Ungulates and Their Impact on Reptiles: A Review of Interspecific Relationships
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search and Paper Selection
2.2. Data Extraction and Analysis
3. Results
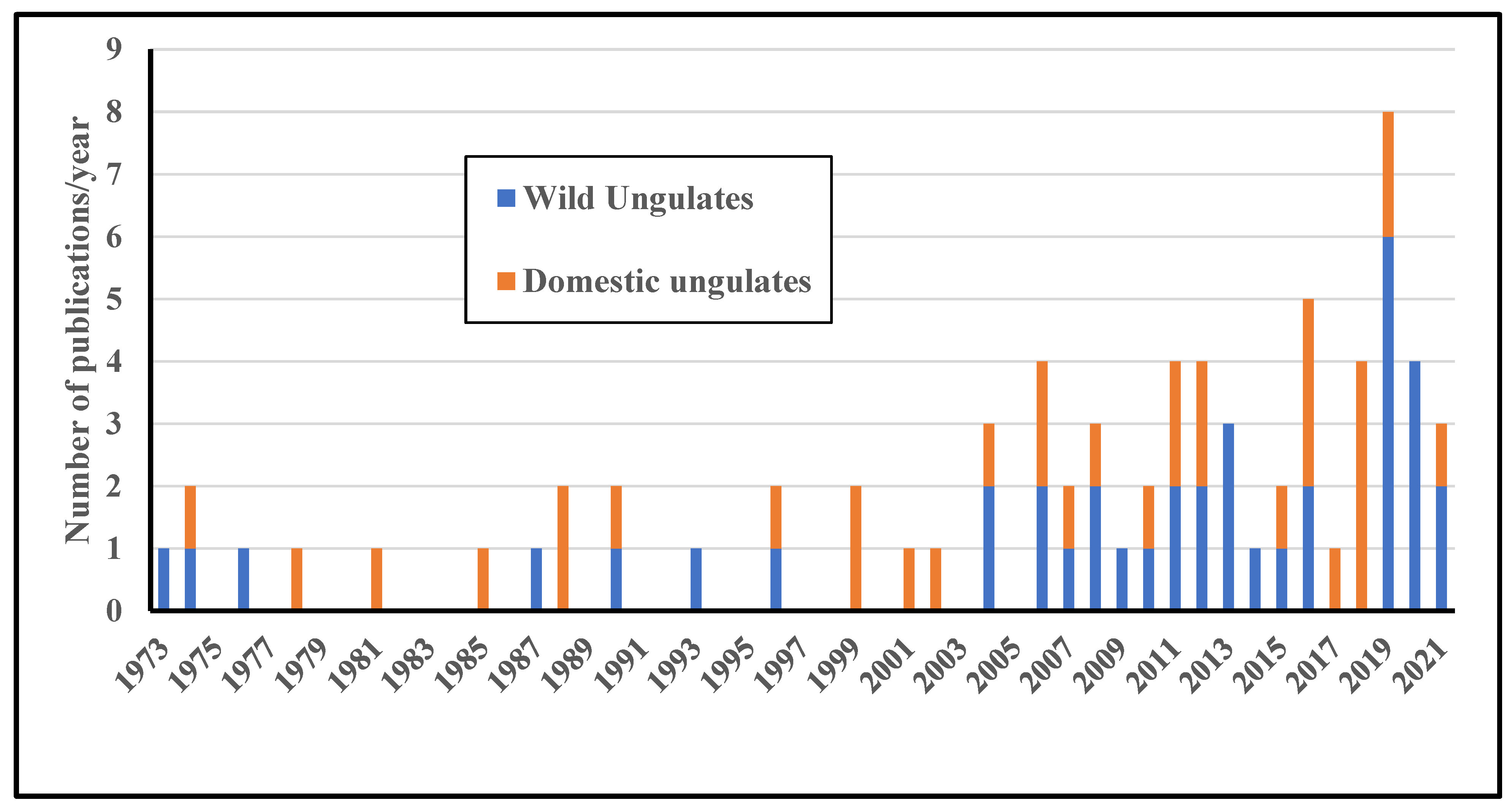
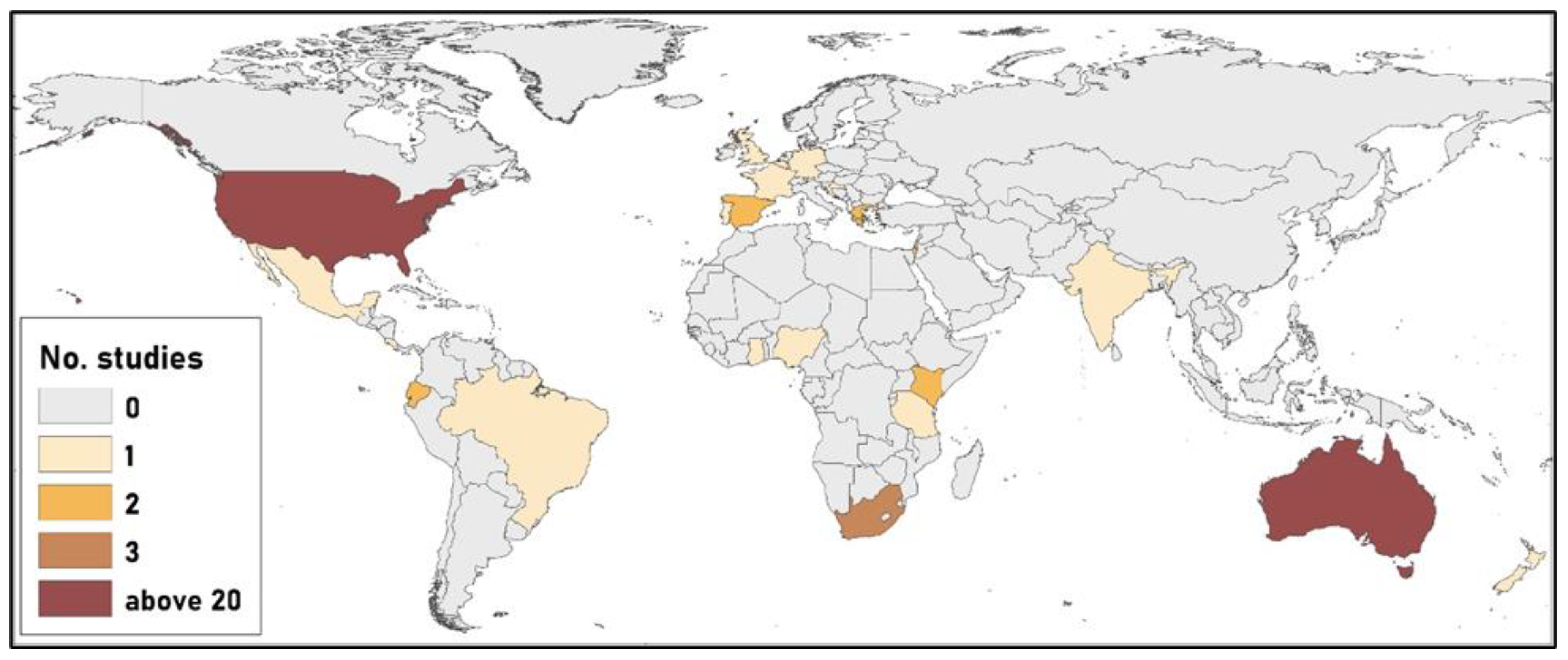
3.1. Spatiotemporal Patterns of Research, Species Involved
3.2. Comparison between Wild and Domestic Ungulates
3.3. Comparison between Ruminants and Monogastric Ungulates
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Ungulate Species (Latin Name) | Ungulate Species (Common Name) | Reptile Species (Latin Name) | Reptile Species (Common Name) | Type of Impact | +/− | Number of Times Pairs Mentioned in Publications | Authors |
|---|---|---|---|---|---|---|---|
| Axis axis | Chital deer | Lacerta viridis LC | Green lizard | (Indirect) Grazing—deer removes understorey (habitat) of lizards | − | 1 | Mohanty et al., 2016 |
| Bos taurus | Cattle | Centrochelys sulcata EN | African spurred tortoise | (Indirect) Grazing—cattle reduce food for spurred tortoise | − | 1 | Petrozzi et al., 2018 |
| Bos taurus | Cattle | Clemmys muhlenbergii CR | Bog turtle | (Indirect) Grazing—cattle remove cover for turtles | − | 1 | Tesauro and Ehrenfeld, 2007 |
| Bos taurus | Cattle | Iguana iguana LC; Lygodactylus sp. LC and Tiliqua scincoides LC; Phrynosoma platyrhinos LC | Green iguana; Dwarf gecko and Common blue-tongued skink; Desert horned lizard | (Indirect) Competition (interference)—for space-use between reptiles and cattles | − | 3 | Mitchell, 1999; Neilly et al., 2018; Newbold and Macmahon, 2008 |
| Capra hircus | Domestic goat | Hemidactylus turcicus LC | Mediterranean house gecko | (Indirect) Grazing—goat removes understorey/cover (habitat) | − | 1 | Pafilis et al., 2013 |
| Equus asinus | Feral burro | Gopherus agassizii CR | Mojave Desert tortoise | (Indirect) Grazing—overgrazing by abundant burros reduce population density of tortoises | − | 1 | Berry et al., 2020 |
| Kobus kob | Kob | Python sebae NT | African rock python | (Direct) Predation—python feeds on kob as prey | + | 1 | Antwi et al., 2019 |
| Loxodonta africana | African elephant | Lygodactylus. spp. LC and Tiliqua scincoides LC | Dwarf gecko and Common bluetongue skink | (Indirect) Grazing—bare soil after grazing increases mortality for gecko and skink | − | 2 | Gordons et al., 2021; Nasseri et al., 2011 |
| Odocoileus virginianus | White-tailed deer | Python bivittatus VU | Burmese python | (Direct) Predation—deer as prey for python (remains of 3 deers in stomach of the python) | + | 2 | Boback et al., 2016; Boback et al., 2020 |
| Odocoileus virginianus | White tailed deer | Thamnophis sirtalis LC | Common garter snake | (Indirect) Presence—ungulates increase abundance of garter snakes through augmenting their invertebrate prey density | + | 1 | Greenwald et al., 2008 |
| Ovis aries and Bos taurus | Sheep and Cattle | Carlia tetradactyla LC, Morethia boulengeri LC and Ctenotus spaldingi LC | Southern rainbow-skink, Boulenger’s snake-eyed skink and Straight-browed ctenotus | (Indirect) Grazing—sheep and cattle remove cover for the skinks | + | 1 | Kay et al., 2017 |
| Ovis aries and Bos taurus | Sheep and Cattle | Cryptoblepharus pannosus LC, Hemiergis talbingoensis LC, Christinus marmoratus LC | Ragged snake-eyed skink, Victoria three-toed earless skink and Marbled gecko | (Indirect) Grazing—sheep and cattle remove cover for the skinks | − | 1 | Kay et al., 2017 |
| Ovis aries | Domestic sheep | Lacerta viridis LC | Green lizard | (Indirect) Grazing—sheep removes cover for the lizards | − | 1 | Smith et al., 1996 |
| Ovis aries | Domestic sheep | Tiliqua adelaidensis EN | Pygmy bluetongue lizard | (Indirect) Grazing—sheep removes cover for the lizards | − | 3 | Brown et al., 2011; Kazmaier, 2001; Nielsen and Bull, 2016 |
| Sus scrofa | Wild boar | Alligator mississippiensis LC | American alligator | (Direct) Predation—nest predated by wild boar | − | 2 | Campos and Mourão, 2014; Elsey et al. 2012 |
| Sus scrofa | Wild boar | Anolis carolinensis LC, Storeria occipitomaculata LC and Sceloporus undulatus LC | Green anole, Red-bellied snake and Eastern fence lizard | (Direct) Predation—reptile species as prey for wild boar | − | 1 | Jolley et al., 2010 |
| Sus scrofa | Wild boar | Blanus cinereus LC and Psammodromus algirus LC | Iberian worm lizard and Algerian sand racer | (Direct) Predation—reptile remains found in stomach content of wild boar | − | 2 | Abáigar, 1993; Briedermann, 1976 |
| Sus scrofa | Wild boar | Chelodina longicollis NT | Eastern long-necked turtle | (Indirect) Rooting, Trampling—wild boar destroys turtle’s habitat | − | 1 | Doupé et al., 2009 |
| Sus scrofa | Wild boar | Chelodina rugosa Ogilby NT | Northern snake-necked turtle | (Direct) Predation—wild boar kills turtles | − | 2 | Fordham et al., 2006 and 2008 |
| Sus scrofa | Wild boar | Malpolon monspessulanus LC | Montpellier snake | (Direct) Predation—Montpellier snake as prey for wild boar | − | 1 | Ballouard et al., 2021 |
| Sus scrofa | Wild boar | Chelonia mydas EN | Green sea turtle | (Direct) Predation—sea turtle as prey for wild boar | − | 2 | Engeman et al., 2019; Nordberg et al., 2019 |
| Sus scrofa | Wild boar | Geochelone elephantopus EN | Galápagos giant tortoise | (Direct) Predation—wild boar killed adult tortoises | − | 1 | MacFarland et al., 1974 |
| Sus scrofa | Wild boar | Kinosternon hirtipes LC | Rough-footed mud turtle | (Direct) Predation—turtle as prey for wild boar | − | 1 | Platt et al., 2019 |
| Sus scrofa | Wild boar | Natator depressus DD, Lepidochelys olivacea VU and Eretmochelys imbricata EN | Flatback turtle, Olive ridley turtle and Hawksbill turtle | (Direct) Predation—turtle nests predated by wild boar | − | 1 | Whytlaw et al., 2013 |
| Sus scrofa | Wild boar | Natrix natrix LC | Grass snake | (Direct) Predation—snake remains found in stomach content of wild boar | − | 1 | Tucak, 1996 |
| Sus scrofa | Wild boar | Storeria occipitomaculata LC | Red-bellied snake | (Direct) Predation—snake remains found in stomach content of wild boar | − | 1 | Scott, 1973 |
| Sus scrofa | Wild boar | Testudo hermanni NT | Hermann’s tortoise | (Direct) Predation—tortoise remains found in stomach content of wild boar | − | 1 | Vilardell et al., 2012 |
| Sus scrofa | Wild boar | Tropidurus jacobii LC and Pseudalsophis steindachneri NT | Santiago lava lizard and Painted racer | (Direct) Predation—reptile remains found in the stomach content of wild boar | − | 1 | Coblentz and Baber, 1987 |
| Sus scrofa | Wild boar | Varanus komodoensis EN | Komodo dragon | (Direct) Predation—wild boar providing prey to dragon | + | 3 | Ariefiandy et al., 2020; Jessop et al., 2019 and 2020 |
| Sus scrofa | Wild boar | Vipera berus LC | Common European viper | (Indirect) Rooting—wild boar foraging behaviour reduces common viper abundance in the area | − | 1 | Graitson et al., 2019 |
| Tapirus terrestrisVU | Lowland tapir | Chelonoidis denticulata VU | Yellow-footed tortoise | (Direct) Predation—tortoise as prey for tapir | − | 1 | Edison and David, 2020 |
References
- Apollonio, M.; Ciuti, S.; Pedrotti, L.; Banti, P. Ungulates and their management in Italy. In European Ungulates and Their Management in the 21st Century, 1st ed.; Apollonio, M., Andersen, R., Putman, R., Eds.; Cambridge University Press: Cambridge, UK, 2010; pp. 475–506. [Google Scholar]
- Carpio, A.J.; Apollonio, M.; Acevedo, P. Wild ungulate overabundance in Europe: Contexts, causes, monitoring and management recommendations. Mamm. Rev. 2021, 51, 95–108. [Google Scholar] [CrossRef]
- Valente, A.M.; Acevedo, P.; Figueiredo, A.M.; Fonseca, C.; Torres, R.T. Overabundant wild ungulate populations in Europe: Management with consideration of socio-ecological consequences. Mamm. Rev. 2020, 50, 353–366. [Google Scholar] [CrossRef]
- Skogland, T. What Are the Effects of Predators on Large Ungulate Populations? Oikos 1991, 61, 401–411. [Google Scholar] [CrossRef]
- Homewood, K.; Lambin, E.F.; Coast, E.; Kariuki, A.; Kikula, I.; Kivelia, J.; Said, M.; Serneels, S.; Thompson, M. Long-term changes in Serengeti-Mara wildebeest and land cover: Pastoralism, population, or policies? Proc. Nat. Acad. Sci. USA 2001, 98, 12544–12549. [Google Scholar] [CrossRef] [PubMed]
- Massei, G.; Kindberg, J.; Licoppe, A.; Gačić, D.; Šprem, N.; Kamler, J.; Baubet, E.; Hohmann, U.; Monaco, A.; Ozoliņš, J.; et al. Wild boar populations up, numbers of hunters down? A review of trends and implications for Europe. Pest Manag. Sci. 2015, 71, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Fons, F. A Review of the Current Status of Relevant Zoonotic Pathogens in Wild Swine (Sus scrofa) Populations: Changes Modulating the Risk of Transmission to Humans. Transbound. Emerg. Dis. 2017, 64, 68–88. [Google Scholar] [CrossRef]
- Su, K.; Ren, J.; Yang, J.; Hou, Y.; Wen, Y. Human-Elephant Conflicts and Villagers’ Attitudes and Knowledge in the Xishuangbanna Nature Reserve, China. Int. J. Environ. Res. Public Health 2020, 17, 8910. [Google Scholar] [CrossRef]
- Loe, L.E.; Liston, G.E.; Pigeon, G.; Barker, K.; Horvitz, N.; Stien, A.; Forchhammer, M.; Getz, W.M.; Irvine, R.J.; Lee, A.; et al. The neglected season: Warmer autumns counteract harsher winters and promote population growth in Arctic reindeer. Glob. Change Biol. 2020, 27, 993–1002. [Google Scholar] [CrossRef]
- Weisberg, P.J.; Thompson Hobbs, N.; Ellis, J.E.; Coughenour, M.B. An ecosystem approach to population management of ungulates. J. Environ. Manag. 2002, 65, 181–197. [Google Scholar] [CrossRef]
- Côté, S.D.; Rooney, T.P.; Tremblay, J.-P.; Dussault, C.; Waller, D.M. Ecological Impacts of Deer Overabundance. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 113–147. [Google Scholar] [CrossRef]
- Servanty, S.; Gaillard, J.-M.; Ronchi, F.; Focardi, S.; Baubet, É.; Gimenez, O. Influence of harvesting pressure on demographic tactics: Implications for wildlife management. J. Appl Ecol. 2011, 48, 835–843. [Google Scholar] [CrossRef]
- Miranda, M.; Sicilia, M.; Bartolomé, J.; Molina-Alcaide, E.; Gálvez-Bravo, L.; Cassinello, J. Contrasting feeding patterns of native red deer and two exotic ungulates in a Mediterranean ecosystem. Wildl. Res. 2012, 39, 171–182. [Google Scholar] [CrossRef]
- Ben-Shahar, R.; Skinner, J.D. Habitat Preferences of African Ungulates Derived by Uni- and Multivariate Analyses. Ecology 1988, 69, 1479–1485. [Google Scholar] [CrossRef]
- Homolka, M. Foraging strategy of large herbivores in forest habitats. Folia Zool. 1996, 45, 127–136. [Google Scholar]
- Zweifel-Schielly, B.; Kreuzer, M.; Ewald, K.C.; Suter, W. Habitat Selection by an Alpine Ungulate: The Significance of Forage Characteristics Varies with Scale and Season. Ecography 2009, 32, 103–113. [Google Scholar] [CrossRef]
- Nichols, R.V.; Cromsigt, J.P.G.M.; Spong, G. DNA left on browsed twigs uncovers bite-scale resource use patterns in European ungulates. Oecologia 2015, 178, 275–284. [Google Scholar] [CrossRef]
- Howland, B.W.A.; Stojanovic, D.; Gordon, I.J.; Fletcher, D.; Snape, M.; Stirnemann, I.A.; Lindenmayer, D.B. Habitat preference of the striped legless lizard: Implications of grazing by native herbivores and livestock for conservation of grassland biota. Austral Ecol. 2016, 41, 455–464. [Google Scholar] [CrossRef]
- Nasseri, N.A.; McBrayer, L.D.; Schulte, B.A. The impact of tree modification by African elephant (Loxodonta africana) on herpetofaunal species richness in northern Tanzania. Afr. J. Ecol. 2011, 49, 133–140. [Google Scholar] [CrossRef]
- Pincheira-Donoso, D.; Bauer, A.M.; Meiri, S.; Uetz, P. Global Taxonomic Diversity of Living Reptiles. PLoS ONE 2013, 8, e59741. [Google Scholar] [CrossRef]
- Shine, R.; Somaweera, R. Last lizard standing: The enigmatic persistence of the Komodo dragon. Glob. Ecol. Conserv. 2019, 18, e00624. [Google Scholar] [CrossRef]
- Bland, L.M.; Böhm, M. Overcoming data deficiency in reptiles., advancing reptile conservation: Addressing knowledge gaps and mitigating key drivers of extinction risk. Biol. Conserv. 2016, 204, 16–22. [Google Scholar] [CrossRef]
- WWF. Living Planet Report 2022—Building a Nature-Positive Society; Almond, R.E.A., Grooten, M., Juffe Bignoli, D., Petersen, T., Eds.; WWF: Gland, Switzerland, 2022. [Google Scholar]
- Meek, R. Anthropogenic sources of mortality in the western whip snake, Hierophis viridiflavus, in a fragmented landscape in Western France. Herpetol. Bull. 2012, 120, 4–8. [Google Scholar]
- Katona, K.; Coetsee, C. Impacts of Browsing and Grazing Ungulates on Faunal Biodiversity. In The Ecology of Browsing and Grazing II; Ecological Studies; Gordon, I.J., Prins, H.H.T., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 277–300. [Google Scholar]
- Kazmaier, R.T.; Hellgren, E.C.; Ruthven, D.C. Habitat selection by the Texas tortoise in a managed thornscrub ecosystem. J. Wildl. Manag. 2001, 65, 653–660. [Google Scholar] [CrossRef]
- McCauley, D.J.; Keesing, F.; Young, T.P.; Allan, B.F.; Pringle, R.M. Indirect Effects of Large Herbivores on Snakes in an African Savanna. Ecology 2006, 87, 2657–2663. [Google Scholar] [CrossRef] [PubMed]
- Ariefiandy, A.; Purwandana, D.; Coulson, G.; Forsyth, D.M.; Jessop, T.S. Monitoring the ungulate prey of the Komodo dragon (Varanus komodoensis): Distance sampling or faecal counts? Wildl. Biol. 2013, 19, 126–137. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef]
- Antwi, R.A.; Ofori, B.Y.; Attuquayefio, D.K.; Owusu, E.H. Predation on the Kob (Kobus kob) by the African rock python (Python sebae) at Shai Hills Resource Reserve, Ghana. Herpetol. Notes 2019, 12, 1181–1183. [Google Scholar]
- Petrozzi, F.; Eniang, E.A.; Akani, G.C.; Amadi, N.; Hema, E.M.; Diagne, T.; Segniagbeto, G.H.; Chirio, L.; Amori, G.; Luiselli, L. Exploring the main threats to the threatened African spurred tortoise (Centrochelys sulcate) in the West African Sahel. Oryx 2018, 52, 544–551. [Google Scholar] [CrossRef]
- Beever, E.A.; Brussard, P.F. Community- and landscape-level responses of reptiles and small mammals to feral-horse grazing in the Great Basin. J. Arid Environ. 2004, 59, 271–297. [Google Scholar] [CrossRef]
- Agresti, A. An Introduction to Categorical Data Analysis; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2019. [Google Scholar]
- Fischer, J.; Lindenmayer, D.B.; Cowling, A. The challenge of managing multiple species at multiple scales: Reptiles in an Australian grazing landscape. J. Appl Ecol. 2004, 41, 32–44. [Google Scholar] [CrossRef]
- Friend, G.R.; Cellier, K.M. Wetland Herpetofauna of Kakadu National Park, Australia: Seasonal Richness Trends, Habitat Preferences and the Effects of Feral Ungulates. J. Tropic Ecol. 1990, 6, 131–152. [Google Scholar] [CrossRef]
- Val, J.; Travers, S.K.; Oliver, I.; Koen, T.B.; Eldridge, D.J. Recent grazing reduces reptile richness but historic grazing filters reptiles based on their functional traits. J. Appl. Ecol. 2019, 56, 833–842. [Google Scholar] [CrossRef]
- Nordberg, E.J.; Macdonald, S.; Zimny, G.; Hoskins, A.; Zimny, A.; Somaweera, R.; Ferguson, J.; Perry, J. An evaluation of nest predator impacts and the efficacy of plastic meshing on marine turtle nests on the western Cape York Peninsula, Australia. Biol. Conserv. 2019, 238, 108201. [Google Scholar] [CrossRef]
- Ariefiandy, A.; Purwandana, D.; Benu, Y.J.; Letnic, M.; Jessop, T.S. Knee deep in trouble: Rusa deer use an aquatic escape behaviour to delay attack by Komodo dragons. Aust. Mammal. 2020, 42, 103–105. [Google Scholar] [CrossRef]
- Bateman, H.L.; Merritt, D.M. Complex riparian habitats predict reptile and amphibian diversity. Glob. Ecol. Conserv. 2020, 22, e00957. [Google Scholar] [CrossRef]
- Graitson, E.; Barbraud, C.; Bonnet, X. Catastrophic impact of wild boars: Insufficient hunting pressure pushes snakes to the brink. Anim. Conserv. 2019, 22, 165–176. [Google Scholar] [CrossRef]
- Platt, S.; Smith, J.; Rainwater, T.; Boeing, W. Notes on the predation of rough-footed mud turtles (Kinosternon hirtipes) in west Texas, USA. West. N. Am. Nat. 2019, 79, 130–134. [Google Scholar] [CrossRef]
- Risch, D.R.; Ringma, J.; Price, M.R. The global impact of wild pigs (Sus scrofa) on terrestrial biodiversity. Sci. Rep. 2021, 11, 13256. [Google Scholar] [CrossRef]
- Larson, A.J.; Paine, R.T. Ungulate herbivory: Indirect effects cascade into the treetops. Proc. Nat. Acad. Sci. USA 2007, 104, 5–6. [Google Scholar] [CrossRef]
- Kay, G.M.; Mortelliti, A.; Tulloch, A.; Barton, P.; Florance, D.; Cunningham, S.A.; Lindenmayer, D.B. Effects of past and present livestock grazing on herpetofauna in a landscape-scale experiment. Conserv. Biol. 2017, 31, 446–458. [Google Scholar] [CrossRef]
- Schieltz, J.M.; Rubenstein, D.I. Evidence based review: Positive versus negative effects of livestock grazing on wildlife. What do we really know? Environ. Res. Lett. 2016, 11, 113003. [Google Scholar] [CrossRef]
- Carpio, A.J.; Castro–López, J.; Guerrero–Casado, J.; Ruiz–Aizpurua, L.; Vicente, J.; Tortosa, F.S. Effect of wild ungulate density on invertebrates in a Mediterranean ecosystem. Anim. Biodivers. Conserv. 2014, 37, 115–125. [Google Scholar] [CrossRef]
- Reider, K.E.; Carson, W.P.; Donnelly, M.A. Effects of collared peccary (Pecari tajacu) exclusion on leaf litter amphibians and reptiles in a Neotropical wet forest, Costa Rica. Biol. Conserv. 2013, 163, 90–98. [Google Scholar] [CrossRef]
- Zakkak, S.; Halley, J.M.; Akriotis, T.; Kati, V. Lizards along an agricultural land abandonment gradient in Pindos Mountains, Greece. Amph. Reptil. 2015, 36, 253–264. [Google Scholar] [CrossRef]
- Cabon, V.; Bùi, M.; Kühne, H.; Seitz, B.; Kowarik, I.; von der Lippe, M.; Buchholz, S. Endangered animals and plants are positively or neutrally related to wild boar (Sus scrofa) soil disturbance in urban grasslands. Sci. Rep. 2022, 12, 16649. [Google Scholar] [CrossRef]


| Wild ungulates | Livestock | χ2 (df) | p | |||
|---|---|---|---|---|---|---|
| N | % | N | % | |||
| Effect | 0.06 (1) | 0.811 | ||||
| Positive | 10 | 13.4 | 7 | 9.3 | ||
| Negative | 30 | 40 | 28 | 37.3 | ||
| Nature of impact *** | 11.17 (1) | 0.000 | ||||
| Direct | 23 | 30.7 | 6 | 8 | ||
| Indirect | 17 | 22.6 | 29 | 38.7 | ||
| Impact type | ||||||
| Ungulate prey | 5 | 6.7 | 0 | 0 | 2.89 (1) | 0.089 |
| Grazing/browsing *** | 8 | 10.7 | 30 | 40 | 29.67 (1) | 0.000 |
| Predation *** | 16 | 21.3 | 1 | 1.3 | 12.65 (1) | 0.000 |
| Overabundance | 4 | 5.4 | 1 | 1.3 | 0.59 (1) | 0.439 |
| Rooting/trampling | 6 | 8 | 3 | 4 | 0.25 (1) | 0.618 |
| Direct | Indirect | |||||
| Effect | 3.03 (1) | 0.08 | ||||
| Positive | 3 | 4 | 14 | 18.7 | ||
| Negative | 26 | 34.7 | 32 | 42.6 | ||
| Comparison | Odds Ratio | 95% Confidence Interval | |
|---|---|---|---|
| Effect: Positive | Wild ungulates | 1.33 | 0.4–3.9 |
| Impact nature: Direct | Wild ungulates | 6.53 | 2.2–19.3 |
| Impact type: Ungulate prey | Wild ungulates | 11 | 0.6–206.6 |
| Impact type: Grazing/browsing | Livestock | 24 | 7.1–81.6 |
| Impact type: Predation | Wild ungulates | 22.67 | 2.8–182.7 |
| Impact type: Overabundance | Wild ungulates | 3.78 | 0.4–35.5 |
| Impact type: Rooting/trampling | Wild ungulates | 1.88 | 0.4–8.2 |
| Impact nature: Direct | Effect: Positive | 0.26 | 2.2–19.3 |
| Ruminants | Omnivores | Hindgut fermenters | χ2 (df) | p | ||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |||
| Domestication status *** | 40.03 (2) | 0.000 | ||||||
| Livestock | 35 | 46 | 0 | 0 | 0 | 0 | ||
| Wild | 12 | 16 | 22 | 29 | 7 | 9 | ||
| Nature of impact *** | 25.14 (2) | 0.000 | ||||||
| Direct | 10 | 13 | 18 | 24 | 1 | 1 | ||
| Indirect | 37 | 49 | 4 | 5 | 6 | 8 | ||
| Effect | 4.69 (2) | 0.09 | ||||||
| Positive | 15 | 20 | 2 | 3 | 1 | 1 | ||
| Negative | 32 | 42 | 20 | 26 | 6 | 8 | ||
| Impact type *** | 53.93 (10) | 0.000 | ||||||
| Ungulate prey | 4 | 5 | 1 | 1 | 0 | 0 | ||
| Grazing/browsing | 34 | 45 | 0 | 0 | 4 | 5 | ||
| Predation | 1 | 1 | 16 | 21 | 1 | 1 | ||
| Overabundance | 4 | 5 | 1 | 1 | 0 | 0 | ||
| Rooting/trampling | 4 | 5 | 3 | 4 | 2 | 3 | ||
| Comparison | Odds Ratio | 95% Confidence Interval | |
|---|---|---|---|
| Foraging group: Ruminant | Livestock | 161.88 | 9.2–2855.3 |
| Foraging group: Omnivore | Impact nature: Direct | 17.6 | 4.9–62.6 |
| Foraging group: Ruminant | Impact nature: Indirect | 7 | 2.5–19.8 |
| Foraging group: Omnivore | Effect: Negative | 4.21 | 0.9–20.2 |
| Foraging group: Ruminant | Effect: Negative | 0.25 | 0.06–0.9 |
| Foraging group: Omnivore | Impact type: Predation | 141.33 | 15.1–1263 |
| Foraging group: Ruminant | Impact type: Grazing/browsing | 16.35 | 4.8–56.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teffo, T.R.; Fehér, Á.; Katona, K. Ungulates and Their Impact on Reptiles: A Review of Interspecific Relationships. Diversity 2023, 15, 28. https://doi.org/10.3390/d15010028
Teffo TR, Fehér Á, Katona K. Ungulates and Their Impact on Reptiles: A Review of Interspecific Relationships. Diversity. 2023; 15(1):28. https://doi.org/10.3390/d15010028
Chicago/Turabian StyleTeffo, Thabang Rainett, Ádám Fehér, and Krisztián Katona. 2023. "Ungulates and Their Impact on Reptiles: A Review of Interspecific Relationships" Diversity 15, no. 1: 28. https://doi.org/10.3390/d15010028
APA StyleTeffo, T. R., Fehér, Á., & Katona, K. (2023). Ungulates and Their Impact on Reptiles: A Review of Interspecific Relationships. Diversity, 15(1), 28. https://doi.org/10.3390/d15010028










