Plant Growth-Promoting Bacteria (PGPB) with Biofilm-Forming Ability: A Multifaceted Agent for Sustainable Agriculture
Abstract
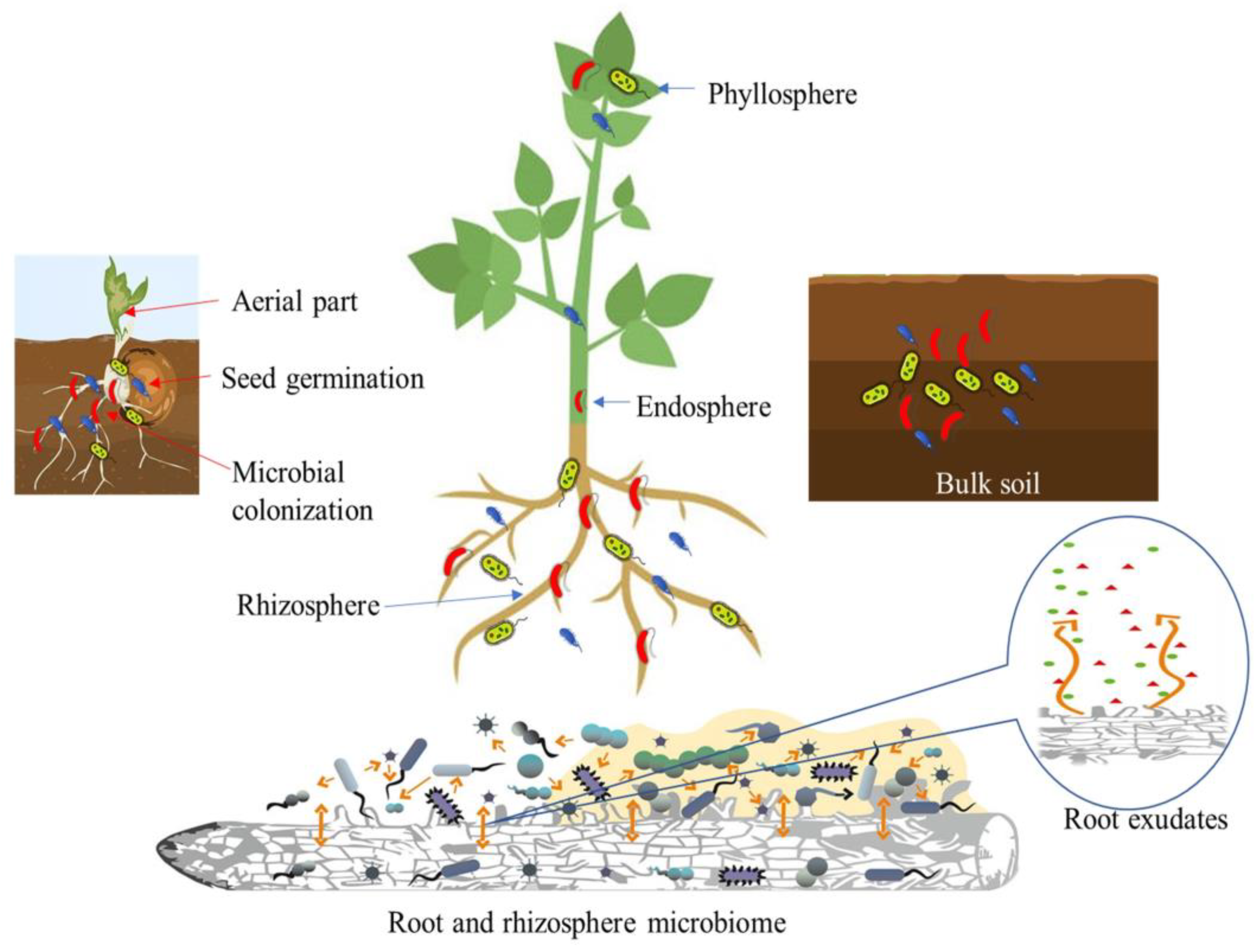
1. Introduction
2. PGPB as Multipotent Bioagents
3. Biofilm Formation by PGPB Communities in Varied Agroecosystems
3.1. Biofilm Structure
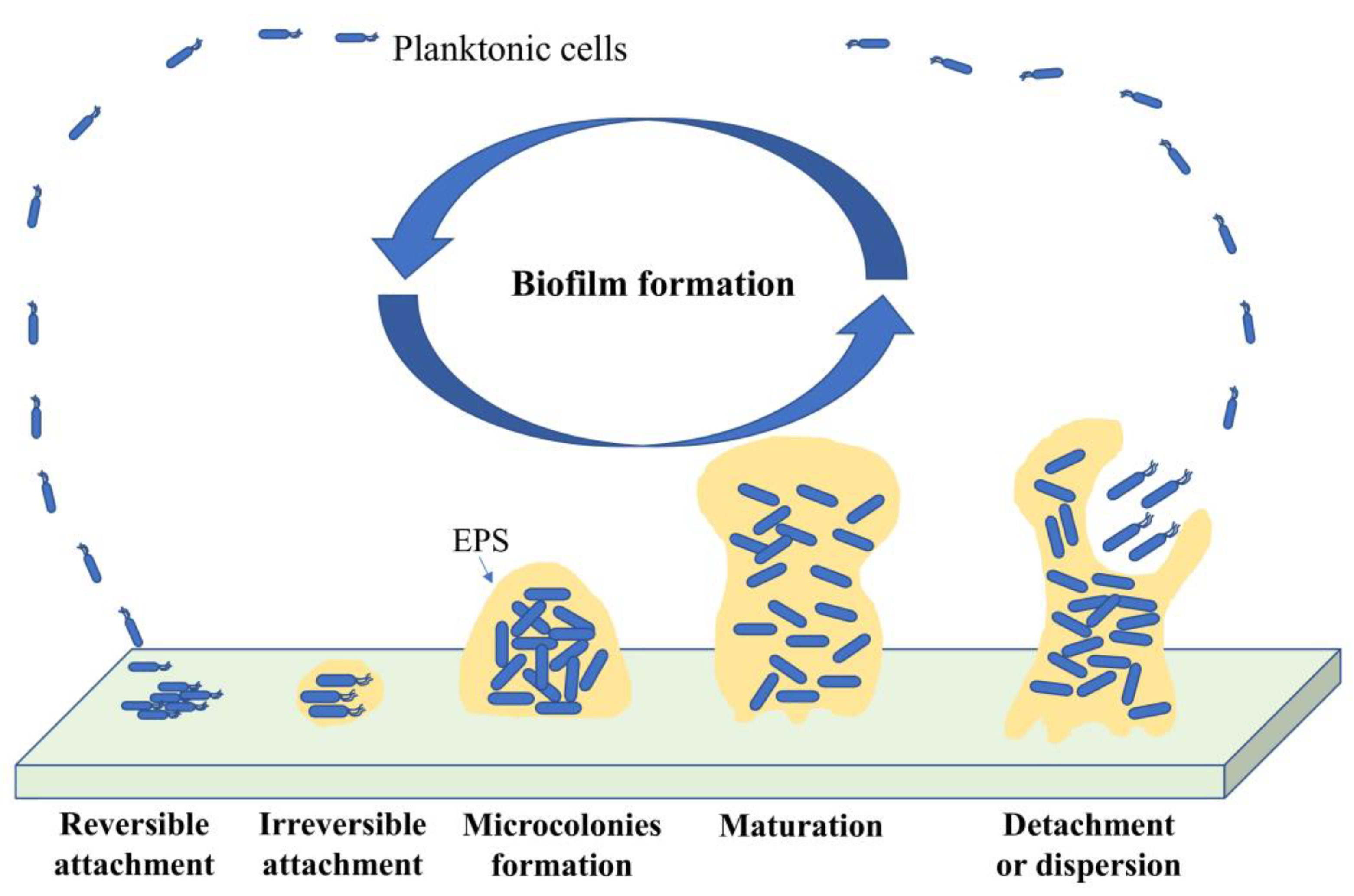
3.2. Biofilm Formation
3.2.1. Bacterial Attachment to a Surface
3.2.2. Adhesion to Surface
3.2.3. Microcolony Formation
3.2.4. Biofilm Maturation
3.2.5. Biofilm Dispersal
3.3. Factors Responsible for Biofilm Formation
4. Role of Biofilm-Forming PGPB in Sustainable Agriculture
4.1. Biocontrol Activity against Plant Pathogens
4.1.1. Root Colonization
4.1.2. Triggering Induced Systemic Resistance (ISR)
4.1.3. Antimicrobial-Producing Biofilm
4.2. Promoting Plant Growth by Biofilm-Forming PGPB
4.3. Mitigating Abiotic Stress in Plants by Biofilm-Producing PGPB
5. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Calicioglu, O.; Flammini, A.; Bracco, S.; Bellù, L.; Sims, R. The Future Challenges of Food and Agriculture: An Integrated Analysis of Trends and Solutions. Sustainability 2019, 11, 222. [Google Scholar] [CrossRef]
- Dhir, B. Biofertilizers and Biopesticides: Eco-Friendly Biological Agents. In Advances in Environmental Biotechnology; Kumar, R., Sharma, A.K., Ahluwalia, S.S., Eds.; Springer Nature Singapore: Singapore, 2017; pp. 1–288. ISBN 9789811040412. [Google Scholar]
- Siebrecht, N. Sustainable Agriculture and Its Implementation Gap—Overcoming Obstacles to Implementation. Sustainability 2020, 12, 3853. [Google Scholar] [CrossRef]
- Zhang, F.; Shen, J.; Zhang, J.; Zuo, Y.; Li, L.; Chen, X. Rhizosphere Processes and Management for Improving Nutrient Use Efficiency and Crop Productivity. Implications for China. In Advances in Agronomy: Volume 107; Sparks, D.L., Ed.; Elsevier Inc.: Oxford, UK, 2010; pp. 1–222. ISBN 9780123810335. [Google Scholar]
- Pii, Y.; Mimmo, T.; Tomasi, N.; Terzano, R.; Cesco, S.; Crecchio, C. Microbial Interactions in the Rhizosphere: Beneficial Influences of Plant Growth-Promoting Rhizobacteria on Nutrient Acquisition Process. A Review. Biol. Fertil. Soils 2015, 51, 403–415. [Google Scholar] [CrossRef]
- Gupta, G.; Parihar, S.S.; Ahirwar, N.; Snehi, S.; Singh, V. Plant Growth Promoting Rhizobacteria (PGPR): Current and Future Prospects for Development of Sustainable Agriculture. J. Microb. Biochem. Technol. 2015, 7, 96–102. [Google Scholar] [CrossRef]
- Mahmood, I.; Imadi, S.; Shazadi, K.; Gul, A.; Hakeem, K. Plant, Soil and Microbes: Volume 1: Implications in Crop Science. In Plant, Soil and Microbes: Volume 1: Implications in Crop Science; Hakeem, K.R., Akhtar, M.S., Abdullah, S.N.A., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–366. ISBN 9783319274553. [Google Scholar]
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical Pesticides and Human Health: The Urgent Need for a New Concept in Agriculture. Front. Public Health 2016, 4, 148. [Google Scholar] [CrossRef] [PubMed]
- Haskett, T.L.; Tkacz, A.; Poole, P.S. Engineering Rhizobacteria for Sustainable Agriculture. ISME J. 2020, 15, 949–964. [Google Scholar] [CrossRef]
- Gebska, M.; Grontkowska, A.; Swiderek, W.; Golebiewska, B. Farmer Awareness and Implementation of Sustainable Agriculture Practices in Different Types of Farms in Poland. Sustainability 2020, 12, 8022. [Google Scholar] [CrossRef]
- Reganold, J.P.; Papendick, R.I.; Parr, J.F. Sustainable Agriculture. Sci. Am. 1990, 262, 112–120. [Google Scholar] [CrossRef]
- Velten, S.; Leventon, J.; Jager, N.; Newig, J. What Is Sustainable Agriculture? A Systematic Review. Sustainability 2015, 7, 7833–7865. [Google Scholar] [CrossRef]
- Farrell, A.; Hart, M. What Does Sustainability Really Mean?: The Search for Useful Indicators. Environment 1998, 40, 4–31. [Google Scholar] [CrossRef]
- Wong, W.S.; Tan, S.N.; Ge, L.; Chen, X.; Yong, J.W.H. The Importance of Phytohormones and Microbes in Biofertilizers. In Bacterial Metabolites in Sustainable Agroecosystem; Sustainable Development and Biodiversity; Maheshwari, D., Ed.; Springer: Cham, Switzerland, 2015; Volume 12, pp. 105–158. ISBN 9783319246543. [Google Scholar]
- Lehman, R.M.; Cambardella, C.A.; Stott, D.E.; Acosta-Martinez, V.; Manter, D.K.; Buyer, J.S.; Maul, J.E.; Smith, J.L.; Collins, H.P.; Halvorson, J.J.; et al. Understanding and Enhancing Soil Biological Health: The Solution for Reversing Soil Degradation. Sustainability 2015, 7, 988–1027. [Google Scholar] [CrossRef]
- Bünemann, E.K.; Bongiorno, G.; Bai, Z.; Creamer, R.E.; De Deyn, G.; de Goede, R.; Fleskens, L.; Geissen, V.; Kuyper, T.W.; Mäder, P.; et al. Soil Quality—A Critical Review. Soil Biol. Biochem. 2018, 120, 105–125. [Google Scholar] [CrossRef]
- Szczepanek, M.; Piotrowska-Dlugosz, A.; Kanopka, I. Sustainable Crop Production Protects the Quality of Soil and Plant Raw Materials. Agronomy 2021, 11, 1178. [Google Scholar] [CrossRef]
- Mumtaz, M.; Ahmad, M.; Jamil, M.; Hussain, T. Zinc Solubilizing Bacillus spp. Potential Candidates for Biofortification in Maize. Microbiol. Res. 2017, 202, 51–60. [Google Scholar] [CrossRef]
- Varma, A.; Choudhary, D.K. Mycorrhizosphere and Pedogenesis; Varma, A., Choudary, D., Eds.; Springer: Singapore, 2019; ISBN 9789811364792. [Google Scholar]
- Gupta, G.; Snehi, S.K.; Singh, V. Role of PGPR in Biofilm Formations and Its Importance in Plant Health. In Biofilms in Plant and Soil Health; Ahmad, I., Husain, F.M., Eds.; John Wiley & Sons Ltd.: London, UK, 2017; ISBN 9781119246329. [Google Scholar]
- Hakim, S.; Naqqash, T.; Nawaz, M.S.; Laraib, I.; Siddique, M.J.; Zia, R.; Mirza, M.S.; Imran, A. Rhizosphere Engineering With Plant Growth-Promoting Microorganisms for Agriculture and Ecological Sustainability. Front. Sustain. Food Syst. 2021, 5, 617157. [Google Scholar] [CrossRef]
- York, L.M.; Carminati, A.; Mooney, S.J.; Ritz, K.; Bennett, M.J. The Holistic Rhizosphere: Integrating Zones, Processes, and Semantics in the Soil Influenced by Roots. J. Exp. Bot. 2016, 67, 3629–3643. [Google Scholar] [CrossRef] [PubMed]
- Sasse, J.; Martinoia, E.; Northen, T. Feed Your Friends: Do Plant Exudates Shape the Root Microbiome? Trends Plant Sci. 2018, 23, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Xiong, C.; Singh, B.K.; He, J.Z.; Han, Y.L.; Li, P.P.; Wan, L.H.; Meng, G.Z.; Liu, S.Y.; Wang, J.T.; Wu, C.F.; et al. Plant Developmental Stage Drives the Differentiation in Ecological Role of the Maize Microbiome. Microbiome 2021, 9, 171. [Google Scholar] [CrossRef]
- Suman, J.; Rakshit, A.; Ogireddy, S.D.; Singh, S.; Gupta, C.; Chandrakala, J. Microbiome as a Key Player in Sustainable Agriculture and Human Health. Front. Soil Sci. 2022, 2, 821589. [Google Scholar] [CrossRef]
- Sehrawat, A.; Sindhu, S.S. Potential of Biocontrol Agents in Plant Disease Control for Improving Food Safety. Def. Life Sci. J. 2019, 4, 220–225. [Google Scholar] [CrossRef]
- Goswami, D.; Thakker, J.N.; Dhandhukia, P.C. Portraying Mechanics of Plant Growth Promoting Rhizobacteria (PGPR): A Review. Cogent Food Agric. 2016, 2, 1127500. [Google Scholar] [CrossRef]
- Galloway, A.F.; Knox, P.; Krause, K. Sticky Mucilages and Exudates of Plants: Putative Microenvironmental Design Elements with Biotechnological Value. New Phytol. 2020, 225, 1461–1469. [Google Scholar] [CrossRef] [PubMed]
- Saleh, D.; Sharma, M.; Seguin, P.; Jabaji, S. Organic Acids and Root Exudates of Brachypodium distachyon: Effects on Chemotaxis and Biofilm Formation of Endophytic Bacteria. Can. J. Microbiol. 2020, 66, 562–575. [Google Scholar] [CrossRef] [PubMed]
- Ahkami, A.; White, R.; Handakumbura, P.; Jansson, C. Rhizosphere Engineering: Enhancing Sustainable Plant Ecosystem Productivity. Rhizosphere 2017, 3, 233–243. [Google Scholar] [CrossRef]
- Zuluaga, M.Y.A.; Milani, K.M.L.; Gonçalves, L.S.A.; De Oliveira, A.L.M. Diversity and Plant Growth-Promoting Functions of Diazotrophic/N-Scavenging Bacteria Isolated from the Soils and Rhizospheres of Two Species of Solanum. PLoS ONE 2020, 15, e0227422. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, W.; Yadav, R.; Li, K. Plant Growth Promoting Bacteria in Agriculture: Two Sides of a Coin. Appl. Soil Ecol. 2019, 138, 10–18. [Google Scholar] [CrossRef]
- del Carmen Orozco-Mosqueda, M.; del Carmen Rocha-Granados, M.; Glick, B.R.; Santoyo, G. Microbiome Engineering to Improve Biocontrol and Plant Growth-Promoting Mechanisms. Microbiol. Res. 2018, 208, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Salomon, M.; Pinter, I.; Piccoli, P.; Bottini, R. Use of Plant Growth-Promoting Rhizobacteria as Biocontrol Agents: Induced Systemic Resistance Against Biotic Stress in Plants. In Microbial Applications; Kalia, V., Ed.; Springer: Cham, Switzerland, 2017; Volume 2, pp. 1–336. ISBN 9783319526690. [Google Scholar]
- Glick, B.R.; Gamalero, E. Recent Developments in the Study of Plant Microbiomes. Microorganisms 2021, 9, 1533. [Google Scholar] [CrossRef]
- Gamalero, E.; Bona, E.; Todeschini, V.; Lingua, G. Saline and Arid Soils: Impact on Bacteria, Plants, and Their Interaction. Biology 2020, 9, 116. [Google Scholar] [CrossRef]
- Jarosch, K.A.; Kandeler, E.; Frossard, E.; Bünemann, E.K. Is the Enzymatic Hydrolysis of Soil Organic Phosphorus Compounds Limited by Enzyme or Substrate Availability? Soil Biol. Biochem. 2019, 139, 107628. [Google Scholar] [CrossRef]
- Samantray, J.; Anand, A.; Dash, B.; Ghosh, M.; Behera, A. Silicate Minerals—Potential Source of Potash—A Review. Miner. Eng. 2022, 179, 107463. [Google Scholar] [CrossRef]
- Santoyo, G.; Sánchez-Yáñez, J.M.; de los Santos-Villalobos, S. Methods for Detecting Biocontrol and Plant Growth-Promoting Traits in Rhizobacteria. In Methods in Rhizosphere Biology Research; Springer Nature Singapore: Singapore, 2019; pp. 133–149. [Google Scholar] [CrossRef]
- Kang, S.M.; Khan, A.L.; Waqas, M.; Asaf, S.; Lee, K.E.; Park, Y.G.; Kim, A.Y.; Khan, M.A.; You, Y.H.; Lee, I.J. Integrated Phytohormone Production by the Plant Growth-Promoting Rhizobacterium Bacillus tequilensis SSB07 Induced Thermotolerance in Soybean. J. Plant Interact. 2019, 14, 416–423. [Google Scholar] [CrossRef]
- Olanrewaju, O.S.; Glick, B.R.; Babalola, O.O. Mechanisms of Action of Plant Growth Promoting Bacteria. World J. Microbiol. Biotechnol. 2017, 33, 197. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Prasad, V.; Lata, C. Bacillus: Plant Growth Promoting Bacteria for Sustainable Agriculture and Environment. In New and Future Developments in Microbial Biotechnology and Bioengineering; Singh, J.S., Singh, D.P., Eds.; Elsevier B.V.: Amsterdam, The Netherlands, 2019; pp. 43–55. ISBN 9780444641915. [Google Scholar]
- Lahlali, R.; Ezrari, S.; Radouane, N.; Kenfaoui, J.; Esmaeel, Q.; El Hamss, H.; Belabess, Z.; Barka, E.A. Biological Control of Plant Pathogens: A Global Perspective. Microorganisms 2022, 10, 596. [Google Scholar] [CrossRef] [PubMed]
- Kenawy, A.; Dailin, D.; Abo-Zaid, G.; Malek, R.; Ambehabati, K.; Zakaria, K.; Sayyed, R.; El Enshasy, H. Biosynthesis of Antibiotics by PGPR and Their Roles in Biocontrol of Plant Diseases. In Plant Growth Promoting Rhizobacteria for Sustainable Stress Management; Sayyed, R., Ed.; Springer Nature Singapore: Singapore, 2019; ISBN 9789811369865. [Google Scholar]
- Zhang, Q.X.; Kong, X.W.; Li, S.Y.; Chen, X.J.; Chen, X.J. Antibiotics of Pseudomonas protegens FD6 Are Essential for Biocontrol Activity. Australas. Plant Pathol. 2020, 49, 307–317. [Google Scholar] [CrossRef]
- Daura-Pich, O.; Hernández, I.; Pinyol-Escala, L.; Lara, J.M.; Martínez-Servat, S.; Fernández, C.; López-García, B. No Antibiotic and Toxic Metabolites Produced by the Biocontrol Agent Pseudomonas putida Strain B2017. FEMS Microbiol. Lett. 2020, 367, fnaa075. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Xiang, W.; Cao, K.X.; Lu, X.; Yao, S.C.; Hung, D.; Huang, R.S.; Li, L.B. Characterization of Volatile Organic Compounds Emitted from Endophytic Burkholderia cenocepacia ETR-B22 by SPME-GC-MS and Their Inhibitory Activity against Various Plant Fungal Pathogens. Molecules 2020, 25, 3765. [Google Scholar] [CrossRef]
- Prigigallo, M.I.; De Stradis, A.; Anand, A.; Mannerucci, F.; L’Haridon, F.; Weisskopf, L.; Bubici, G. Basidiomycetes Are Particularly Sensitive to Bacterial Volatile Compounds: Mechanistic Insight Into the Case Study of Pseudomonas protegens Volatilome Against Heterobasidion Abietinum. Front. Microbiol. 2021, 12, 684664. [Google Scholar] [CrossRef]
- Ossowicki, A.; Jafra, S.; Garbeva, P. The Antimicrobial Volatile Power of the Rhizospheric Isolate Pseudomonas donghuensis P482. PLoS ONE 2017, 12, e0174362. [Google Scholar] [CrossRef]
- Bonaterra, A.; Badosa, E.; Daranas, N.; Francés, J.; Roselló, G.; Montesinos, E. Bacteria as Biological Control Agents of Plant Diseases. Microorganisms 2022, 10, 1759. [Google Scholar] [CrossRef]
- Jadhav, H.; Shaikh, S.S.; Sayyed, R.Z. Rhizotrophs: Plant Growth Promotion to Bioremediation. In Rhizotrophs Plant Growth Promot. to Bioremediation; Mehnaz, S., Ed.; Springer Nature Singapore: Singapore, 2017. [Google Scholar] [CrossRef]
- Rasul, M.; Yasmin, S.; Zubair, M.; Mahreen, N.; Yousaf, S.; Arif, M.; Iqbal, Z.; Sajjad, M. Phosphate Solubilizers as Antagonists for Bacterial Leaf Blight with Improved Rice Growth in Phosphorus Deficit Soil. Biol. Control 2019, 136, 103997. [Google Scholar] [CrossRef]
- Oleńska, E.; Małek, W.; Wójcik, M.; Swiecicka, I.; Thijs, S.; Vangronsveld, J. Beneficial Features of Plant Growth-Promoting Rhizobacteria for Improving Plant Growth and Health in Challenging Conditions: A Methodical Review. Sci. Total Environ. 2020, 743, 140682. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Pandey, S. ACC Deaminase Producing Bacteria with Multifarious Plant Growth Promoting Traits Alleviates Salinity Stress in French Bean (Phaseolus vulgaris) Plants. Front. Microbiol. 2019, 10, 1506. [Google Scholar] [CrossRef]
- Singh, P.; Singh, R.K.; Zhou, Y.; Wang, J.; Jiang, Y.; Wang, Y.; Yang, L.; Jiang, M. Unlocking the Strength of Plant Growth Promoting Pseudomonas in Improving Crop Productivity in Normal and Challenging Environments: A Review. J. Plant Interact. 2022, 17, 220–238. [Google Scholar] [CrossRef]
- Abdelaal, K.; Alkahtani, M.; Attia, K.; Hafez, Y. The Role of Plant Growth-Promoting Bacteria in Alleviating the Adverse Effecys of Drought on Plants. Biology 2021, 10, 520. [Google Scholar] [CrossRef]
- Kasim, W.A.; Gaafar, R.M.; Abou-Ali, R.M.; Omar, M.N.; Hewait, H.M. Effect of Biofilm Forming Plant Growth Promoting Rhizobacteria on Salinity Tolerance in Barley. Ann. Agric. Sci. 2016, 61, 217–227. [Google Scholar] [CrossRef]
- Altaf, M.M.; Khan, M.; Abulreesh, H.; Ahmad, I. Quorum Sensing in Plant Growth-Promoting Rhizobacteria and Its Impact on Plant-Microbe Interaction. In Plant-Microbe Interactions in Agro-Ecological Perspectives; Singh, D.P., Singh, H.B., Prabha, R., Eds.; Springer Nature Singapore: Singapore, 2017; Volume 1, pp. 1–657. ISBN 9789811058134. [Google Scholar]
- Muhammad, M.H.; Idris, A.L.; Fan, X.; Guo, Y.; Yu, Y.; Jin, X.; Qiu, J.; Guan, X.; Huang, T. Beyond Risk: Bacterial Biofilms and Their Regulating Approaches. Front. Microbiol. 2020, 11, 928. [Google Scholar] [CrossRef]
- Pandit, A.; Adholeya, A.; Cahill, D.; Brau, L.; Kochar, M. Microbial Biofilms in Nature: Unlocking Their Potential for Agricultural Applications. J. Appl. Microbiol. 2020, 129, 199–211. [Google Scholar] [CrossRef]
- Azulay, D.N.; Spaeker, O.; Ghrayeb, M.; Wilsch-Bräuninger, M.; Scoppola, E.; Burghammer, M.; Zizak, I.; Bertinetti, L.; Politi, Y.; Chai, L. Multiscale X-Ray Study of Bacillus subtilis Biofilms Reveals Interlinked Structural Hierarchy and Elemental Heterogeneity. Proc. Natl. Acad. Sci. USA 2022, 119, e2118107119. [Google Scholar] [CrossRef] [PubMed]
- Karygianni, L.; Ren, Z.; Koo, H.; Thurnheer, T. Biofilm Matrixome: Extracellular Components in Structured Microbial Communities. Trends Microbiol. 2020, 28, 668–681. [Google Scholar] [CrossRef]
- del Mar Cendra, M.; Torrents, E. Pseudomonas aeruginosa Biofilms and Their Partners in Crime. Biotechnol. Adv. 2021, 49, 107734. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wingender, J. The Biofilm Matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Berlanga, M.; Guerrero, R. Living Together in Biofilms: The Microbial Cell Factory and Its Biotechnological Implications. Microb. Cell Fact. 2016, 15, 165. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An Emergent Form of Bacterial Life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Pinto, R.M.; Soares, F.A.; Reis, S.; Nunes, C.; Van Dijck, P. Innovative Strategies Toward the Disassembly of the EPS Matrix in Bacterial Biofilms. Front. Microbiol. 2020, 11, 952. [Google Scholar] [CrossRef] [PubMed]
- Soler-Arango, J.; Figoli, C.; Muraca, G.; Bosch, A.; Brelles-Mariño, G. The Pseudomonas aeruginosa Biofilm Matrix and Cells Are Drastically Impacted by Gas Discharge Plasma Treatment: A Comprehensive Model Explaining Plasma-Mediated Biofilm Eradication. PLoS ONE 2019, 14, e0216817. [Google Scholar] [CrossRef]
- Kungwani, N.; Shukla, S.K.; Rao, T.; Das, S. Biofilm-Mediated Bioremediation of Polycyclic Aromatic Hydrocarbons: Current Status and Future Perspectives. In Microbial Biodegradation and Bioremediation, 2nd ed.; Das, S., Dash, H.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 547–570. [Google Scholar]
- Bhagat, N.; Raghav, M.; Dubey, S.; Bedi, N. Bacterial Exopolysaccharides: Insight into Their Role in Plant Abiotic Stress Tolerance. J. Microbiol. Biotechnol. 2021, 31, 1045–1059. [Google Scholar] [CrossRef] [PubMed]
- Limoli, D.H.; Jones, C.J.; Wozniak, D.J. Bacterial Extracellular Polysaccharides in Biofilm Formation and Function. Microbiol. Spectr. 2015, 3, 1–30. [Google Scholar] [CrossRef]
- Lahiri, D.; Nag, M.; Dutta, B.; Dey, A.; Sarkar, T.; Pati, S.; Edinur, H.A.; Kari, Z.A.; Noor, N.H.M.; Ray, R.R. Bacterial Cellulose: Production, Characterization and Application as Antimicrobial Agent. Int. J. Mol. Sci. 2021, 22, 12984. [Google Scholar] [CrossRef]
- Ryder, C.; Byrd, M.; Wozniak, D.J. Role of Polysaccharides in Pseudomonas aeruginosa Biofilm Development. Curr. Opin. Microbiol. 2016, 10, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Floyd, K.A.; Eberly, A.R.; Hadjifrangiskou, M. Adhesion of Bacteria to Surfaces and Biofilm Formation on Medical Devices. In Biofilms and Implantable Medical Devices: Infection and Control; Deng, Y., Lv, W., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; pp. 47–95. ISBN 9780081003985. [Google Scholar]
- Molina-Santiago, C.; de Vicente, A.; Romero, D. Bacterial Extracellular Matrix as a Natural Source of Biotechnologically Multivalent Materials. Comput. Struct. Biotechnol. J. 2021, 19, 2796–2805. [Google Scholar] [CrossRef] [PubMed]
- Chin, J.S.F.; Sinha, S.; Nalaparaju, A.; Yam, J.K.H.; Qin, Z.; Ma, L.; Liang, Z.X.; Lu, L.; Bhattacharjya, S.; Yang, L. Pseudomonas aeruginosa Psl Exopolysaccharide Interacts with the Antimicrobial Peptide LG21. Water 2017, 9, 681. [Google Scholar] [CrossRef]
- Lee, K.; Yoon, S.S. Pseudomonas aeruginosa Biofilm, a Programmed Bacterial Life for Fitness. J. Microbiol. Biotechnol. 2017, 27, 1053–1064. [Google Scholar] [CrossRef]
- Fong, J.N.C.; Yildiz, F.H. Biofilm Matrix Proteins. Microb. Biofilms 2015, 3, 201–222. [Google Scholar] [CrossRef]
- Fulaz, S.; Vitale, S.; Quinn, L.; Casey, E. Nanoparticle–Biofilm Interactions: The Role of the EPS Matrix. Trends Microbiol. 2019, 27, 915–926. [Google Scholar] [CrossRef]
- Altaf, M.; Ahmad, I. Biofilm Formation on Plant Surfaces by Rhizobacteria: Impact on Plant Growth and Ecological Significance. Handb. Microb. Bioresour. 2016, 5, 81–95. [Google Scholar] [CrossRef]
- Carniello, V.; Peterson, B.W.; van der Mei, H.C.; Busscher, H.J. Physico-Chemistry from Initial Bacterial Adhesion to Surface-Programmed Biofilm Growth. Adv. Colloid Interface Sci. 2018, 261, 1–14. [Google Scholar] [CrossRef]
- Bennett, R.R.; Lee, C.K.; De Anda, J.; Nealson, K.H.; Yildiz, F.H.; O’Toole, G.A.; Wong, G.C.L.; Golestanian, R. Species-Dependent Hydrodynamics of Flagellum-Tethered Bacteria in Early Biofilm Development. J. R. Soc. Interface 2016, 13, 20150966. [Google Scholar] [CrossRef] [PubMed]
- Conrad, J.C.; Gibiansky, M.L.; Jin, F.; Gordon, V.D.; Motto, D.A.; Mathewson, M.A.; Stopka, W.G.; Zelasko, D.C.; Shrout, J.D.; Wong, G.C.L. Flagella and Pili-Mediated near-Surface Single-Cell Motility Mechanisms in P. aeruginosa. Biophys. J. 2011, 100, 1608–1616. [Google Scholar] [CrossRef]
- Ligthart, K.; Belzer, C.; de Vos, W.M.; Tytgat, H.L.P. Bridging Bacteria and the Gut: Functional Aspects of Type IV Pili. Trends Microbiol. 2020, 28, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Gibiansky, M.L.; Wang, J.; Wang, C.; Lux, R.; Li, Y.; Wong, G.C.L.; Shi, W. Interplay between Type IV Pili Activity and Exopolysaccharides Secretion Controls Motility Patterns in Single Cells of Myxococcus xanthus. Sci. Rep. 2016, 6, 17790. [Google Scholar] [CrossRef][Green Version]
- Colin, R.; Ni, B.; Laganenka, L.; Sourjik, V. Multiple Functions of Flagellar Motility and Chemotaxis in Bacterial Physiology. FEMS Microbiol. Rev. 2021, 45, fuab038. [Google Scholar] [CrossRef]
- López-Farfán, D.; Reyes-Darias, J.A.; Matilla, M.A.; Krell, T. Concentration Dependent Effect of Plant Root Exudates on the Chemosensory Systems of Pseudomonas putida KT2440. Front. Microbiol. 2019, 10, 78. [Google Scholar] [CrossRef] [PubMed]
- O’Neal, L.; Vo, L.; Alexandre, G. Specific Root Exudate Compounds Sensed by Dedicated Chemoreceptors Shape Azospirillum brasilense Chemotaxis in the Rhizosphere. Appl. Environ. Microbiol. 2020, 86, e01026-20. [Google Scholar] [CrossRef] [PubMed]
- Velmourougane, K.; Prasanna, R.; Saxena, A.K. Agriculturally Important Microbial Biofilms: Present Status and Future Prospects. J. Basic Microbiol. 2017, 57, 548–573. [Google Scholar] [CrossRef] [PubMed]
- Costa, O.Y.A.; Raaijmakers, J.M.; Kuramae, E.E. Microbial Extracellular Polymeric Substances: Ecological Function and Impact on Soil Aggregation. Front. Microbiol. 2018, 9, 1636. [Google Scholar] [CrossRef] [PubMed]
- Ghafoor, A.; Hay, I.D.; Rehm, B.H.A. Role of Exopolysaccharides in Pseudomonas aeruginosa Biofilm Formation and Architecture. Appl. Environ. Microbiol. 2011, 77, 5238–5246. [Google Scholar] [CrossRef]
- Jahid, I.K.; Ha, S. Do Inactivation Kinetics of Various Chemical Disinfectants on Aeromonas hydrophila Planktonic Cells and Biofilms. Foodborne Pathog. Dis. 2014, 11, 346–353. [Google Scholar] [CrossRef]
- Klausen, M.; Aaes-Jørgensen, A.; Molin, S.; Tolker-Nielsen, T. Involvement of Bacterial Migration in the Development of Complex Multicellular Structures in Pseudomonas aeruginosa Biofilms. Mol. Microbiol. 2003, 50, 61–68. [Google Scholar] [CrossRef]
- Barken, K.B.; Pamp, S.J.; Yang, L.; Gjermansen, M.; Bertrand, J.J.; Klausen, M.; Givskov, M.; Whitchurch, C.B.; Engel, J.N.; Tolker-Nielsen, T. Roles of Type IV Pili, Flagellum-Mediated Motility and Extracellular DNA in the Formation of Mature Multicellular Structures in Pseudomonas aeruginosa Biofilms. Environ. Microbiol. 2008, 10, 2331–2343. [Google Scholar] [CrossRef]
- Otzen, D.E. Biosurfactants and Surfactants Interacting with Membranes and Proteins: Same but Different? Biochim. Biophys. Acta Biomembr. 2017, 1859, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Źuñiga, A.; Donoso, R.A.; Ruiz, D.; Ruz, G.A.; Gonźalez, B. Quorum-Sensing Systems in the Plant Growth-Promoting Bacterium Paraburkholderia phytofirmans PsJN Exhibit Cross-Regulation and Are Involved in Biofilm Formation. Mol. Plant-Microbe Interact. 2017, 30, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Rabin, N.; Zheng, Y.; Opoku-Temeng, C.; Du, Y.; Bonsu, E.; Sintim, H.O. Biofilm Formation Mechanisms and Targets for Developing Antibiofilm Agents. Futur. Med. Chem. 2015, 7, 493–512. [Google Scholar] [CrossRef] [PubMed]
- Pamp, S.; Tolker-Nielsen, T. Multiple Roles of Biosurfactants in Structural Biofilm Development by Pseudomonas aeruginosa. J. Bacteriol. 2007, 189, 2531–2539. [Google Scholar] [CrossRef] [PubMed]
- Sheraton, M.V.; Yam, J.K.H.; Tan, C.H.; Oh, H.S.; Mancini, E.; Yang, L.; Sloot, P.M.A. Mesoscopic Energy Minimization Drives Pseudomonas stratification of Antibiotic Activity Based on Cell Metabolism. Antimicrob. Agents Chemother. 2018, 62, e02544–e17. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari, A.; Dehghany, J.; Schwebs, T.; Müsken, M.; Häussler, S.; Meyer-Hermann, M. Inoculation Density and Nutrient Level Determine the Formation of Mushroom-Shaped Structures in Pseudomonas aeruginosa Biofilms. Sci. Rep. 2016, 6, 32097. [Google Scholar] [CrossRef]
- Wille, J.; Coenye, T. Biofilm Dispersion: The Key to Biofilm Eradication or Opening Pandora’s Box? Biofilm 2020, 2, 100027. [Google Scholar] [CrossRef]
- Guilhen, C.; Miquel, S.; Charbonnel, N.; Joseph, L.; Carrier, G.; Forestier, C.; Balestrino, D. Colonization and Immune Modulation Properties of Klebsiella pneumoniae Biofilm-Dispersed Cells. npj Biofilms Microbiomes 2019, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Ansari, F.A.; Jafri, H.; Ahmad, I.; Abulreesh, H.H. Factors Affecting Biofilm Formation in in Vitro and in the Rhizosphere. In Biofilms in Plant and Soil Health; Ahmad, I., Husain, F.M., Eds.; John Wiley & Sons Ltd: London, UK, 2017; pp. 275–290. ISBN 9781119246343. [Google Scholar] [CrossRef]
- Lee, B.H.; Cole, S.; Badel-Berchoux, S.; Guillier, L.; Felix, B.; Krezdorn, N.; Hébraud, M.; Bernardi, T.; Sultan, I.; Piveteau, P. Biofilm Formation of Listeria monocytogenes Strains Under Food Processing Environments and Pan-Genome-Wide Association Study. Front. Microbiol. 2019, 10, 2698. [Google Scholar] [CrossRef] [PubMed]
- Samrot, A.V.; Mohamed, A.A.; Faradjeva, E.; Jie, L.S.; Sze, C.H.; Arif, A.; Sean, T.C.; Michael, E.N.; Mun, C.Y.; Qi, N.X.; et al. Mechanisms and Impact of Biofilms and Targeting of Biofilms Using Bioactive Compounds—A Review. Medicina 2021, 57, 839. [Google Scholar] [CrossRef] [PubMed]
- Savijoki, K.; Nyman, T.A.; Kainulainen, V.; Miettinen, I.; Siljamäki, P.; Fallarero, A.; Sandholm, J.; Satokari, R.; Varmanen, P. Growth Mode and Carbon Source Impact the Surfaceome Dynamics of Lactobacillus rhamnosus GG. Front. Microbiol. 2019, 10, 1272. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.; Liu, D. Effects of Carbon Sources and Temperature on the Formation and Structural Characteristics of Food-Related Staphylococcus epidermidis Biofilms. Food Sci. Hum. Wellness 2020, 9, 370–376. [Google Scholar] [CrossRef]
- Wang, D.; Xu, A.; Elmerich, C.; Ma, L.Z. Biofilm Formation Enables Free-Living Nitrogen-Fixing Rhizobacteria to Fix Nitrogen under Aerobic Conditions. ISME J. 2017, 11, 1602–1613. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Li, L.J.; Shi, Q.S.; Ouyang, Y.S.; Chen, Y.B.; Hu, W.F. Effects of Nutritional and Environmental Conditions on Planktonic Growth and Biofilm Formation of Citrobacter werkmanii BF-6. J. Microbiol. Biotechnol. 2013, 23, 1673–1682. [Google Scholar] [CrossRef]
- Haque, M.M.; Mosharaf, M.K.; Haque, M.A.; Tanvir, M.Z.H.; Alam, M.K. Biofilm Formation, Production of Matrix Compounds and Biosorption of Copper, Nickel and Lead by Different Bacterial Strains. Front. Microbiol. 2021, 12, 615113. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, G.F.; Bukhari, M.A. Factors Influencing Bacterial Biofilm Formation and Development. Am. J. Biomed. Sci. Res. 2021, 12, 617–626. [Google Scholar] [CrossRef]
- Helman, Y.; Chernin, L. Silencing the Mob: Disrupting Quorum Sensing as a Means to Fight Plant Disease. Mol. Plant Pathol. 2015, 16, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, S.K.; Chowdhury, I.; Singh, R. Understanding the Mechanism of Bacterial Biofilms Resistance to Antimicrobial Agents. Open Microbiol. J. 2017, 11, 53–62. [Google Scholar] [CrossRef]
- Harjai, K.; Sabharwal, N. Biofilm Formation and Quorum Sensing in Rhizosphere. In Biofilms in Plant and Soil Health; Ahmad, I., Husain, F.M., Eds.; John Wiley & Sons Ltd: London, UK, 2017; pp. 111–130. ISBN 9781119246343. [Google Scholar] [CrossRef]
- Talagrand-Reboul, E.; Jumas-Bilak, E.; Lamy, B. The Social Life of Aeromonas through Biofilm and Quorum Sensing Systems. Front. Microbiol. 2017, 8, 37. [Google Scholar] [CrossRef]
- Clements, T.; Ndlovu, T.; Khan, S.; Khan, W. Biosurfactants Produced by Serratia Species: Classification, Biosynthesis, Production and Application. Appl. Microbiol. Biotechnol. 2019, 103, 589–602. [Google Scholar] [CrossRef]
- Luo, C.; Zhou, H.; Zou, J.; Wang, X.; Zhang, R.; Xiang, Y.; Chen, Z. Bacillomycin L and Surfactin Contribute Synergistically to the Phenotypic Features of Bacillus subtilis 916 and the Biocontrol of Rice Sheath Blight Induced by Rhizoctonia solani. Appl. Microbiol. Biotechnol. 2015, 99, 1897–1910. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Wu, X.; Wang, Y.; Dai, Y. Role of Biofilm Formation by Bacillus pumilus HR10 in Biocontrol against Pine Seedling Damping-Off Disease Caused by Rhizoctonia solani. Forests 2020, 11, 652. [Google Scholar] [CrossRef]
- Sayyed, R.; Seifi, S.; Patel, P.R.; Shaikh, S.S.; Jadhav, H.P.; Enshasy, H. El Siderophore Production in Groundnut Rhizosphere Isolate, Achromobacter sp. RZS2 Influenced by Physicochemical Factors and Metal Ions. Environ. Sustain. 2019, 2, 117–124. [Google Scholar] [CrossRef]
- Mishra, P.; Mishra, J.; Dwivedi, S.K.; Arora, N. Microbial Enzymes in Biocontrol of Phytopathogens. In Microbial Enzymes: Roles and Applications in Industries; Arora, N., Mishra, J., Mishra, V., Eds.; Springer: Singapore, 2020; pp. 259–285. ISBN 9789811517105. [Google Scholar] [CrossRef]
- Ahmed, E.; Holmström, S.J.M. Minireview Siderophores in Environmental Research: Roles and Applications. Microb. Biotechnol. 2014, 7, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Luo, X.; Liu, J.; Lu, H. Novel Functional Metabolites That Affect Biofilm Formation Are Regulated by Bioavailable Iron with Siderophore-Dependent Pathway. SSRN Electron. J. 2020. [Google Scholar] [CrossRef]
- Harrison, F.; Buckling, A. Siderophore Production and Biofilm Formation as Linked Social Traits. ISME J. 2009, 3, 632–634. [Google Scholar] [CrossRef]
- Tovi, N.; Frenk, S.; Hadar, Y.; Minz, D. Host Specificity and Spatial Distribution Preference of Three Pseudomonas Isolates. Front. Microbiol. 2019, 10, 3263. [Google Scholar] [CrossRef]
- Haggag, W.M.; Timmusk, S. Colonization of Peanut Roots by Biofilm-Forming Paenibacillus polymyxa Initiates Biocontrol against Crown Rot Disease. J. Appl. Microbiol. 2008, 104, 961–969. [Google Scholar] [CrossRef]
- Harting, R.; Nagel, A.; Nesemann, K.; Höfer, A.M.; Bastakis, E.; Kusch, H.; Stanley, C.E.; Stöckli, M.; Kaever, A.; Hoff, K.J.; et al. Pseudomonas Strains Induce Transcriptional and Morphological Changes and Reduce Root Colonization of Verticillium spp. Front. Microbiol. 2021, 12, 652468. [Google Scholar] [CrossRef]
- Stoll, A.; Salvatierra-Martínez, R.; González, M.; Araya, M. The Role of Surfactin Production by Bacillus velezensis on Colonization, Biofilm Formation on Tomato Root and Leaf Surfaces and Subsequent Protection (ISR) against Botrytis cinerea. Microorganisms 2021, 9, 2251. [Google Scholar] [CrossRef]
- Azri, M.H.; Ismail, S.; Abdullah, R. An Endophytic Bacillus Strain Promotes Growth of Oil Palm Seedling by Fine Root Biofilm Formation. Rhizosphere 2018, 5, 1–7. [Google Scholar] [CrossRef]
- Hazarika, S.N.; Saikia, K.; Borah, A.; Thakur, D. Prospecting Endophytic Bacteria Endowed With Plant Growth Promoting Potential Isolated From Camellia sinensis. Front. Microbiol. 2021, 12, 738058. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Yang, D.; Wang, D.; Miao, Y.; Shao, J.; Zhou, X.; Xu, Z.; Li, Q.; Feng, H.; Li, S.; et al. Whole Transcriptomic Analysis of the Plant-Beneficial Rhizobacterium Bacillus amyloliquefaciens SQR9 during Enhanced Biofilm Formation Regulated by Maize Root Exudates. BMC Genom. 2015, 16, 685. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, R.L.; Vismans, G.; Yu, K.; Song, Y.; De Jonge, R.; Burgman, W.P.; Burmølle, M.; Herschend, J.; Bakker, P.A.H.M.; Pieterse, C.M.J. Disease-Induced Assemblage of a Plant-Beneficial Bacterial Consortium. ISME J. 2018, 12, 1496–1507. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.; Mosharaf, K.; Khatun, M.; Shozib, H.B.; Miah, M.U.; Molla, A.H. Biofilm Producing Rhizobacteria With Multiple Plant Growth-Promoting Traits Promote Growth of Tomato Under Water-Deficit Stress. Front. Microbiol. 2020, 11, 542053. [Google Scholar] [CrossRef]
- Mahdi, I.; Fahsi, N.; Hafidi, M.; Allaoui, A.; Biskri, L. Plant Growth Enhancement Using Rhizospheric Halotolerant Phosphate Solubilizing Bacterium Bacillus licheniformis Qa1 and Enterobacter asburiae Qf11 Isolated from Chenopodium quinoa Willd. Microorganisms 2020, 8, 948. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Jiang, C.; Zhang, L.; Chen, L.; Zhang, X. Biofilms Positively Contribute to Bacillus amyloliquefaciens 54-Induced Drought Tolerance in Tomato Plants. Int. J. Mol. Sci. 2019, 20, 6271. [Google Scholar] [CrossRef] [PubMed]
- Ansari, F.A.; Jabeen, M.; Ahmad, I. Pseudomonas azotoformans FAP5, a Novel Biofilm-Forming PGPR Strain, Alleviates Drought Stress in Wheat Plant. Int. J. Environ. Sci. Technol. 2021, 18, 3855–3870. [Google Scholar] [CrossRef]
- Yang, N.; Nesme, J.; Røder, H.L.; Li, X.; Zuo, Z.; Petersen, M.; Burmølle, M.; Sørensen, S.J. Emergent Bacterial Community Properties Induce Enhanced Drought Tolerance in Arabidopsis. NPJ Biofilms Microbiomes 2021, 7, 82. [Google Scholar] [CrossRef]
- Costerton, J.; Geesey, G.; Cheng, K. How Bacteria Stick. Sci. Am. 1978, 238, 86–95. [Google Scholar] [CrossRef]
- Lam, J.; Chan, R.; Lam, K.; Costerton, J. Production of Mucoid Microcolonies by Pseudomonas aeruginosa within Infected Lungs in Cystic Fibrosis. Infect. Immun. 1980, 28, 546–556. [Google Scholar] [CrossRef]
- Ahmad, I.; Khan, M.; Altaf, M.; Qais, A.; Ansari, F.; Rumbaugh, K. Biofilms: An Overview of Their Significance in Plant and Soil Health. In Biofilms in Plant and Soil Health; Ahmad, I., Husain, F., Eds.; John Wiley and Sons Ltd: London, UK, 2017; p. 585. ISBN 9781119246343. [Google Scholar]
- Branda, S.; Gonzalez-Pator, J.; Ben-Yehuda, S.; Losick, R.; Kolter, R. Fruiting Body Formation by Bacillus subtilis. Proc. Natl. Acad. Sci. USA 2001, 98, 11621–11626. [Google Scholar] [CrossRef]
- Kinsinger, R.F.; Shirk, M.C.; Fall, R. Rapid Surface Motility in Bacillus subtilis Is Dependent on Extracellular Surfactin and Potassium Ion. J. Bacteriol. 2003, 185, 5627–5631. [Google Scholar] [CrossRef] [PubMed]
- López, D.; Fischbach, M.A.; Chu, F.; Losick, R.; Kolter, R. Structurally Diverse Natural Products That Cause Potassium Leakage Trigger Multicellularity in Bacillus subtilis. Proc. Natl. Acad. Sci. USA 2009, 106, 280–285. [Google Scholar] [CrossRef] [PubMed]
- McLoon, A.L.; Guttenplan, S.B.; Kearns, D.B.; Kolter, R.; Losick, R. Tracing the Domestication of a Biofilm-Forming Bacterium. J. Bacteriol. 2011, 193, 2027–2034. [Google Scholar] [CrossRef] [PubMed]
- Zeriouh, H.; de Vicente, A.; Pérez-García, A.; Romero, D. Surfactin Triggers Biofilm Formation of Bacillus subtilis in Melon Phylloplane and Contributes to the Biocontrol Activity. Environ. Microbiol. 2014, 16, 2196–2211. [Google Scholar] [CrossRef]
- Bais, H.; Fall, R.; Vivanco, J.M. Biocontrol of Bacillus subtilis against Infection of Arabidopsis Roots by Pseudomonas syringae Is Facilitated by Biofilm Formation and Surfactin Production. Plant Physiol. 2004, 134, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Selin, C.; Habibian, R.; Poritsanos, N.; Athukorala, S.N.P.; Fernando, D.; De Kievit, T.R. Phenazines Are Not Essential for Pseudomonas Chlororaphis PA23 Biocontrol of Sclerotinia sclerotiorum, but Do Play a Role in Biofilm Formation. FEMS Microbiol. Ecol. 2010, 71, 73–83. [Google Scholar] [CrossRef]
- Sang, M.K.; Kim, K.D. Biocontrol Activity and Root Colonization by Pseudomonas corrugata Strains CCR04 and CCR80 against Phytophthora Blight of Pepper. BioControl 2014, 59, 437–448. [Google Scholar] [CrossRef]
- Timmusk, S.; Van West, P.; Gow, N.A.R.; Paul Huffstutler, R. Paenibacillus polymyxa Antagonizes Oomycete Plant Pathogens Phytophthora palmivora and Pythium aphanidermatum. J. Appl. Microbiol. 2009, 106, 1473–1481. [Google Scholar] [CrossRef]
- Ruiu, L. Plant-Growth-Promoting Bacteria (PGPB) against Insects and Other Agricultural Pests. Agronomy 2020, 10, 861. [Google Scholar] [CrossRef]
- Fan, B.; Borriss, R.; Bleiss, W.; Wu, X. Gram-Positive Rhizobacterium Bacillus amyloliquefaciens FZB42 Colonizes Three Types of Plants in Different Patterns. J. Microbiol. 2012, 50, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Romera, F.J.; García, M.J.; Lucena, C.; Martínez-Medina, A.; Aparicio, M.A.; Ramos, J.; Alcántara, E.; Angulo, M.; Pérez-Vicente, R. Induced Systemic Resistance (ISR) and Fe Deficiency Responses in Dicot Plants. Front. Plant Sci. 2019, 10, 287. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Ryu, C. Understanding Plant Social Networking System: Avoiding Deleterious Microbiota but Calling Beneficials. Int. J. Mol. Sci. 2021, 22, 3319. [Google Scholar] [CrossRef] [PubMed]
- Timmermann, T.; Poupin, M.J.; Vega, A.; Urrutia, C.; Ruz, G.A.; González, B. Gene Networks Underlying the Early Regulation of Paraburkholderia phytofirmans PsJN Induced Systemic Resistance in Arabidopsis. PLoS ONE 2019, 14, e0221358. [Google Scholar] [CrossRef] [PubMed]
- Nie, P.; Li, X.; Wang, S.; Guo, J.; Zhao, H.; Niu, D. Induced Systemic Resistance against Botrytis cinerea by Bacillus cereus AR156 through a JA/ET- and NPR1-Dependent Signaling Pathway and Activates PAMP-Triggered Immunity in Arabidopsis. Front. Plant Sci. 2017, 8, 238. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, C.M.J.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.M.; Bakker, P.A.H.M. Induced Systemic Resistance by Beneficial Microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef] [PubMed]
- Niu, D.D.; Liu, H.X.; Jiang, C.H.; Wang, Y.P.; Wang, Q.Y.; Jin, H.L.; Guo, J.H. The Plant Growth-Promoting Rhizobacterium Bacillus cereus AR156 Induces Systemic Resistance in Arabidopsis thaliana by Simultaneously Activating Salicylate- and Jasmonate/Ethylene-Dependent Signaling Pathways. Mol. Plant-Microbe Interact. 2011, 24, 533–542. [Google Scholar] [CrossRef]
- Zebelo, S.; Song, Y.; Kloepper, J.W.; Fadamiro, H. Rhizobacteria Activates (+)-δ-Cadinene Synthase Genes and Induces Systemic Resistance in Cotton against Beet Armyworm (Spodoptera exigua). Plant Cell Environ. 2016, 39, 935–943. [Google Scholar] [CrossRef]
- Fazle Rabbee, M.; Baek, K.H. Antimicrobial Activities of Lipopeptides and Polyketides of Bacillus velezensis for Agricultural Applications. Molecules 2020, 25, 4973. [Google Scholar] [CrossRef] [PubMed]
- Hazarika, D.J.; Goswami, G.; Gautom, T.; Parveen, A.; Das, P.; Barooah, M.; Boro, R.C. Lipopeptide Mediated Biocontrol Activity of Endophytic Bacillus subtilis against Fungal Phytopathogens. BMC Microbiol. 2019, 19, 71. [Google Scholar] [CrossRef]
- Taktek, S.; St-Arnaud, M.; Piché, Y.; Fortin, J.A.; Antoun, H. Igneous Phosphate Rock Solubilization by Biofilm-Forming Mycorrhizobacteria and Hyphobacteria Associated with Rhizoglomus irregulare DAOM 197198. Mycorrhiza 2017, 27, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Hettiarachchi, R.P.; Dharmakeerthi, R.S.; Seneviratne, G.; Jayakody, A.N.; De Silva, K.E.; Gunathilake, T.; Thewarapperuma, A. Determination of Desirable Properties of Bacteria, Fungi and Their Biofilm Associated with Rubber Rhizosphere. Trop. Agric. Res. 2016, 27, 399. [Google Scholar] [CrossRef]
- Bandara, W.M.M.S.; Seneviratne, G.; Kulasooriya, S.A. Interactions among Endophytic Bacteria and Fungi: Effects and Potentials. J. Biosci. 2006, 31, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Browning, M.; Wallace, D.B.; Dawson, C.; Alm, S.R.; Amador, J.A. Potential of Butyric Acid for Control of Soil-Borne Fungal Pathogens and Nematodes Affecting Strawberries. Soil Biol. Biochem. 2006, 38, 401–404. [Google Scholar] [CrossRef]
- Ren, D.; Madsen, J.S.; Sørensen, S.J.; Burmølle, M. High Prevalence of Biofilm Synergy among Bacterial Soil Isolates in Cocultures Indicates Bacterial Interspecific Cooperation. ISME J. 2015, 9, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, R.; Bidyarani, N.; Babu, S.; Hossain, F.; Shivay, Y.S.; Nain, L. Cyanobacterial Inoculation Elicits Plant Defense Response and Enhanced Zn Mobilization in Maize Hybrids. Cogent Food Agric. 2015, 1, 998507. [Google Scholar] [CrossRef]
- Amaya-Gómez, C.V.; Porcel, M.; Mesa-Garrig, L.; Gómez-Álvarez, M.I. A Framework for the Selection of Plant Growth-Promoting Rhizobacteria Based on Bacterial Competence Mechanisms. Appl. Environ. Microbiol. 2020, 86, e00760-20. [Google Scholar] [CrossRef]
- Yasmeen, T.; Ahmad, A.; Arif, M.S.; Mubin, M.; Rehman, K.; Shahzad, S.M.; Iqbal, S.; Rizwan, M.; Ali, S.; Alyemeni, M.N.; et al. Biofilm Forming Rhizobacteria Enhance Growth and Salt Tolerance in Sunflower Plants by Stimulating Antioxidant Enzymes Activity. Plant Physiol. Biochem. 2020, 156, 242–256. [Google Scholar] [CrossRef]
- Timmusk, S.; Abd El-Daim, I.A.; Copolovici, L.; Tanilas, T.; Kännaste, A.; Behers, L.; Nevo, E.; Seisenbaeva, G.; Stenström, E.; Niinemets, Ü. Drought-Tolerance of Wheat Improved by Rhizosphere Bacteria from Harsh Environments: Enhanced Biomass Production and Reduced Emissions of Stress Volatiles. PLoS ONE 2014, 9, e96086. [Google Scholar] [CrossRef]
- Nocelli, N.; Bogino, P.C.; Banchio, E.; Giordano, W. Roles of Extracellular Polysaccharides and Biofilm Formation in Heavy Metal Resistance of Rhizobia. Materials 2016, 9, 418. [Google Scholar] [CrossRef]
- Ozdemir, G.; Ceyhan, N.; Manav, E. Utilization in Alginate Beads for Cu(II) and Ni(II) Adsorption of an Exopolysaccharide Produced by Chryseomonas luteola TEM05. World J. Microbiol. Biotechnol. 2005, 21, 163–167. [Google Scholar] [CrossRef]

| Functional Trait | PGPB | Plant Species | Inoculation of PGPB | Enhancement | Reference |
|---|---|---|---|---|---|
| Biocontrol | P. polymyxa B5 | Peanut (Arachis hypogaea L.) | Seed | 96.7% biocontrol efficacy 43.5% plant yield | [125] |
| B. pumilus HR10 | Masson pine (Pinus massoniana) | Roots | 76.8% biocontrol efficacy | [118] | |
| P. synxantha, P. brassicacearum | Arabidopsis thaliana | Roots | 81% and 82% biocontrol efficacy, respectively | [126] | |
| B. subtilis 916 | Rice (Oryza sativa L.) | Rice sheaths | 60% biocontrol efficacy | [117] | |
| B. velezensis BBC047 | Tomato (Solanum lycopersicum L.) | Roots; leaves | ±66% and ±53% biocontrol efficacy, respectively | [127] | |
| Plant growth promotion | B. salmalaya 139SI | Oil palm (Elaeis guineensis Jacq.) | Seedling and soil | 55.4% stem height 66.7% stem dry weight | [128] |
| Consortium of Pseudomonas sp. M45 and Stenotrophomonas sp. K96 | Tea (Camellia sinensis) | Roots | 4.85-fold shoot length 4.65-fold root length | [129] | |
| B. amyloliquefaciens SQR9 | Maize (Zea mays L.) | Roots | 42–60% biomass 32–46% shoot height 33–49% root length | [130] | |
| Plant growth promotion, biocontrol | Consortium of Microbacterium, Stenotrophomonas, Xanthomonas | Arabidopsis thaliana L. | Soil | ±31% shoot fresh weight ±36% biocontrol efficacy | [131] |
| Plant growth promotion, drought tolerance | B. aryabhattai ESB6, P. azotoformans ESR4, P. cedrina ESR12, P. chlororaphis ESR15, P. gessardii ESR9, P. poae ESR6, P. veronii ESR21, S. Maltophilia ESR20 | Tomato (Solanum lycopersicum L.) | Roots | 11%, 14%, 7%, 6%, 8%, 10%, 3%, and 12% plant height, respectively 18%, 33%, 22%, 18%, 3%, 29%, 17%, and 2.5% root dry weight, respectively | [132] |
| Plant growth promotion, salinity tolerance | B. licheniformis QA1, E. asburiae QF11 | Quinoa (Chenopodium quinoa Willd.) | Seeds and soil | ±42% and ±46% root length, respectively ±46% and ±13% shoot length, respectively | [133] |
| Drought tolerance | B. amyloliquefaciens 54 | Tomato (Solanum lycopersicum L.) | Roots | ±15% relative water content of leaves | [134] |
| P. azotoforman FAP5 | Wheat (Triticum aestivum L.) | Seeds | 9% shoot length 14% root length 10% shoot dry weight 16% root dry weight | [135] | |
| Consortium of Microbacterium oxydans, Paenibacillus amylolyticus, Stenotrophomonas rhizophila, Xanthomonas retroflexus | Arabidopsis thaliana | Rhizosphere | 2-fold fresh weight 1.5-fold diameter of rosettes 1.5-fold chlorophylls content | [136] | |
| Salinity tolerance | B. amyloliquifaciens | Barley (Hordeum vulgare L.) | Seeds and soil | 23% root dry weight 43% shoot dry weight | [57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ajijah, N.; Fiodor, A.; Pandey, A.K.; Rana, A.; Pranaw, K. Plant Growth-Promoting Bacteria (PGPB) with Biofilm-Forming Ability: A Multifaceted Agent for Sustainable Agriculture. Diversity 2023, 15, 112. https://doi.org/10.3390/d15010112
Ajijah N, Fiodor A, Pandey AK, Rana A, Pranaw K. Plant Growth-Promoting Bacteria (PGPB) with Biofilm-Forming Ability: A Multifaceted Agent for Sustainable Agriculture. Diversity. 2023; 15(1):112. https://doi.org/10.3390/d15010112
Chicago/Turabian StyleAjijah, Nur, Angelika Fiodor, Alok Kumar Pandey, Anuj Rana, and Kumar Pranaw. 2023. "Plant Growth-Promoting Bacteria (PGPB) with Biofilm-Forming Ability: A Multifaceted Agent for Sustainable Agriculture" Diversity 15, no. 1: 112. https://doi.org/10.3390/d15010112
APA StyleAjijah, N., Fiodor, A., Pandey, A. K., Rana, A., & Pranaw, K. (2023). Plant Growth-Promoting Bacteria (PGPB) with Biofilm-Forming Ability: A Multifaceted Agent for Sustainable Agriculture. Diversity, 15(1), 112. https://doi.org/10.3390/d15010112







