Abstract
Cetaceans can be considered good natural samplers of biodiversity due to the different hunting strategies they adopt. In this study, the stomach contents of 28 Tursiops truncatus (15 females and 13 males), stranded along Tuscany coasts, NW Mediterranean, between 2008 and 2021, were analyzed. The prey items were identified at the lowest taxonomic level possible, and assessed in terms of abundance, weight, and frequency of occurrence. The index of relative importance (IRI) was also computed. Overall, 2201 bony fishes and 406 cephalopods were identified. The trophic spectrum resulted in high diversity (69 taxa) and the prey species, 53 fishes and 16 cephalopods, live at different levels of the water column. Predation was mainly based on European hake, Merluccius merluccius (%IRI 26.9), and conger eel, Conger conger (%IRI 25.1). The abundant presence of nocturnal species, such as Conger and Ophidion, indicates the nocturnal hunting activity of the bottlenose dolphin. Furthermore, evidence is presented of the dolphins’ ability to capture fish at night, taking advantage of the sound produced by these fish to locate them. Diet did not show any statistical differences among sexes, except that females preyed upon a significantly higher quantity of octopods than males.
1. Introduction
The bottlenose dolphin, Tursiops truncatus (Montagu, 1821), is a cosmopolitan species occurring in inshore and offshore waters of tropical and temperate seas, showing a high level of ecological plasticity, adapted to a variety of marine and estuarine habitats, including rivers [1,2]. The bottlenose dolphin is a regular species seen in the Mediterranean Sea and is classified as least concern, according to IUCN Red List of Threatened Species in the basin [3]. The bottlenose dolphin is widely distributed along all coasts of the Mediterranean Sea and its presence is usually limited to shallow waters of the continental shelf [4,5,6], although it may also be found in deeper waters [7]. Studies conducted in Pelagos Sanctuary suggest that this dolphin prefers shallow waters (less than 100 m deep) and displays a residential habit [8]. The bottlenose dolphin is considered a Species of Community Interest, included in Annex II of the Habitat Directive (Council Directive 92/43/EEC). Furthermore, the largest Site of Community Importance (SIC) in the Mediterranean dedicated to bottlenose dolphins was recently established in Tuscany waters, Ligurian Sea, with Regional Council Resolution no. 2, dated 14 January 2020.
The diet of bottlenose dolphins has been described from many areas of the world, including North [9,10,11,12] and South America [13,14,15], Australia [16,17], and South Africa [18]. The diet of T. truncatus consists of a variety of prey items, including fish, cephalopods, and occasionally crustaceans [7]. Many dietary studies conducted in European waters from the northeast Atlantic [19,20,21,22,23,24,25,26], Black Sea [27], and Mediterranean Sea [28,29,30,31,32,33,34,35,36] indicate that bottlenose dolphins feed on a great variety of demersal and pelagic species. Nevertheless, the dominance of Gadiformes fish in the diet of T. truncatus from European waters was reported in the northeast Atlantic [19,22,23], while European hake (Merlucciidae) was a significant portion of the diet of this dolphin in the western and northwestern Mediterranean Sea [28,29,30,31,32,33], but not for its eastern coasts [34,35,36]. The adaptability of T. truncatus allows it to exploit different food resources depending on local availability as well as on sexual and ontogenetic aspects of the predator [2,32]. Due to its coastal habit, the bottlenose dolphin often interacts with fisheries’ activities to facilitate prey capture in a variety of ways [2], e.g., following bottom trawlers (e.g., [37]), hunting on the set nets of small scale fisheries (e.g., [38,39]), and feeding around fish farm structures (e.g., [40,41]).
The present work aims to investigate the diet of bottlenose dolphins in the waters of Tuscany (northwestern Mediterranean Sea) by means of analysis of stomach contents of stranded animals. Our study includes the analyses by Scuderi et al. [33], Neri [42], and Pedà et al. [43], which have been verified and then coupled with new data collected from 2015 to 2021. An indirect estimate of the size and weight of the prey items, computed by means of regression parameters available or created ex novo, was carried out to better characterize the food spectrum of bottlenose dolphins. The diet of T. truncatus was also investigated according to the sex and size of each dolphin.
2. Materials and Methods
The study was carried out in the Ligurian Sea, northwestern Mediterranean, in the area off the coast of Tuscany. The coastline spreads for ca. 600 km (44°02′ N, 10°01′ E to 42°22′ N, 11°26′ E), and the open sea includes the islands of the Tuscan Archipelago. The northern part of this area is characterized by sandy bottoms, where the mouths of the three major rivers of Tuscany (Arno, Serchio, and Magra) are present; the central-southern coasts alternate between rocky and sandy bottoms. The coastline, highly urbanized, industrialized, and affected by intense maritime traffic and tourism, is included in the Pelagos Sanctuary, the largest area established in Mediterranean Sea to preserve cetaceans and the marine environment in the northwest Mediterranean [44].
The Sea Turtle and Cetacean Stranding Network is a program in force since 1980 in Tuscan waters. Monitoring operations have been implemented since 2007, thanks to the current Tuscany Observatory for Biodiversity (OTB), Regional Law 30/2015 art. 11, a regional network of strandings, sightings, and recoveries of cetaceans, sea turtles, and elasmobranchs. The Tuscany Region has created a coordinated and synergic system among its technical Agency ARPAT (Environmental Protection Agency, Tuscany Region), universities, research centers, museums, aquaria, environmental associations, and fishers.
A total of 53 stomachs of T. truncatus were collected by the personnel of ARPAT (Livorno, Italy) from specimens stranded along the Tuscany coasts from 2005 and 2021 (Figure 1).
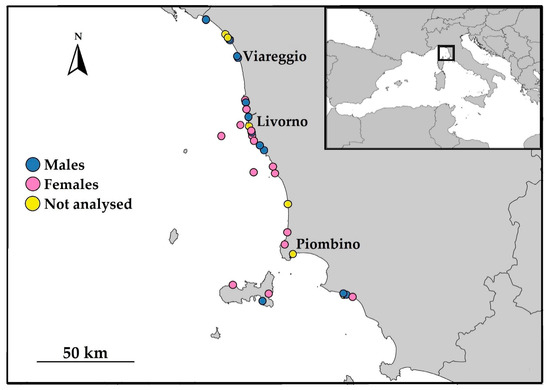
Figure 1.
Map of the Tuscany coast showing the stranding locations of T. truncatus analyzed (males and females) for dietary studies as well as those specimens not analyzed, such as calves and individuals with empty stomachs.
For this study, large dolphins from 2.5 m length upwards were considered sexually mature adults (A), according to Mead and Potter [9], while the others were evaluated as juveniles (J). During necropsies, small bottlenose dolphins without teeth or with remains of milk in their stomachs were considered neonates or calves.
After necropsies, stomachs were frozen at −20 °C. In laboratory, they were successively defrosted and opened; the contents, after being washed in a sieve with 0.3 mm mesh, were preserved in a solution of 75% ethanol. The taxonomic identification of the stomach contents was carried out under a stereoscope. The prey items were identified to the lowest taxonomic level possible. In most cases, the remains of prey were found highly digested: e.g., otoliths of bony fishes and beaks of cephalopod mollusks were often the only items found testifying to predation. Therefore, the prey identification was performed based on the morphological features of otoliths and beaks, following Clarke [45] and Tuset et al. [46]. Reference collections of otoliths and beaks were also used for species identification.
The contribution of each prey item to the trophic spectrum of T. truncatus was assessed as follows: the percentage in number (%N), weight (%W), and the frequency of occurrence (%F), e.g., the percentage of stomachs with at least one item of the given prey, were computed. These indices, combined with each other, were used to compute the Index of Relative Importance [47,48]:
thus providing a more global picture of dietary importance; the IRI was expressed in absolute terms and in percentage.
IRI = %F × (%N + %W),
Dietary diversity in prey number was investigated using the Shannon–Wiener index (H’) [49]:
in which pi is the proportion of numerical abundance corresponding to the i-th species attributes and s is the number of species, thus attributing a “weight” to each prey item for presence and abundance.
The trophic spectrum and the diversity in prey numbers were also investigated between sexes and size classes of bottlenose dolphins.
Furthermore, the unpaired two-samples Wilcoxon test, a non-parametric test, was used to analyze the contribution in weight, number, and size of the most important prey items compared between sexes and dolphin size. Data analyses were carried out using the package R version 4.1.3 [50].
As stated above, most prey were found highly digested. The size and weight of these was estimated based on the parameters of the relationships between otolith or beak sizes vs. total length or total weight for fish and cephalopods, respectively.
The available allometric relationships were used for several species of bony fishes to estimate both total length [51,52,53] and total weight [45,54,55,56,57,58,59,60,61]. Table S1 (Supplementary Materials) shows the regression coefficients used to estimate the sizes of bony fishes, while Table S2 (Supplementary Materials) illustrates the equations utilized to estimate total weight of cephalopods; whenever possible, the available equations based on data collected in the Mediterranean Sea were applied.
For the other prey species, lengths and weights were estimated ex novo using specimens sampled off Tuscany coasts during the biological sampling of DCF (Data Collection Framework, Reg. UE 2017/1004) in 2020 and 2021.
Regarding bony fishes, the collected specimens were weighed (to 0.1 g) and measured for total length (TL, to 0.5 cm); the left otoliths (sagittae) were measured as major axis length (OL) (Figure 2a) to the nearest 0.1 mm using a dissection microscope provided with a micrometer eyepiece. Cephalopods were measured for dorsal mantle length (DML, to 0.5 cm) and weighed (to 0.1 g). The beaks were removed and measured: lower rostral length (LRL) of the lower beak for Myopsida and Oegopsida cephalopods (Figure 2b), and lower hood length (LHL) of the lower beak for Octopoda (Figure 2c) were measured according to Clarke [45]. These measurements were taken using a digital caliper mod. CDC STORM with a resolution of 0.01 mm.

Figure 2.
Images of otoliths and beaks, with relative measures: (a) major axis length (OL) of M. merluccius left otolith; (b) lower rostral length (LRL) of I. coindetii lower beak; (c) lower hood length (LHL) of O. vulgaris lower beak.
The relationships among body length or weight vs. otolith or beak size were computed using linear or power equations to estimate the a and b parameters [62]. In this way, it was possible to estimate size and weight for all the species identified as prey in the stomachs of T. truncatus, except for the fish Ariosoma balearicum (Delaroche, 1809), for which no bibliographic data exist nor were sufficient specimens in the biological sample available.
3. Results
A total of 684 fishes and 267 cephalopods was collected to estimate the values of a and b of relationships among otolith/beak length and fish/cephalopod length and weight. The characteristics of the samples collected are shown in Table S3 (Supplementary Materials).
The dolphins comprised 24 males and 29 females, ranging from 93 to 315 cm TL. Fourteen specimens (9 females and 5 males) were identified as calves and their stomachs were empty or held traces of milk. The average size of calves was 140.5 cm, while the larger calf measured 172 cm TL. Stomachs of six females and four males, ranging in size from 167 to 300 cm, were found empty.
In total, 28 individuals (15 females and 13 males) of 53 collected dolphins showed remains of food in their stomachs and were retained for further analyses (Table 1). The smallest dolphin with food matter in the stomach measured 170 cm TL.

Table 1.
Data of T. truncatus analyzed for dietary study. TL, total length; N, number of prey items.
Table 1 summarizes the main characteristics of the 28 individuals of T. truncatus analyzed. In only in a few cases, prey species were almost intact.
Table 2 provides the trophic spectrum of T. truncatus, listing the prey species found in the stomachs and their contribution in terms of percentage of frequency of occurrence, in number and in weight. A more detailed trophic spectrum subdivided according to sex and size class is provided in Supplementary Material Table S4. A total of 2607 prey, belonging to 2 major taxa, were identified; 2201 of them belonged to 53 taxa of bony fishes (%N = 84.4; %F = 100.0) and 406 belonged to 16 taxa of cephalopods (%N = 15.6; %F = 82.1).

Table 2.
Trophic spectrum of T. truncatus from the Tuscany area (northwestern Mediterranean Sea). For each prey item: %N, percentage in number; %F, percentage frequency of occurrence; %W, percentage in biomass; %IRI = percentage of the index of relative importance. TL: total length (cm); DML: dorsal mantle length (cm); TW: total weight (g); n.a.: not available; H: habit; B: benthic; D: demersal; P: pelagic. (* ≤ 0.01).
More than half the prey belongs to species of demersal habit (%N = 53.9), and 37.7% possess benthic habits; only 8.4% of prey are pelagic species.
Concerning the %IRI, Osteichthyes were, by far, the most important prey category (%IRI = 80.4), followed by cephalopods (%IRI = 19.6). Despite the large number of prey items found in the stomachs, the diet was based on a restricted number of species. The most important fishes were European hake, M. merluccius (%IRI = 26.9), and conger eel, C. conger (25.1%); indeed, these two species accounted for approximately 34% of the total number of prey items (Figure 3) and 30% of the total weight (Figure 4). Furthermore, the conger eel was the species most frequently found in the stomachs of T. truncatus (%F = 92.9). For bony fishes, the annular sea bream, D. annularis (%IRI = 5.9), the snake blenny, O. barbatum (%IRI = 5.4), and the common Pandora, P. erythrinus (%IRI = 5.1), were important additional prey species. Sparidae (%IRI = 18.3) was found to be the third most important family in the diet of T. truncatus, while Merluccidae (%IRI = 21.8) and Congridae (%IRI = 20.7) were the most important. Despite the minor importance of mullets, Mugilidae (%IRI = 4.9), this prey is abundant in weight: indeed, the biomass of thinlip grey mullet, C. ramada, and unidentified Mugilidae constituted approximately 12% of the total weight (Figure 4).
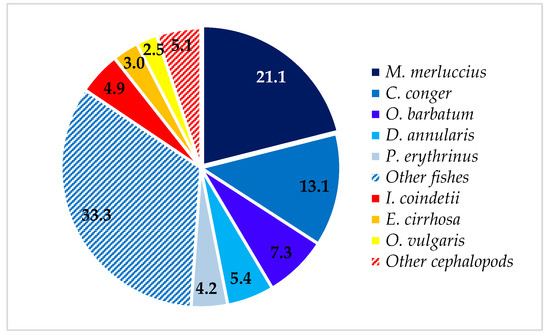
Figure 3.
Numerical percentage of the main prey species found in T. truncatus stomachs.
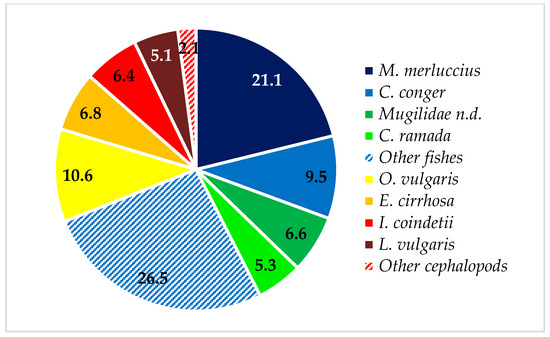
Figure 4.
Weight percentage of main prey species found in T. truncatus stomachs.
Concerning the size of bony fish prey, sizes ranged from 2 cm for species such as Lesueurigobius sp. to 60 cm for some specimens of conger eel. The abundance of fishes per size class is shown in Figure 5. The graph shows that more than 50% of specimens preyed upon by bottlenose dolphins measured 10.0–19.9 cm and 67.3% of these fishes have a demersal habit.
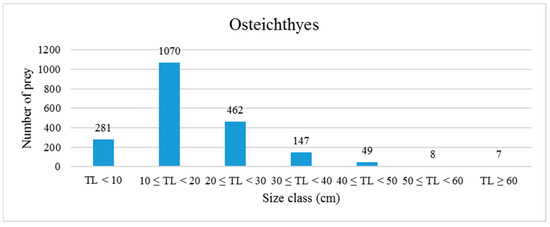
Figure 5.
Frequency distribution of prey (fishes) according to size classes. TL: Total length.
Cephalopods are mostly represented by the shortfin broad tail squid, I. coindetii (%IRI = 7.2), and by the common octopus, O. vulgaris (%IRI = 4.9). Illex coindetii was the most abundant (Figure 5) and frequent among cephalopods; as a matter of fact, ca. half T. truncatus fed upon I. coindetii (Table 2). Nevertheless, the common octopus represented 10.6% of the total weight (Figure 4) and Octopodidae (%IRI = 11.4%) were found to be the most important cephalopod family in the diet of analyzed bottlenose dolphins.
Regarding the biomass of prey, Figure 6 shows that 93.8% of the prey items weighed less than 200 g; the estimated weight of individual prey ranged from 0.08 g for a specimen of Lesueurigobius sp. to 1535.40 g for a specimen of European hake.
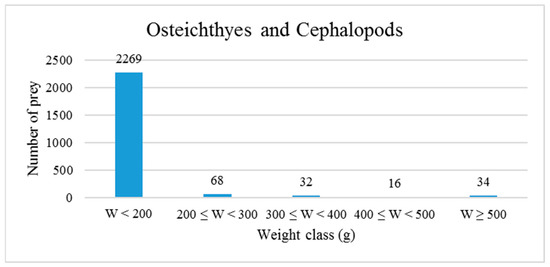
Figure 6.
Frequency distribution of prey (fishes and cephalopods) according to weight classes. W: weight.
Regarding the comparison of the food spectrum between males and females (Supplementary Material Table S4), bony fishes were important in the diets of both sexes, although they were found in different proportions. Fish were very important (%IRI = 93.4) in the diet of males and constituted 88.4% in weight of the ingested prey, while in females they were less important (%IRI = 70.6; %W = 58.3). More than half of the prey belonging to species with demersal habits were identified in both male (%N = 54.7) and female diets (%N = 53.4). Prey with benthic habits were more abundant in females (%N = 43.0) than in males (%N = 30.4), while only 3.7% prey of females and 14.9% prey items of males were pelagic species.
The European hake and conger eel were the most important species in both males (%IRI M. merluccius = 38.1; %IRI C. conger = 23.9) and females (%IRI M. merluccius = 18.35; %IRI C. conger = 23.7). Other significant fish families in the diet of males were Sparidae (%IRI = 11.4) and Mugilidae (%IRI = 8.6). Sparids were also very important in the diet of females (%IRI = 22.5) and in the number of predated species. As a matter of fact, there were nine species of sparids found in females and only four in males. Other fishes such as Carangidae and the snake blenny were important additional prey items for males and females, respectively (Table S4).
Cephalopods formed a very important portion of the diet of females (%IRI = 29.4), constituting 41.7% in weight of the ingested prey items. The common octopus was the more important species (%IRI = 8.3), while Octopodidae was the more important family (%IRI = 16.0) in females. On the other hand, octopods were less important in the diet of males (Supplementary Materials Table S4), while the shortfin broad tail squid contributed to around 6% for both sexes.
Concerning feeding comparisons by size class (Supplementary Material Table S4), bony fishes were very important in both groups, especially for small bottlenose dolphins (%IRI = 90.8). Cephalopods were prevalent in the diet of adults (%IRI = 22.9), especially octopods (%IRI = 19.7), while only one young male contained the remains of horned octopus (E. cirrhosa). The shortfin broad tail squid was the only cephalopod species relevant in the trophic spectrum of juveniles and had similar importance to that of adults (%IRI = (A) 6.7; (J) 7.2). European hake (%IRI = (A) 23.6; (J) 30.8) and conger eel (%IRI = (A) 22.7; (J) 25.5) were the most important prey species in both groups of dolphins. Sparids were the most important prey for small dolphins (%IRI = 33.2) and the common pandora (%IRI = 9.4) was the most important species of this family.
The diversity index showed a broad diet for the bottlenose dolphins analyzed (H’ = 2.4 ± 0.8), without any particular differences by sex or size classes (Supplementary Material Table S5).
The Wilcoxon test, applied to compare the values in number, weight, and size of the most important prey in the diet of T. truncatus, did not show any significant difference (p-value > 0.05) between males/females, adults/juveniles, and adult males/adult females (Supplementary Material Table S6). Only the contribution of Octopodidae was significantly different: higher in the diet of females than males, both in number (Figure 7a) and in weight (Figure 7b).
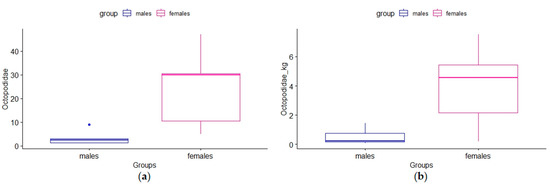
Figure 7.
Boxplots of the contribution of Octopodidae in the diet of males and females of bottlenose dolphins: (a) numerical and (b) weight contribution.
Moreover, it is worth mentioning that during the stomach contents analysis some pieces of set nets (e.g., trammel nets, gill nets) were found in two stomachs, and both animals were found stranded, one dolphin with the head wrapped in a piece of net, while the other with obvious signs on the body of entrapment in a net.
4. Discussion
Stomach content analysis, a classic methodology applied in fishery ecology, is still the most widely approach used to provide detailed information on trophic spectra and to estimate trophic indices. Although expensive, in terms of time and expertise needed, stomach content analysis can be a suitable method to implement wide spatiotemporal monitoring. As a matter of fact, stomach content analysis is a routine method applied to monitor fish species’ trophic relationships in ICES contexts for decades, and recently it has been included in the National Work Plans for fishery data collection (under the EU Data Collection Framework, see Regulation (EU) 2017/1004) in the Mediterranean and Black Seas [63].
Similarly, stomach content analysis remains the most widely used technique for evaluating cetacean diet [64,65] and it can be useful to facilitate the interpretation of stable isotope data [66].
Our study was focused on the analysis of stomach contents of bottlenose dolphins stranded along the Tuscan coasts. One of the limitations of studying the diet from stomach contents in animals found dead (either by natural causes or by an interaction with anthropogenic activities) is the time elapsed from the moment of death to that of discovery. In fact, during this period, the digestion of the stomach content continues after death [67]. As in this study, most of the stomach contents are frequently found highly digested and, in many cases only otoliths, cephalopods beaks and bones (such as opercles, jaws with teeth and rocker bones) are observed. For this reason, the outcomes of the analysis of the stomach contents can provide information mainly for the last meals of the predator, except for the beaks of cephalopods, for which resistance to digestion means they can remain in the stomach for a longer period than flesh, bones, and otoliths [64,68]. Moreover, the otoliths of fish in the stomach contents are digested, leading to a possible underestimation of the predated biomass. Another limitation could be finding calves with empty stomachs. In fact, although lactation is the main source of nutrition in the first year of life of the calf, solid food has been found with milk in the stomachs of calves only a few months old. Maternal investment for calves can extend for ca. 3–6 years [2]. Consequently, the size of weaning can also vary. In our study, the smallest dolphin with food remains in its stomach was 170 cm TL, while the largest calf with milk in the stomach measured 172 cm TL. This agrees with research from other diet studies conducted in European waters [20,22,34], while stable isotope analysis indicated that five calves from 130 to 179 cm were suckling animals [26]. This suggests that weaning is a gradual process, also possibly depending on the geographic region, during which the calf continues to nurse for years.
Food and feeding analysis of bottlenose dolphins stranded in the Tuscan Archipelago, presented in this study, highlights a diversified trophic spectrum. Despite the rather low number of stomachs analyzed (28), 69 different taxa have been identified, belonging to bony fishes and to cephalopods. This wide diversity is in agreement with findings of other studies performed along European waters [21,22,24,25,27,28,29,31,32].
The predominant role of fishes in the diet of T. truncatus is in line with what was reported in other areas of the Mediterranean and Black Seas [27,28,29,30,31,32,33,34,35,36], as well as of the eastern Atlantic [19,20,21,22,23,24,25,26]. In this study, the bulk of the predation is based on a few species, such as the European hake and the conger eel. The importance of European hake in the diet of T. truncatus in north Atlantic coasts [21,22,23,25], in the Adriatic Sea [31], and in the western Mediterranean Sea [28,29,30,32,33] has been previously reported by several authors. Similarly, Santos et al. [19,22] and Arronte et al. [23] reported the dominance of the Gadiformes and Gadidae, especially in the stomachs of bottlenose dolphins in the northeast Atlantic. Regarding the dimension of hakes, both Blanco et al. [32] and Santos et al. [22] found that adult bottlenose dolphins had eaten larger hake than had the juveniles. On the contrary, no size preferences in relation to sex or size class of dolphins were highlighted in our study.
The occurrence of conger eels in the diet of T. truncatus in Mediterranean Sea was previously reported by Salomon et al. [30] and Blanco et al. [32] from Spanish Mediterranean coasts, by Orsi Relini et al. [29] and Scuderi et al. [33] as regards the Italian coasts of Pelagos Sanctuary (Ligurian Sea), by Miokovic et al. [31] for the Croatian coast, and by Milani et al. [35] from the North Aegean Sea. On the other hand, the presence of conger eels is rare for bottlenose dolphins from the eastern Atlantic [22,24], while the Balearic conger, A. balearicum, is the most important prey in the Levantine basin [34]. In the Gulf of Cadiz, C. conger was reported as the most important ingested prey by Giménez et al. [25] according to stomach content analysis. Nevertheless, stable isotope analyses showed that the assimilated diet consisted mainly of different Sparidae species and a mixture of other species such as European hakes, mackerels, conger eels and other species [25]. Overall, Sparidae could be considered as prey of secondary relevance in our study, despite the high number of species found, while it was more significant for T. truncatus in the eastern Mediterranean Sea [35,36]. However, in our study, sparids are an important component in the diet of small bottlenose dolphins, like the results of Scheinin et al. [34] in the Levantine Basin.
Octopodidae were found to be the most important cephalopod prey. The occurrence of octopods in the diet of bottlenose dolphins has also been reported in other areas [25,28,30,32,43]. Our results highlighted a clear preference for octopods in the diet of large females (TL ≥ 250 cm), similarly reported by Blanco et al. [32] for Spanish Mediterranean waters. According to Wells and Scott [2], this interesting aspect could be due to differences in behavior among the two sexes. During nursing, females may be less mobile and frequent different areas than do males; therefore, their diet could be concentrated on more sedentary species, such as the common octopus.
The snake blenny, O. barbatum, was the third most abundant fish prey species (%N = 7.3) in the bottlenose dolphins’ diet. The presence of Ophidiidae in the diet of T. truncatus in European waters was only found in some studies on the Mediterranean Sea [30,32,35], which reported an amount similar to ours study, while only one specimen of O. barbatum was found by Giménez et al. [25] and Sheinin et al. [34]. The snake blenny has been reported as a nocturnal predator that lives buried in the sandy and muddy bottoms at depths from a few to 150 m [69,70,71]. Ophidion barbatum is a species in which adult males can produce sounds with a specific sonic apparatus. One of the elements that constitutes the sonic apparatus of this species is the rocker bone [72,73], a hard kidney-shaped structure [69,73,74]. These aspects indicate that bottlenose dolphins can hunt at night, taking advantage of the sound produced by O. barbatum to locate them. Gannon et al. [75] reported that bottlenose dolphins changed their direction of travel, turning towards the source of sound production, using passive listening during the foraging process; the important presence of soniferous species in the diet of bottlenose dolphin was previously reported [10,76].
The abundance of European hake in the stomachs of bottlenose dolphins reflects the large quantity of this fish present in the investigated area. As a matter of fact, in the waters of Tuscany, the most important area of concentration of juvenile hake for the Mediterranean Sea was reported [77]. The European hake is one of the most important target species of the demersal fisheries in western Mediterranean. This species suffers from a chronic overexploitation due to increased overfishing with high fishing mortality on the first age classes [78,79]. Therefore, it is important to regularly assess the status of this important stock. Currently, scarce and scattered information is available on the role of M. merluccius as a prey; the findings of this study can contribute useful insight to assess its natural mortality (M).
On the contrary, according to the outcomes from experimental trawl surveys (e.g., the EU international MEDITS trawl survey) and the landing monitoring program performed in Tuscany in the last 30 years [60], the conger eel and the snake blenny, C. conger and O. barbatum, are not as abundant as the stomach contents of T. truncatus suggest. The experimental survey and most commercial fishing are carried out in the investigated area during daytime hours [80], when these species of Conger and Ophidion spend most of their time hidden or burrowed, and are, therefore, less accessible to trawl nets. They are more active during the night as nocturnal predators [70,81], and there is evidence that the two species are more abundant in the landings of the few hauls performed during night hours [82].
The prey identified in the stomachs of bottlenose dolphins in this study belong to species living at different levels of the water column, from typically benthic to strictly pelagic ones, as well as species characterized by different degrees of mobility, from sedentary burrowing (e.g., conger eel, snake blenny) to actively swimming ones (e.g., European hake). Moreover, the presence of less important species which have different habits may be due to secondary predation. The secondary ingestion of prey consists of remains of small prey found in the stomachs of a larger predator [65]. Due to the digestion state of the stomach content analyzed, we cannot exclude the existence of prey from secondary ingestions. Unless they were recovered intact, or slightly digested, in the stomach, the bias is avoidable [65].
The finding of remains of set nets in the stomachs of two dolphins confirms the opportunistic interaction of T. truncatus with fishing activities that can be the cause of injury or death. All these aspects reflect the ability and plasticity of this predator to search, to hunt, and to feed on prey items in very different situations and conditions.
Our study provides new information on the bottlenose dolphin diet in Tuscan waters and increases the overall knowledge on the trophic ecology of this species. Moreover, significant sexual intraspecific differences in the diet of this dolphin have been highlighted. Due to the diversified food spectrum, the stomach content analysis of T. truncatus provides information on the coastal biodiversity of the investigated areas. Cetaceans can be considered good natural samplers, and this aspect could be exploited to integrate data from scientific surveys at sea [68]. Therefore, we suggest the continuation of the collection and analysis of stomachs of this species in order to better investigate possible differences related to size and sex, and to better investigate spatial and temporal aspects. Furthermore, it would be useful to conduct sighting surveys of opportunistic feeding of this species during commercial fishing activities. The bottlenose dolphin is classified as an “ecologically relevant” species for the European Marine Strategy Framework directive (MSFD, 2008/56/EC); therefore, it would be desirable to formally include the trophic ecology of this species in the monitoring protocols of the Marine Strategy.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d15010021/s1, Table S1: Regression coefficients used to estimate total length and total weight of Osteichthyes, available from literature. OL: otolith length. TL: total length. TW: total weight. Table S2: Equations used to estimate total weight of cephalopods, available from literature. LHL: lower hood length; LRL: lower rostral length. Table S3: Relationships between the otolith/beak length and the fish/cephalopod body length and between the beak length and the cephalopod body weight. N, number of specimens; TL: total length (bony fishes); DML: dorsal mantle length (cephalopods); OL: otolith length; LRL: lower rostral length (beaks); LHL: lower hood length (beaks); W: total body weight; PE: power equation; LE: linear equation. Table S4: Trophic spectrum of T. truncatus (males and females; males; females; adults; juveniles) from the Tuscany area (northwestern Mediterranean Sea). For each prey item: N, number of prey; %N, percentage in number; F, frequency of occurrence; %F, percentage frequency of occurrence; W, biomass (g); %W, percentage in biomass; %IRI, percentage of the index of relative importance. TL: total length (cm); DML: dorsal mantle length (cm); TW: total weight (g); n.a.: not available; H: habit; B: benthic; D: demersal; P: pelagic. (* ≤ 0.01). Table S5: Shannon–Wiener diversity index (H’) for T. truncatus related to sex and size class. N: number of bottlenose dolphins. Table S6: Results of Wilcoxon test for numerical, size, and weight contribution of the most important taxa. N: number of bottlenose dolphins; TL: total length; DML: dorsal mantle length; M: males; F: females; A: adults; J: juveniles; AM: adults males; AF: adults females; n.s.: no significant difference.
Author Contributions
Conceptualization, A.N., P.S. and L.M.; methodology, A.N. and P.S.; formal analysis, A.N.; investigation, A.N.; resources, A.N. and C.M.; data curation, A.N. and C.M.; writing—original draft preparation, A.N.; writing—review and editing, A.N., P.S., A.V., C.M. and L.M.; funding acquisition, P.S. and L.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was carried out within the framework of the PhD project of A. Neri, which was co-funded by the University of Siena (UniSi) and the Consorzio per il Centro Interuniversitario di Biologia Marina ed Ecologia Applicata (CIBM) of Livorno.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We wish to thank Alessandro Ligas for helping with statistical analysis using the package R. Thanks to Andrea Massaro for helping with the otoliths collection. Thank you to Claudia Musumeci for drawing the map.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jefferson, T.A.; Leatherwood, S.; Webber, M.A. FAO Species Identification Guide. Marine Mammals of the World; FAO: Rome, Italy, 1993; pp. 154–155. [Google Scholar]
- Wells, R.S.; Scott, M.D. Common Bottlenose Dolphin: Tursiops truncatus. In Encyclopedia of Marine Mammals, 2nd ed.; Perrin, W.F., Wursig, B., Thewissen, J.G.M., Eds.; Academic Press: San Diego, CA, USA, 2009; pp. 249–255. [Google Scholar]
- Natoli, A.; Genov, T.; Kerem, D.; Gonzalvo, J.; Lauriano, G.; Holcer, D.; Labach, H.; Marsili, L.; Mazzariol, S.; Moura, A.E.; et al. Tursiops truncatus (Mediterranean Subpopulation). The IUCN Red List of Threatened Species in 2021. Available online: https://www.iucnredlist.org/species/16369383/215248781 (accessed on 6 October 2022).
- Bearzi, G.; Notarbartolo Di Sciara, G.; Politi, E. Social ecology of bottlenose dolphins in the Kvarneric (northern Adriatic Sea). Mar. Mammal Sci. 1997, 13, 650–668. [Google Scholar] [CrossRef]
- Genov, T.; Kotnjek, P.; Lesjak, J.; Hace, A.; Fortuna, C.M. Bottlenose dolphins (Tursiops truncatus) in Slovenian and adjacent waters (northern Adriatic Sea). Ann. Ser. Hist. Nat. 2008, 18, 227–244. [Google Scholar]
- Marini, C.; Fossa, F.; Paoli, C.; Bellingeri, M.; Gnone, G.; Vassallo, P. Predicting bottlenose dolphin distribution along Liguria coast (northwestern Mediterranean Sea) through different modeling techniques and indirect predictors. J. Environ. Manage. 2015, 150, 9–20. [Google Scholar] [CrossRef]
- ACCOBAMS. Conserving Whales, Dolphins and Porpoises in the Mediterranean Sea, Black Sea and Adjacent Areas: An ACCOBAMS Status Report; Notarbartolo di Sciara, G., Tonay, A.M., Eds.; ACCOBAMS: Monaco, 2021. [Google Scholar]
- Gnone, G.; Bellingeri, M.; Dhermain, F.; Dupraz, F.; Nuti, S.; Bedocchi, D.; Moulins, A.; Rosso, M.; Alessi, J.; Mccrea, R.S.; et al. Distribution, abundance, and movements of the bottlenose dolphin (Tursiops truncatus) in the Pelagos Sanctuary MPA (north-west Mediterranean Sea). Aquat. Conserv. Mar. Freshw. Ecosyst. 2011, 21, 372–388. [Google Scholar] [CrossRef]
- Mead, J.G.; Potter, C.W. Natural History of Bottlenose Dolphins Along the Central Atlantic Coast of the United States. In The Bottlenose Dolphin; Leatherwood, S., Reeves, R.R., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 165–195. [Google Scholar] [CrossRef]
- Barros, N.B.; Wells, R.S. Prey and feeding patterns of resident bottlenose dolphins (Tursiops truncatus) in Sarasota Bay, Florida. J. Mammal 1998, 79, 1045–1059. [Google Scholar] [CrossRef]
- McCabe, E.J.B.; Gannon, D.P.; Barros, N.B.; Wells, R.S. Prey selection by resident common bottlenose dolphins (Tursiops truncatus) in Sarasota Bay, Florida. Mar. Biol. 2010, 157, 931–942. [Google Scholar] [CrossRef]
- Bowen, S.R. Diet of Bottlenose Dolphins Tursiops truncatus in the Northwest Florida Panhandle and Foraging. Master’s Thesis, Savannah State University, Savannah, GA, USA, May 2011. [Google Scholar]
- Van Waerebeek, K.; Reyes, J.C.; Read, A.J.; McKinnon, J.S. Preliminary Observations of Bottlenose Dolphins from the Pacific Coast of South America. In The Bottlenose Dolphin; Leatherwood, S., Reeves, R.R., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 143–154. [Google Scholar] [CrossRef]
- García-Godos, I.; Van Waerebeek, K.; Reyes, J.C.; Alfaro-Shigueto, J.; Arias-Schreiber, M. Prey occurrence in the stomach contents of four small cetacean species in Peru. LAJAM 2007, 6, 171–183. [Google Scholar] [CrossRef]
- Milmann, L.; Danilewicz, D.; Machado, R.; Santos, R.A.D.; Ott, P.H. Feeding ecology of the common bottlenose dolphin, Tursiops truncatus, in southern Brazil: Analyzing its prey and the potential overlap with fisheries. Braz. J. Oceanogr. 2016, 64, 415–422. [Google Scholar] [CrossRef]
- Corkeron, P.J.; Bryden, M.M.; Hedstrom, K.E. Feeding by Bottlenose Dolphins in Association with Trawling Operations in Moreton Bay, Australia. In The Bottlenose Dolphin; Leatherwood, S., Reeves, R.R., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 329–336. [Google Scholar] [CrossRef]
- Gibbs, S.E.; Harcourt, R.G.; Kemper, C.M. Niche differentiation of bottlenose dolphin species in South Australia revealed by stable isotopes and stomach contents. Wildl. Res. 2011, 38, 261–270. [Google Scholar] [CrossRef]
- Cockcroft, V.G.; Ross, G.J.B. Food and Feeding of the Indian Ocean Bottlenose Dolphin off Southern Natal, South Africa. In The Bottlenose Dolphin; Leatherwood, S., Reeves, R.R., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 295–308. [Google Scholar] [CrossRef]
- Santos, M.B.; Pierce, G.J.; Reid, R.J.; Patterson, I.A.P.; Ross, H.M.; Mente, E. Stomach contents of bottlenose dolphins (Tursiops truncatus) in Scottish waters. J. Mar. Biol. Assoc. UK 2001, 81, 873–878. [Google Scholar] [CrossRef]
- De Pierrepont, J.F.; Dubois, B.; Desormonts, S.; Santos, M.B.; Robin, J.P. Stomach contents of English Channel cetaceans stranded on the coast of Normandy. J. Mar. Biol. Assoc. UK 2005, 85, 1539–1546. [Google Scholar] [CrossRef]
- Spitz, J.; Rousseau, Y.; Ridoux, V. Diet overlap between harbour porpoise and bottlenose dolphin: An argument in favour of interference competition for food? Estuar. Coast. Shelf Sci. 2006, 70, 259–270. [Google Scholar] [CrossRef]
- Santos, M.B.; Fernández, R.; López, A.; Martínez, J.A.; Pierce, G.J. Variability in the diet of bottlenose dolphin, Tursiops truncatus, in Galician waters, north-western Spain, 1990–2005. J. Mar. Biol. Assoc. UK 2007, 87, 231–241. [Google Scholar] [CrossRef]
- Arronte, J.C.; Valdés, P.; Pérez, C. Diet of the Bottlenose dolphin (Tursiops truncatus) in the central Cantabrian Sea. In Proceedings of the 23rd Annual Conference of the European Cetacean Society, Istanbul, Turkey, 2–4 March 2009. [Google Scholar]
- Hernandez-Milian, G.; Berrow, S.; Santos, M.B.; Reid, D.; Rogan, E. Insights into the trophic ecology of bottlenose dolphins (Tursiops truncatus) in Irish waters. Aquat. Mammal 2015, 41, 226–239. [Google Scholar] [CrossRef]
- Giménez, J.; Marçalo, A.; Ramírez, F.; Verborgh, P.; Gauffier, P.; Esteban, R.; Nicolau, L.; González-Ortegón, E.; Baldó, F.; Vilas, C.; et al. Diet of bottlenose dolphins (Tursiops truncatus) from the Gulf of Cadiz: Insights from stomach content and stable isotope analyses. PLoS ONE 2017, 12, e0184673. [Google Scholar] [CrossRef]
- Fernández, R.; García-Tiscar, S.; Santos, M.B.; López, A.; Martínez-Cedeira, J.A.; Newton, J.; Pierce, G.J. Stable isotope analysis in two sympatric populations of bottlenose dolphins Tursiops truncatus: Evidence of resource partitioning? Mar. Biol. 2011, 158, 1043–1055. [Google Scholar] [CrossRef]
- Gladilina, E.V.; Gol’din, P.E. New prey fishes in diet of black sea bottlenose dolphins, Tursiops truncatus (mammalia, cetacea). Vestn. Zool. 2014, 48, 83–92. [Google Scholar] [CrossRef]
- Voliani, A.; Volpi, C. Stomach content analysis of a stranded individual of Tursiops truncatus. Rapp. Comm. Int. Mer Médit. 1990, 32, 238. [Google Scholar]
- Orsi Relini, L.; Capello, M.; Poggi, R. The stomach content of some bottlenose dolphins (Tursiops truncatus) from the Ligurian Sea. In Proceedings of the 8th Annual Conference of the European Cetacean Society, Montpellier, France, 2–5 March 1994. [Google Scholar]
- Salomon, O.; Blanco, C.; Raga, J.A. Diet of the bottlenose dolphin (Tursiops truncatus) in the Gulf of Valencia (Western Mediterranean). In Proceedings of the Eleventh Annual Conference of the European Cetacean Society, Stralsund, Germany, 10–12 March 1997. [Google Scholar]
- Miokovic, D.; Kovacic, D.; Pribanic, S. Stomach content analysis of one bottlenose dolphin (Tursiops truncatus, Montague 1821) from the Adriatic Sea. Nat. Croat. 1999, 8, 61–65. [Google Scholar]
- Blanco, C.; Salomón, O.; Raga, J.A. Diet of the bottlenose dolphin (Tursiops truncatus) in the western Mediterranean Sea. J. Mar. Biol. Assoc. UK 2001, 81, 1053–1058. [Google Scholar] [CrossRef]
- Scuderi, A.; Voliani, A.; Mancusi, C.; Pedà, C.; Romeo, T. Stomach contents of bottlenose dolphins stranded along the coasts of Tuscany (North Western Mediterranean Sea). In Proceedings of the 25th Annual Conference of the European Cetacean Society, Cádiz, Spain, 21–23 March 2011. [Google Scholar] [CrossRef]
- Scheinin, A.P.; Kerem, D.; Lojen, S.; Liberzon, J.; Spanier, E. Resource partitioning between common bottlenose dolphin (Tursiops truncatus) and the Israeli bottom trawl fishery? Assessment by stomach contents and tissue stable isotopes analysis. J. Mar. Biol. Assoc. UK 2014, 94, 1203–1220. [Google Scholar] [CrossRef]
- Milani, C.B.; Vella, A.; Vidoris, P.; Christidis, A.; Koutrakis, E.; Frantzis, A.; Miliou, A.; Kallianiotis, A. Cetacean stranding and diet analyses in the North Aegean Sea (Greece). J. Mar. Biol. Assoc. UK 2018, 98, 1011–1028. [Google Scholar] [CrossRef]
- Borrell, A.; Vighi, M.; Genov, T.; Giovos, I.; Gonzalvo, J. Feeding ecology of the highly threatened common bottlenose dolphin of the Gulf of Ambracia, Greece, through stable isotope analysis. Mar. Mammal Sci. 2021, 37, 98–110. [Google Scholar] [CrossRef]
- Gonzalvo, J.; Valls, M.; Cardona, L.; Aguilar, A. Factors determining the interaction between common bottlenose dolphins and bottom trawlers off the Balearic Archipelago (western Mediterranean Sea). J. Exp. Mar. Biol. Ecol. 2008, 367, 47–52. [Google Scholar] [CrossRef]
- Pennino, M.G.; Rotta, A.; Pierce, G.J.; Bellido, J.M. Interaction between bottlenose dolphin (Tursiops truncatus) and trammel nets in the Archipelago de La Maddalena, Italy. Hydrobiologia 2015, 747, 69–82. [Google Scholar] [CrossRef]
- Revuelta, O.; Domènech, F.; Fraija-Fernández, N.; Gozalbes, P.; Novillo, O.; Penadés-Suay, J.; Tomás, J. Interaction between bottlenose dolphins (Tursiops truncatus) and artisanal fisheries in the Valencia region (Spanish Mediterranean Sea). Ocean Coast. Manag. 2018, 165, 117–125. [Google Scholar] [CrossRef]
- Díaz López, B. Interactions between Mediterranean bottlenose dolphins (Tursiops truncatus) and gillnets off Sardinia, Italy. ICES J. Mar. Sci. 2006, 63, 944–951. [Google Scholar] [CrossRef]
- Díaz López, B.; Bernal Shirai, J.A. Bottlenose dolphin (Tursiops truncatus) presence and incidental capture in a marine fish farm on the north-eastern coast of Sardinia (Italy). J. Mar. Biol. Assoc. UK 2007, 87, 113–117. [Google Scholar] [CrossRef]
- Neri, A. Confronto della Dieta di Stenella coeruleoalba (Meyen, 1833) e Tursiops truncatus (Montagu, 1821) (Cetartiodactyla, Delphinidae) Negli Esemplari Spiaggiati Lungo le Coste della Toscana. Master’s Thesis, University of Trieste, Trieste, Italy, 26 February 2015. [Google Scholar]
- Pedà, C.; Battaglia, P.; Scuderi, A.; Voliani, A.; Mancusi, C.; Andaloro, F.; Romeo, T. Cephalopod prey in the stomach contents of odontocete cetaceans stranded in the western Mediterranean Sea. Mar. Biol. Res. 2015, 11, 593–602. [Google Scholar] [CrossRef]
- Santuario Pelagos. Available online: https://www.sanctuaire-pelagos.org/it/ (accessed on 10 August 2022).
- Clarke, M.R. A Handbook for the Identification of Cephalopods Beaks; Clarendon Press: Oxford, UK, 1986. [Google Scholar]
- Tuset, V.M.; Lombarte, A.; Assis, C.A. Otolith atlas for the western Mediterranean, north and central eastern Atlantic. Sci. Mar. 2008, 72, 7–198. [Google Scholar] [CrossRef]
- Pinkas, L.; Oliphant, S.; Iverson, I.L.K. Food habits of albacore, bluefin tuna and bonito in California waters. Fish Bull. 1971, 152, 1–105. [Google Scholar]
- Hyslop, E.J. Stomach contents analysis—A review of methods and their application. J. Fish Biol. 1980, 17, 411–429. [Google Scholar] [CrossRef]
- Shannon, C.E. A Mathematical Theory of Communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 10 March 2022).
- Viva, C.; Sartor, P.; Bertolini, D.; De Ranieri, S.; Ligas, A. Relationship of otolith length to fish total length in six demersal species from the NW Mediterranean Sea. J. Appl. Ichthyol. 2015, 31, 973–974. [Google Scholar] [CrossRef]
- Giménez, J.; Manjabacas, A.; Tuset, V.M.; Lombarte, A. Relationships between otolith and fish size from Mediterranean and north-eastern Atlantic species to be used in predator-prey studies. J. Fish Biol. 2016, 89, 2195–2202. [Google Scholar] [CrossRef]
- Russo, L. Analisi Comparative delle Caratteristiche Morfologiche degli Otoliti della Specie Pagellus erythrinus nel Mar Ligure e Mar Tirreno Centro-Settentrionale. Master’s Thesis, University of Pisa, Pisa, Italy, 14 December 2021. [Google Scholar]
- Wolff, G.A. Identification and Estimation of Size from the Beaks of 18 Species of Cephalopods from the Pacific Ocean; NOAA Technical Report NMFS 17; U.S. Department of Commerce, NOAA/National Marine Fisheries Service: Washington, DC, USA, 1984; pp. 1–50. [Google Scholar]
- Lu, C.C.; Ickeringill, R. Cephalopod beak identification and biomass estimation techniques: Tools for dietary studies of southern Australian finfishes. Mus. Vic. Sci. Rep. 2002, 6, 1–65. [Google Scholar] [CrossRef]
- De Ranieri, S. Programma Nazionale Italiano per la Raccolta di Dati alieutici 2010. Campionamento Biologico delle Catture; Sezioni C ed E. Rapporto Finale. Final Technical Report. 2011; 261p, unpublished work. (In Italian) [Google Scholar]
- Romeo, T.; Battaglia, P.; Pedà, C.; Perzia, P.; Consoli, P.; Esposito, V.; Andaloro, F. Pelagic cephalopods of the central Mediterranean Sea determined by the analysis of the stomach content of large fish predators. Helgol. Mar. Res. 2012, 66, 295–306. [Google Scholar] [CrossRef]
- Ligas, A.; Mannini, A.; Carpentieri, P.; Mancusi, C.; Sartor, P.; De Ranieri, S. Lenght weigth relationship in demersal species from Ligurian and northern-central Tyrrhenian Sea. Biol. Mar. Mediterr. 2012, 19, 212–213. [Google Scholar]
- De Ranieri, S. Programma Nazionale Italiano per la Raccolta di Dati Alieutici 2012. Campionamento Biologico delle Catture; Sezioni C ed E. Rapporto Finale. Final Technical Report. 2013; 306p, unpublished work. (In Italian) [Google Scholar]
- Maiorano, P.; Sabatella, R.F.; Marzocchi, B.M. (Eds.) Annuario sullo Stato delle Risorse e sulle Strutture Produttive dei Mari Italiani; 2019; 432p, (In Italian). Available online: http://www.nisea.eu/dir/wp-content/uploads/2019/09/Annuario-20142016_2019_08_05 (accessed on 15 June 2021).
- FishBase. World Wide Web Electronic Publication. Available online: www.fishbase.org (accessed on 15 June 2021).
- Ricker, W.E. Linear Regressions in Fishery Research. J. Fish. Res. Board Can. 1973, 30, 409–434. [Google Scholar] [CrossRef]
- Ligas, A. Strengthening Regional Cooperation in the Area of Fisheries Biological Data Collection in the Mediterranean and Black Sea (STREAM); MARE/2016/22 SI2.770115. Final Report. 2019. Available online: https://www.coispa.it/it/progetti/41/stream-strengthening-regional-cooperation-in-the-area-of-fisheries-biological-data-collection-in-the-mediterranean-and-black-sea.html (accessed on 28 August 2021).
- Pierce, G.J.; Santos, M.B.; Learmonth, J.; Mente, E.; Stowasser, G. Methods for dietary studies on marine mammals. In Proceedings of the Investigating the roles of cetaceans in marine ecosystems. In Proceedings of the CIESM Workshop Monographs n°25, Venice, Italy, 28–31 January 2004. [Google Scholar]
- Pierce, G.J.; Boyle, P.R. A review of methods for diet analysis in piscivorous marine mammals. Oceanogr. Mar. Biol. Annu. Rev. 1991, 29, 409–486. [Google Scholar]
- Cresson, P.; Ruitton, S.; Ourgaud, M.; Harmelin-Vivien, M. Contrasting perception of fish trophic level from stomach content and stable isotope analyses: A Mediterranean artificial reef experience. J. Exp. Mar. Biol. Ecol. 2014, 452, 54–62. [Google Scholar] [CrossRef]
- Wijnsma, G.; Pierce, G.J.; Santos, M.B. Assessment of errors in cetacean diet analysis: In vitro digestion of otoliths. J. Mar. Biol. Assoc. UK 1999, 79, 573–575. [Google Scholar] [CrossRef]
- Clarke, M.R. Cephalopods in the diet of odontocetes. In Research on Dolphins; Bryden, M.M., Harrison, R., Eds.; Oxford Science Publications: New York, NY, USA, 1986; pp. 281–321. [Google Scholar]
- Tortonese, E. Osteichthyes (Pesci ossei). Parte II; Calderini: Bologna, Italy, 1975; pp. 402–408. [Google Scholar]
- Matallanas, J.; Riba, G. Aspectos biolo’gicos de Ophidion barbatum Linnaeus, 1758 y O. rochei Muller, 1845 (Pisces, Ophidiidae) de la costa catalana. Investig. Pesquera 1980, 44, 399–406. [Google Scholar]
- Nielsen, J.G. Ophidiidae. In Fishes of the North-Eastern Atlantic and the Mediterranean; Whitehead, P.J.P., Bauchot, M.L., Hureau, C.J., Nielsen, J., Tortonese, E., Eds.; UNESCO: Paris, France, 1986; Volume 3, pp. 1158–1166. [Google Scholar]
- Casadevall, M.; Matallanas, J.; Carrasson, M.; Muñoz, M. Morphometric, meristic and anatomical differences between Ophidion barbatum L., 1758 and O. rochei Müller, 1845 (Pisces, Ophidiidae). Publ. Espec. Inst. Esp. Oceanogr. 1996, 21, 45–61. [Google Scholar]
- Parmentier, E.; Fontenelle, N.; Fine, M.L.; Vandewalle, P.; Henrist, C. Functional morphology of the sonic apparatus in Ophidion barbatum (Teleostei, Ophidiidae). J. Morphol. 2006, 267, 1461–1468. [Google Scholar] [CrossRef]
- Parmentier, E.; Compère, P.; Casadevall, M.; Fontenelle, N.; Cloots, R.; Henrist, C. The rocker bone: A new kind of mineralised tissue? Cell Tissue Res. 2008, 334, 67–79. [Google Scholar] [CrossRef][Green Version]
- Gannon, D.P.; Barros, N.B.; Nowacek, D.P.; Read, A.J.; Waples, D.M.; Wells, R.S. Prey detection by bottlenose dolphins, Tursiops truncatus: An experimental test of the passive listening hypothesis. Anim. Behav. 2005, 69, 709–720. [Google Scholar] [CrossRef]
- Barros, N.B.; Myrberg, A.A. Prey detection by means of passive listening in bottlenose dolphins (Tursiops truncatus). J. Acoust. Soc. Am. 1987, 82, S65. [Google Scholar] [CrossRef]
- Bartolino, V.; Ottavi, A.; Colloca, F.; Ardizzone, G.D.; Stefánsson, G. Bathymetric preferences of juvenile European hake (Merluccius merluccius). ICES J. Mar. Sci. 2008, 65, 963–969. [Google Scholar] [CrossRef]
- Froese, R.; Garilao, C.; Winker, H.; Coro, G.; Demirel, N.; Tsikliras, A.; Dimarchopoulou, D.; Scarcella, G.; Sampang-Reyes, A. Exploitation and Status of European Stocks. World Wide Web Electronic Publication. Available online: https://europe.oceana.org/reports/exploitation-and-status-european-stocks/ (accessed on 1 September 2022).
- STECF 2019. Stock Assessments: Demersal Stocks in the Western Mediterranean Sea (STECF-19-10); JRC119055; Publications Office of the European Union: Luxembourg, 2019. [Google Scholar] [CrossRef]
- Spedicato, M.T.; Massutí, E.; Mérigot, B.; Tserpes, G.; Jadaud, A.; Relini, G. The MEDITS trawl survey specifications in an ecosystem approach to fishery management. Sci. Mar. 2019, 83, 9–20. [Google Scholar] [CrossRef]
- Levy, A.; Able, K.W.; Grimes, C.B.; Hood, P. Biology of the conger eel Conger oceanicus in the Mid-Atlantic Bight—II. Foods and feeding ecology. Mar. Biol. 1988, 98, 597–600. [Google Scholar] [CrossRef]
- Sartor, P.; (Consorzio per il Centro Interuniversitario di Biologia Marina ed Ecologia Applicata “G. Bacci” (CIBM), Livorno, Italy). Personal communication, 2022.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).