13C-Labeled Artificial Root Exudates Are Immediately Respired in a Peat Mesocosm Study
Abstract
:1. Introduction
2. Materials and Methods
3. Results
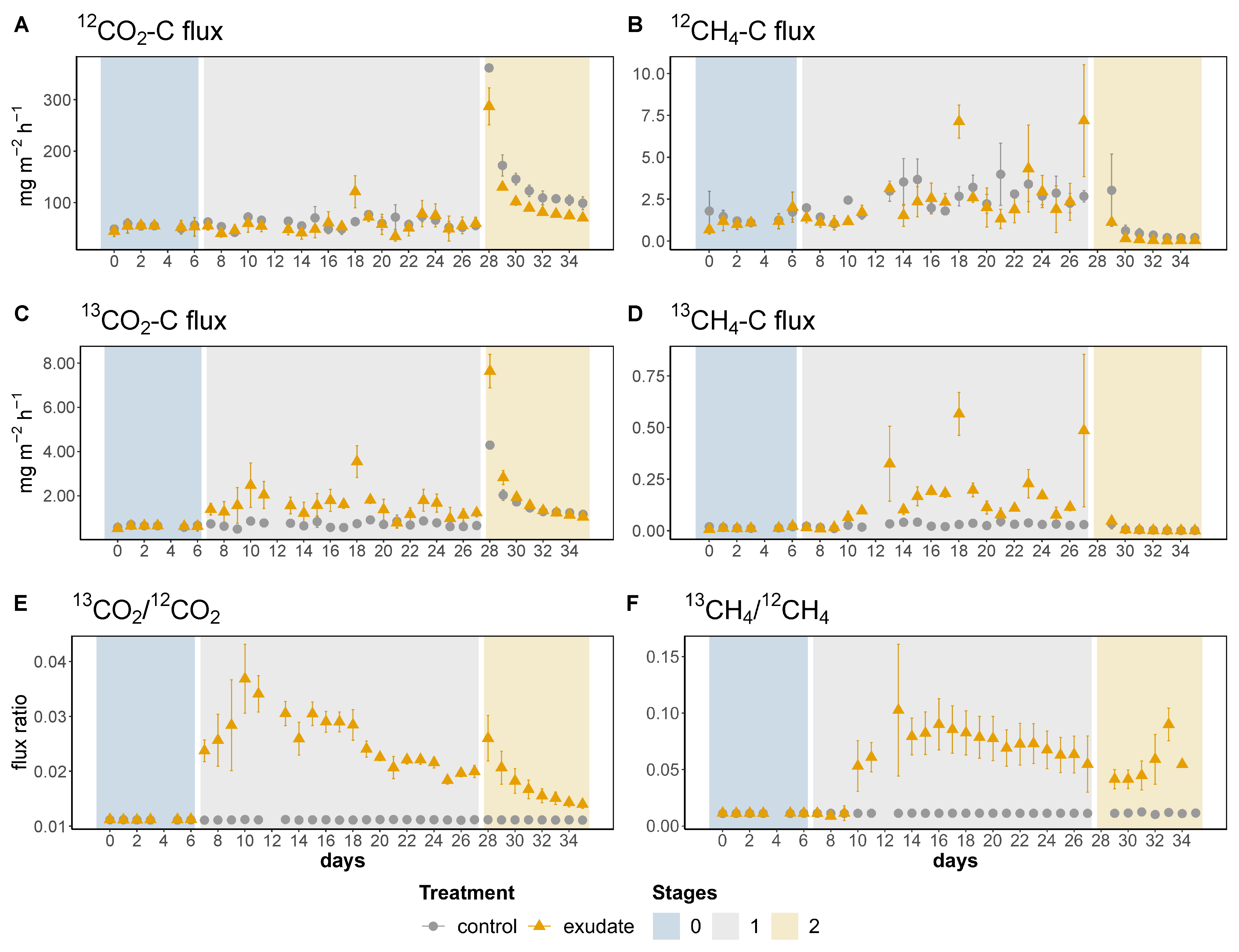
3.1. Gaseous Carbon Release and Stable Isotopic Flux Ratios
3.1.1. Stage 0: Pre-Treatment Phase
3.1.2. Stage 1: Treatment Phase
3.1.3. Stage 2: Drainage Phase
3.2. Stable Carbon Isotopes in Solid Peat
3.3. DOC and Microbial Biomass Carbon
3.4. Fate of 13C-Labeled Artificial Root Exudates
4. Discussion
4.1.
4.2.
4.3. Stable Carbon Isotope Ratios of Solid Peat
4.4. DOC and Microbial Biomass
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Xu, J.; Morris, P.J.; Liu, J.; Holden, J. PEATMAP: Refining estimates of global peatland distribution based on a meta-analysis. Catena 2018, 160, 134–140. [Google Scholar] [CrossRef]
- Yu, Z.; Loisel, J.; Brosseau, D.P.; Beilman, D.W.; Hunt, S.J. Global peatland dynamics since the Last Glacial Maximum. Geophys. Res. Lett. 2010, 37, 1–5. [Google Scholar] [CrossRef]
- Nichols, J.E.; Peteet, D.M. Rapid expansion of northern peatlands and doubled estimate of carbon storage. Nat. Geosci. 2019, 12, 917–921. [Google Scholar] [CrossRef]
- Canarini, A.; Kaiser, C.; Merchant, A.; Richter, A.; Wanek, W. Root Exudation of Primary Metabolites: Mechanisms and Their Roles in Plant Responses to Environmental Stimuli. Front. Plant Sci. 2019, 10, 157. [Google Scholar] [CrossRef]
- Yan, F.; Schubert, S.; Mengel, K. Soil pH increase due to biological decarboxylation of organic anions. Soil Biol. Biochem. 1996, 28, 617–624. [Google Scholar] [CrossRef]
- Girkin, N.T.; Turner, B.L.; Ostle, N.; Craigon, J.; Sjögersten, S. Root exudate analogues accelerate CO2 and CH4 production in tropical peat. Soil Biol. Biochem. 2018, 117, 48–55. [Google Scholar] [CrossRef]
- Kuzyakov, Y. Priming effects: Interactions between living and dead organic matter. Soil Biol. Biochem. 2010, 42, 1363–1371. [Google Scholar] [CrossRef]
- Keiluweit, M.; Bougoure, J.J.; Nico, P.S.; Pett-Ridge, J.; Weber, P.K.; Kleber, M. Mineral protection of soil carbon counteracted by root exudates. Nat. Clim. Chang. 2015, 5, 588–595. [Google Scholar] [CrossRef]
- Ström, L.; Ekberg, A.; Mastepanov, M.; Røjle Christensen, T. The effect of vascular plants on carbon turnover and methane emissions from a tundra wetland. Glob. Chang. Biol. 2003, 9, 1185–1192. [Google Scholar] [CrossRef]
- Crow, S.E.; Wieder, R.K. Sources of CO2 Emission from a Northern Peatland: Root Respiration, Exudation, and Decomposition. Ecology 2005, 86, 1825–1834. [Google Scholar] [CrossRef]
- Mastný, J.; Bárta, J.; Kaštovská, E.; Picek, T. Decomposition of peatland DOC affected by root exudates is driven by specific r and K strategic bacterial taxa. Sci. Rep. 2021, 11, 18677. [Google Scholar] [CrossRef]
- Basiliko, N.; Stewart, H.; Roulet, N.T.; Moore, T.R. Do Root Exudates Enhance Peat Decomposition? Geomicrobiol. J. 2012, 29, 374–378. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, L.; Xu, H.; Ren, K.; Xu, Z.; Tang, Y.; Xiao, J. The effects of warming on root exudation and associated soil N transformation depend on soil nutrient availability. Rhizosphere 2021, 17, 100263. [Google Scholar] [CrossRef]
- Phillips, R.P.; Finzi, A.C.; Bernhardt, E.S. Enhanced root exudation induces microbial feedbacks to N cycling in a pine forest under long-term CO2 fumigation. Ecol. Lett. 2011, 14, 187–194. [Google Scholar] [CrossRef]
- Drollinger, S.; Kuzyakov, Y.; Glatzel, S. Effects of peat decomposition on δ13C and δ15N depth profiles of Alpine bogs. Catena 2019, 178, 1–10. [Google Scholar] [CrossRef]
- Rochette, P.; Ellert, B.; Gregorich, E.G.; Desjardins, R.L.; Pattey, E.; Lessard, R.; Johnson, B.G. Description of a dynamic closed chamber for measuring soil respiration and its comparison with other techniques. Can. J. Soil Sci. 1997, 77, 195–203. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Makarov, M.I.; Malysheva, T.I.; Menyailo, O.V.; Soudzilovskaia, N.A.; van Logtestijn, R.S.P.; Cornelissen, J.H.C. Effect of K2SO4 concentration on extractability and isotope signature (δ13C and δ15N) of soil C and N fractions. Eur. J. Soil Sci. 2015, 66, 417–426. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2020. [Google Scholar]
- Wickham, H.; François, R.; Henry, L.; Müller, K. Dplyr: A Grammar of Data Manipulation. 2020. Available online: https://CRAN.R-project.org/package=dply (accessed on 1 September 2022).
- Kassambara, A. Ggpubr: ’Ggplot2’ Based Publication Ready Plots. 2020. Available online: https://CRAN.R-project.org/package=ggpubr (accessed on 1 September 2022).
- Grolemund, G.; Wickham, H. Dates and Times Made Easy with lubridate. J. Stat. Softw. 2011, 40, 1–25. [Google Scholar] [CrossRef]
- Kassambara, A. Rstatix: Pipe-Friendly Framework for Basic Statistical Tests. 2021. Available online: https://CRAN.R-project.org/package=rstatix (accessed on 1 September 2022).
- Aaltonen, H.; Zhu, X.; Khatun, R.; Laurén, A.A.; Palviainen, M.; Könönen, M.; Peltomaa, E.; Beringer, F.; Köster, K.; Ojala, A.; et al. The effects of glucose addition and water table manipulation on peat quality of drained peatland forests with different management practices. Soil Sci. Soc. Am. J. 2022, 2022, 1–32. [Google Scholar] [CrossRef]
- Yan, W.; Wang, Y.; Ju, P.; Huang, X.; Chen, H. Water level regulates the rhizosphere priming effect on SOM decomposition of peatland soil. Rhizosphere 2022, 21, 100455. [Google Scholar] [CrossRef]
- Ellis, T.; Hill, P.W.; Fenner, N.; Williams, G.G.; Godbold, D.; Freeman, C. The interactive effects of elevated carbon dioxide and water table draw-down on carbon cycling in a Welsh ombrotrophic bog. Ecol. Eng. 2009, 35, 978–986. [Google Scholar] [CrossRef]
- Blodau, C. Carbon cycling in peatlands—A review of processes and controls. Environ. Rev. 2002, 10, 111–134. [Google Scholar] [CrossRef]
- Conrad, R. Methane Production in Soil Environments-Anaerobic Biogeochemistry and Microbial Life between Flooding and Desiccation. Microorganisms 2020, 8, 881. [Google Scholar] [CrossRef] [PubMed]
- Whalen, S.C. Biogeochemistry of Methane Exchange between Natural Wetlands and the Atmosphere. Environ. Eng. Sci. 2005, 22, 73–94. [Google Scholar] [CrossRef]
- Lai, D. Methane Dynamics in Northern Peatlands: A Review. Pedosphere 2009, 19, 409–421. [Google Scholar] [CrossRef]
- Galand, P.E.; Fritze, H.; Conrad, R.; Yrjälä, K. Pathways for methanogenesis and diversity of methanogenic archaea in three boreal peatland ecosystems. Appl. Environ. Microbiol. 2005, 71, 2195–2198. [Google Scholar] [CrossRef]
- Kotsyurbenko, O.R.; Chin, K.J.; Glagolev, M.V.; Stubner, S.; Simankova, M.V.; Nozhevnikova, A.N.; Conrad, R. Acetoclastic and hydrogenotrophic methane production and methanogenic populations in an acidic West-Siberian peat bog. Environ. Microbiol. 2004, 6, 1159–1173. [Google Scholar] [CrossRef]
- Moscôso, J.S.C.; Da Silva, L.S.; Pujol, S.B.; Giacomini, S.J.; Severo, F.F.; Marzari, L.B.; Molin, G.D. Methane emission induced by short-chain organic acids in lowland soil. Rev. Bras. Ciênc. Solo 2019, 43, 1–15. [Google Scholar] [CrossRef]
- Luo, D.; Li, Y.; Yao, H.; Chapman, S.J. Effects of different carbon sources on methane production and the methanogenic communities in iron rich flooded paddy soil. Sci. Total Environ. 2022, 823, 153636. [Google Scholar] [CrossRef]
- Segers, R. Methane production and methane consumption: A review of processes underlying wetland methane fluxes. Biogeochemistry 1998, 41, 23–51. [Google Scholar] [CrossRef]
- Ye, R.; Keller, J.K.; Jin, Q.; Bohannan, B.J.; Bridgham, S.D. Peatland types influence the inhibitory effects of a humic substance analog on methane production. Geoderma 2016, 265, 131–140. [Google Scholar] [CrossRef]
- Estop-Aragonés, C.; Blodau, C. Effects of experimental drying intensity and duration on respiration and methane production recovery in fen peat incubations. Soil Biol. Biochem. 2012, 47, 1–9. [Google Scholar] [CrossRef]
- Hoag, R.S.; Price, J.S. The effects of matrix diffusion on solute transport and retardation in undisturbed peat in laboratory columns. J. Contam. Hydrol. 1997, 28, 193–205. [Google Scholar] [CrossRef]
- McCarter, C.; Rezanezhad, F.; Quinton, W.L.; Gharedaghloo, B.; Lennartz, B.; Price, J.; Connon, R.; van Cappellen, P. Pore-scale controls on hydrological and geochemical processes in peat: Implications on interacting processes. Earth Sci. Rev. 2020, 207, 103227. [Google Scholar] [CrossRef]
- Di Lonardo, D.P.; de Boer, W.; Zweers, H.; van der Wal, A. Effect of the amount of organic trigger compounds, nitrogen and soil microbial biomass on the magnitude of priming of soil organic matter. PLoS ONE 2019, 14, e0216730. [Google Scholar] [CrossRef]
- Schimel, J. The implications of exoenzyme activity on microbial carbon and nitrogen limitation in soil: A theoretical model. Soil Biol. Biochem. 2003, 35, 549–563. [Google Scholar] [CrossRef]
| Time (Week) | DOC Control Group (mg L−1) | DOC Treatment Group (mg L−1) |
|---|---|---|
| 1 (stage 0) | 113.6 ± 22.5 | 129.8 ± 37.6 |
| 2 (stage 1) | 115.4 ± 20.4 ns | 122.9 ± 35.5 ns |
| 3 (stage 1) | 119.2 ± 22.5 ns | 128.7 ± 38.6 ns |
| 4 (stage 1) | 95.7 ± 20.5 ns | 109.8 ± 34.5 ns |
| 5 (stage 1) | 93.1 ± 21.9 ns | 94.1 ± 35.4 ns |
| 6 (stage 2) | 104.3 ± 8.4 ns | 87.9 ± 9.7 ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Müller, R.; Maier, A.; Inselsbacher, E.; Peticzka, R.; Wang, G.; Glatzel, S. 13C-Labeled Artificial Root Exudates Are Immediately Respired in a Peat Mesocosm Study. Diversity 2022, 14, 735. https://doi.org/10.3390/d14090735
Müller R, Maier A, Inselsbacher E, Peticzka R, Wang G, Glatzel S. 13C-Labeled Artificial Root Exudates Are Immediately Respired in a Peat Mesocosm Study. Diversity. 2022; 14(9):735. https://doi.org/10.3390/d14090735
Chicago/Turabian StyleMüller, Raphael, Andreas Maier, Erich Inselsbacher, Robert Peticzka, Gang Wang, and Stephan Glatzel. 2022. "13C-Labeled Artificial Root Exudates Are Immediately Respired in a Peat Mesocosm Study" Diversity 14, no. 9: 735. https://doi.org/10.3390/d14090735
APA StyleMüller, R., Maier, A., Inselsbacher, E., Peticzka, R., Wang, G., & Glatzel, S. (2022). 13C-Labeled Artificial Root Exudates Are Immediately Respired in a Peat Mesocosm Study. Diversity, 14(9), 735. https://doi.org/10.3390/d14090735








