Antiparasitic Potential of Methanol Extract of Brown Alga Sargassum polycystum (Phaeophyceae) and Its LC-MS/MS Metabolite Profiling
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Sample Collection
2.3. Solvent Extraction
2.4. Antiparasitic Bioassay
2.5. Observation of Leech Behavior
2.6. Liquid Chromatography–Tandem Mass Spectrometry (LC-MS/MS) Acquisition
2.7. Data Analysis
2.8. Statistical Analysis
3. Results
3.1. Antiparasitic Properties of Sargassum polycystum
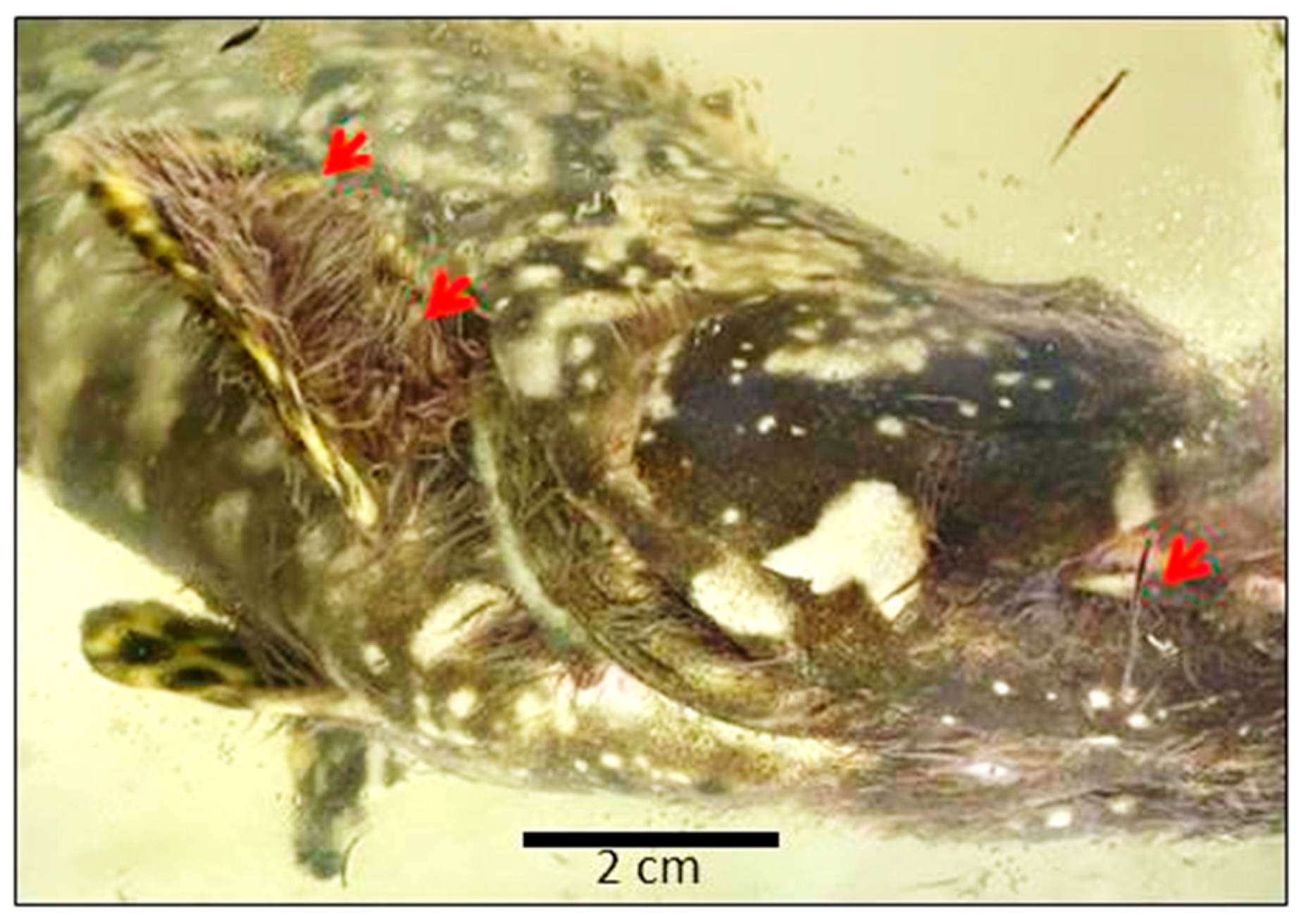
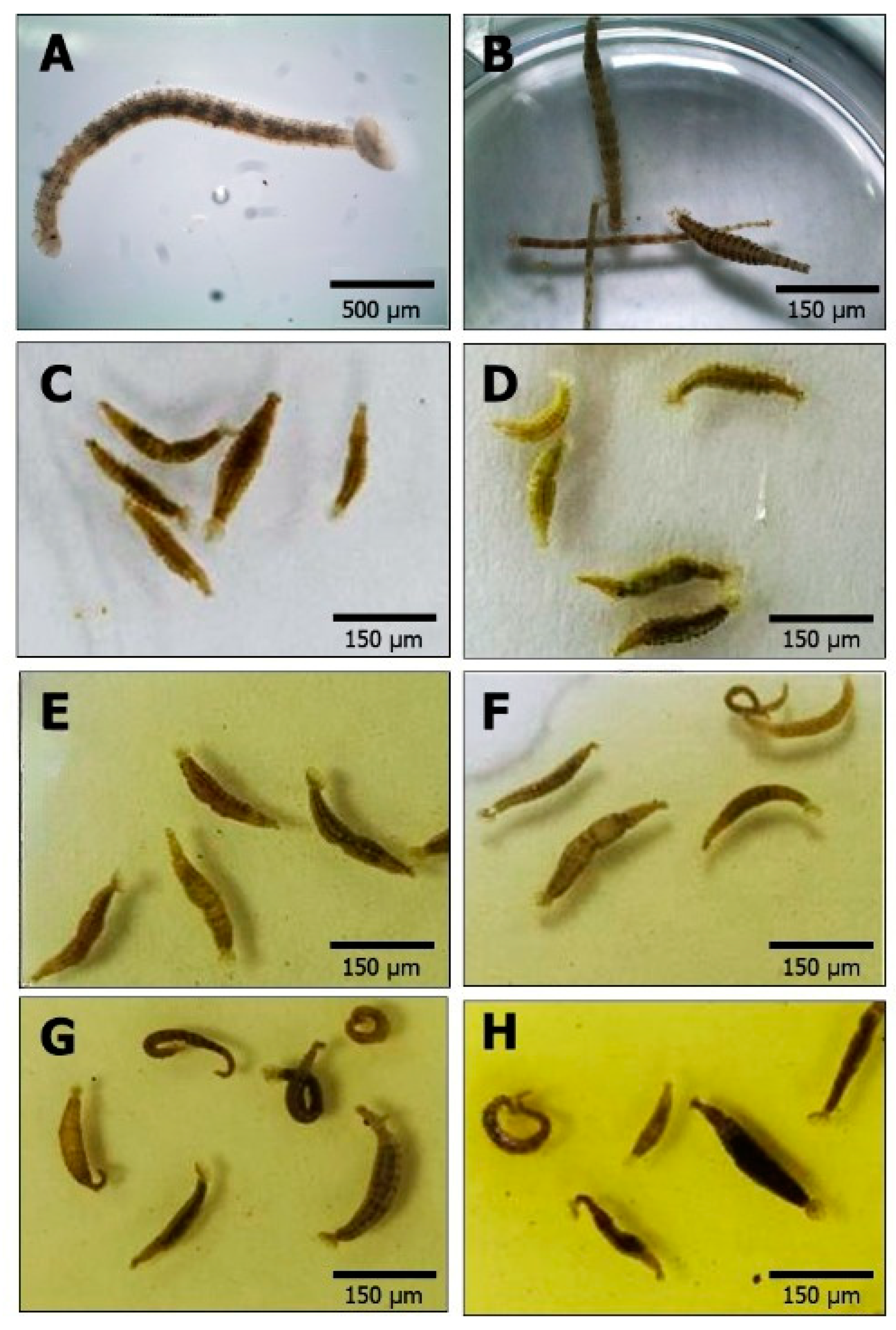
3.2. Behavior of Z. arugamensis
3.3. Physicochemical Parameters of Leeches Treated with Solutions
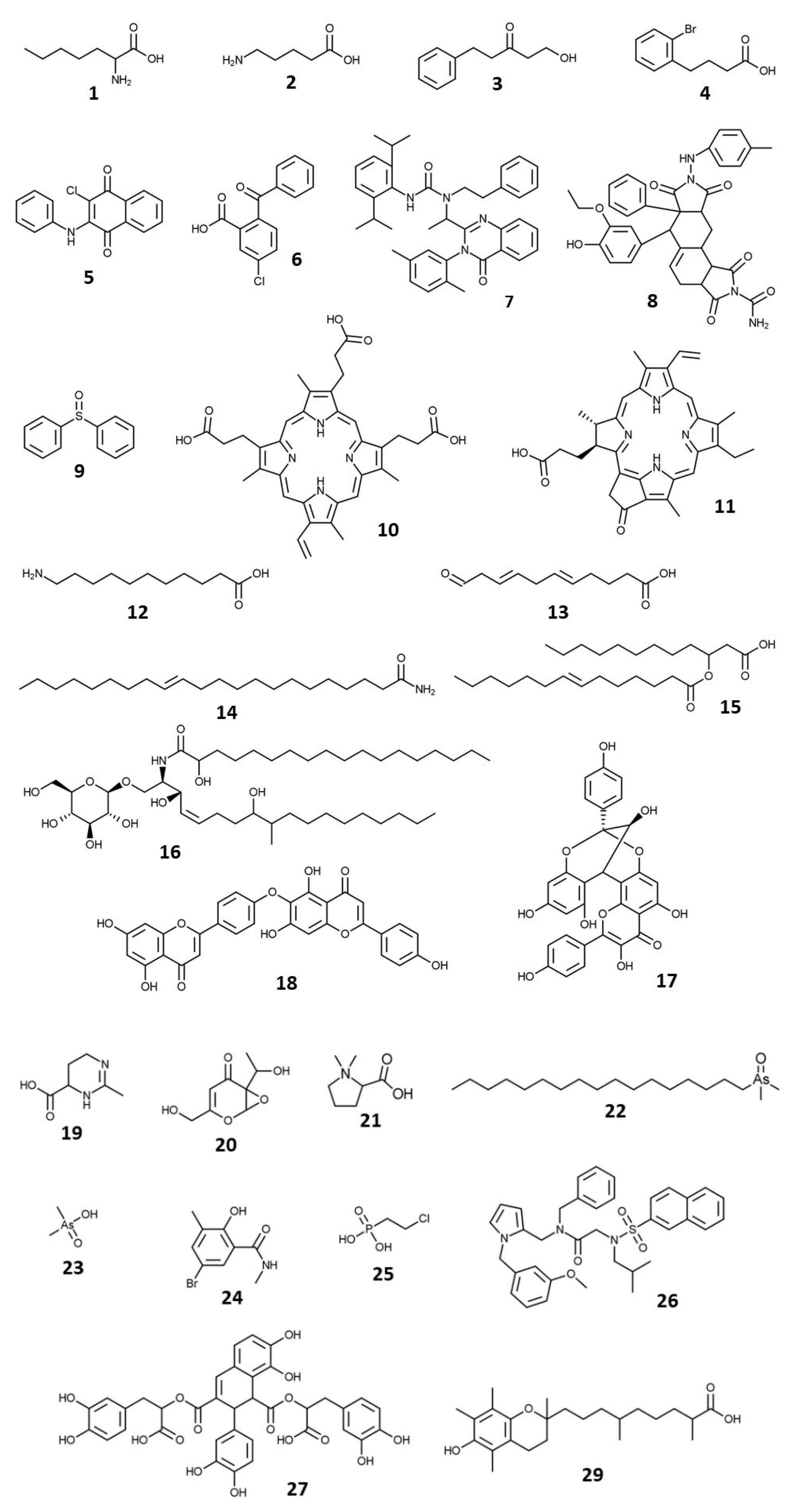
3.4. LC-MS Analysis and Metabolite Identification
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bergmann, W. Contributions to the study of marine products: II. The sterols of mollusks. J. Biol. Chem. 1934, 104, 317–328. [Google Scholar] [CrossRef]
- Shah, M.D.; Venmathi Maran, B.A.; Shaleh, S.R.M.; Zuldin, W.H.; Gnanaraj, C.; Yong, Y.S. Therapeutic potential and nutraceutical profiling of North Bornean seaweeds: A review. Mar. Drugs 2022, 20, 101. [Google Scholar] [CrossRef] [PubMed]
- Venmathi Maran, B.A.; Iqbal, M.; Gangadaran, P.; Ahn, B.C.; Rao, P.V.; Shah, M.D. Hepatoprotective potential of Malaysian medicinal plants: A review on phytochemicals, oxidative stress, and antioxidant mechanisms. Molecules 2022, 27, 1533. [Google Scholar] [CrossRef] [PubMed]
- Salomon, C.E.; Magarvey, N.A.; Sherman, D.H. Merging the potential of microbial genetics with biological and chemical diversity: An even brighter future for marine natural product drug discovery. Nat. Prod. Rep. 2004, 21, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Kamthania, M.C.; Kumar, A. Bioactive compounds and properties of seaweeds-A review. Open Access Libr. J. 2014, 1, 1–17. [Google Scholar] [CrossRef]
- Chiang, Y.-M.; Yoshida, T.; Ajisaka, T.; Trono, G., Jr.; Tseng, C.K.; Baoren, L. Distribution and variation in Sargassum polycystum C. A. Agardh (Fucales, Phaeophyta). In Taxonomy of Economic Seaweeds with Reference to Some Pacific and Western Atlantic Species; California Sea Grant College: San Diego, CA, USA, 1992; pp. 35–42. [Google Scholar]
- Ajisaka, T.; Phang, S.M.; Yoshida, T. Preliminary report of Sargassum species collected from Malaysian coasts. In Taxonomy of Economic Seaweeds with Reference to Some Pacific Species; Abbott, I.A., Ed.; California Sea Grant College System: San Diego, CA, USA, 1999; pp. 23–41. [Google Scholar]
- Palanisamy, S.; Vinosha, M.; Marudhupandi, T.; Rajasekar, P.; Prabhu, N.M. Isolation of fucoidan from Sargassum polycystum brown algae: Structural characterization, in vitro antioxidant and anticancer activity. Int. J. Biol. Macromol. 2017, 102, 405–412. [Google Scholar] [CrossRef]
- Chua, T.E.; Teng, S.K. Floating fishpens for rearing fishes in coastal waters, reservoirs and mining pools in Malaysia. In Fisheries Bulletin No.2; Ministry of Agriculture: Kuala Lumpur, Malaysia, 1977; Volume 20, pp. 1–36. [Google Scholar]
- Leong, T.-S.; Wong, S.-Y. A comparative study of the parasite fauna of wild and cultured grouper (Epinephelus malabaricus Bloch et Schneider) in Malaysia. Aquaculture 1988, 68, 203–207. [Google Scholar] [CrossRef]
- Liong, P.C.; Hanafi, H.B.; Merican, Z.O.; Nagaraj, G. Aquaculture development in Malaysia. In Proceedings of the Seminar on Aquaculture Development in Southeast Asia, Iloilo City, Philippines, 8–12 September 1987; Juario, J.V., Benitez, L.V., Eds.; SEAFDEC, Aquaculture Department: Iloilo City, Philippines, 1987; pp. 73–90. [Google Scholar]
- Kua, B.C.; Azmi, M.A.; Hamid, N.K.A. Life cycle of the marine leech (Zeylanicobdella arugamensis) isolated from sea bass (Lates calcarifer) under laboratory conditions. Aquaculture 2010, 302, 153–157. [Google Scholar] [CrossRef]
- Sadovy, Y. Regional Survey for Fry/Fingerling Supply and Current Practices for Grouper Mariculture: Evaluating Current Status and Long-Term Prospects for Grouper Mariculture in Southeast Asia. In Final Report to the Collaborative APEC Grouper Research and Development Network (FWG 01/99); Hong Kong University Press: Hong Kong, China, 2000. [Google Scholar]
- Ch’Ng, C.L.; Senoo, S. Egg and larval development of a new hybrid grouper, Tiger grouper Epinephelus fuscoguttatus x Giant grouper E. lanceolatus. Aquac. Sci. 2008, 56, 505–512. [Google Scholar]
- Othman, A.R.; Kawamura, G.; Senoo, S.; Ching, F.F. Effects of different salinities on growth, feeding performance and plasma cortisol level in hybrid TGGG (Tiger grouper, Epinephelus fuscoguttatus x Giant grouper, Epinephelus lanceolatus) juveniles. Int. Res. J. Biol. Sci. 2015, 4, 15–20. [Google Scholar]
- Venmathi Maran, B.A.; Leong, T.S.; Ohtsuka, S.; Nagasawa, K. Records of Caligus (Crustacea: Copepoda: Caligidae) from marine fish cultured in floating cages in Malaysia with a redescription of the male of Caligus longipedis Bassett-Smith, 1898. Zool. Stud. 2009, 48, 797–807. [Google Scholar]
- Gopalakrishnan, A.; Venmathi Maran, B.A.; Puvanendran, V.; Rajkumar, M.; Balasubramanian, T.; Ferguson, H. Neoplasia in the Indian oil sardine, Sardinella longiceps (Valenciennes), and the Great barracuda, Sphyraena barracuda (Edwards), from the south-east coast of India. J. Fish Dis. 2011, 34, 881–885. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Lacierda, E.R.; Toledo, J.D.; Tan-Fermin, J.D.; Burreson, E.M. Marine leech (Zeylanicobdella arugamensis) infestation in cultured Orange-spotted grouper, Epinephelus coioides. Aquaculture 2000, 185, 191–196. [Google Scholar] [CrossRef]
- Leal, J.F.; Neves, M.G.P.M.S.; Santos, E.B.H.; Esteves, V.I. Use of formalin in intensive aquaculture: Properties, application and effects on fish and water quality. Rev. Aquac. 2016, 10, 281–295. [Google Scholar] [CrossRef]
- Hutson, K.S.; Mata, L.; Paul, N.A.; De Nys, R. Seaweed extracts as a natural control against the monogenean ectoparasite, Neobenedenia sp., infecting farmed barramundi (Lates calcarifer). Int. J. Parasitol. 2012, 42, 1135–1141. [Google Scholar] [CrossRef]
- Ayoub, Z.; Malik, S.B.; Mehta, A. In vitro and in vivo anti-cancer efficacy of Adiantum capillus-veneris L. against some selected human cancer cell lines and on EAC mouse model. Int. J. Pharm. Sci. Drug Res. 2020, 12, 267–274. [Google Scholar] [CrossRef]
- Shah, M.D.; Venmathi Maran, B.A.; Haron, F.K.; Ransangan, J.; Ching, F.F.; Shaleh, S.R.M.; Shapawi, R.; Yong, Y.S.; Ohtsuka, S. Antiparasitic potential of Nephrolepis biserrata methanol extract against the parasitic leech Zeylanicobdella arugamensis (Hirudinea) and LC-QTOF analysis. Sci. Rep. 2020, 10, 22091. [Google Scholar] [CrossRef]
- Venmathi Maran, B.A.; Josmeh, D.; Tan, J.K.; Yong, Y.S.; Shah, M.D. Efficacy of the aqueous extract of Azadirachta indica against the marine parasitic leech and its phytochemical profiling. Molecules 2021, 26, 1908. [Google Scholar] [CrossRef]
- Shah, M.D.; Venmathi Maran, B.A.; Tan, J.K.; Yong, Y.S.; Ching, F.F.; Shaleh, S.R.M.; Shapawi, R. The anti-leech potential of the solvent extract of Bornean neem leaves and ultra-high performance liquid chromatography-high-resolution mass spectrometry profiling. J. King Saud Univ. Sci. 2021, 33, 101541. [Google Scholar] [CrossRef]
- Pluskal, T.; Castillo, S.; Villar-Briones, A.; Orešič, M. MZmine 2: Modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinform. 2010, 11, 395. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vázquez-Fresno, R.; Sajed, T.; Johnson, D.; Li, C.; Karu, N.; et al. HMDB 4.0: The human metabolome database for 2018. Nucleic Acids Res. 2018, 46, D608–D617. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Guo, A.; Oler, E.; Wang, F.; Anjum, A.; Peters, H.; Dizon, R.; Sayeeda, Z.; Tian, S.; Lee, B.L.; et al. HMDB 5.0: The human metabolome database for 2022. Nucleic Acids Res. 2022, 50, D622–D631. [Google Scholar] [CrossRef] [PubMed]
- Sud, M.; Fahy, E.; Cotter, D.; Brown, A.; Dennis, E.A.; Glass, C.K.; Merrill, A.H.; Murphy, R.C.; Raetz, C.R.H.; Russell, D.W.; et al. LMSD: LIPID MAPS structure database. Nucleic Acids Res. 2007, 35, D527–D532. [Google Scholar] [CrossRef]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and community curation of mass spectrometry data with global natural products social molecular networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Murphy, R.C. Tandem Mass Spectrometry of Lipids: Molecular Analysis of Complex Lipids; Gaskell, S.J., Ed.; The Royal Society of Chemistry: Piccadilly, UK, 2015; ISBN 9781849738279. [Google Scholar]
- Chiao-Wei, C.; Ling, H.S.; Wong, C.-L. Antibacterial activity of Sargassum polycystum C. Agardh and Padina australis Hauck (Phaeophyceae). Afr. J. Biotechnol. 2011, 10, 14125–14131. [Google Scholar] [CrossRef]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.D.; Venmathi Maran, B.A.; Iqbal, M.; Ching, F.F.; Lal, M.M.T.; Othman, R.; Shapawi, R. Antiparasitic activity of the medicinal plant Dillenia suffruticosa against the marine leech Zeylanicobdella arugamensis (Hirudinea) and its phytochemical composition. Aquac. Res. 2020, 51, 215–221. [Google Scholar] [CrossRef]
- Gholami-Ahangaran, M.; Bahmani, M.; Zia-Jahromi, N. Comparing of anti-leech effect of Vitis vinifera L. plant and niclosamide on different forms of Limnatis nilotica. World Appl. Sci. J. 2012, 20, 1082–1085. [Google Scholar] [CrossRef]
- Thing, C.Y.; Ransangan, J.; Hatai, K. Antiparasitic effect of formalin, trichlorfon, hydrogen peroxide, and copper sulfate on the parasitic isopod Caecognathia coralliophila. Fish Pathol. 2016, 51, 125–127. [Google Scholar] [CrossRef]
- Sharp, N.J.; Diggles, B.K.; Poortenaar, C.W.; Willis, T.J. Efficacy of aqui-s, formalin and praziquantel against the monogeneans, Benedenia seriolae and Zeuxapta seriolae, infecting yellowtail kingfish Seriola lalandi lalandi in New Zealand. Aquaculture 2004, 236, 67–83. [Google Scholar] [CrossRef]
- Rowland, S.J.; Mifsud, C.; Nixon, M.; Read, P.; Landos, M. Use of formalin and copper to control ichthyophthiriosis in the Australian freshwater fish silver perch (Bidyanus bidyanus Mitchell). Aquac. Res. 2008, 40, 44–54. [Google Scholar] [CrossRef]
- Andrade-Porto, S.M.; Affonso, E.G.; Kochhann, D.; Malta, J.C.O.; Roque, R.; Ono, E.A.; Araújo, C.S.O.; Tavares-Dias, M. Antiparasitic efficacy and blood effects of formalin on Arapaima gigas (Pisces: Arapaimidae). Aquaculture 2017, 479, 38–44. [Google Scholar] [CrossRef]
- Kim, I.S.; Park, Y.J.; Yoon, S.J.; Lee, H.B. Ephedrannin A and B from roots of Ephedra sinica inhibit lipopolysaccharide-induced inflammatory mediators by suppressing nuclear factor -κB activation in RAW 264.7 macrophages. Int. Immunopharmacol. 2010, 10, 1616–1625. [Google Scholar] [CrossRef] [PubMed]
- Kunert, O.; Swamy, R.C.; Kaiser, M.; Presser, A.; Buzzi, S.; Appa Rao, A.V.N.; Schühly, W. Antiplasmodial and leishmanicidal activity of biflavonoids from Indian Selaginella bryopteris. Phytochem. Lett. 2008, 1, 171–174. [Google Scholar] [CrossRef]
- Mu, W.; Cheng, X.; Zhang, X.; Liu, Y.; Lv, Q.; Liu, G.; Zhang, J.; Li, X. Hinokiflavone induces apoptosis via activating mitochondrial ROS/JNK/caspase pathway and inhibiting NF -κB activity in hepatocellular carcinoma. J. Cell. Mol. Med. 2020, 24, 8151–8165. [Google Scholar] [CrossRef] [PubMed]
- Shim, S.-Y.; Lee, S.; Lee, M. Biflavonoids isolated from Selaginella tamariscina and their anti-inflammatory activities via ERK1/2 signaling. Molecules 2018, 23, 926. [Google Scholar] [CrossRef] [PubMed]
- Stiboller, M.; Freitas, F.P.; Francesconi, K.A.; Schwerdtle, T.; Nogueira, A.J.A.; Raber, G. Lipid-soluble arsenic species identified in the brain of the marine fish skipjack tuna (Katsuwonus pelamis) using a sequential extraction and HPLC/mass spectrometry. J. Anal. At. Spectrom. 2019, 34, 2440–2450. [Google Scholar] [CrossRef]
- Taleshi, M.S.; Seidler-Egdal, R.K.; Jensen, K.B.; Schwerdtle, T.; Francesconi, K.A. Synthesis and characterization of arsenolipids: Naturally occurring arsenic compounds in fish and algae. Organometallics 2014, 33, 1397–1403. [Google Scholar] [CrossRef]
- Zheng, Y.; Tian, C.; Dong, L.; Tian, L.; Glabonjat, R.A.; Xiong, C. Effect of arsenic-containing hydrocarbon on the long-term potentiation at Schaffer Collateral-CA1 synapses from infantile male rat. Neurotoxicology 2021, 84, 198–207. [Google Scholar] [CrossRef]
- Kumar, A.; Basu, S.; Rishu, A.K.; Kumar, G. Revisiting the mechanisms of arsenic uptake, transport and detoxification in plants. Environ. Exp. Bot. 2022, 194, 104730. [Google Scholar] [CrossRef]
- Snodgrass, W.R. Heavy metal toxicology. Encycl. Food Health 2016, 328–331. [Google Scholar]
- Besednova, N.N.; Zaporozhets, T.S.; Andryukov, B.G.; Kryzhanovsky, S.P.; Ermakova, S.P.; Kuznetsova, T.A.; Voronova, A.N.; Shchelkanov, M.Y. Antiparasitic effects of sulfated polysaccharides from marine hydrobionts. Mar. Drugs 2021, 19, 637. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, A.; Rajkumar, M.; Sun, J.; Parida, A.; Venmathi Maran, B.A. Integrated biological control of the weed water hyacinth using the novel method of grass carp Ctenopharyngodon idella (Valenciennes, 1844) and the weevil Neochetina spp. Chin. J. Oceanol. Limnol. 2011, 29, 162–166. [Google Scholar] [CrossRef]

| No. | Group | Mortality Time (min) Mean ± S.D. |
|---|---|---|
| 1 | * Normal control | 0.00 ± 00 |
| 2 | # Positive control | 1.59 ± 0.30 a |
| 3 | 6.25 (mg/mL) | 30.18 ± 7.69 a,b |
| 4 | 12.5 (mg/mL) | 11.10 ± 2.52 a,b,c |
| 5 | 25 (mg/mL) | 6.93 ± 2.44 a,b,c,d |
| 6 | 50 (mg/mL) | 2.77 ± 1.40 a,b,c,d,e |
| 7 | 100 (mg/mL) | 0.93 ± 0.44 a,b,c,d,e,f,g |
| Group | pH | Salinity (ppt) | Dissolved Oxygen (mg/mL) | Temperature (°C) |
|---|---|---|---|---|
| * Normal control | 6.53 | 23.1 | 7.91 | 26.2 |
| # Positive control | 6.36 | 15.3 | 7.90 | 26.2 |
| 6.25 (mg/mL) | 6.27 | 24.0 | 8.08 | 25.5 |
| 12.5 (mg/mL) | 6.23 | 18.5 | 7.81 | 25.1 |
| 25 (mg/mL) | 6.01 | 29.1 | 8.04 | 25.2 |
| 50 (mg/mL) | 5.76 | 34.7 | 8.03 | 25.4 |
| 100 (mg/mL) | 5.43 | 46.9 | 7.85 | 25.9 |
| No. | Matched Metabolites | Molecular Formula | m/z | Mass Error (ppm) | Class |
|---|---|---|---|---|---|
| 1 | 2-Aminoheptanoic acid | C7H15NO2 | 146.1176 | 0.42 | Amino Acid |
| 2 | 5-Aminopentanoic acid | C5H11NO2 | 118.0866 | 3.08 | Amino Acid |
| 3 | 1-Hydroxy-5-phenyl-3-pentanone | C11H14O2 | 179.1069 | 1.47 | Aromatic |
| 4 | 2-(4-Bromophenyl) butanoic acid | C10H11BrO2 | 243.0005 | −3.88 | Aromatic |
| 5 | 2-Anilino-3-chloro-1,4-naphthoquinone | C16H10ClNO2 | 284.0466 | −2.26 | Aromatic |
| 6 | 2-Benzoyl-5-chlorobenzoic acid | C14H9ClO3 | 261.0306 | −2.46 | Aromatic |
| 7 | 3-(2,6-Diisopropylphenyl)-1-{1-[3-(2,5-dimethylphenyl)-4-oxo-3,4-dihydro-2-quinazolinyl]ethyl}-1-(2-phenylethyl)urea | C39H44N4O2 | 601.3529 | −1.23 | Aromatic |
| 8 | 6-(3-Ethoxy-4-hydroxyphenyl)-8-[(4-methylphenyl)amino]-1,3,7,9-tetraoxo-6a-phenyl-3,3a,4,6,6a,7,8,9,9a,10,10a,10b-dodecahydroisoindolo [5,6-e]isoindole-2(1H)-carboxamide | C36H34N4O7 | 635.2504 | 0.73 | Aromatic |
| 9 | Diphenyl sulfoxide | C12H10OS | 203.0529 | 2.28 | Aromatic |
| 10 | Harderoporphyrin | C35H36N4O6 | 609.2706 | −0.23 | Chlorophyll Breakdown Product |
| 11 | Pyrophaeophorbide a | C33H34N4O3 | 535.2708 | 0.86 | Chlorophyll Breakdown Product |
| 12 | 11-Amino-undecanoic acid | C11H23NO2 | 202.1803 | 0.80 | Fatty Acyl |
| 13 | 11-Oxo-undeca-5,8-dienoic acid | C11H16O3 | 197.1175 | 1.84 | Fatty Acyl |
| 14 | Erucamide | C22H43NO | 338.3416 | −0.41 | Fatty Acyl |
| 15 | 3-(7-tetradecenoyloxy)-dodecanoic acid | C26H48O4 | 425.3625 | −0.09 | Fatty Acyl |
| 16 | Termitomycesphin F | C43H83NO10 | 774.6094 | 0.60 | Fatty Acyl |
| 17 | Ephedrannin A | C30H20O11 | 557.1078 | −0.07 | Flavonoid |
| 18 | Hinokiflavone | C30H18O10 | 539.0977 | 0.86 | Flavonoid |
| 19 | Ectoine | C6H10N2O2 | 143.0815 | 0.43 | Heterocyclic |
| 20 | Erinapyrone C | C8H10O5 | 187.0603 | 1.40 | Heterocyclic |
| 21 | Stachydrine | C7H13NO2 | 144.1020 | 1.13 | Heterocyclic |
| 22 | 1-Dimethylarsinoyl-heptadecane | C19H41OAs | 361.2446 | 0.17 | Organoarsenic |
| 23 | Cacodylic acid | C2H7AsO2 | 138.9736 | 1.17 | Organoarsenic |
| 24 | 5-Bromo-2-hydroxy-N,3-dimethylbenzamide | C9H10BrNO2 | 243.9971 | 1.49 | Phenolic |
| 25 | Ethephon | C2H6ClO3P | 144.9822 | 4.59 | Plant Growth Regulator |
| 26 | N-Benzyl-N~2~-isobutyl-N-{[1-(3-methoxybenzyl)-1H-pyrrol-2-yl]methyl}-N~2~-(2-naphthylsulfonyl)glycinamide | C36H39N3O4S | 610.2743 | 1.58 | Polyaromatic |
| 27 | Salvianolic acid L | C36H30O16 | 719.1609 | 0.36 | Polyphenolic |
| 28 | Steroidal compound | C29H49NO2 | 444.3839 | 0.81 | Steroid |
| 29 | α-Carboxydimethyloctylhydroxychroman | C24H38O4 | 391.2842 | −0.10 | Vitamin E |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haron, F.K.; Shah, M.D.; Yong, Y.S.; Tan, J.K.; Lal, M.T.M.; Venmathi Maran, B.A. Antiparasitic Potential of Methanol Extract of Brown Alga Sargassum polycystum (Phaeophyceae) and Its LC-MS/MS Metabolite Profiling. Diversity 2022, 14, 796. https://doi.org/10.3390/d14100796
Haron FK, Shah MD, Yong YS, Tan JK, Lal MTM, Venmathi Maran BA. Antiparasitic Potential of Methanol Extract of Brown Alga Sargassum polycystum (Phaeophyceae) and Its LC-MS/MS Metabolite Profiling. Diversity. 2022; 14(10):796. https://doi.org/10.3390/d14100796
Chicago/Turabian StyleHaron, Fatin Khairah, Muhammad Dawood Shah, Yoong Soon Yong, Jen Kit Tan, Mohammad Tamrin Mohamad Lal, and Balu Alagar Venmathi Maran. 2022. "Antiparasitic Potential of Methanol Extract of Brown Alga Sargassum polycystum (Phaeophyceae) and Its LC-MS/MS Metabolite Profiling" Diversity 14, no. 10: 796. https://doi.org/10.3390/d14100796
APA StyleHaron, F. K., Shah, M. D., Yong, Y. S., Tan, J. K., Lal, M. T. M., & Venmathi Maran, B. A. (2022). Antiparasitic Potential of Methanol Extract of Brown Alga Sargassum polycystum (Phaeophyceae) and Its LC-MS/MS Metabolite Profiling. Diversity, 14(10), 796. https://doi.org/10.3390/d14100796








