. Sreekala, M.A. Rafeeq, K.M. Remia, and Shamiyath for their support, Rishiddh Jhaveri for insights into edge effects, and the Department of Forests and Wildlife, Kerala, for permissions and support to conduct this study. We thank the reviewers for helpful comments on an earlier version of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Basset, Y.; Cizek, L.; Cuenoud, P.; Didham, R.K.; Novotny, V.; Odegaard, F.; Roslin, T.; Tishechkin, A.K.; Schmidl, J.; Winchester, N.N.; et al. Arthropod distribution in a tropical rainforest: Tackling a four dimensional puzzle. PLoS ONE 2015, 10, e0144110. [Google Scholar] [CrossRef] [PubMed]
- Molleman, F. Moving beyond phenology: New directions in the study of temporal dynamics of tropical insect communities. Curr. Sci. 2018, 114, 982–986. [Google Scholar] [CrossRef]
- Molleman, F.; Kop, A.; Brakefield, P.; De Vries, P.; Zwaan, B. Vertical and temporal patterns of biodiversity of fruit-feeding butterflies in a tropical forest in Uganda. Biodivers. Conserv. 2006, 15, 107–121. [Google Scholar] [CrossRef]
- DeVries, P.J.; Murray, D.; Lande, R. Species diversity in vertical, horizontal, and temporal dimensions of a fruit-feeding butterfly community in an Ecuadorian rainforest. Biol. J. Linn. Soc. 1997, 62, 343–364. [Google Scholar] [CrossRef]
- Grøtan, V.; Lande, R.; Chacon, I.A.; DeVries, P.J. Seasonal cycles of diversity and similarity in a Central American rainforest butterfly community. Ecography 2014, 37, 509–516. [Google Scholar] [CrossRef]
- Primack, R.B.; Corlett, R.T. Tropical Rain Forests: An Ecological and Biogeographical Comparison; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Devries, P.J.; Alexander, L.G.; Chacon, I.A.; Fordyce, J.A. Similarity and difference among rainforest fruit-feeding butterfly communities in Central and South America. J. Anim. Ecol. 2012, 81, 472–482. [Google Scholar] [CrossRef]
- Kishimoto-Yamada, K.; Itioka, T. How much have we learned about seasonality in tropical insect abundance since Wolda (1988)? Entomol. Sci. 2015, 18, 407–419. [Google Scholar] [CrossRef]
- dos Santos, J.P.; Iserhard, C.A.; Carreira, J.Y.O.; Freitas, A.V.L. Monitoring fruit-feeding butterfly assemblages in two vertical strata in seasonal Atlantic Forest: Temporal species turnover is lower in the canopy. J. Trop. Ecol. 2017, 33, 345–355. [Google Scholar] [CrossRef]
- MacArthur, R.H.; Wilson, E.O. The Theory of Island Biogeography; Princeton University Press: Princeton, NJ, USA, 1967; p. 203. [Google Scholar]
- Luk, C.-L.; Hadi, U.K.; Ziegler, T.; Waltert, M. Vertical and horizontal habitats of fruit-feeding butterflies (Lepidoptera) on Siberut, Mentawai Islands, Indonesia. Ecotropica 2011, 17, 79–90. [Google Scholar]
- Basset, Y.; Novotny, V.; Miller, S.E.; Kitching, R.L. (Eds.) Arthropods of Tropical Forests. Spatio-Temporal Dynamics and Resource Use in the Canopy; Cambridge University Press: Cambridge, UK, 2003; p. 474. [Google Scholar]
- DeVries, P.J. Stratification of fruit-feeding Nymphalid butterflies in a Costa Rican rainforest. J. Res. Lepid. 1988, 26, 98–108. [Google Scholar]
- DeVries, P.J.; Walla, T.R. Species diversity and community structure in neotropical fruit- feeding butterflies. Biol. J. Linn. Soc. 2001, 74, 1–15. [Google Scholar] [CrossRef]
- Fermon, H.; Waltert, M.; Vane-Wright, R.I.; Muhlenberg, M. Forest use and vertical stratification in fruit-feeding butterflies of Sulawesi, Indonesia: Impacts for conservation. Biodivers. Conserv. 2005, 14, 333–350. [Google Scholar] [CrossRef]
- Hill, J.K.; Hamer, K.C.; Tangah, J.; Dawood, M. Ecology of tropical butterflies in rainforest gaps. Oecologia 2001, 128, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Watt, A.S. Pattern and process in the plant community. Ecology 1947, 35, 1–22. [Google Scholar] [CrossRef]
- Feener Jr, D.H.; Schupp, E.W. Effect of treefall gaps on the patchiness and species richness of Neotropical ant assemblages. Oecologia 1998, 116, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Blake, J.G.; Hoppes, W.G. Influence of resource abundance on use of treefall gaps by birds in an isolated woodlot. Auk 1986, 103, 328–340. [Google Scholar] [CrossRef]
- Blau, W.S. The effect of environmental disturbance on a tropical butterfly population. Ecology 1980, 61, 1005–1012. [Google Scholar] [CrossRef]
- Arihafa, A.; Mack, A.L. Treefall gap dynamics in a tropical rain forest in Papua New Guinea. Pac. Sci. 2013, 67, 47–58. [Google Scholar] [CrossRef]
- Seifert, C.L.; Schulze, C.H.; Dreschke, T.C.T.; Frötscher, H.; Fiedler, K. Day vs. night predation on artificial caterpillars in primary rainforest habitats—An experimental approach. Entomol. Exp. Appl. 2016, 158, 54–59. [Google Scholar] [CrossRef]
- Rutowski, R.L. The evolution of male mate-locating behavior in butterflies. Am. Nat. 1991, 138, 1121–1139. [Google Scholar] [CrossRef]
- Davies-Colley, R.J.; Payne, G.W.; van Elswijk, M. Microclimate gradients across a forest edge. N. Z. J. Ecol. 2000, 24, 111–121. [Google Scholar]
- Bernaschini, M.L.; Valladares, G.; Salvo, A. Edge effects on insect–plant food webs: Assessing the influence of geographical orientation and microclimatic conditions. Ecol. Entomol. 2020, 45, 806–820. [Google Scholar] [CrossRef]
- Meiners, S.J.; Handel, S.N.; Pickett, S.T.A. Tree seedling establishment under insect herbivory: Edge effects and interannual variation. Plant Ecol. 2000, 151, 161–170. [Google Scholar] [CrossRef]
- Bossart, J.; Opuni-Frimpong, E. Distance from edge determines fruit-feeding butterfly community diversity in Afrotropical forest fragments. Environ. Entomol. 2009, 38, 43–52. [Google Scholar] [CrossRef]
- Kemp, D.J. Visual mate-searching behaviour in the evening brown butterfly, Melanitis leda (L.) (Lepidoptera: Nymphalidae). Aust. J. Entomol. 2002, 41, 300–305. [Google Scholar] [CrossRef]
- Valtonen, A.; Molleman, F.; Chapman, C.A.; Carey, J.R.; Ayres, M.P.; Roininen, H. Tropical phenology: Bi-annual rhythms and interannual variation in an Afrotropical butterfly assemblage. Ecosphere 2013, 4, 36. [Google Scholar] [CrossRef]
- Grøtan, V.; Lande, R.; Engen, S.; Saether, B.E.; DeVries, P.J. Seasonal cycles of species diversity and similarity in a tropical butterfly community. J. Anim. Ecol. 2012, 81, 714–723. [Google Scholar] [CrossRef]
- Kunte, K. The Wildlife (Protection) Act and conservation prioritization of butterflies of the Western Ghats, southwestern India. Curr. Sci. 2008, 94, 729–735. [Google Scholar]
- Hamer, K.C.; Hill, J.K.; Lace, L.A.; Langan, A.M. Ecological and biogeographical effects of forest disturbance on tropical butterflies of Sumba, Indonesia. J. Biogeogr. 1997, 24, 67–75. [Google Scholar] [CrossRef]
- Lucci Freitas, A.V.; Agra Iserhard, C.; Pereira Santos, J.; Oliveira CarreiraI, J.Y.; Bandini Ribeiro, D.; Alves Melo, D.H.; Batista Rosa, A.H.; Marini-Filho, O.J.; Mattos Accacio, G.; Uehara-Prado, M. Studies with butterfly bait traps: An overview. Rev. Colomb. Entomol. 2014, 40, 203–212. [Google Scholar]
- Devries, P.J.; Hamm, C.A.; Fordyce, J.A. A Standardized Sampling Protocol for Fruit-Feeding Butterflies (Nymphalidae). In Core Standardized Methods for Rapid Biological Field Assessment; Larsen, T.H., Ed.; Conservation International: Arlington, VA, USA, 2016; pp. 139–148. [Google Scholar]
- Naik, D.; Rao, R.; Kunte, K.; Mustak, M.S. Ecological monitoring and indicator taxa: Butterfly communities in heterogeneous landscapes of the Western Ghats and Malabar coast, India. J. Insect Conserv. 2022, 26, 107–119. [Google Scholar] [CrossRef]
- Kunte, K.J. Seasonal patterns in butterfly abundance and species diversity in four tropical habitats in northern Western Ghats. J. Biosci. 1997, 22, 593–603. [Google Scholar] [CrossRef]
- Mittermeier, R.A.; Gil, P.; Hoffman, M.; Pilgrim, J.; Brooks, T.; Mittermeier, C.; Lamoreux, J.; Da Fonseca, G.; Saligmann, P. Hotspots Revisited: Earth’s Biologically Richest and Most Endangered Terrestrial Ecoregions Cemex; Conservation International: Mexico City, Mexico, 2004; Volume 392. [Google Scholar]
- Champion, H.G.; Seth, S.K. A Revised Survey of the Forest Types of India; Manager of Publications: Delhi, India, 1968. [Google Scholar]
- Udvardy, M.D.; Udvardy, M. A Classification of the Biogeographical Provinces of the World; International Union for Conservation of Nature and Natural Resources Morges: Morges, Switzerland, 1975; Volume 8. [Google Scholar]
- Norris, M.J. The feeding-habits of the adult Lepidoptera Heteroneura. Trans. R. Entomol. Soc. Lond. 1936, 85, 61–90. [Google Scholar] [CrossRef]
- Molleman, F.; Grunsven, R.; Liefting, M.; Zwaan, B.; Brakefield, P. Is male puddling behaviour of tropical butterflies targeted at sodium for nuptial gifts or activity? Biol. J. Linn. Soc. 2005, 86, 345–361. [Google Scholar] [CrossRef]
- Molleman, F. Puddling: From natural history to understanding how it affects fitness. Entomol. Exp. Appl. 2010, 134, 107–113. [Google Scholar] [CrossRef]
- Runkle, J.R. Guidelines and Sample Protocol for Sampling Forest Gaps; US Department of Agriculture, Forest Service, Pacific Northwest Research Station: Washington, DC, USA, 1992; Volume 283.
- Rydon, A. Notes on the use of butterfly traps in East Africa. J. Lepid. Soc. 1964, 18, 51–58. [Google Scholar]
- Evans, W.H. Identification of Indian Butterflies; Bombay Natural History Society: Bombay, India, 1932. [Google Scholar]
- Wynter-Blyth, M.A. Butterflies of the Indian Region; Bombay Natural History Society: Mumbai, India, 1957. [Google Scholar]
- Kunte, K. India, a Lifescape: Butterflies of Peninsular India; Universities Press: Hyderabad, India, 2000. [Google Scholar]
- Magurran, A.E. Ecological Diversity and Its Measurement; Princeton University Press: Princeton, NJ, USA, 1988. [Google Scholar]
- Heck Jr, K.L.; van Belle, G.; Simberloff, D. Explicit calculation of the rarefaction diversity measurement and the determination of sufficient sample size. Ecology 1975, 56, 1459–1461. [Google Scholar] [CrossRef]
- Schulze, C.H.; Linsenmair, K.E.; Fiedler, K. Understorey versus canopy: Patterns of vertical stratification and diversity among Lepidoptera in a Bornean rain forest. Plant Ecol. 2001, 153, 133–152. [Google Scholar] [CrossRef]
- Cordeiro, N. Geographical consistency in vertical stratification preferences of butterfly species in eastern Africa. Afr. Entomol. 2017, 25, 550–553. [Google Scholar] [CrossRef]
- Aduse-Poku, K.; Molleman, F.; Oduro, W.; Oppong, S.K.; Lohman, D.J.; Etienne, R.S. Relative contribution of neutral and deterministic processes in shaping fruit-feeding butterfly assemblages in Afrotropical forests. Ecol. Evol. 2018, 8, 296–308. [Google Scholar] [CrossRef]
- Rodrigues, E.N.L.; Mendonça, M.D.S.; Costa-Schmidt, L.E. Spider diversity responds strongly to edge effects but weakly to vegetation structure in riparian forests of Southern Brazil. Arthropod-Plant Interact. 2014, 8, 123–133. [Google Scholar] [CrossRef]
- Padhye, A.; Dahanukar, N.; Paingankar, M.; Deshpande, M.; Deshpande, D. Season and landscape wise distribution of butterflies in Tamhini, northern Western Ghats, India. Zoos’ Print J. 2006, 21, 2175–2181. [Google Scholar] [CrossRef]
- Arun, P. Seasonality of swallowtail butterfly community (Lepidoptera: Papilionidae) of Siruvani forest, Western Ghats, Southern India. In Proceedings of the Seminar on Wonderful World of Insects, Thane, India, 3 December 2008; pp. 66–71. [Google Scholar]
- Wolda, H. Fluctuations in abundance of tropical insects. Am. Nat. 1978, 112, 1017–1045. [Google Scholar] [CrossRef]
- Novotny, V.; Basset, Y. Seasonality of sap sucking insects (Auchenorrhyncha, Hemiptera) feeding on Ficus (Moraceae) in a lowland rain forest in New Guinea. Oecologia 1998, 9, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Tangah, J.; Hill, J.; Hamer, K.; Dawood, M. Vertical distribution of fruit-feeding butterflies in Sabah, Borneo. Sepilok Bull. 2004, 1, 17–27. [Google Scholar]
- de Brito Freire Jr, G.; Ribeiro, D.B.; de Carvalho Santos, A.; Silva, T.; Dias, J.P.; Rodrigues, H.P.; Diniz, I.R. Horizontal and vertical variation in the structure of fruit-feeding butterfly (Nymphalidae) assemblages in the Brazilian Cerrado. Insect Conserv. Divers. 2022, 15, 226–235. [Google Scholar] [CrossRef]
- Araujo, P.F.; Freitas, A.V.L.; Gonçalves, G.A.d.S.; Ribeiro, D.B. Vertical stratification on a small scale: The distribution of fruit-feeding butterflies in a semi-deciduous Atlantic forest in Brazil. Stud. Neotrop. Fauna Environ. 2021, 56, 10–39. [Google Scholar] [CrossRef]
- Mohamed, R.; Rosmidi, F.H.; Adanan, N.A.; Ahmad, A.; Abdullah, M.T. Vertical stratification of fruit-feeding butterflies in Tasik Kenyir. In Greater Kenyir Landscapes; Springer Nature: Cham, Switzerland, 2019; pp. 131–142. [Google Scholar]
- Ribeiro, D.B.; Williams, M.R.; Specht, A.; Freitas, A.V.L. Vertical and temporal variability in the probability of detection of fruit-feeding butterflies and moths (Lepidoptera) in tropical forest. Austral Entomol. 2016, 55, 112–120. [Google Scholar] [CrossRef]
- Fermon, H.; Waltert, M.; Muhlenberg, M. Movement and vertical stratification of fruit-feeding butterflies in a managed West African rainforest. J. Insect Conserv. 2003, 7, 7–19. [Google Scholar] [CrossRef]
- Aduse-Poku, K.; William, O.; Oppong, S.K.; Larsen, T.; Ofori-Boateng, C.; Molleman, F. Spatial and temporal variation in butterfly biodiversity in a West African forest: Lessons for establishing efficient rapid monitoring programmes. Afr. J. Ecol. 2012, 50, 326–334. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).