A New Species of Parasitic Copepod, Nemesis santhadevii (Siphonostomatoida: Eudactylinidae) from the Gills of the Coral Catshark Atelomycterus marmoratus, from Kota Kinabalu, Malaysia †
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Taxonomy
3.1.1. Type—Host
3.1.2. Type—Locality
3.1.3. Type—Material
3.1.4. Site on Host
3.1.5. Etymology
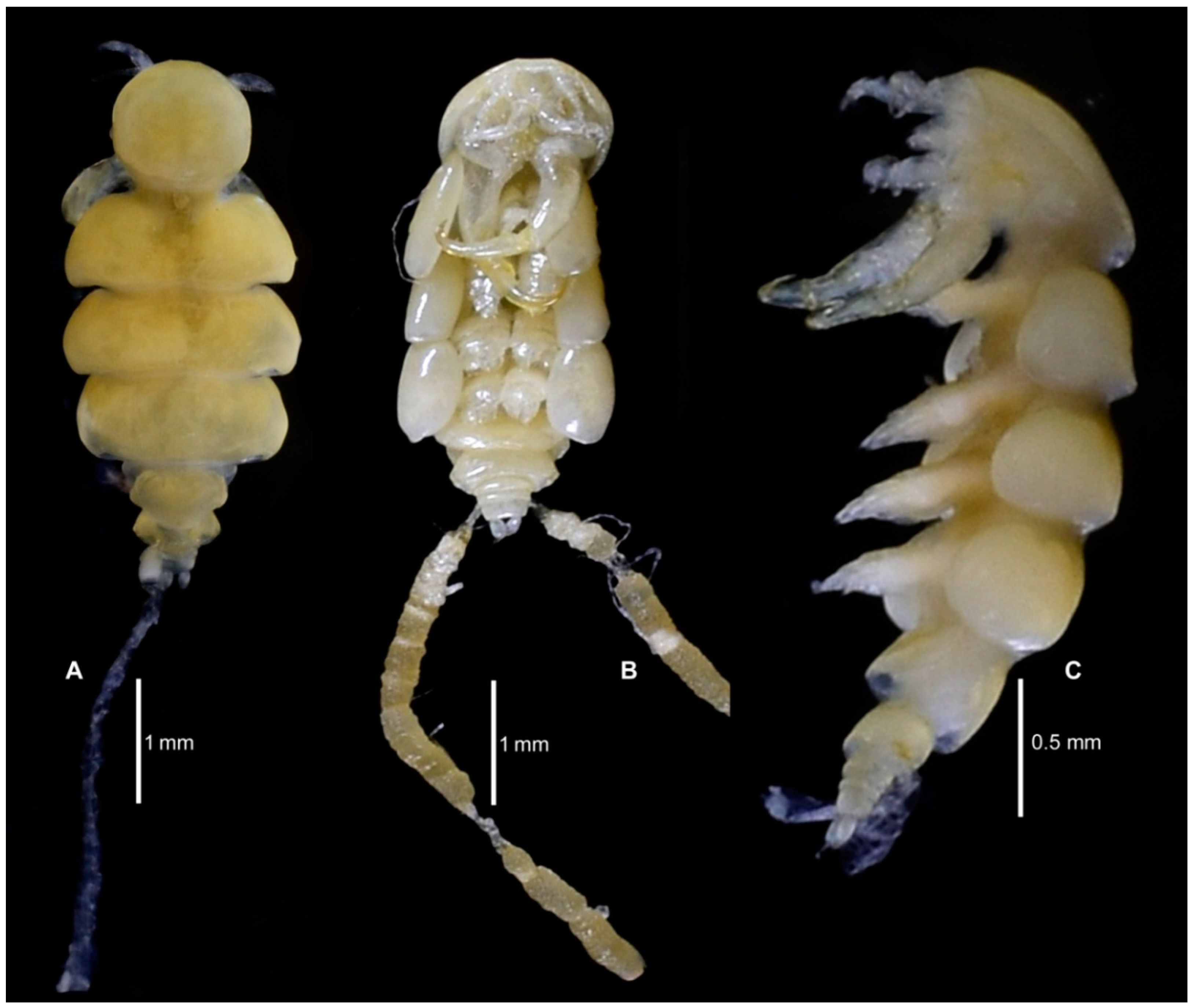
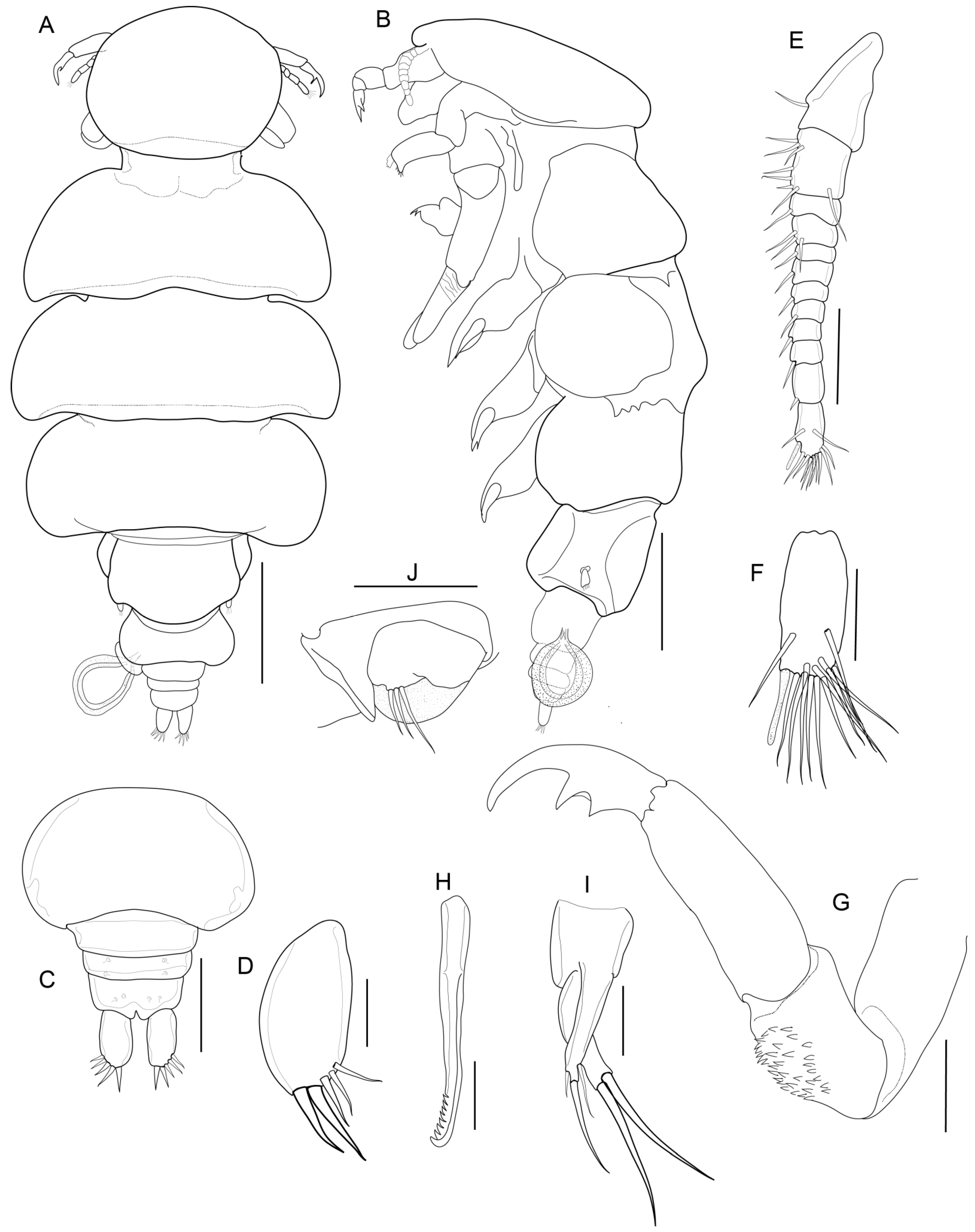
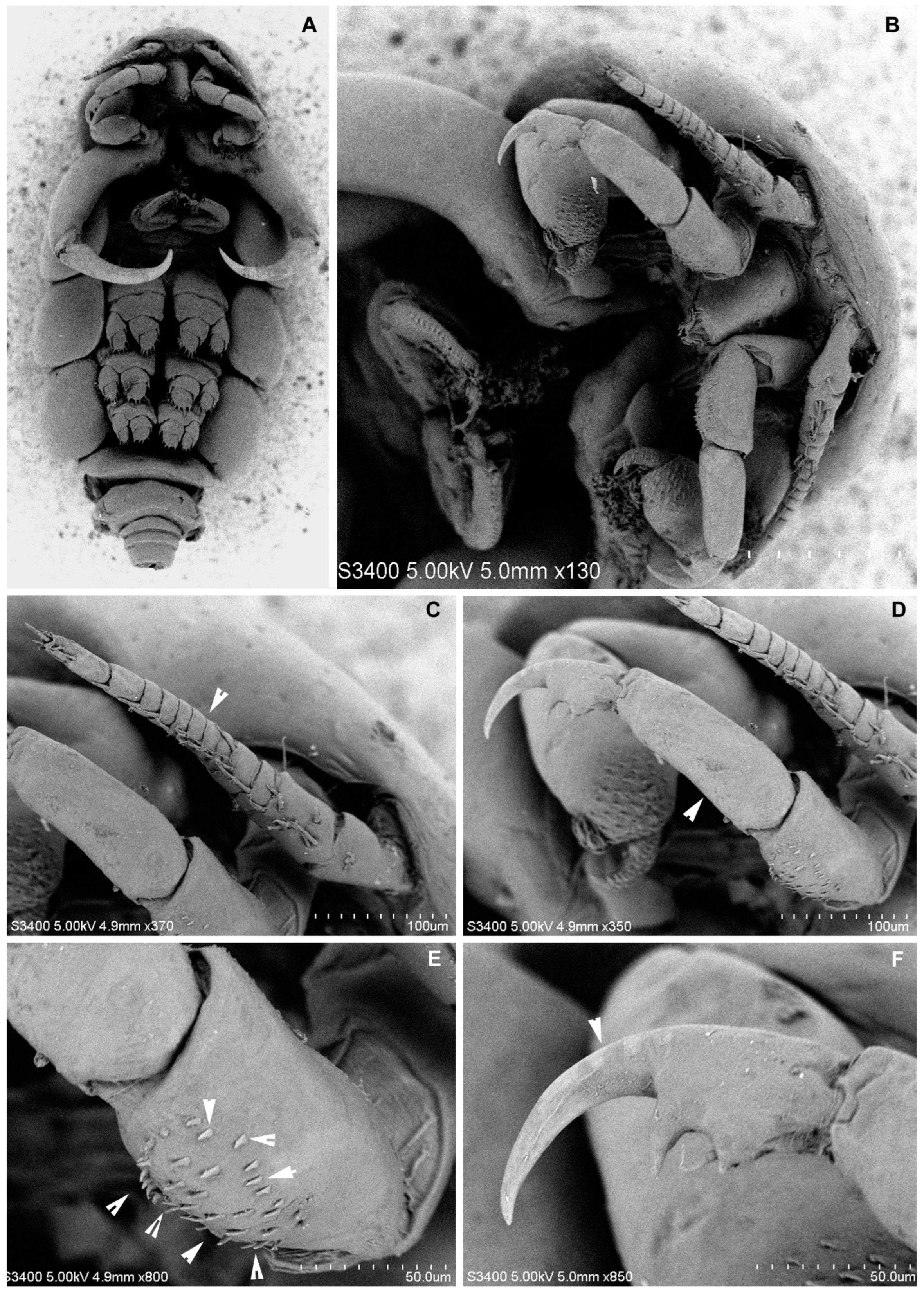
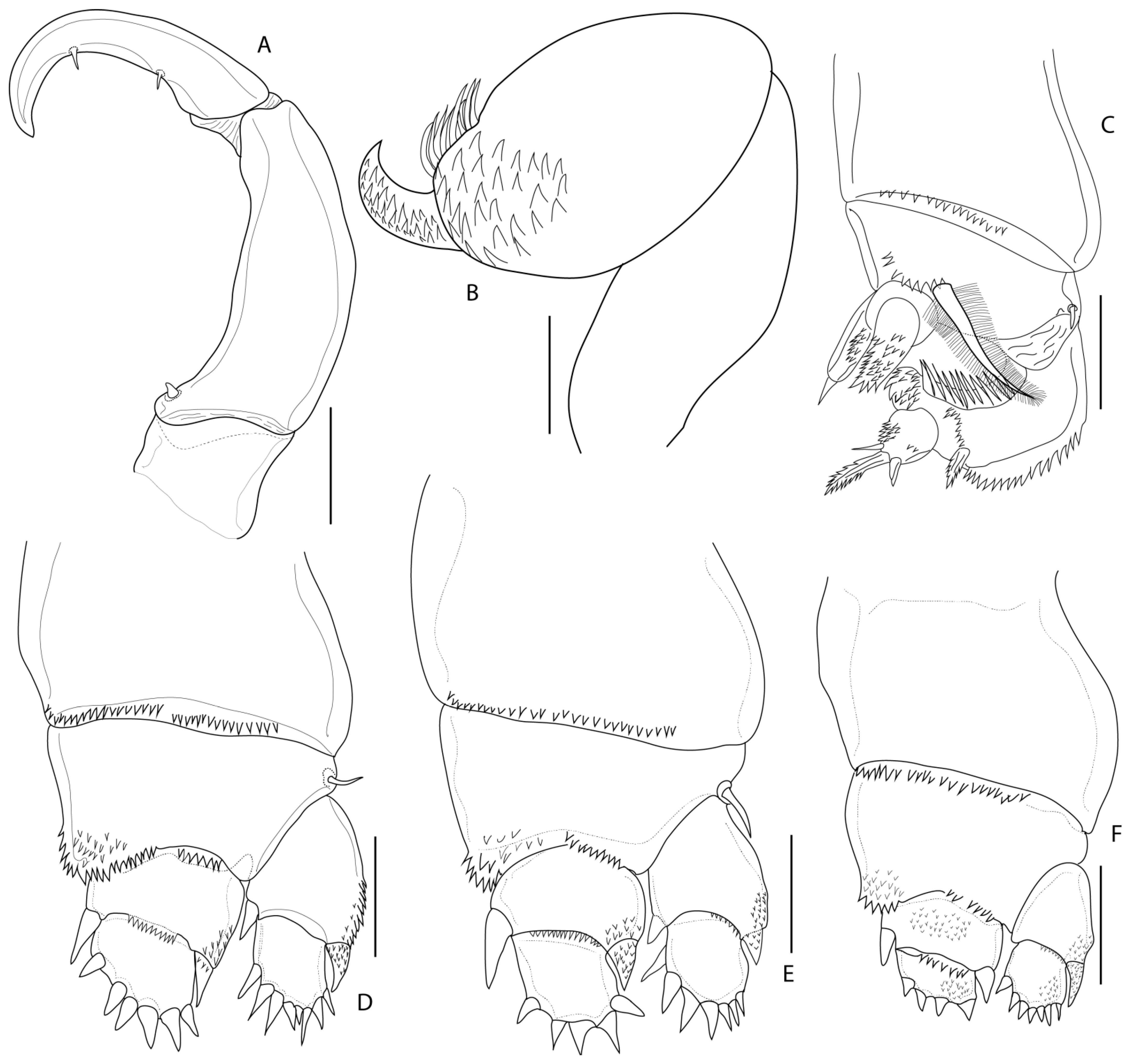
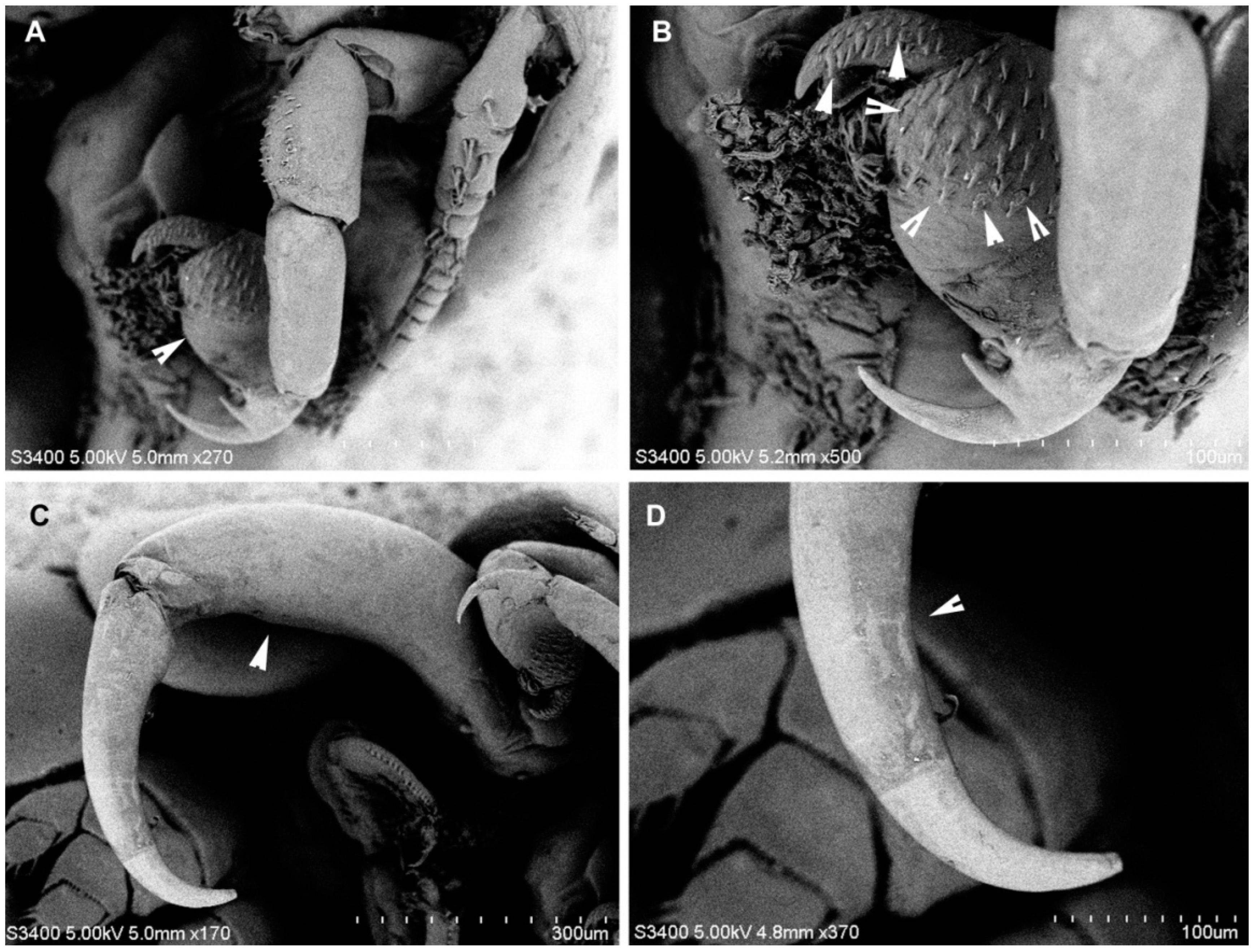
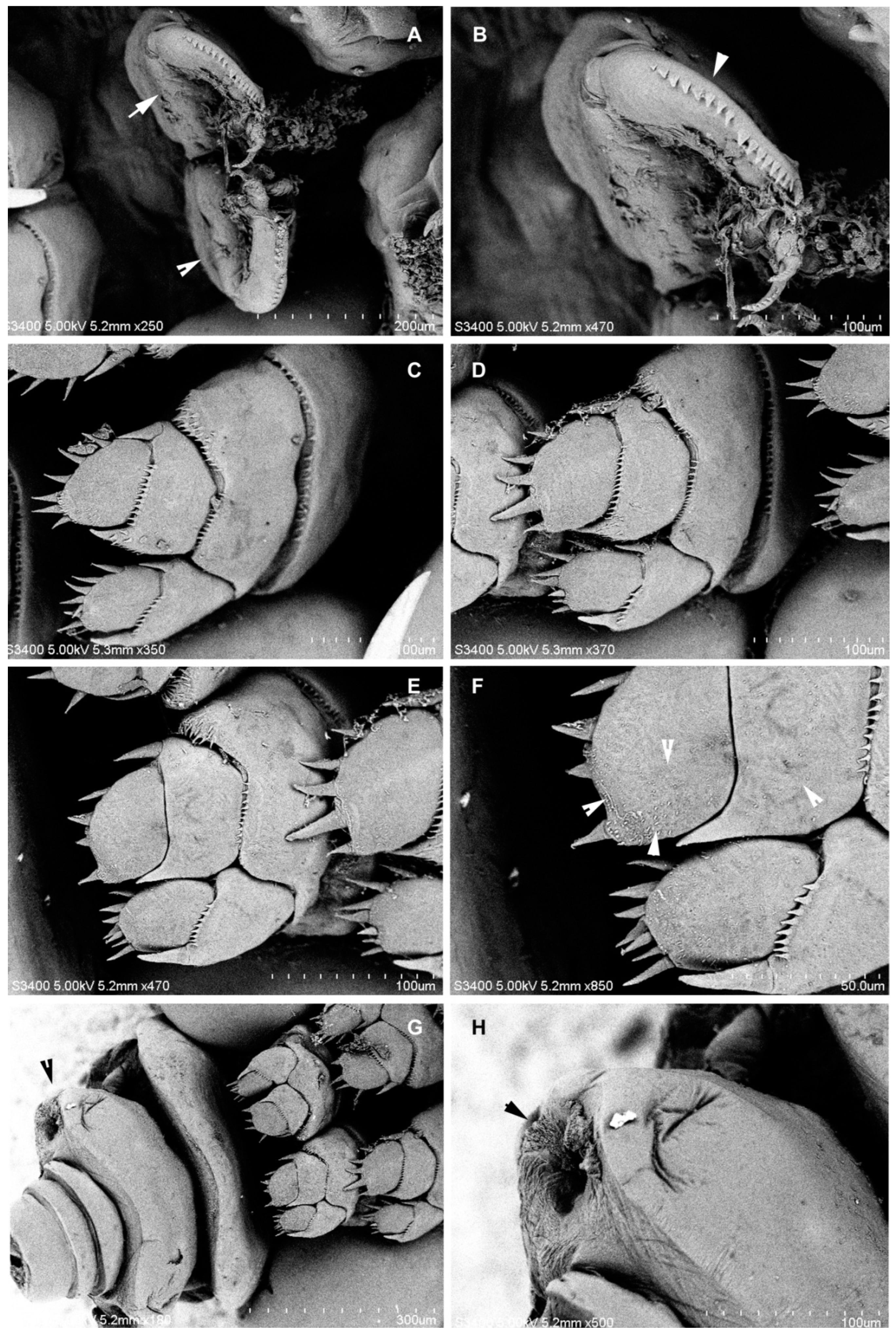
3.1.6. Description
3.1.7. Distribution
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walter, T.C.; Boxshall, G.A. World of Copepods Database. 2022. Available online: http://www.marinespecies.org/copepoda (accessed on 5 September 2022).
- Risso, A. Histoire Naturelle des Principales Productions de 1 ‘Europe Meridionale et Particulierement de Celles des Environs de Nice et des Alpes Maritimes; Levrault: Paris, France, 1826; Volume 5, pp. 1–402. [Google Scholar]
- Wilson, C.B. Crustacean parasites of West Indian fishes and land crabs with descriptions of new genera and species. Proc. U. S. Nat. Mus. 1913, 44, 189–277. [Google Scholar] [CrossRef]
- Wilson, C.B. North American Parasitic Copepods Belonging to the Family Dichelesthiidae; US Government Printing Office: Washington, DC, USA, 1922; Volume 60, pp. 1–100.
- Scott, A. The copepod parasites of Irish Sea fishes. Rep. Lancashire Sea Fish Lab. 1929, 81–118. [Google Scholar]
- Wilson, C.B. The Copepoda of the Woods Hole region, Massachusetts. Bull. U. S. Natl. Mus. 1932, 158, 1–635. [Google Scholar]
- Pearse, A.S. Parasitic Crustacea from Bimini Bahamas. Proc. U. S. Natl. Mus. 1951, 101, 341–372. [Google Scholar] [CrossRef]
- Pearse, A.S. Parasitic Crustacea from the Texas coast. Pubis. Inst. Mar. Sci. Univ. Tex. 1952, 2, 5–42. [Google Scholar]
- Shiino, S.M. Copepods parasitic on Japanese fishes. 15. Eudactylinidae and Dichelesthiidae. Rep. Faculty Fish. Prefect. Univ. Mie. 1957, 2, 392–410. [Google Scholar]
- Cressey, R.F. Caligoid copepods parasitic on sharks of the Indian Ocean. Proc. U. S. Natl. Mus. 1967, 12, 1–21. [Google Scholar] [CrossRef]
- Cressey, R.F. Copepods parasitic on sharks from the east coast of Florida. Smith. Contr. Zool. 1970, 38, 1–30. [Google Scholar] [CrossRef]
- Rangnekar, M.P. Parasitic Copepods from Marine Fishes of Bombay; Crustaceana, Supplement, No. 7, Studies on Copepoda II; Brill: Leiden, The Netherlands, 1984; pp. 344–351. [Google Scholar]
- Froese, R.; Pauly, D. (Eds.) FishBase. World Wide Web Electronic Publication, Version (09/2009). 2022. Available online: www.fishbase.org (accessed on 20 August 2022).
- Humes, A.G.; Gooding, R.U. A method for studying the external anatomy of copepods. Crustaceana 1964, 6, 238–240. [Google Scholar] [CrossRef]
- Huys, R.; Boxshall, G.A. Copepod Evolution; The Ray Society: London, UK, 1991; p. 468. [Google Scholar]
- Fricke, R.; Eschmeyer, W.N.; van der Laan, R. (Eds.) Catalog of Fishes: Genera, Species, References. 2020. Available online: http://research.calacademy.org/research/ichthyology/catalog/fishcatmain.asp (accessed on 10 March 2022).
- Yamaguti, S. Parasitic Copepoda and Branchiura of Fishes; Interscience Publishers, John Wiley: New York, NY, USA, 1963; p. 723. [Google Scholar]
- Pillai, N.K. The Fauna of India: Copepod parasites of marine fishes. In The Fauna of India. Zoological Society of India; Calcutta; Government of India: New Delhi, India, 1985; p. 900. [Google Scholar]
- Hesse, C.E. Crustaces. Raresounoveaux des cotes de France. Ann. Sci. Natl. 1883, 15, 1–48. [Google Scholar]
- Raux, P. Crustaces de la Mediterraneeet de son Littoral; Levrault: Paris, France, 1828; p. 176. [Google Scholar]
- Heller, C. Crustaceen. In Zoologischer Teil in Reise der Oesterreichischen Fregatte Novara; Steindachner: Berlin, Germany, 1868; Volume 2, p. 280. [Google Scholar]
- Heegaard, P.E. Parasitic Copepoda from Australian waters. Rec. Aust. Mus. 1962, 25, 149–234. [Google Scholar] [CrossRef]
- Hewitt, G.C. Some New Zealand Parasitic Copepoda of the Family Eudactylinidae; Victoria University of Wellington: Wellington, New Zealand, 1969; Volume 49, pp. 1–31. [Google Scholar]
- Beneden, P.J.V. Note surun nouveau genre de Crustace parasite Eudactylina (E. acuta). Bull. Acad. R. Belg. 1853, 20, 235–239. [Google Scholar]
| Coxa | Basis | Exopod | Endopod | |||
|---|---|---|---|---|---|---|
| Proximal | Distal | Proximal | Distal | |||
| Leg 2 | 0-0 | 1-0 | 0-II | 0-VIII | 0-II | 0-VI |
| Leg 3 | 0-0 | 1-0 | 0-II | 0-VII | 0-II | 0-V |
| Leg 4 | 0-0 | 0-0 | 0-I | 0-VII | 0-II | 0-IV |
| Species | Morphological Characteristics | References | ||||
|---|---|---|---|---|---|---|
| Cephalothoracic Shield: Body Length | Comparative Size of second to fifth Pedigerous Somites | Fifth Pedigerous Somite | Nature of Spinules of Segments in Antenna | |||
| 1 | N. aggregatus Cressey, 1967 | 0.33:1 | Somites 2–4 equal in size, somite 5 is slightly narrower than the others | Slightly narrower than the fourth somite | A patch of 10–12 spinules on segment 2 | [10,18] |
| 2 | N. atlantica Wilson C.B., 1922 | 0.28:1 | Subequal in size | Abruptly narrower than the fourth somite | Patches of 25–40 spinules on segment 2 | [3,10] |
| 3 | N. carchariaeglauci (Hesse, 1883) | - | - | - | - | [19] |
| 4 | N. lamna Risso, 1826 | 0.25:1 | Equal in size | Equal to the fourth somite | Patches of 25–40 spinules on segment 2 | [2,5,6,10,11,18,20,21,22,23] |
| 5 | N. macrocephalus Shiino, 1957 | 0.39:1 | Decreasing in size posteriorly | Slightly narrower than the fourth somite | Patches of 25–40 spinules on segment 2 | [9] |
| 6 | N. pallida Wilson C.B., 1932 | 0.29:1 | Subequal in size | Slightly narrower than the fourth somite | Patches of 25–40 spinules on segment 2 | [6] |
| 7 | N. pilosus Pearse, 1951 | 0.33:1 | Decreasing in size posteriorly | Slightly narrower than the fourth somite | Patches of 25–40 spinules on segment 2 | [7,11] |
| 8 | N. robusta (Beneden, 1851) | 0.29:1 | Subequal in size | Abruptly narrower than the fourth somite | Patches of 25–40 spinules on segment 2 | [10,18,21,22,23,24] |
| 9 | N. santhadevii sp. nov. | 0.20:1 | Somites 2 and 3 are equal in size, somite 4 is slightly narrower than somite 3 | 0.4 times the width of the fourth somite | Patches of 34–38 short spinules on segment 2. It is stouter than segment 3. Segment 3 is unarmed. | Present study |
| 10 | N. sphyrnae Rangnekar, 1984 | 0.33:1 | Decreasing in size posteriorly | Abruptly narrower than the fourth somite | Two rows of 12–14 spinules on segment 2 | [12] |
| 11 | N. spinulosus Cressey, 1970 | 0.28:1 | Decreasing in size posteriorly | Slightly narrower than the fourth somite | Segment 2 with two patches of spinules, the inner patch composed of heavier spinules than the outer patch; segment 3 has a patch of spinules along the inner border | [11] |
| 12 | N. tiburo Pearse, 1952 | - | - | - | A row of spinules on segments 2 and 3 | [8] |
| 13 | N. versicolor Wilson C.B., 1913 | 0.29:1 | Subequal in size | Abruptly narrower than the fourth somite | A row of spinules on segments 2 and 3 | [3,10,17,18] |
| List of Species | Host | Distribution | References |
|---|---|---|---|
| N. aggregatus Cressey, 1967 | Alopias vulpinus (Bonnaterre, 1788) Alopias pelagicus (Nakamura, 1935) | Indian Ocean | [10,18] |
| N. atlantica Wilson C.B., 1922 | Rhizoprionodon terraenovae (Richardson, 1836), Carcharhinus brevipinna (Müller and Henle, 1839), Carcharhinus leucas (Müller and Henle, 1839), Carcharhinus limbatus (Müller and Henle, 1839), Carcharhinus acronotus (Poey, 1860), Sphyrna mokarran (Rüppell, 1837) | Beaufort, Florida | [3,10] |
| N. carchariaeglauci Hesse, 1883 | Prionace glauca (Linnaeus, 1758) Triakis semifasciata (Girard, 1855) | By generic transfer [19] | |
| N. lamna Risso, 1826 | Cetorhinus maximus (Gunnerus, 1765), Isurus oxyrinchus (Rafinesque, 1810), Carcharodon carcharias (Linnaeus, 1758), Lamna nasus (Bonnaterre, 1788), Alopias vulpinus, Odontaspis ferox (Risso, 1810), Lichia amia (Linnaeus, 1758) | Mediterranean, European Sea, California coast, Kerala Coast, India | [2,5,6,10,11,18,20,21,22,23] |
| N. macrocephalus Shiino, 1957 | Carcharhinus melanopterus (Quoy and Gaimard, 1824) | Hamazima, Mie Prefecture | [9] |
| N. pallida Wilson C.B., 1932 | Alopias vulpinus, Carcharhinus plumbeus (Nardo, 1827), Carcharias taurus (Rafinesque, 1810), Galeocerdo cuvier (Péron and Lesueur, 1822), Carcharodon carcharias, Carcharhinus obscurus (Lesueur, 1818) | Martha’s Vineyard (Atlantic) | [6] |
| N. pilosus Pearse, 1951 | Carcharias taurus (= Carcharias littoralis) (Type host) Negaprion brevirostris (Poey, 1868), Carcharhinus brevipinna, Carcharhinus limbatus | Bahamas (type locality) | [7,11] |
| N. robusta Beneden, 1851 | Carcharodon carcharias Lamna nasus | - | By generic transfer [10,18,21,22,23,24] |
| N. santhadeviisp. nov. | Atelomycterus marmoratus (Anonymous (Bennett), 1830) | Kota Kinabalu, Malaysia (Type locality) | Present study |
| N. sphyrnae Rangnekar, 1984 | Sphyrna zygaena (Linnaeus, 1758) | Bombay, India | [12] |
| N. spinulosus Cressey, 1970 | Carcharhinus milberti (Müller and Henle, 1839) (= Carcharhinus plumbeus ) (Type host), Carcharhinus obscurus | Sarasota, Florida | [11] |
| N. tiburo Pearse, 1952 | Sphyrna tiburo (Linnaeus, 1758) | Bahamas | [8] |
| N. versicolor Wilson C.B., 1913 | Sphyrna zygaena, Carcharhinus brevipinna | West Indies, Madagascar | [3,10,17,18] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venmathi Maran, B.A.; Aneesh, P.T.; Moon, S.Y. A New Species of Parasitic Copepod, Nemesis santhadevii (Siphonostomatoida: Eudactylinidae) from the Gills of the Coral Catshark Atelomycterus marmoratus, from Kota Kinabalu, Malaysia. Diversity 2022, 14, 759. https://doi.org/10.3390/d14090759
Venmathi Maran BA, Aneesh PT, Moon SY. A New Species of Parasitic Copepod, Nemesis santhadevii (Siphonostomatoida: Eudactylinidae) from the Gills of the Coral Catshark Atelomycterus marmoratus, from Kota Kinabalu, Malaysia. Diversity. 2022; 14(9):759. https://doi.org/10.3390/d14090759
Chicago/Turabian StyleVenmathi Maran, Balu Alagar, Panakkool Thamban Aneesh, and Seong Yong Moon. 2022. "A New Species of Parasitic Copepod, Nemesis santhadevii (Siphonostomatoida: Eudactylinidae) from the Gills of the Coral Catshark Atelomycterus marmoratus, from Kota Kinabalu, Malaysia" Diversity 14, no. 9: 759. https://doi.org/10.3390/d14090759
APA StyleVenmathi Maran, B. A., Aneesh, P. T., & Moon, S. Y. (2022). A New Species of Parasitic Copepod, Nemesis santhadevii (Siphonostomatoida: Eudactylinidae) from the Gills of the Coral Catshark Atelomycterus marmoratus, from Kota Kinabalu, Malaysia. Diversity, 14(9), 759. https://doi.org/10.3390/d14090759







