Pollen Types Reveal Floral Diversity in Natural Honeys from Campeche, Mexico
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Honey Sampling
2.3. Quantitative Pollen Analysis
- X = the number of pollen grains in each gram of honey.
- A = the arithmetic mean of pollen grains in the two compartments of the Neubauer chamber (N1 + N2/2).
2.4. Qualitative Pollen Analysis
2.5. Statistical Analyses
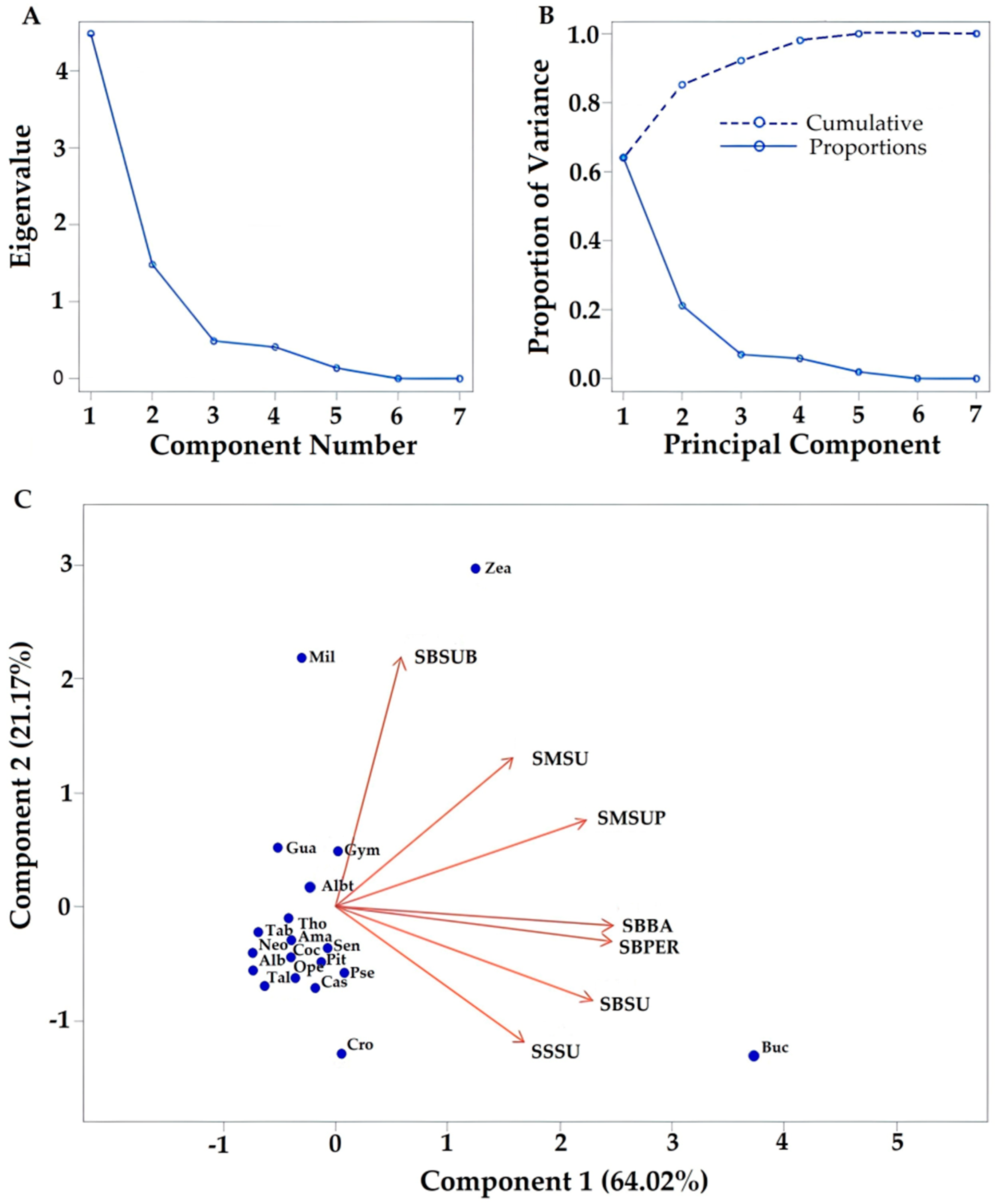
3. Results
3.1. Pollen Qualification
3.2. Flora Identification
3.3. Relationship between Flora Identified through Pollen Types and Ecosystem Sampling
3.4. Honey Classification
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Majewska, E.; Drużyńska, B.; Wołosiak, R. Determination of the Botanical Origin of Honeybee Honeys Based on the Analysis of Their Selected Physicochemical Parameters Coupled with Chemometric Assays. Food Sci. Biotechnol. 2019, 28, 1307–1314. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.; Pauli, N.; Biggs, E.; Barbour, L.; Boruff, B. Why Bees Are Critical for Achieving Sustainable Development. Ambio 2021, 50, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Aparna, A.R.; Rajalakshmi, D. Honey—Its Characteristics, Sensory Aspects, and Applications. Food Rev. Int. 1999, 15, 455–471. [Google Scholar] [CrossRef]
- Campbell, A.J.; Gigante Carvalheiro, L.; Gastauer, M.; Almeida-Neto, M.; Giannini, T.C. Pollinator Restoration in Brazilian Ecosystems Relies on a Small but Phylogenetically-Diverse Set of Plant Families. Sci. Rep. 2019, 9, 17383. [Google Scholar] [CrossRef] [PubMed]
- González-Suárez, M.; Mora-Olivo, A.; Villanueva-Gutiérrez, R.; Lara-Villalón, M.; Vanoye-Eligio, V.; Guerra-Pérez, A.; González-Suárez, M.; Mora-Olivo, A.; Villanueva-Gutiérrez, R.; Lara-Villalón, M.; et al. Diversidad de la flora de interés apícola en el estado de Tamaulipas, México. Rev. Mex. De Cienc. Pecu. 2020, 11, 914–932. [Google Scholar] [CrossRef]
- Attique, R.; Zafar, M.; Ahmad, M.; Zafar, S.; Ghufran, M.A.; Mustafa, M.R.U.; Yaseen, G.; Ahmad, L.; Sultana, S.; Nabila; et al. Pollen Morphology of Selected Melliferous Plants and Its Taxonomic Implications Using Microscopy. Microsc. Res. Tech. 2022, 85, 2361–2380. [Google Scholar] [CrossRef]
- Mărgăoan, R.; Topal, E.; Balkanska, R.; Yücel, B.; Oravecz, T.; Cornea-Cipcigan, M.; Vodnar, D.C. Monofloral Honeys as a Potential Source of Natural Antioxidants, Minerals and Medicine. Antioxidants 2021, 10, 1023. [Google Scholar] [CrossRef]
- Villanueva-Gutiérrez, R.; Moguel-Ordóñez, Y.B.; Echazarreta-González, C.M.; Arana-López, G. Monofloral Honeys in the Yucatán Peninsula, Mexico. Grana 2009, 48, 214–223. [Google Scholar] [CrossRef]
- Magaña-Magaña, M.A.; Tavera-Cortés, M.E.; Salazar-Barrientos, L.L.; Sanginés-García, J.R.; Magaña-Magaña, M.A.; Tavera Cortés, M.E.; Salazar Barrientos, L.L.; Sanginés García, J.R. Productividad de la apicultura en México y su impacto sobre la rentabilidad. Rev. Mex. De Cienc. Agrícolas 2016, 7, 1103–1115. [Google Scholar] [CrossRef]
- Secretaría de Agricultura y Desarrollo Rural Crecen Producción y Exportaciones de Miel en México al Cierre de 2021. Available online: http://www.gob.mx/agricultura/prensa/crecen-produccion-y-exportaciones-de-miel-en-mexico-al-cierre-de-2021-agricultura-293944?idiom=es (accessed on 5 September 2022).
- Coh-Martínez, M.E.; Cetzal-Ix, W.; Martínez-Puc, J.F.; Basu, S.K.; Noguera-Savelli, E.; Cuevas, M.J. Perceptions of the Local Beekeepers on the Diversity and Flowering Phenology of the Melliferous Flora in the Community of Xmabén, Hopelchén, Campeche, Mexico. J. Ethnobiol. Ethnomed. 2019, 15, 16. [Google Scholar] [CrossRef]
- Real-Luna, N.; Alcántara-Salinas, G.; Rivera-Hernández, J.E.; Zalazar-Marcial, E.; Pérez-Sato, J.A. The Melliferous Flora of Veracruz, Mexico. Agro Product. 2021, 14, 65–80. [Google Scholar] [CrossRef]
- Zerrouk, S.; Seijo, M.C.; Boughediri, L.; Escuredo, O.; Rodríguez-Flores, M.S. Palynological Characterisation of Algerian Honeys According to Their Geographical and Botanical Origin. Grana 2014, 53, 147–158. [Google Scholar] [CrossRef]
- Homrani, M.; Escuredo, O.; Rodríguez-Flores, M.S.; Fatiha, D.; Mohammed, B.; Homrani, A.; Seijo, M.C. Botanical Origin, Pollen Profile, and Physicochemical Properties of Algerian Honey from Different Bioclimatic Areas. Foods 2020, 9, 938. [Google Scholar] [CrossRef]
- Güemes-Ricalde, F.J.; Echazarreta-González, C.; Villanueva-G, R.; Pat-Fernández, J.M.; Gómez-Álvarez, R. La apicultura en la Península de Yucatán. Rev. Mex. Del Caribe 2003, 16, 117–132. [Google Scholar]
- Martínez-Puc, J.F.; Cetzal-Ix, W.; González-Valdivia, N.A.; Casanova-Lugo, F.; Saikat-Kumar, B. Caracterización de la actividad apícola en los principales municipios productores de miel en Campeche, México. J. Selva Andin. Anim. Sci. 2018, 5, 44–53. [Google Scholar] [CrossRef]
- Etxegarai-Legarreta, O.; Sanchez-Famoso, V. The Role of Beekeeping in the Generation of Goods and Services: The Interrelation between Environmental, Socioeconomic, and Sociocultural Utilities. Agriculture 2022, 12, 551. [Google Scholar] [CrossRef]
- Jacinto-Pimienta, S.Y.; Mendoza-Hernández, J.H.R.; Zaldivar-Cruz, J.M.; Sol-Sánchez, Á.; Vargas-Villamil, L.M.; Reyes-Sánchez, C.A.; Jacinto-Pimienta, S.Y.; Mendoza-Hernández, J.H.R.; Zaldivar-Cruz, J.M.; Sol-Sánchez, Á.; et al. El uso de componentes principales en la clasificación melisopalinológica de la miel de Apis mellifera L. Rev. Mex. De Cienc. Agrícolas 2016, 7, 2831–2840. [Google Scholar]
- CONABIO Portal de Información Geográfica—CONABIO. Available online: http://www.conabio.gob.mx/informacion/gis/ (accessed on 5 September 2022).
- Retana-Guiascón, O.G.; Aguilar-Nah, M.S.; Niño-Gómez, G. Uso de la vida silvestre de alternativas de manejo integral el caso de la comunidad maya de Pich, Campeche, México. Trop. Subtrop. Agroecosystem 2011, 14, 885–890. [Google Scholar]
- Gutiérrez-Báez, C.; Zamora-Crescencio, P.; Cabrera-Mis, G.G. Estructura y Composición Florística de La Selva Mediana Subperennifolia El Remate, Calkiní, Campeche, México. For. Veracruzana 2016, 18, 1–12. [Google Scholar]
- Zamora-Crescencio, P.; Rico-Gray, V.; Barrientos-Medina, R.C.; Puc-Garrido, E.C.; Villegas, P.; del Domínguez-Carrasco, M.R.; Gutiérrez-Báez, C.; Zamora-Crescencio, P.; Rico-Gray, V.; Barrientos-Medina, R.C.; et al. Estructura y composición florística de la selva mediana subperennifolia en Bethania, Campeche, México. Polibotánica 2017, 43, 67–86. [Google Scholar] [CrossRef]
- Zamora-Crescencio, P. Contribución al estudio florístico y descripción de la vegetación del municipio de Tenabo, Campeche, México. Polibotanica 2003, 15, 1–40. [Google Scholar]
- Dzib-Castillo, B.; Chanatásig-Vaca, C.; González-Valdivia, N.A. Estructura y composición en dos comunidades arbóreas de la selva baja caducifolia y mediana subcaducifolia en Campeche, México. Rev. Mex. De Biodivers. 2014, 85, 167–178. [Google Scholar] [CrossRef]
- Palacio-Aponte, A.G.; Noriega-Trejo, R.; Zamora-Crescencio, P. Caracterización físico-geográfica del paisaje conocido como “bajos inundables”: El caso del Área Natural Protegida Balamkín, Campeche. Investig. Geográficas 2002, 49, 57–73. [Google Scholar] [CrossRef]
- Erdtman, G. The Acetolysis Method-a Revised Description. Sven. Bot. Tidskr. 1960, 54, 516–564. [Google Scholar]
- Hassanien, M.M.; El-Sherif, M.E.M.; Salem, A.a.a.A.; Ali, M.a.M. Quantitative Pollen Analyses of Bee Honey at Certain Apiaries in Qualyubia Governorate and Available Honey in the Local Market, Egypt. Arab Univ. J. Agric. Sci. 2018, 26, 303–311. [Google Scholar] [CrossRef]
- Louveaux, J.; Maurizio, A.; Vorwohl, G. Methods of Melissopalynology. Bee World 1978, 59, 139–157. [Google Scholar] [CrossRef]
- Alfaro-Bates, R.G.; González-Acereto, J.Á.; Ortiz-Díaz, J.J.; Viera-Castro, F.A.; Burgos-Pérez, A.I.; Martínez-Hernández, E.; Ramírez-Arriaga, E. Caracterización Palinológica de las Mieles de la Península de YuCatán, 1st ed.; Comisión Nacional para el Conocimiento y Uso de la Biodiversidad: Mérida, Yucatán, Mexico, 2010; ISBN 978-607-7573-42-5. [Google Scholar]
- Ramos-Díaz, A.; San Roman-Avila, D.; Noriega-Trejo, R.; Góngora-Chin, R.; Sanchez, A.; Rodriguez-Buenfil, I. Catálogo de Los Principales Tipos Polínicos Encontrados En Las Mieles Producidas En La Península de Yucatán, 1st ed.; SIIES, CIATEJ y EDESU: Mérida, Yucatán, Mexico, 2015; ISBN 978-607-8424-10-8. [Google Scholar]
- Song, X.-Y.; Yao, Y.-F.; Yang, W.-D. Pollen Analysis of Natural Honeys from the Central Region of Shanxi, North China. PLoS ONE 2012, 7, e49545. [Google Scholar] [CrossRef]
- SAS Institute Inc. SAS® 9.4 Statements, 1st ed.; SAS Campus Drive: Cary, NC, USA, 2011. [Google Scholar]
- CONABIO Selvas Húmedas. Available online: https://www.biodiversidad.gob.mx/ecosistemas/selvaHumeda (accessed on 1 March 2022).
- CONABIO La biodiversidad en Campeche: Estudio de Estado. Available online: https://www.biodiversidad.gob.mx/region/EEB/estudios/ee_campeche (accessed on 1 March 2022).
- CYCI Flora de La Península de Yucatán. Available online: https://www.cicy.mx/sitios/flora%20digital/ficha_virtual.php?especie=966 (accessed on 1 March 2022).
- Ramírez-Arriaga, E.; Navarro-Calvo, L.A.; Díaz-Carbajal, E. Botanical Characterisation of Mexican Honeys from a Subtropical Region (Oaxaca) Based on Pollen Analysis. Grana 2011, 50, 40–54. [Google Scholar] [CrossRef]
- Castillo Cázares, A.V.; Moguel Ordóñez, Y.B.; Cortés Cruz, M.A.; Espinosa Huerta, E.; Arechavaleta Velasco, M.E.; Mora Avilés, M.A.; Castillo Cázares, A.V.; Moguel Ordóñez, Y.B.; Cortés Cruz, M.A.; Espinosa Huerta, E.; et al. Composición botánica de mieles de la península de Yucatán, mediante qPCR y análisis de curvas de disociación. Rev. Mex. De Cienc. Pecu. 2016, 7, 489–505. [Google Scholar] [CrossRef]
- Granados-Argüello, R.I.; Villanueva-Gutiérrez, R.; Martínez-Hernández, E.; García-Mayoral, L.E.; González de la Torre, J.E. Análisis melisopalinológico de mieles de Apis mellifera L. en la zona centro de Veracruz, México. Polibotánica 2020, 147–163. [Google Scholar] [CrossRef]
- Uzcanga-Pérez, N.G.; Cano-González, A.; Medina-Méndez, J.; Espinoza-Arellan, J. Caracterización de Los Productores de Maíz de Temporal En El Estado de Campeche, México. Rev. Mex. Agronegocio 2015, 36, 1295–1305. [Google Scholar]
- Secretaría de Agricultura y Desarrollo Rural Maíz el cultivo de México. Available online: http://www.gob.mx/agricultura/articulos/maiz-el-cultivo-de-mexico (accessed on 29 December 2021).
- Chai, Q.; Nemecek, T.; Liang, C.; Zhao, C.; Yu, A.; Coulter, J.A.; Wang, Y.; Hu, F.; Wang, L.; Siddique, K.H.M.; et al. Integrated Farming with Intercropping Increases Food Production While Reducing Environmental Footprint. Proc. Natl. Acad. Sci. USA 2021, 118, e2106382118. [Google Scholar] [CrossRef] [PubMed]
- Espina-Peréz, D.; Ordext, G.S. Apicultura Tropical, 4th ed.; Editorial Tecnológica de Costa Rica: Cartago, Costa Rica, 1984. [Google Scholar]
- Sajwani, A.; Farooq, S.A.; Patzelt, A.; Eltayeb, E.A.; Bryant, V.M. Melissopalynological Studies from Oman. Palynology 2007, 31, 63–79. [Google Scholar] [CrossRef]
- Feás, X.; Pires, J.; Estevinho, M.L.; Iglesias, A.; De Araujo, J.P.P. Palynological and Physicochemical Data Characterisation of Honeys Produced in the Entre-Douro e Minho Region of Portugal. Int. J. Food Sci. Technol. 2010, 45, 1255–1262. [Google Scholar] [CrossRef]
- Addi, A.; Bareke, T. Botanical Origin and Characterization of Monofloral Honeys in Southwestern Forest of Ethiopia. Food Sci. Nutr. 2021, 9, 4998–5005. [Google Scholar] [CrossRef] [PubMed]
- Da Bandeira, M.S.F.; de Novais, J.S. Melissopalynological Characterization of Honeys from the Discovery Coast, Brazil. Palynology 2020, 44, 539–550. [Google Scholar] [CrossRef]
- Anklam, E. A Review of the Analytical Methods to Determine the Geographical and Botanical Origin of Honey. Food Chem. 1998, 63, 549–562. [Google Scholar] [CrossRef]
- Villanueva-Gutiérrez, R. Polliniferous Plants Aud Foraging Strategles Of Apis Mellifera (Hyínenoptera: Apidae) in the Yucatán Peninsula, Mexico. Rev. Biol. Trop. 2002, 50, 1035–1044. [Google Scholar]
- Shukla, V.; Rao, K.S.; Tripathi, D. Pollen Diversity of Honey from Northern and Southern Prayagraj District Uttar Pradesh, India. Grana 2022, 61, 148–160. [Google Scholar] [CrossRef]
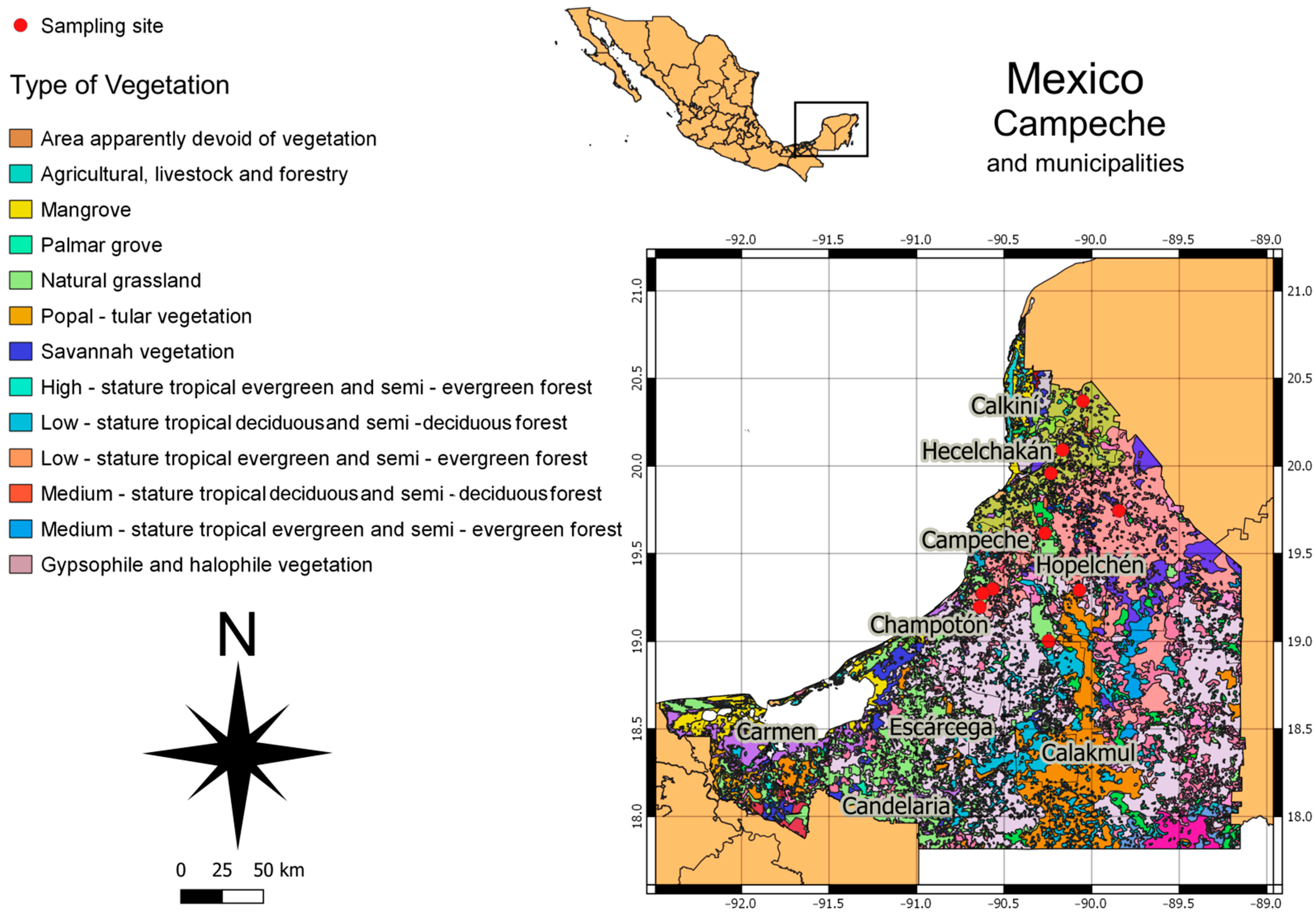
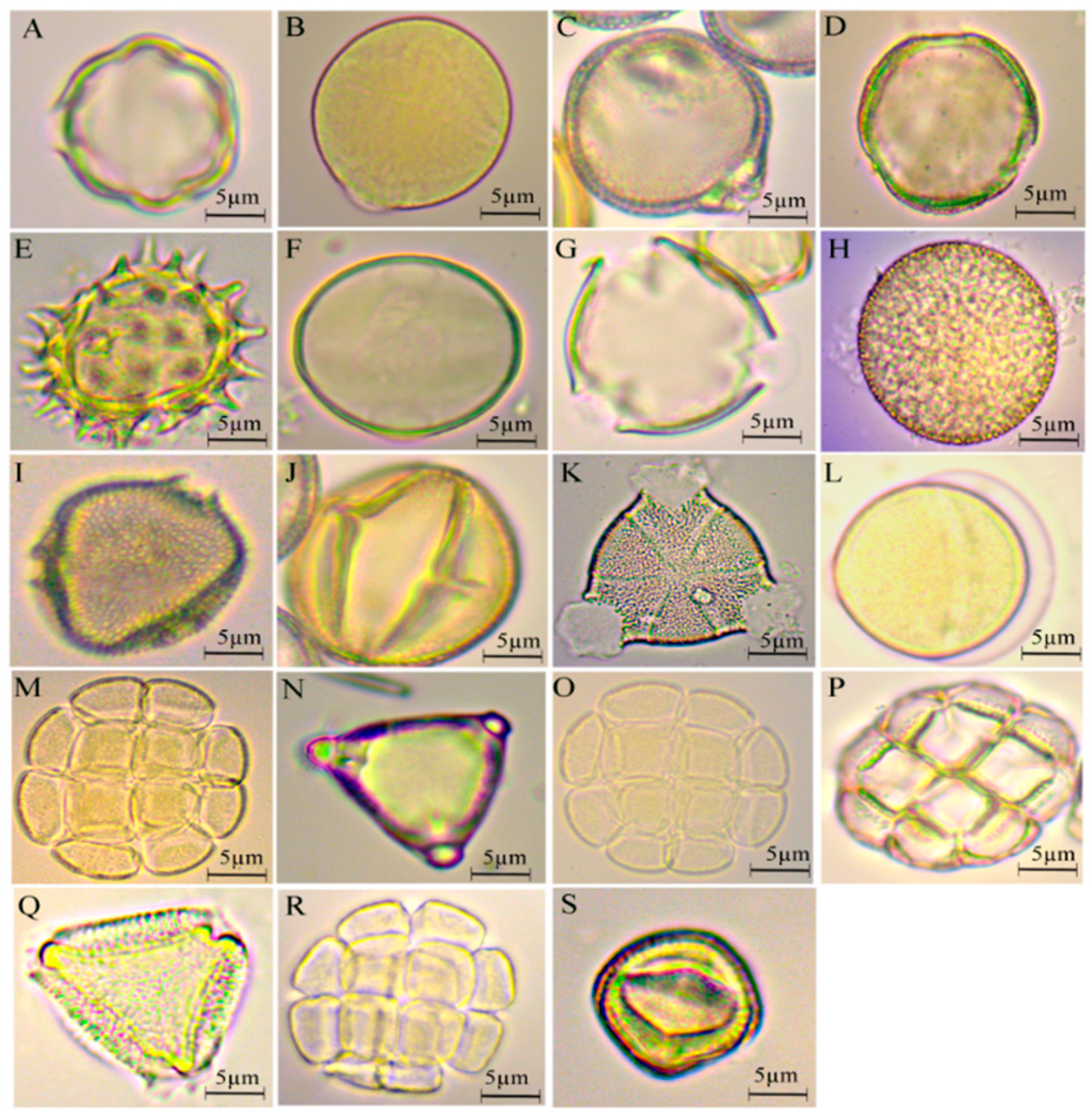
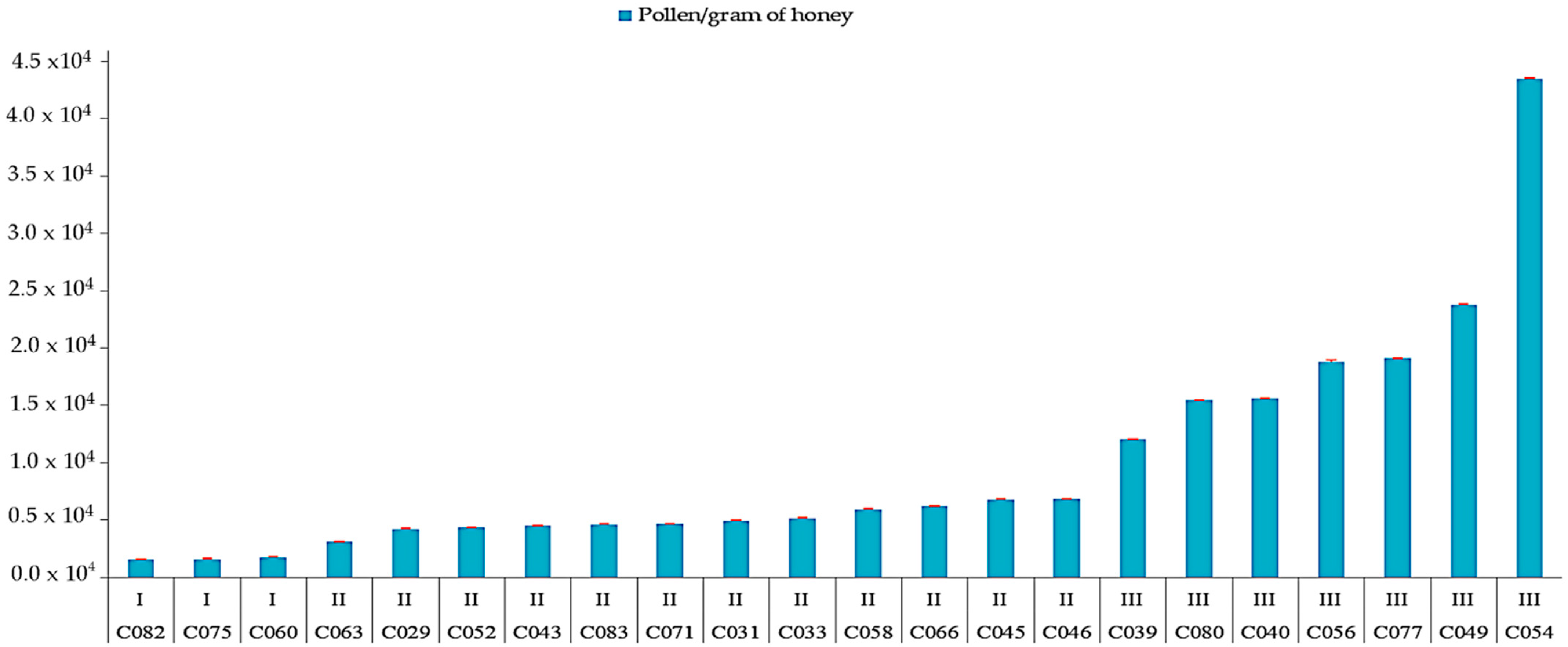
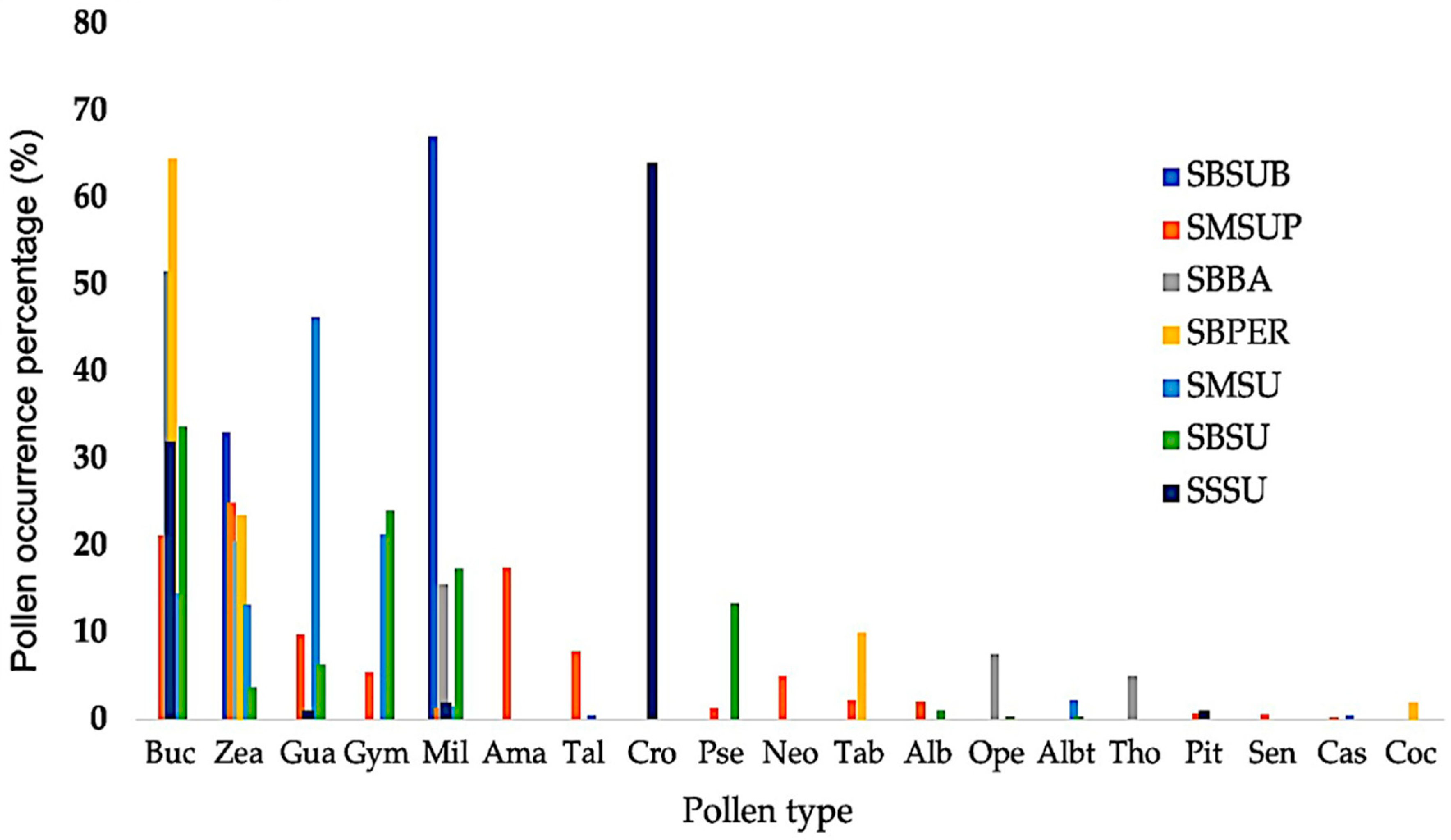
| Sample | Location | Time Collection | Ecosystem | Coordinates | References |
|---|---|---|---|---|---|
| C029 | Pich | August 2021 | Low deciduous forest | N 19°29′11″/O 90°07′05″ | [20] |
| C031 | Calkiní | February 2021 | Medium-stature tropical forest | N 20°22′21″/O 90°03′03″ | [21] |
| C033 | Hopelchén | April 2021 | Low-stature tropical forest | N 19°32′30″/O 89°36′30″ | [20] |
| C039 | Calkiní | February 2021 | Medium deciduous forest | N 20°22′21″/O 90°03′03″ | [21] |
| C040 | Calkiní | February 2021 | Medium deciduous forest | N 20°22′21″/O 90°03′03″ | |
| C043 | Calkiní | February 2021 | Medium deciduous forest | N 20°22′21″/O 90°03′03″ | |
| C045 | Calkiní | February 2021 | Medium deciduous forest | N 20°22′21″/O 90°03′03″ | |
| C046 | Calkiní | February 2021 | Low inundated tropical forest | N 19°29′77″/O 90°56′52″ | |
| C049 | Hopelchén | April 2021 | Low-stature tropical forest | N 19°32′30″/O 89°36′30″ | [21] |
| C052 | Calkiní | February 2021 | Medium deciduous forest | N 20°22′21″/O 90°03′03″ | [22] |
| C054 | Campeche | August 2021 | Medium deciduous forest | N 19°50′55″/O 90°31′ | [22] |
| C056 | Campeche | August 2021 | Medium deciduous forest | N 19°50′55″/O 90°31′ | |
| C058 | Tenabo | March 2021 | Low deciduous forest | N 20°04′09″/O 90°22′55″ | [23] |
| C060 | Tenabo | March 2021 | Low deciduous forest | N 19°50′55″/O 90°31′ | |
| C063 | Tenabo | March 2021 | Low deciduous forest | N 19°50′55″/O 90°31′ | |
| C066 | Hecelchakán | July 2021 | Medium deciduous forest | N 20°10′37″/O 90°08′02″ | [24] |
| C071 | Hecelchakán | July 2021 | Medium deciduous forest | N 20°22′21″/O 90°03′03″ | |
| C075 | Canasayab | May 2021 | Low inundated tropical forest | N 19°29′77″/O 90°56′52″ | [25] |
| C077 | La nueva Esperanza | June 2021 | Secondary forest | N 19°19′55″/O 90°63′97″ | [23] |
| C080 | Ulumali | February 2021 | Medium deciduous forest | N 19°27′25″/O 90°62′33″ | |
| CO82 | Ah Kim Pech, | March 2021 | Medium deciduous forest | N 18°99′88″/O 90°24′44″ | |
| CO83 | Chilam Balam | March 2021 | Medium deciduous forest | N 19°00′30″/O 90°56′52″ |
| Taxa | ABB | SMSUP | SBBA | SBPER | SMSU | SBSU | SSSU | SBSUB |
|---|---|---|---|---|---|---|---|---|
| Albizia lebbeck (L.) Benth. | Alb | <10% | <10% | |||||
| Albizia tomentosa (Micheli) Standl. | Albt | <10% | <10% | |||||
| Amaranthus spinosus L. | Ama | <20% | ||||||
| Cassia fistula L. | Cas | <10% | <10% | |||||
| Coccoloba reflexiflora Standl. | Coc | <10% | ||||||
| Croton icche Lundell | Cro | >60% | ||||||
| Guazuma ulmifolia Lam. | Gua | <10% | >40% | <10% | <10% | |||
| Gymnopodium floribundum Rolfe | Gym | <10% | >20% | |||||
| Milleria quinqueflora L. | Mil | <10% | <20% | <10% | <20% | <10% | >60% | |
| Neomillspaughia emarginata (H. Gross) S.F Blake | Neo | <10% | ||||||
| Operculina pinnatifida (Kunth) O’ Donell | Ope | <10% | ||||||
| Pithecellobium lanceolatum (Willd.) Benth. | Pit | <10% | <10% | |||||
| Pseudobombax ellipticum (Kunth) Dugand | Pse | <10% | <10% | |||||
| Senegalia gaumeri (SF Blake) Britton and Rose | Sen | <10% | ||||||
| Tabebuia rosea (Bertol.) DC. | Tab | <10% | <10% | |||||
| Talisia floresii Standl. | Tal | <10% | <10% | |||||
| Terminalia buceras (L.) C. Wright | Buc | ~20% | ~50% | >60% | >10% | ~30% | ~30% | - |
| Thouinia paucidentata Radlk. | Tho | <10% | ||||||
| Zea mays L. | Zea | >20% | ~20% | >20% | >10% | <10% | >30% |
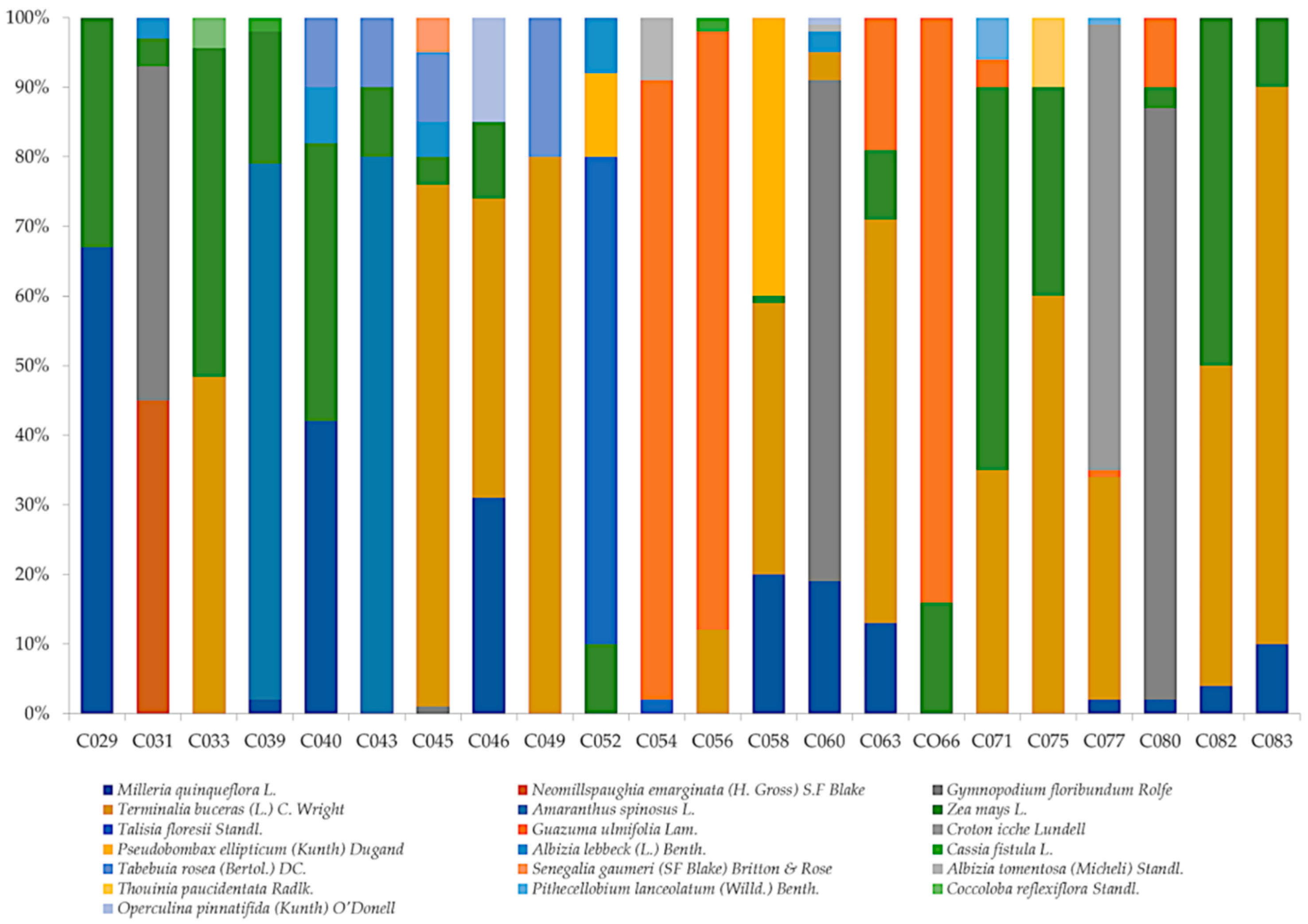
| Sample | APC/1 g Honey | Class (Louveaux et al. [28]) | Shannon–Weaver Diversity Index | Nature of Honey | Floral Source: Nectariferous (N) or Polleniferous (P) | |
|---|---|---|---|---|---|---|
| Monofloral | N | P | ||||
| C029 | 43,500 | II | 0.27 | Milleria quinqueflora (67%) | x | x |
| Zea mays (33%) | x | |||||
| C031 | 50,500 | II | 0.36 | Neomillspaughia emarginata (45%) | x | x |
| Gymnopodium floribundum (48%) | x | x | ||||
| Amaranthus spinosus (4%) | x | |||||
| Albizia lebbeck (3%) | x | x | ||||
| C033 | 52,500 | II | 0.83 | Termilaria buceras (49%) | x | x |
| Zea mays (47%) | x | |||||
| Coccoloba reflexiflora (4%) | x | x | ||||
| C039 | 121,500 | III | 0.67 | Amaranthus spinosus (77%) | x | |
| Zea mays (19%) | x | |||||
| Cassia fistula (2%) | x | x | ||||
| Milleria quinqueflora (2%) | x | x | ||||
| C043 | 46,000 | II | 0.28 | Amaranthus spinosus (80%) | x | |
| Zea mays (10%) | x | |||||
| Tabebuia rosea (10%) | x | x | ||||
| C045 | 69,000 | II | 0.92 * | Termilaria buceras (75%) | x | x |
| Zea mays (4%) | x | |||||
| Albizia lebbeck (5%) | x | x | ||||
| Gymnopodium floribundum (1%) | x | x | ||||
| C049 | 239,000 | 0.50 | Termilaria buceras (80%) | x | x | |
| Tabebuia rosea (20%) | x | x | ||||
| C052 | 44,500 | II | 0.94 | Talisia floresii (70%) | x | x |
| Pseudobombax ellipticum (12%) | x | x | ||||
| Zea mays (10%) | x | |||||
| Albizia lebbeck (8%) | x | x | ||||
| C054 | 436,000 | III | 0.40 | Guazuma ulmifolia (89%) | x | x |
| Thouinia paucidentata (9%) | x | x | ||||
| Talisia floresii (2%) | x | x | ||||
| C056 | 189,000 | III | 0.46 | Guazuma ulmifolia (86%) | x | x |
| Termilaria buceras (12%) | x | x | ||||
| C060 | 18,500 | I | 0.83 | Gymnopodium floribundum (72%) | x | x |
| Milleria quinqueflora (19%) | x | x | ||||
| Termilaria buceras (4%) | x | x | ||||
| Albizia lebbeck (3%) | x | x | ||||
| Albizia tomentosa (1%) | x | x | ||||
| C063 | 32,000 | II | 1.13 | Termilaria buceras (58%) | x | x |
| Guazuma ulmifolia (19%) | x | x | ||||
| Milleria quinqueflora (13%) | x | x | ||||
| Zea mays (10%) | x | |||||
| C066 | 63,000 | II | 0.44 | Guazuma ulmifolia (84%) | x | x |
| Zea mays (16%) | x | |||||
| C075 | 17,000 | I | 0.90 | Termilaria buceras (60%) | x | x |
| Zea mays (30%) | x | |||||
| Thouinia paucidentata (10%) | x | x | ||||
| C077 | 191,900 | III | 0.82 | Croton icche (64%) | x | x |
| Termilaria buceras (32%) | x | x | ||||
| Milleria quinqueflora (2%) | x | x | ||||
| Pithecellobium lanceolatum (1%) | x | x | ||||
| C080 | 155,500 | III | 0.55 | Gymnopodium floribundum (85%) | x | x |
| Guazuma ulmifolia (10%) | x | x | ||||
| Zea mays (3%) | x | |||||
| Milleria quinqueflora (2%) | x | x | ||||
| C082 | 16,500 | I | 0.83 | Zea mays (50%) | x | |
| Termilaria buceras (46%) | x | x | ||||
| Milleria quinqueflora (4%) | x | x | ||||
| C083 | 47,000 | II | 0.64 | Zea mays (50%) | x | |
| Termilaria buceras (46%) | x | x | ||||
| Milleria quinqueflora (4%) | x | x | ||||
| Multifloral | ||||||
| C040 | 157,000 | III | 1.16 * | Milleria quinqueflora (42%) | x | x |
| Zea mays (40%) | x | |||||
| Tabebuia rosea (10%) | x | x | ||||
| Albizia lebbeck (8%) | x | x | ||||
| C046 | 69,500 | II | 0.97 * | Termilaria buceras (43%) | x | x |
| Milleria quinqueflora (31%) | x | x | ||||
| Zea mays (11%) | x | |||||
| C058 | 60,500 | II | 1.10 * | Pseudobombax ellipticum (40%) | x | x |
| Termilaria buceras (39%) | x | x | ||||
| Milleria quinqueflora (20%) | x | x | ||||
| C071 | 47,500 | II | 0.99 * | Zea mays (55%) | x | |
| Termilaria buceras (35%) | x | x | ||||
| Pithecellobium lanceolatum (6%) | x | x | ||||
| Guazuma ulmifolia (4%) | x | x | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villalpando-Aguilar, J.L.; Quej-Chi, V.H.; López-Rosas, I.; Cetzal-Ix, W.; Aquino-Luna, V.Á.; Alatorre-Cobos, F.; Martínez-Puc, J.F. Pollen Types Reveal Floral Diversity in Natural Honeys from Campeche, Mexico. Diversity 2022, 14, 740. https://doi.org/10.3390/d14090740
Villalpando-Aguilar JL, Quej-Chi VH, López-Rosas I, Cetzal-Ix W, Aquino-Luna VÁ, Alatorre-Cobos F, Martínez-Puc JF. Pollen Types Reveal Floral Diversity in Natural Honeys from Campeche, Mexico. Diversity. 2022; 14(9):740. https://doi.org/10.3390/d14090740
Chicago/Turabian StyleVillalpando-Aguilar, José Luis, Víctor Hugo Quej-Chi, Itzel López-Rosas, William Cetzal-Ix, Víctor Ángel Aquino-Luna, Fulgencio Alatorre-Cobos, and Jesús Froylán Martínez-Puc. 2022. "Pollen Types Reveal Floral Diversity in Natural Honeys from Campeche, Mexico" Diversity 14, no. 9: 740. https://doi.org/10.3390/d14090740
APA StyleVillalpando-Aguilar, J. L., Quej-Chi, V. H., López-Rosas, I., Cetzal-Ix, W., Aquino-Luna, V. Á., Alatorre-Cobos, F., & Martínez-Puc, J. F. (2022). Pollen Types Reveal Floral Diversity in Natural Honeys from Campeche, Mexico. Diversity, 14(9), 740. https://doi.org/10.3390/d14090740






