Pollen Morphology of Rosa sericea Complex and Their Taxonomic Contribution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pollen Sample Collection
2.2. Taxonomic Investigation through Light and Scanning Electron Microscopy
2.3. Statistical Analysis
P/E Ratio
3. Results
3.1. General Pollen Morphology of R. sericea Complex
3.2. Qualitative Morphological Characteristics of Pollen of R. sericea Complex
3.3. General Description
3.3.1. Rosa omeiensis
3.3.2. Rosa sericea
3.3.3. Rosa sikangensis
3.3.4. Rosa mairei
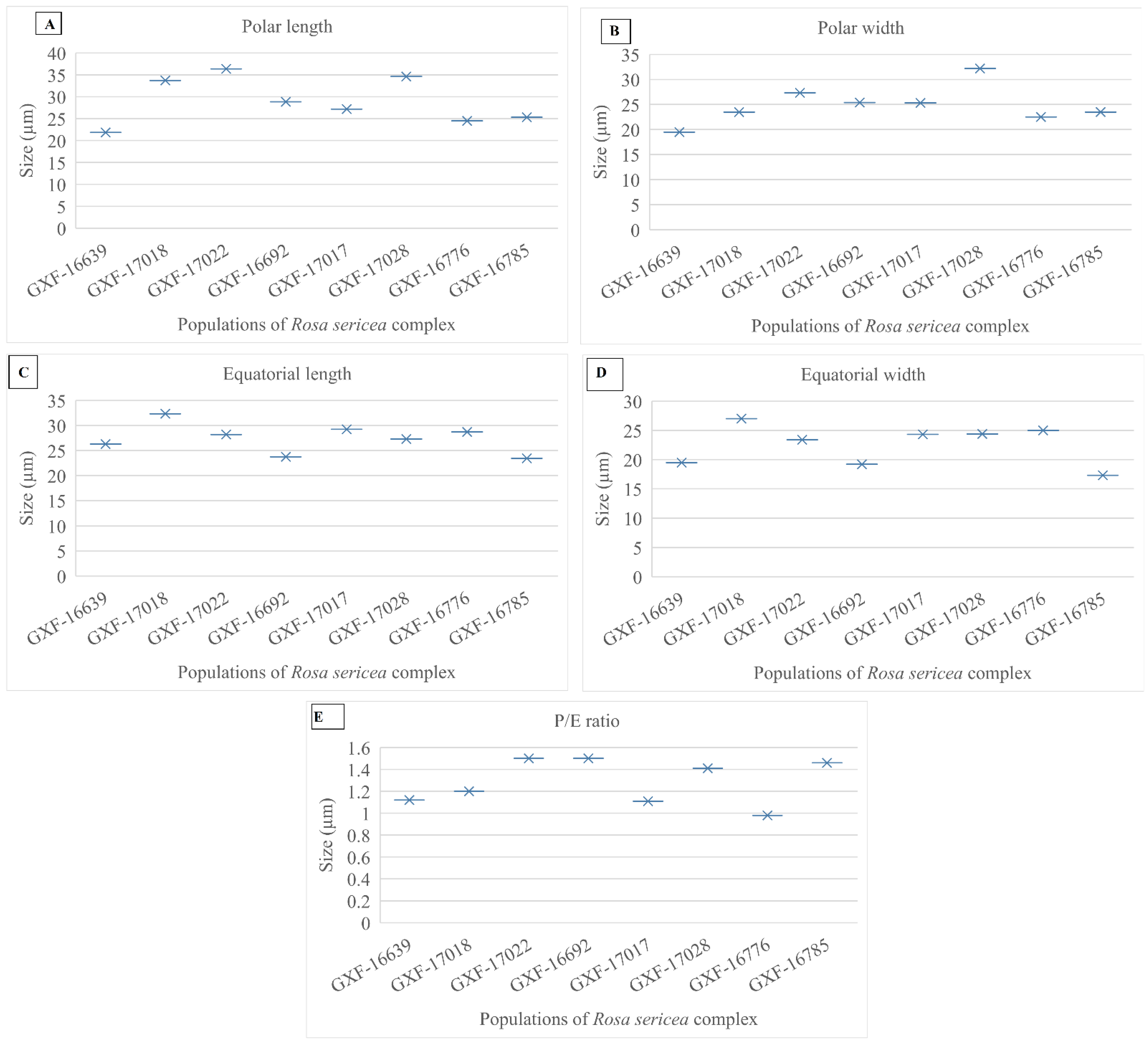
3.4. Quantitative Features of Pollen of R. sericea Complex
3.4.1. Pollen Size
3.4.2. P/E Ratio
3.5. Variation within the Populations of the Species of R. sericea Complex
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kalkman, C. Rosaceae. In Flowering Plants Dicotyledons; Springer: Berlin/Heidelberg, Germany, 2004; pp. 343–386. [Google Scholar]
- Mabberley, D. Mabberley’s Plant-Book: A Portable Dictionary of Plants, Their Classifications, and Uses; Cambridge University Press: Cambridge, UK, 2008; p. 1021. [Google Scholar]
- Schulze-Menz, G.K. Rosaceae. In Engler’s Syllabus der Pflanzenfamilien II, 12th ed.; Melchior, H., Ed.; Gebru der Borntraeger: Berlin, Germany, 1964; pp. 209–218. [Google Scholar]
- Potter, D.; Eriksson, T.; Evans, R.C.; Oh, S.; Smedmark, J.; Morgan, D.R.; Kerr, M.; Robertson, K.R.; Arsenault, M.; Dickinson, T.A. Phylogeny and classification of Rosaceae. Plant Syst. Evol. 2007, 266, 5–43. [Google Scholar] [CrossRef]
- Shah, S.N.; Ahmad, M.; Zafar, M.; Ullah, F.; Zaman, W.; Malik, K.; Rashid, N.; Gul, S. Taxonomic importance of spore morphology in Thelypteridaceae from Northern Pakistan. Microsc. Res. Tech. 2019, 82, 1326–1333. [Google Scholar] [CrossRef] [PubMed]
- Jehanzeb, S.; Zafar, M.; Ahmad, M.; Sultana, S.; Zaman, W.; Ullah, F. Comparative petioler anatomy of tribe Mentheae subfamily Nepetoideae, Lamiaceae from Pakistan. Feddes Repert. 2020, 131, 163–174. [Google Scholar] [CrossRef]
- Usma, A.; Ahmad, M.; Zafar, M.; Sultana, S.; Lubna; Kalsoom, N.; Zaman, W.; Ullah, F. Micromorphological variations and taxonomic implications of caryopses of some grasses from Pakistan. Wulfenia 2020, 27, 86–96. [Google Scholar]
- Gul, S.; Ahmad, M.; Zafar, M.; Bahadur, S.; Zaman, W.; Ayaz, A.; Shuaib, M.; Butt, M.A.; Ullah, F.; Saqib, S. Palynological characteristics of selected Lamioideae taxa and its taxonomic significance. Microsc. Res. Tech. 2021, 84, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Erdtman, G. Pollen morphology and plant taxonomy. Svensk. Bot. Tidskr. 1945, 38, 163–168. [Google Scholar]
- Erdtman, G. Plant Morphology and Plant Taxonomy of Angiosperm—An Introduction to Palynology; Almqvist & Wiksell: Stockholm, Sweden; Chronica Botanica: Waltham, MA, USA, 1952; Volume 539. [Google Scholar]
- Nowicke, J.W.; Skvarla, J.J. Pollen morphology: The potential influence in higher order systematics. Ann. Mo. Bot. Gard. 1979, 66, 633–700. [Google Scholar] [CrossRef]
- Pacini, E.; Franchi, G.G. Pollen biodiversity—Why are pollen grains different despite having the same function? A review. Bot. J. Linn. Soc. 2020, 193, 141–164. [Google Scholar] [CrossRef]
- Van Geel, B.; Coope, G.R.; Van Der Hammen, T. Palaeoecology and stratigraphy of the Lateglacial type section at Usselo (The Netherlands). Rev. Palaeobot. Palynol. 1989, 60, 25–129. [Google Scholar] [CrossRef]
- Ullah, F.; Gao, Y.; Jiao, R.-F.; Gao, X.-F. Comparative taxonomic variation in fruits and seeds’ surface morphology among populations of alpine Rosa sericea complex (Rosaceae). Microsc. Res. Tech. 2021, 84, 2337–2350. [Google Scholar] [CrossRef]
- Ullah, F.; Gao, Y.; Sari, İ.; Jiao, R.-F.; Saqib, S.; Gao, X.-F. Macro-Morphological and Ecological Variation in Rosa sericea Complex. Agronomy 2022, 12, 1078. [Google Scholar] [CrossRef]
- Hebda, R.J.; Chinnappa, C. Studies on pollen morphology of Rosaceae. Acta Bot. Gall. 1994, 141, 183–193. [Google Scholar] [CrossRef] [Green Version]
- Heywood, V. Flowering Plants of the World. Mayflower Book; Elsevier: New York, NY, USA, 2007; pp. 141–144. [Google Scholar]
- Crepet, W.L.; Nixon, K.C.; Gandolfo, M.A. Fossil evidence and phylogeny: The age of major angiosperm clades based on mesofossil and macrofossil evidence from Cretaceous deposits. Am. J. Bot. 2004, 91, 1666–1682. [Google Scholar] [CrossRef] [PubMed]
- Hummer, K.E.; Janick, J. Rosaceae: Taxonomy, economic importance, genomics. In Genetics and Genomics of Rosaceae; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1–17. [Google Scholar]
- Soltis, D.; Soltis, P.; Endress, P.; Chase, M. Phylogeny and Evolution of Angiosperms Sinauer Associates; Sunderland Holding Inc.: Sunderland, MA, USA, 2005; pp. 237–248. [Google Scholar]
- Zieliński, J.; Guzicka, M.; Tomaszewski, D.; Maciejewska-Rutkowska, I. Pericarp anatomy of wild roses (Rosa L., Rosaceae). Flora-Morphol. Distrib. Funct. Ecol. Plants 2010, 205, 363–369. [Google Scholar] [CrossRef]
- Adamczak, A.; Buchwald, W.; Zieliński, J.; Mielcarek, S. Flavonoid and organic acid content in rose hips (Rosa L., sect. Caninae DC. EM. Christ.). Acta Biol. Crac. Ser. Botánica 2012, 54, 105–112. [Google Scholar] [CrossRef]
- Nybom, H.; Carlson-Nilsson, U.; Werlemark, G.; Uggla, M. Different levels of morphometric variation in three heterogamous dogrose species (Rosa sect. Caninae, Rosaceae). Plant Syst. Evol. 1997, 204, 207–224. [Google Scholar] [CrossRef]
- Wissemann, V.; Ritz, C.M. The genus Rosa (Rosoideae, Rosaceae) revisited: Molecular analysis of nrITS-1 and atp B-rbc L intergenic spacer (IGS) versus conventional taxonomy. Bot. J. Linn. Soc. 2005, 147, 275–290. [Google Scholar] [CrossRef]
- Rehder, A. Manual of cultivated trees and shrubs hardy in North America. Nature 1940, 148, 7. [Google Scholar]
- Rehder, A. Bibliography of Cultivated Trees and Shrubs Hardy in the Cooler Temperate Regions of the Northern Hemisphere; Arnold Arboretum of Harvard Univ.: Jamaica Plain, MA, USA, 1949. [Google Scholar]
- Rowley, G.D. Typification of the genus Rosa L. Taxon 1976, 25, 181. [Google Scholar] [CrossRef]
- Jarvis, C. Seventy-two proposals for the conservation of types of selected Linnaean generic names, the report of Subcommittee 3C on the lectotypification of Linnaean generic names. Taxon 1992, 41, 552–583. [Google Scholar] [CrossRef]
- Gao, Y.-D.; Zhang, Y.; Gao, X.-F.; Zhu, Z.-M. Pleistocene glaciations, demographic expansion and subsequent isolation promoted morphological heterogeneity: A phylogeographic study of the alpine Rosa sericea complex (Rosaceae). Sci. Rep. 2015, 5, 11698. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Gao, X.; Harris, A. Species boundaries and parapatric speciation in the complex of alpine shrubs, Rosa sericea (Rosaceae), based on population genetics and ecological tolerances. Front. Plant Sci. 2019, 10, 321. [Google Scholar] [CrossRef] [PubMed]
- Erdtman, G. Handbook of Palynology: Morphology, Taxonomy, Ecology; Hafner: New York, NY, USA, 1969. [Google Scholar]
- Sampson, F.B. Pollen diversity in some modern magnoliids. Int. J. Plant Sci. 2000, 161, S193–S210. [Google Scholar] [CrossRef]
- Reitsma, T. Pollen morphology of some European Rosaceae. Acta Bot. Neerl. 1966, 15, 290–307. [Google Scholar] [CrossRef]
- Teppner, H. Zur kenntnis der gattung Waldsteinia, I. schlussel zum bestimmen von rosaceen-pollen einschliesslich ahnlicher pollenformen aus anderen familien. Phyton 1966, 11, 224–238. [Google Scholar]
- Wrońska-Pilarek, D.; Boratyńska, K. Pollen morphology of Rosa gallica L. (Rosaceae) from southern Poland. Acta Soc. Bot. Pol. 2005, 74, 297–304. [Google Scholar] [CrossRef]
- Ueda, Y. Pollen surface morphology in the genus Rosa and related genera. Jap. J. Palynol. 1992, 38, 94–105. [Google Scholar]
- Popek, R. Biosystematyczne Studia nad Rodzajem Rosa L. w Polsce i Krajach Ościennych; Wydawnictwo Naukowe Wyższej Szkoły Pedagogicznej: Kraków, Czech Republic, 1996. [Google Scholar]
- Bai, J.; Zhang, Q.; Luo, L.; Pan, H.; Yu, C. Pollen morphology of some Chinese traditional roses. Bull. Bot. Res. 2011, 31, 15–23. [Google Scholar]
- Eide, F.y. Key for northwest European Rosaceae pollen. Grana 1981, 20, 101–118. [Google Scholar] [CrossRef]
- Hebda, R.J.; Chinnappa, C. Studies on pollen morphology of Rosaceae in Canada. Rev. Palaeobot. Palynol. 1990, 64, 103–108. [Google Scholar] [CrossRef]
- Hebda, R.; Chinnappa, C.; Smith, B. Pollen morphology of the Rosaceae of Western Canada: I. Agrimonia to Crataegus. Grana 1988, 27, 95–113. [Google Scholar] [CrossRef]
- Shinwari, M.I.; Khan, M. Pollen morphology of wild roses from Pakistan. Hamdard Med. 2004, 47, 5–13. [Google Scholar]
- Wrońska-Pilarek, D.; Jagodziński, A.M. Systematic importance of pollen morphological features of selected species from the genus Rosa (Rosaceae). Plant Syst. Evol. 2011, 295, 55–72. [Google Scholar] [CrossRef]
- Xiong, X.H.; Zhou, X.M.; Li, M.; Xu, B.; Deng, H.N.; Yu, Q.; Gao, X.F. Pollen morphology in Rubus (Rosaceae) and its taxonomic implications. Plant Syst. Evol. 2019, 305, 705–716. [Google Scholar] [CrossRef]
- Dickinson, T.; Evans, R.; Campbell, C. Rosaceae classification and phylogeny: Introduction and overview. Plant Syst. Evol. 2007, 266, 5–43. [Google Scholar]
- Challice, J. Rosaceae chemotaxonomy and the origins of the Pomoideae. Bot. J. Linn. Soc. 1974, 69, 239–259. [Google Scholar] [CrossRef]
- Morgan, D.R.; Soltis, D.E.; Robertson, K.R. Systematic and evolutionary implications of rbcL sequence variation in Rosaceae. Am. J. Bot. 1994, 81, 890–903. [Google Scholar] [CrossRef]
- Zielinski, J. Studia nad rodzajem Rosa L. Systematyka sekcji Caninae DC em. Christ. Arbor. Kórnicki 1985, 30, 3–109. [Google Scholar]
- Gustafsson, Ä. The constitution of the Rosa canina complex. Hereditas 1944, 30, 405–428. [Google Scholar] [CrossRef]
- Zhou, L.; Wei, Z.; Wu, Z. Pollen morphology of Rosoideae (Rosaceae) of China. Acta Bot. Yunnanica 1999, 21, 455–460. [Google Scholar]
- Ghosh, A.; Saha, I. Pollen morphological study of some selected Indian taxa of Rosaceae. Indian J. Appl. Pure Bio 2017, 32, 121–130. [Google Scholar]
- Zhou, L.; Wei, Z.; Wu, Z. Pollen morphology of Maloideae of China (Rosaceae). Acta Bot. Yunnanica 2000, 22, 47–52. [Google Scholar]
- Zhou, L.; Wei, Z.; Wu, Z. Pollen morphology of Prunoideae of China (Rosaceae). Acta Bot. Yunnanica 1999, 21, 207–211. [Google Scholar]
- Song, J.-H.; Moon, H.-K.; Hong, S.-P. Pollen morphology of the tribe Sorbarieae (Rosaceae). Plant Syst. Evol. 2016, 302, 853–869. [Google Scholar] [CrossRef]
- Wronska-Pilarek, D.; Lira, J. Pollen morphology of Polish species of the genus Rosa-I. Rosa pendulina. Dendrobiology 2006, 55, 65–73. [Google Scholar]
- Fatemi, N.; Attar, F.; Assareh, M.H.; Hamzehee, B. Pollen morphology of the genus Rosa L. (Rosaceae) in Iran. Iran. J. Bot. 2012, 18, 284–293. [Google Scholar]
- Wronska-Pilarek, D. Pollen morphology of Polish native species of the Rosa genus (Rosaceae) and its relation to systematics. Acta Soc. Bot. Pol. 2011, 80, 221. [Google Scholar] [CrossRef]
- Jacob, Y.; Pierret, V. Pollen size and ploidy level in the genus Rosa. In Proceedings of the XIX International Symposium on Improvement of Ornamental Plants 508, Angers, France, 27–30 July 1998; pp. 289–292. [Google Scholar]
- Reitsma, T. Suggestions towards unification of descriptive terminology of angiosperm pollenn grains. Rev. Palaeobot. Palynol. 1970, 10, 39–60. [Google Scholar] [CrossRef]
- Punt, W.; Hoen, P.; Blackmore, S.; Nilsson, S.; Le Thomas, A. Glossary of pollen and spore terminology. Rev. Palaeobot. Palynol. 2007, 143, 1–81. [Google Scholar] [CrossRef]
- Erdtman, G. Pollen Morphology and Plant Taxonomy; McGraw-Hill: New York, NY, USA, 1952; pp. 6–24. [Google Scholar]
- Agudo, J.S.; Rico, E.; Sánchez, J.S. Palynological study of Potentilla subg. Potentilla (Rosaceae) in the western Mediterranean. Grana 1998, 37, 276–284. [Google Scholar] [CrossRef]
- Kolodziejek, J.; Gabara, B. Palynological study of Polish taxa of Potentilla subsect. Collinae (Rosaceae). Acta Bot. Croat. 2008, 67, 139–146. [Google Scholar]
- Wronska-Pilarek, D.; Jagodzinski, A.M. Pollen morphological variability of Polish native species of Rosa L. (Rosaceae). Dendrobiology 2009, 62, 71–82. [Google Scholar]
- Wrońska-Pilarek, D.; Jagodziński, A.M.; Bocianowski, J.; Janyszek, M. The optimal sample size in pollen morphological studies using the example of Rosa canina L. (Rosaceae). Palynology 2015, 39, 56–75. [Google Scholar] [CrossRef]
- Wrońska-Pilarek, D.; Bocianowski, J.; Jagodziński, A.M. Comparison of pollen grain morphological features of selected species of the genus Crataegus (Rosaceae) and their spontaneous hybrids. Bot. J. Linn. Soc. 2013, 172, 555–571. [Google Scholar] [CrossRef]
- Tahir, S.S. Pollen morphology of Sibbaldia species (Rosaceae). Pak. J. Bot. 2005, 37, 7–13. [Google Scholar]
- Ueda, Y.; Okada, Y. Discrimination of rose cultivar groups by pollen surface structure. J. Hortic. Sci. 1994, 69, 601–607. [Google Scholar] [CrossRef]
- Fischer, H.; Darrow, G.; Perlmutter, F. Raspberry and blackberry breeding: Production of tetraploid raspberries. Proc. Am. Soc. Hort. Sci. 1943, 42, 447–456. [Google Scholar]
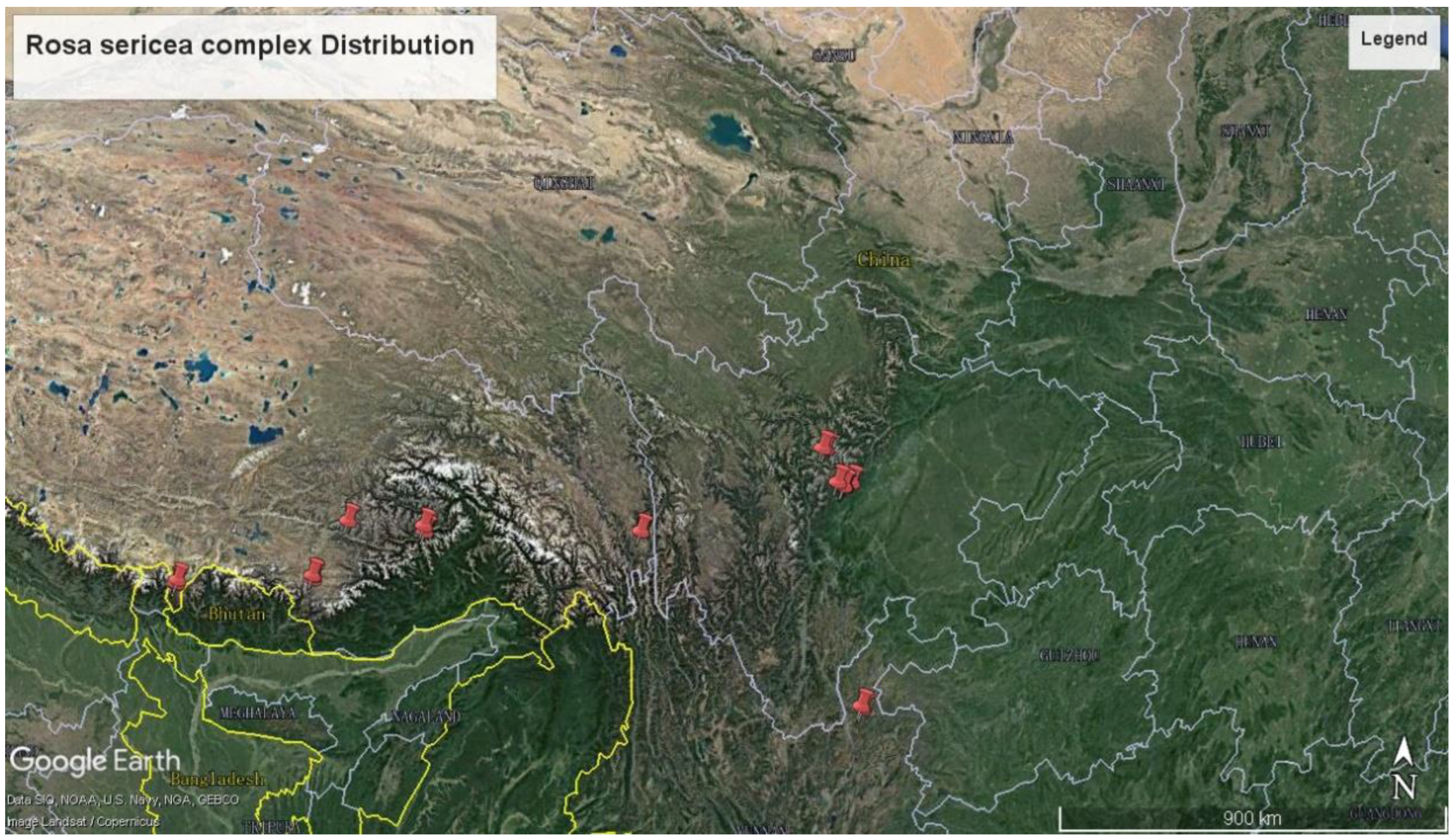




| Taxon | Population Code | Altitude | Location | Latitude | Longitude |
|---|---|---|---|---|---|
| R. sericea | GXF-16734 | 3188 | Xizang, Gyaca | 29.13 | 92.69 |
| R.omeiensis | GXF-16639 | 3800 | Xizang, Yadong | 27.57 | 89.02 |
| R. sericea | GXF-16646 | 2806 | Xizang, Yadong | 27.41 | 88.94 |
| R.omeiensis | GXF-16751 | 3588 | Xizang, Mainling | 29.49 | 94.91 |
| R. sericea | GXF-16771 | 3704 | Xizang, Mainling | 29.49 | 94.92 |
| R.omeiensis | GXF-17018 | 3127 | Sichuan, Baoxing | 30.83 | 102.72 |
| R.omeiensis | GXF-17022 | 3593 | Sichuan, Xianojin | 30.88 | 102.65 |
| R. sericea | GXF-16692 | 2912 | Xizang, Cuona | 27.91 | 91.80 |
| R. sericea | GXF-17017 | 2325 | Sichuan, Baoxing | 30.77 | 102.72 |
| R. sericea | GXF-17028 | 3591 | Sichuan, Xiaojin | 30.88 | 102.65 |
| R.sikangensis | GXF-16776 | Xizang, Bangda | 30.31 | 96.55 | |
| R.mairei | GXF-16785 | 2185 | Yunnan, Huize | 26.48 | 103.58 |
| R. sericea | GXF-16692 | 2935 | Xizang, Cuona | 27.91 | 91.80 |
| R.omeiensis | GXF-16639 | 3800 | Xizang, Yadong | 27.57 | 89.02 |
| R.omeiensis | GXF-17018 | 3127 | Sichuan, Baoxing | 30.83 | 102.72 |
| R.omeiensis | GXF-17022 | 3593 | Sichuan, Xianojin | 30.88 | 102.65 |
| R. sericea | GXF-16692 | 2912 | Xizang, Cuona | 27.91 | 91.80 |
| R. sericea | GXF-17017 | 2325 | Sichuan, Baoxing | 30.77 | 102.72 |
| R. sericea | GXF-17028 | 3591 | Sichuan, Xiaojin | 30.88 | 102.65 |
| R.sikangensis | GXF-16776 | Xizang, Bangda | 30.31 | 96.55 | |
| R.mairei | GXF-16785 | 2185 | Yunnan, Huize | 26.48 | 103.58 |
| Taxon | Polar View | Equatorial View | Sculpture | Pollen Outline | Apertures |
|---|---|---|---|---|---|
| R. omeiensis | 1,3-lobed spherical, with obtuse poles | Spheroidal to prolate | Striae slightly bent, 0.15 µm in diam; 0.18 µm above channels; channels 0.29 µm wide; 7.71 perforations/µm2, round, 0.02–0.05 µm in diam. | The polar view is almost circular, rarely triangular obtuse, equatorial view often elliptic, rarely circular. | Tri-ectocolpi, long, deep and sharply tapering, regularly arranged, longitudinal pattern. Having variable width, greater at equatorial region with sculpturing of ectocolpus membrane. Operculum on aperture membrane, usually situated symmetrically. |
| R. sericea | 1,3-lobed spherical, with round poles | Spheroidal to prolate | Striae slightly bent, different in length, 0.12 µm in diam; 0.20 µm above channels; channels 0.15 µm wide; 7.13 perforations/µm2, round, 0.02–0.08 µm in diam. | Polar view a bit longitudinal, almost circular, rarely obtuse or triangular, equatorial view spheroidal to prolate, sometimes elliptic. | Tri ectocolpi, long deep and sharp tapering, often elliptical, long in outline, deeply set into the exine, ending sharply. Operculum in central part of ectocopus, elongated, narrow and psilate. |
| R. sikangensis | 1,3-lobed spherical, with obtuse poles | Subspheroid al to prolate | Striae straight, 0.10–0.15 μm in diam., strongly divaricated; 0.09 µm above channels; channels 0.22 μm wide; 1.13 perforations/μm2, ovate, different in size, polar axis ca. 0.1 μm long. | Polar view circular, triangular obtuse apices, sometimes elliptical, equatorial view circular, rarely elliptical. | Tri ectocolpi, deep and sharp tapering, ectocolpus is regulated. Operculum on aperture membrane, operculum sculpture psilate. |
| R. mairei | 1,3-lobed spherical, with round poles | Spheroidal to prolate | Striae straight, 0.15 μm in diam., strongly divaricated; 0.18 µm above channels; channels 0.11–0.25 μm wide; 6.64 perforations/μm2, nearly round, 0.04–0.11 μm. | The polar view is almost circular, obtuse apices, narrowly elliptical, and the equatorial view is almost circular and spheroidal to prolate. | Tri ectocolpi, deeply and sharp tapering, ectocolpus regular. Operculum is positioned in the middle of the ectocolpi and covers it partly or completely. |
| Taxa | Polar Diameter (µm) | Equatorial Diameter (µm) | P/E (µm) | Colpi Length | Colpi Width | Distance between Colpi |
|---|---|---|---|---|---|---|
| R. omeiensis | 32.39 (21.86–36.37) | 28.58 (26.27–32.35) | 1.13 | 22.5 µm | 2.5 µm | 20 µm |
| R. sericea | 29.83 (25.39–34.61) | 26.67 (23.73–29.22) | 1.11 | 24 µm | 2.6 µm | 17 µm |
| R. sikangensis | 25.06 (21.90–28.83) | 17.97 (15.20–20.37) | 0.98 | 20.4 µm | 1.9 µm | 15 µm |
| R. mairei | 27.10 (22.94–30.20) | 22.72 (15.29–28.14) | 1.46 | 23.5 µm | 2.3 µm | 18 µm |
| Taxon | Population Code | Polar Length | Polar Width | Equatorial Length | Equatorial Width | P/E | Shape | Aperture | Exine |
|---|---|---|---|---|---|---|---|---|---|
| R. omeiensis | GXF-16639 | 21.86 µm | 19.5 µm | 26.27 µm | 19.5 µm | 1.12 | Colpus | 3 | Thick |
| R. omeiensis | GXF-17018 | 33.7 µm | 23.5 µm | 32.35 µm | 27 µm | 1.2 | Colpus | 3 | Thin |
| R. omeiensis | GXF-17022 | 36.37 µm | 27.35 µm | 28.2 µm | 23.4 µm | 1.5 | Colpus | 3 | Thin |
| R. sericea | GXF-16692 | 28.83 µm | 25.4 µm | 23.73 µm | 19.2 µm | 1.5 | Colpus | 3 | Thick |
| R. sericea | GXF-17017 | 27.20 µm | 25.3 µm | 29.22 µm | 24.32 µm | 1.11 | Colpus | 3 | Thin |
| R. sericea | GXF-17028 | 34.61 µm | 32.20 µm | 27.30 µm | 24.4 µm | 1.41 | Colpus | 3 | Thick |
| R. sikangensis | GXF-16776 | 24.5 µm | 22.5 µm | 28.7 µm | 25 µm | 0.98 | Colpus | 3 | Thin |
| R. mairei | GXF-16785 | 25.3 µm | 23.5 µm | 23.43 µm | 17.3 µm | 1.46 | Colpus | 3 | Thick |
| Population Code | Colpus on Polar | Colpus on Equatorial | Polar Diameter | Equatorial Diameter | P/E | Size Category | Polar View | Equatorial View | Pole of Pollen | Figure |
|---|---|---|---|---|---|---|---|---|---|---|
| GXF-16639-2 | 2 | 1 | 2 | 1 | 1 | 2 | 1 | 1 | 1 | 1A, 1B, 1C |
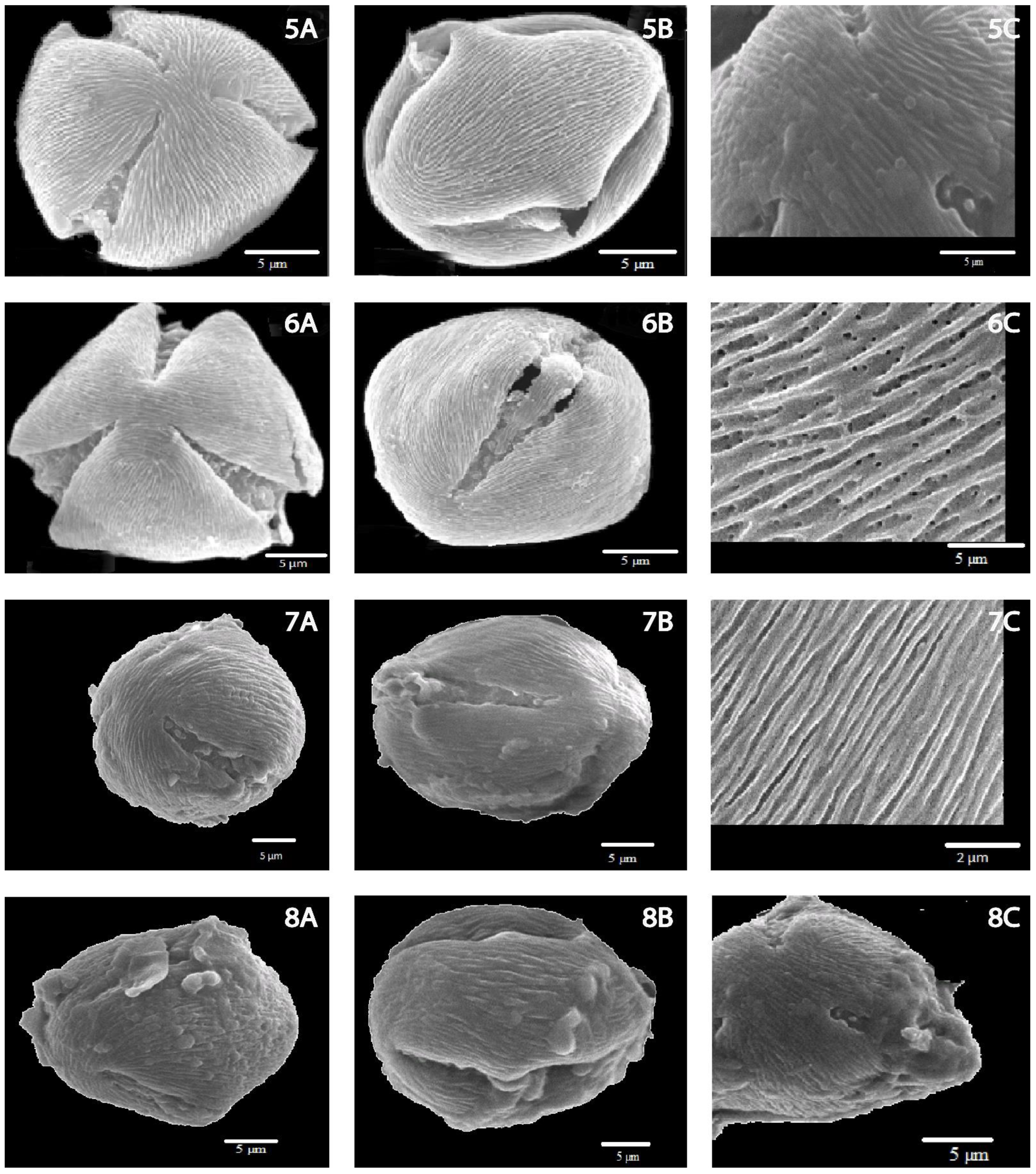
| GXF-16639-1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7A, 7B, 7C |
| GXF-16639-3 | 1 | 1 | 1 | 1 | 1 | 1 | ½ | 2 | 1 | 8A, 8B, 8C |
| GXF-16639-4 | 2 | 2 | 2 | 2 | 1 | 2 | 1 | 1 | 1 | 9A, 9B, 9C |
| GXF-17018-1 | 2 | 1 | 2 | 2 | 2 | 2 | 1 | 2 | 1 | 17A, 17B, 17C |
| GXF-17018-2 | 2 | 1 | 2 | 2 | 1 | 2 | 1 | 1 | 1 | 18A, 18B, 18C |
| GXF-17022-2 | 2 | 2 | 2 | 1 | 1 | 2 | 1 | 1 | 1 | 19A, 19B, 19C |
| GXF-17022-3 | 2 | 1 | 2 | 1 | 2 | 2 | 1 | 2 | 1 | 20A, 20B, 20C |
| GXF-16692-4 | 2 | 2 | 2 | 2 | 1 | 2 | 2/1 | 1 | 2 | 2A, 2B, 2C |
| GXF-16692-1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 2 | 12A, 12B, 12C |
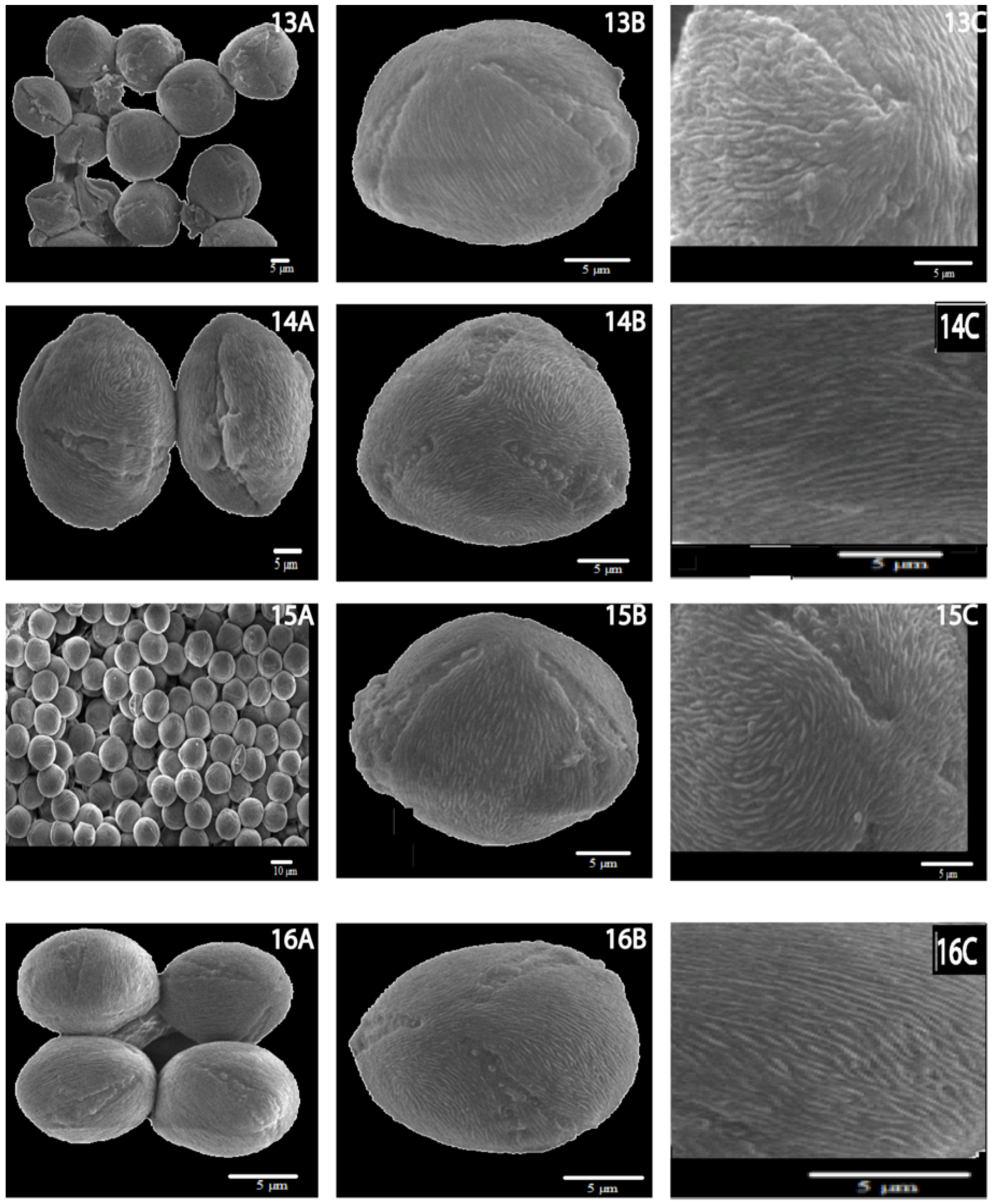
| GXF-16692-2 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 2 | 13A, 13B, 13C |
| GXF-16692-3 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 14A, 14B, 14C |
| GXF-16692-5 | 2 | 1 | 2 | 2 | 2 | 2 | 1 | 1 | 2 | 15A, 15B, 15C |
| GXF-17017-1 | 2 | 2 | 1 | 2 | 1 | 2 | 1 | 1 | 2 | 16A, 16B, 16C |
| GXF-17028-1 | 2 | 1 | 2 | 2 | 1 | 2 | 2/1 | 1 | 2 | - |
| GXF-17028-2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 2 | - |
| GXF-16785-2 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 3A, 3B, 3C |
| GXF-16785-1 | 2 | 2 | 1 | 1 | 2 | 1 | 1 | 1 | 2 | 5A, 5B, 5C |
| GXF-16785-3 | 2 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 2 | 6A, 6B, 6C |
| GXF-16676-1 | 2 | 1 | 1 | 2 | 2 | 2 | 1 | 1 | 1 | 4A, 4B, 4C |
| GXF-16676-2 | 2 | 1 | 1 | 2 | 2 | 2 | 1 | 1 | 1 | 10A, 10B, 10C |
| GXF-16676-3 | 2 | 1 | 1 | 2 | 2 | 2 | 1 | 1 | 1 | 11A, 11B, 11C |
| Keys | 1 = one/three colpi 2 = three colpi | 1 = one/three colpi 2 = three colpi | 1 = less than 29 µm 2 = more than 29 µm | 1 = less than 24 µm 2 = more than 24 µm | 1 = less than 1.14 µm 2 = more than 1.14 µm | 1 = small 2 = medium | 1 = spherical 2 = semi-spherical | 1 = spheroidal 2 = prolate | 1 = obtuse pole 2 = round pole |
| PC | Eigenvalue | % Variance |
|---|---|---|
| 1 | 0.836 | 35.777 |
| 2 | 0.425 | 18.209 |
| 3 | 0.404 | 17.304 |
| 4 | 0.227 | 9.732 |
| 5 | 0.188 | 8.042 |
| 6 | 0.163 | 6.984 |
| 7 | 0.067 | 2.903 |
| 8 | 0.024 | 1.045 |
| 9 | 5.382 | 2.302 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, F.; Gao, Y.-D.; Zaman, W.; Gao, X.-F. Pollen Morphology of Rosa sericea Complex and Their Taxonomic Contribution. Diversity 2022, 14, 705. https://doi.org/10.3390/d14090705
Ullah F, Gao Y-D, Zaman W, Gao X-F. Pollen Morphology of Rosa sericea Complex and Their Taxonomic Contribution. Diversity. 2022; 14(9):705. https://doi.org/10.3390/d14090705
Chicago/Turabian StyleUllah, Fazal, Yun-Dong Gao, Wajid Zaman, and Xin-Fen Gao. 2022. "Pollen Morphology of Rosa sericea Complex and Their Taxonomic Contribution" Diversity 14, no. 9: 705. https://doi.org/10.3390/d14090705
APA StyleUllah, F., Gao, Y.-D., Zaman, W., & Gao, X.-F. (2022). Pollen Morphology of Rosa sericea Complex and Their Taxonomic Contribution. Diversity, 14(9), 705. https://doi.org/10.3390/d14090705








