Ecology and Biology of the Rare Endemic Land Leech Xerobdella anulata (Xerobdellidae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling and Identification
2.3. Molecular Identification
2.4. Vegetation and Soil Analysis
2.5. Data Analysis
2.5.1. Distribution
2.5.2. Soil Properties
2.5.3. Seasonal Activity
2.5.4. Phylogenetic Analysis
3. Results and Discussion
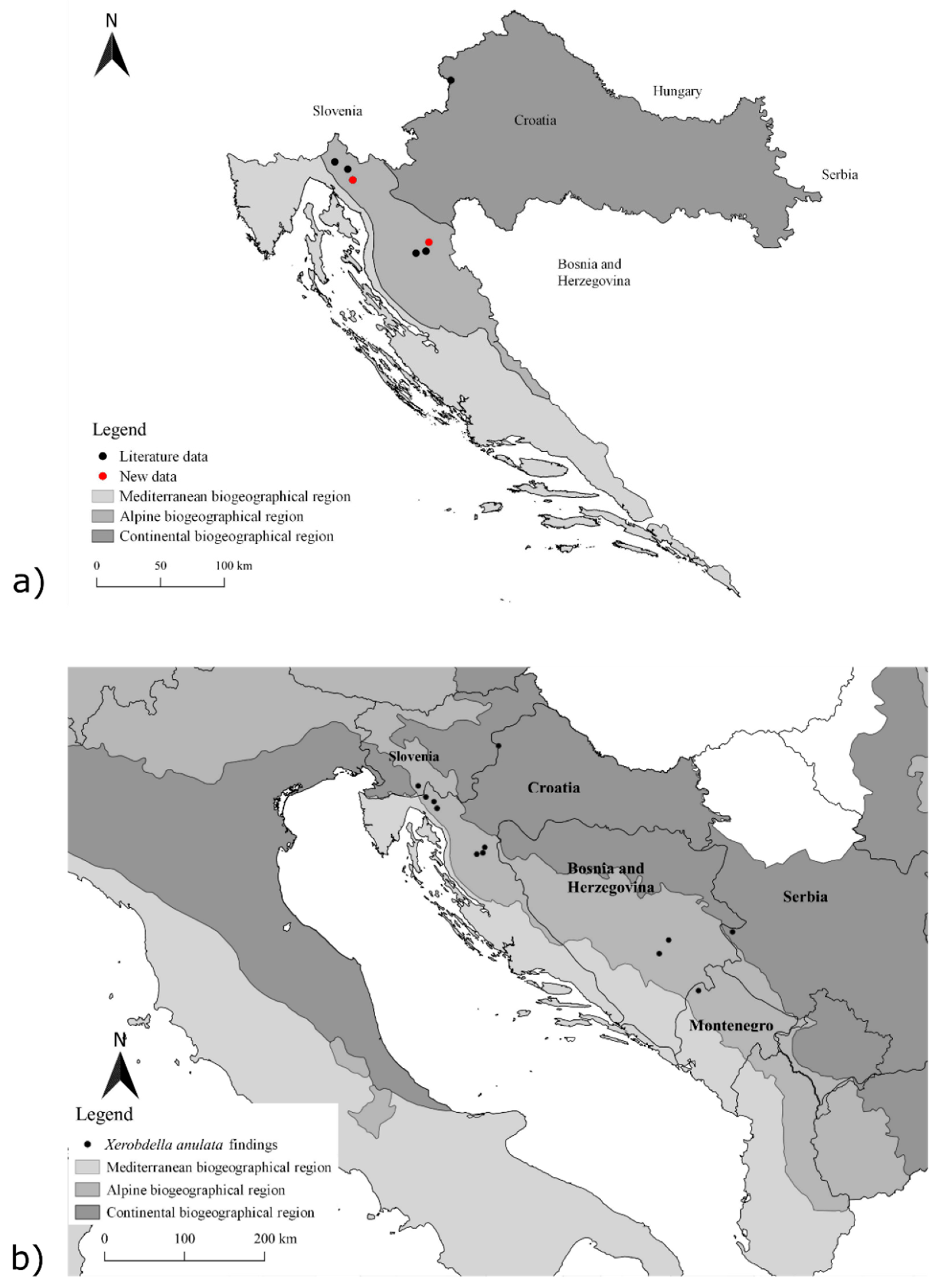
3.1. Distribution
3.2. Morphology
3.3. Habitat
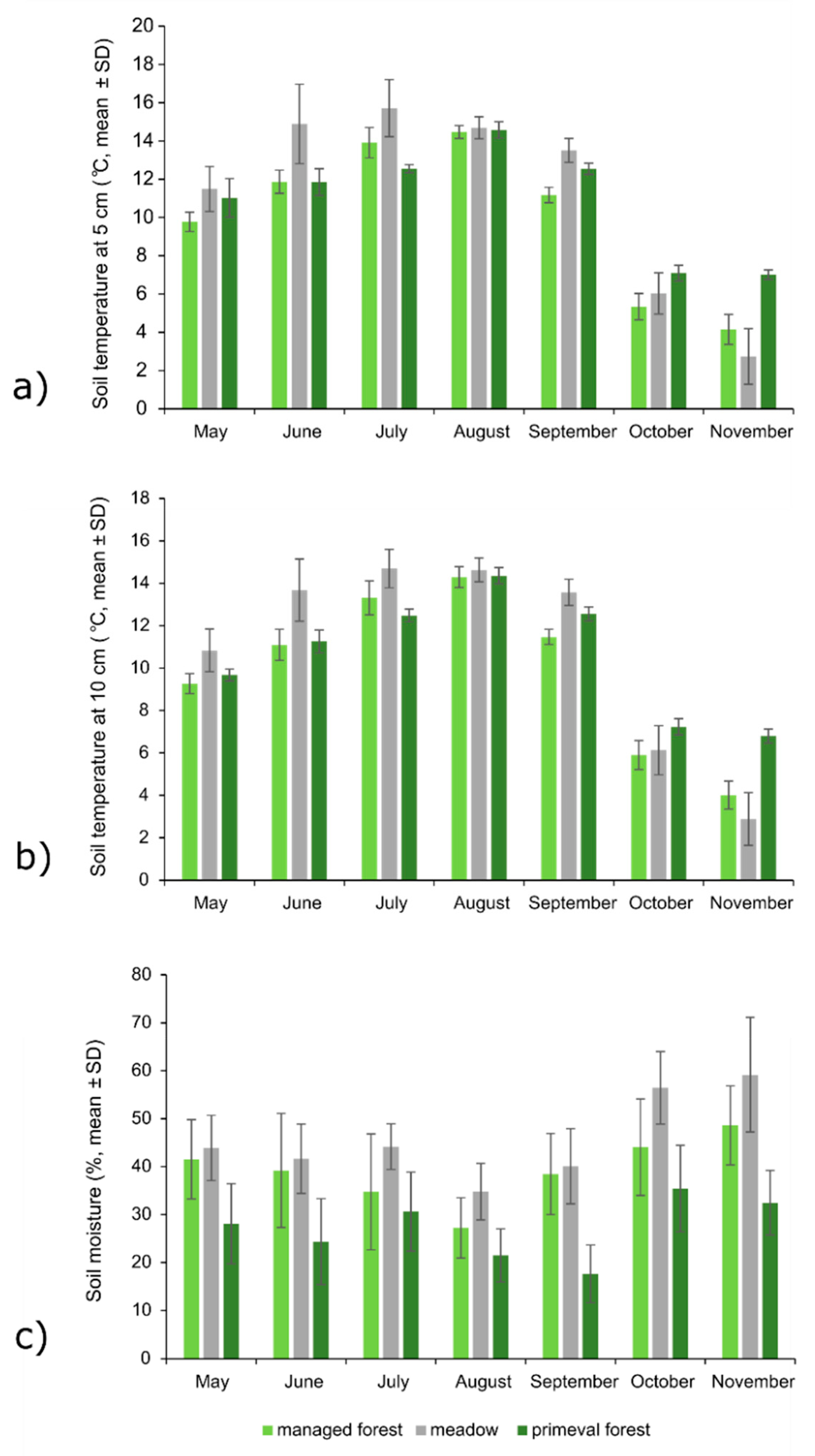
3.4. Soil Properties
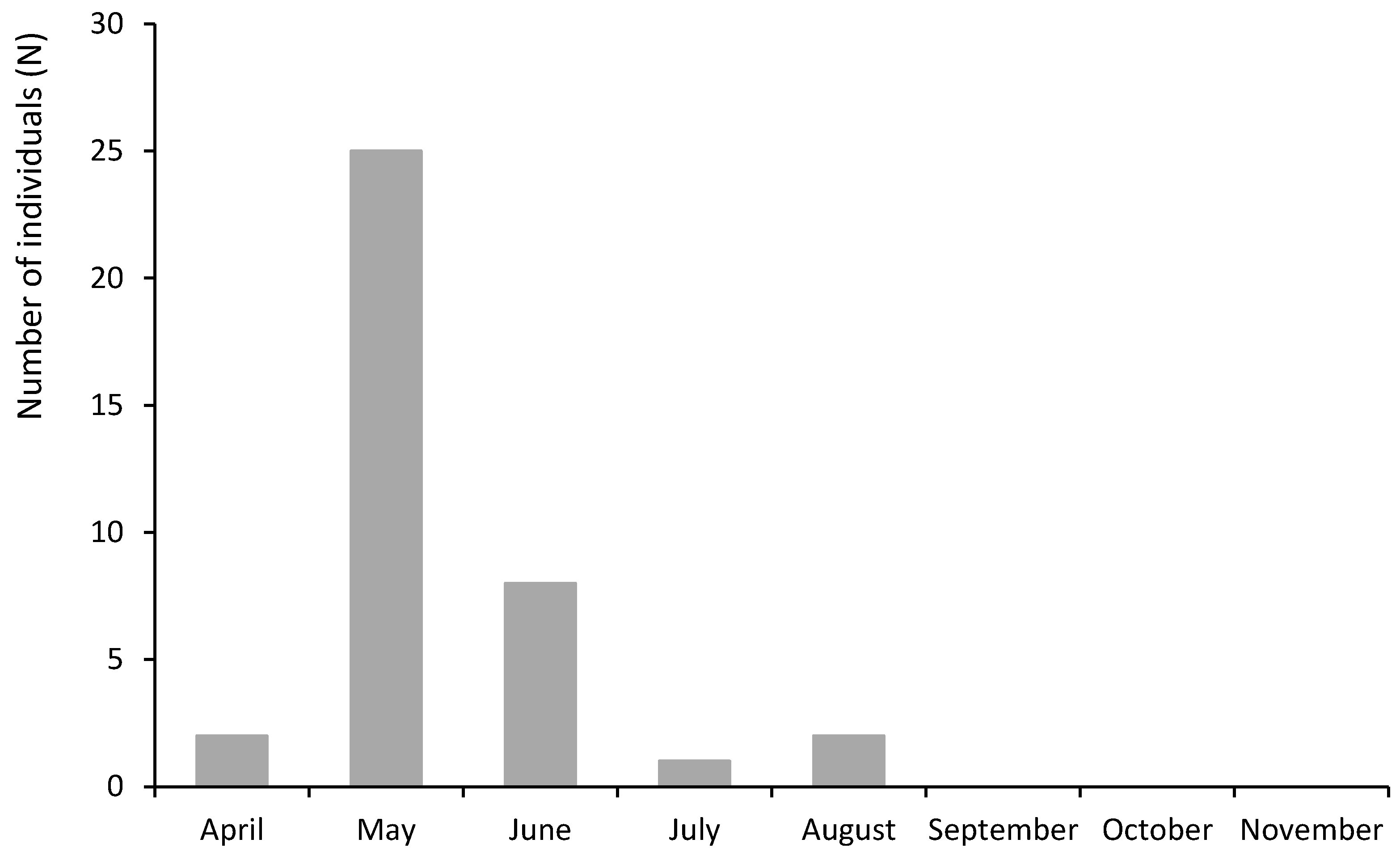
3.5. Seasonal Distribution
3.6. Feeding Habits
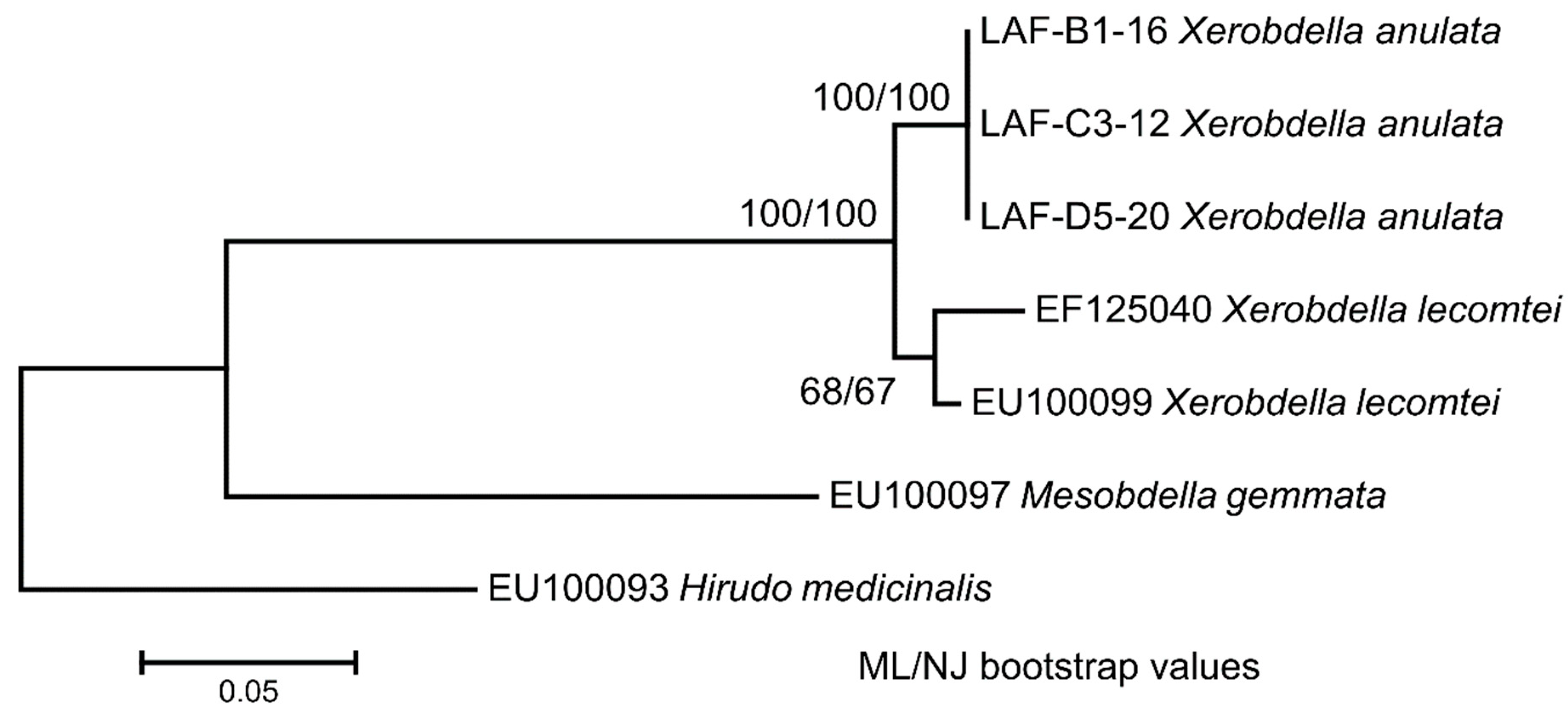
3.7. Phylogenetic Relationships
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Country | Biogeographical Region | Location | Habitat Type | Plant Association, Based on Historic Literature Data | Valid Name of Plant Association | Number of Individuals | Date | Data Source |
|---|---|---|---|---|---|---|---|---|
| Croatia | Alpine | Babin potok, Vrhovine (Lika region) | Forest | Helleboreto-Pinetum | Helleboro nigri-Pinetum sylvestris Horvat 1958 | 1 | 11.5.1962 | [31] |
| Croatia | Alpine | Babin potok, Vrhovine (Lika region) | Forest | Helleboreto-Pinetum | Helleboro nigri-Pinetum sylvestris Horvat 1958 | 2 | 30.5.1963 | [31] |
| Croatia | Alpine | Babin potok, Vrhovine (Lika region) | Forest | Piceetum dolomiticum | Suballiance Vaccinio-Piceeion | 1 | 11.5.1962 | [31] |
| Croatia | Alpine | Babin potok, Vrhovine (Lika region) | Forest | Piceetum dolomiticum | Suballiance Vaccinio-Piceeion | 1 | 26.6.1962 | [31] |
| Croatia | Continental | Cesergradska Gora, Klanjec (Hrvatsko zagorje region) | Forest | Fagetum croaticum montanum | Lamio orvalae-Fagetum sylvaticae (Horvat 1938) Borhidi 1963 | 1 | 19.5.1962 | [31] |
| Croatia | Alpine | Bijeli Vrh, Vrhovine (Lika region) | Forest | Fagetum croaticum abietetosum | Omphalodo-Fagetum (Tregubov 1957/Marinček et al. 1993) | 2 | 26.4.1963 | [31] |
| Croatia | Alpine | Bijeli Vrh, Vrhovine (Lika region) | Forest | Fagetum croaticum abietetosum | Omphalodo-Fagetum (Tregubov 1957/Marinček et al. 1993) | 1 | 29.5.1963 | [31] |
| Croatia | Alpine | Frk, Crni Lug (Gorski kotar region) | Forest | Blechno-Abietetum | Blechno-Abietetum Horvat /1938/1950 | 1 | 17.7.1964 | [31] |
| Croatia | Alpine | Snježnik (Gorski kotar region) | Forest | Fagetum croaticum subalpinum | Ranunculo platanifolii-Fagetum Marinček et al. 1993 | 1 | 19.8.1964 | [31] |
| Croatia | Alpine | Snježnik (Gorski kotar region) | Forest | Pinetum mugho croaticum | Hyperico grisebachii-Pinetum mugi (Horvat 1938) ex Zupančič et al. 2004 | 2 | 28.5.1964 | [31] |
| Croatia | Alpine | Snježnik (Gorski kotar region) | Forest | Pinetum mugho croaticum | Hyperico grisebachii-Pinetum mugi (Horvat 1938) ex Zupančič et al. 2004 | 1 | 18.8.1964 | [31] |
| Croatia | Alpine | Bukovica, Ravna gora (Gorski kotar region) | Managed forest | Omphalodo-Fagetum (Tregubov 1957/Marinček et al. 1993) | 3 | 13.5.2009 | New data | |
| Croatia | Alpine | Bukovica, Ravna gora (Gorski kotar region) | Managed forest | Omphalodo-Fagetum (Tregubov 1957/Marinček et al. 1993) | 6 | 29.5.2010 | New data | |
| Croatia | Alpine | Bukovica, Ravna gora (Gorski kotar region) | Meadow | Alchemillo-Trisetetum Horvat 1962, Festuco-Agrostetum Horvat 1962 | 6 | 10.6.2009 | New data | |
| Croatia | Alpine | Bukovica, Ravna gora (Gorski kotar region) | Meadow | Alchemillo-Trisetetum Horvat 1962, Festuco-Agrostetum Horvat 1962 | 3 | 13.5.2009 | New data | |
| Croatia | Alpine | Bukovica, Ravna gora (Gorski kotar region) | Meadow | Alchemillo-Trisetetum Horvat 1962, Festuco-Agrostetum Horvat 1962 | 3 | 29.5.2010 | New data | |
| Croatia | Alpine | Čorkova uvala, NP Plitvice Lakes (Lika region) | Primeval forest | Omphalodo-Fagetum (Tregubov 1957/Marinček et al. 1993) | 1 | 12.6.2009 | New data | |
| Serbia | Continental | Rastište, Tara Mt. | Forest | - | 2 | 24.5.2019 | [7] | |
| Slovenia | Alpine | Snežnik | Forest | - | - | - | [11] | |
| Bosnia and Herzegovina | Alpine | Bjelašnica | Forest | - | - | - | [11] | |
| Bosnia and Herzegovina | Alpine | Sarajevo | Forest | - | - | - | [11] | |
| Montenegro | Alpine | Plužine, Piva River | - | - | - | 8.5.2018 | [32] |
References
- Borda, E.; Siddall, M.E. Arhynchobdellida (Annelida: Oligochaeta: Hirudinida): Phylogenetic Relationships and Evolution. Mol. Phylogenet. Evol. 2004, 30, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Kutschera, U.; Pfeiffer, I.; Ebermann, E. The European Land Leech: Biology and DNA-Based Taxonomy of a Rare Species That Is Threatened by Climate Warming. Naturwissenschaften 2007, 94, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Trontelj, P.; Sket, B.; Steinbrück, G. Molecular Phylogeny of Leeches: Congruence of Nuclear and Mitochondrial RDNA Data Sets and the Origin of Bloodsucking. J. Zool. Syst. Evol. Res. 1999, 37, 141–147. [Google Scholar] [CrossRef]
- Minelli, A. Hirudinea. In Fauna d’Italia; Calderini: Bologna, Italia, 1979; ISBN 978-88-7019-020-5. [Google Scholar]
- Soós, Á. Az Európai Szárazföldi Piócákról (Hirudinoidea: Xerobdellidae). Különlenyomat Az Állattani Közlemények 1973, 60, 103–109. [Google Scholar]
- Jueg, U. Xerobdella praealpina Minelli, 1971 (Hirudinea, Xerobdellidae) in Österreich und Slowenien. Lauterbornia 2015, 79, 145–149. [Google Scholar]
- Grosser, C. First Record of Xerobdella anulata Autrum, 1958 (Hirudinida: Xerobdellidae) from Serbia. Biol. Serb. 2020, 42, 10–13. [Google Scholar] [CrossRef]
- Autrum, H. Hirudinea, Egel. In Die Tierwelt Mitteleuropas, 1st ed.; Brohmer, P., Ehrmann, P., Ulmer, G., Eds.; Quelle & Meyer: Leipzig, Germany, 1958. [Google Scholar]
- Borda, E.; Oceguera-Figueroa, A.; Siddall, M.E. On the Classification, Evolution and Biogeography of Terrestrial Haemadipsoid Leeches (Hirudinida: Arhynchobdellida: Hirudiniformes). Mol. Phylogenet. Evol. 2008, 46, 142–154. [Google Scholar] [CrossRef]
- Reisinger, E. Lebensweise und Verbreitung des Europäischen Landblutegels (Xerobdella lecomtei Frauenfeld). Carinthia II. 141 1951, 61, 110–124. [Google Scholar]
- Sket, B. K Poznavanju favne pijavk (Hirudinea) v Jugoslaviji; Razprave, Razred za Prirodoslovne in Medicinske vede; Slovenska Akademija znanosti in umetnosti: Ljubljana, Slovenia, 1968; Volume 4. [Google Scholar]
- Soós, Á. Milyen Pióca Fajok Várhatók Még a Magyar Faunában? Különlenyomat az Állattani Közlemények 1964, 51, 125–133. [Google Scholar]
- Soós, Á. A Zoogeographical Sketch of the Fresh-Water and Terrestrial Leeches (Hirudinoidea). Opusc. Zool. Budapest 1970, 10, 313–323. [Google Scholar]
- Vukelić, J.; Mikac, S.; Baričević, D.; Bakšić, D.; Rosavec, R. Šumska staništa i šumske zajednice u Hrvatskoj; Nacionalna Ekološka Mreža; Državni zavod za zaštitu Prirode: Zagreb, Republika Hrvatska, 2008; p. 263. [Google Scholar]
- Jelaska, S.D. Floristic and Ecological Characteristics of the Beech-Fir Virgin Forest in Croatia. In Proceedings of the Scientific Symposium “Virgin Forest Ecosystems of Dinaric Karst and Nature-Based Forest Management in Croatia”, Zagreb, Croatia, 27–28 September 2007; Matić, S., Anić, I., Eds.; Croatian Academy of Science and Art: Zagreb, Croatia, 2009; pp. 91–100. [Google Scholar]
- Vukelić, J.; Mikac, S. Znanstvena analiza značajki prašuma sjevernog Velebita i uspostavljanje monitoringa kao polazišta za praćenje promjena u šumskim staništima, posebice unutar Ekološke Mreže NATURA 2000; Šumarski fakultet Sveučilišta u Zagrebu: Zagreb, Croatia, 2010. [Google Scholar]
- Alegro, A.; Papp, B.; Szurdoki, E.; Šegota, V.; Šapić, I.; Vukelić, J. Contributions to the Bryophyte Flora of Croatia III. Plitvička Jezera National Park and Adjacent Areas. Studia Bot. Hung. 2014, 45, 49–65. [Google Scholar] [CrossRef] [Green Version]
- Šegota, T.; Filipčić, A. Köppenova podjela klima i hrvatsko nazivlje. Geoadria 2017, 8, 17–37. [Google Scholar] [CrossRef] [Green Version]
- Zaninović, K.; Gajić-Čapka, M.; Perčec Tadić, M.; Vučetić, M.; Milković, J.; Bajić, A.; Cindric, K.; Cvitan, L.; Katušin, Z.; Kaučić, D.; et al. Klimatski atlas Hrvatske—Climate Atlas of Croatia: 1961–1990. 1971–2000; Meteorological and Hydrological Service of Croatia: Zagreb, Croatia, 2008. [Google Scholar]
- Brigić, A.; Starčević, M.; Hrašovec, B.; Elek, Z. Old Forest Edges May Promote the Distribution of Forest Species in Carabid Assemblages (Coleoptera: Carabidae) in Croatian Forests. Eur. J. Entomol. 2014, 111, 715–725. [Google Scholar] [CrossRef] [Green Version]
- Casquet, J.; Thebaud, C.; Gillespie, R.G. Chelex without Boiling, a Rapid and Easy Technique to Obtain Stable Amplifiable DNA from Small Amounts of Ethanol-stored Spiders. Mol. Ecol. Resour. 2012, 12, 136–141. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoehn, W.; Lutz, R.; Vrijenhoek, R. DNA Primers for Amplification of Mitochondrial Cytochrome c Oxidase Subunit I from Diverse Metazoan Invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Braun-Blanquet, J. Pflanzensoziologie, Grundzüge Der Vegetationskunde, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 1964; ISBN 978-3-7091-8111-9. [Google Scholar]
- Barkman, J.J.; Doing, H.; Segal, S. Kritische Bemerkungen und Vorschläge zur Quantitativen Vegetationsanalyse. Acta Bot. Neerl. 1964, 13, 394–419. [Google Scholar] [CrossRef]
- Euro+Med PlantBase—The Information Resource for Euro-Mediterranean Plant Diversity. Available online: http://ww2.bgbm.org/EuroPlusMed/ (accessed on 18 May 2022).
- EEA (European Environment Agency). 2010. Available online: http://www.eea.europa.eu/soer/europe-and-the-world (accessed on 18 May 2022).
- Croatian Geodetic Administration (CGA). Središnji registar prostornih jedinica RH (GIS Shapefileovi); Central Registry of Spatial Units in the Republic of Croatia (GIS Shapefiles): Zagreb, Croatia, 2022. [Google Scholar]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [Green Version]
- Kimura, M. A Simple Method for Estimating Evolutionary Rates of Base Substitutions through Comparative Studies of Nucleotide Sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The Neighbor-Joining Method: A New Method for Reconstructing Phylogenetic Trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Dresscher, G.N.; Engel, H.; Van Der Spoel, S. Neue Fundorte und Variabilität der Xerobdella anulata Autrum, 1958 (Hirudinea, Haemadipsidae). Beaufortia Misc. Publ. Zool. Mus. Univ. Amst. 1966, 162, 213–219. [Google Scholar]
- Marinković, N.; Zorić, K.; Atanacković, A.; Tubić, B.; Paunović, M.; Pešić, V.; Raković, M. First Record of Terrestrial Leech Xerobdella anulata Autrum, 1958 from Montenegro. In Proceedings of the 49th Annual Conference of the Serbian Water Pollution Control Society Water 2020 Conference Proceedings, Serbian Water Pollution Control Society, Trebinje, Bosnia and Herzegovina, 19–20 November 2020; pp. 167–170, ISBN 978-86-916753-7-0. [Google Scholar]
- Melbourne, B.A. Bias in the Effect of Habitat Structure on Pitfall Traps: An Experimental Evaluation. Austral. Ecol. 1999, 24, 228–239. [Google Scholar] [CrossRef] [Green Version]
- Nuutinen, V.; Butt, K.R.; Jauhiainen, L.; Shipitalo, M.J.; Sirén, T. Dew-Worms in White Nights: High-Latitude Light Constrains Earthworm (Lumbricus terrestris) Behaviour at the Soil Surface. Soil Biol. Biochem. 2014, 72, 66–74. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence Limits on Phylogenies: An Approach Using the Bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, M.; Haring, E.; Sattmann, H.; Grosser, C.; Pešić, V. DNA Barcoding for Species Delimitation of the Freshwater Leech Genus Glossiphonia from the Western Balkan (Hirudinea, Glossiphoniidae). Biodivers. Data J. 2021, 9, e66347. [Google Scholar] [CrossRef]



| Vegetation Analysis | Managed Forest | Meadow | Primeval Forest |
|---|---|---|---|
| Plant associations | Omphalodo-Fagetum/Tregubov 1957/Marinček et al. 1993 | Alchemillo-Trisetetum Horvat 1962, Festuco-Agrostetum Horvat 1962 | Omphalodo-Fagetum /Tregubov 1957/Marinček et al. 1993 |
| Tree layer | |||
| Dominant species | Fagus sylvatica L. | Fagus sylvatica L. | |
| Abies alba Mill. | Abies alba Mill. | ||
| Picea abies (L.) H. Karst. | A. pseudoplatanus | ||
| Acer pseudoplatanus L. | Fraxinus excelsior L. | ||
| Sorbus aucuparia L. | |||
| Plant species richness (total) | 5 | 10 | |
| Shrub layer | |||
| Dominant species | F. sylvatica | Acer pseudoplatanus L. | |
| A. alba | |||
| Rhamnus alpinus L. spp. Fallax | |||
| Rubus hirtus Waldst. et. Kit. | |||
| Daphne mezereum L. | |||
| Lonicera xylosteum L. | |||
| Lonicera nigra L. | |||
| Rubus idaeus L. | |||
| Daphne laureola L. | |||
| Plant species richness (total) | 14 | 26 | |
| Herb layer | |||
| Dominant species | Omphalodes verna Moench | Festuca pratensis Huds. | Dryopteris filix-mas (L.) Scott. |
| Allium ursinum L. | Festuca rupicola Heuff. | Cardamine kitaibelii Becherer | |
| Galium odoratum (L.) Scop. | Brachypodium pinnatum (L.) P. Beauv. | Stellaria nemorum L. ssp. glochidiosperma Murb. | |
| Oxalis acetosella L. | Dactylis glomerata L. | ||
| Anemone nemorosa L. | Galium mollugo L. | ||
| Cardamine trifolia L. | Briza media L. | ||
| Lamium orvala L. | Centaurea nigrescens Willd. | ||
| Achillea millefolium L. | |||
| Plant species richness (total) | 38 | 92 | 39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brigić, A.; Medak, K.; Rebrina, F.; Jelić, M.; Alegro, A.; Kerovec, M. Ecology and Biology of the Rare Endemic Land Leech Xerobdella anulata (Xerobdellidae). Diversity 2022, 14, 701. https://doi.org/10.3390/d14090701
Brigić A, Medak K, Rebrina F, Jelić M, Alegro A, Kerovec M. Ecology and Biology of the Rare Endemic Land Leech Xerobdella anulata (Xerobdellidae). Diversity. 2022; 14(9):701. https://doi.org/10.3390/d14090701
Chicago/Turabian StyleBrigić, Andreja, Kristian Medak, Fran Rebrina, Mišel Jelić, Antun Alegro, and Mladen Kerovec. 2022. "Ecology and Biology of the Rare Endemic Land Leech Xerobdella anulata (Xerobdellidae)" Diversity 14, no. 9: 701. https://doi.org/10.3390/d14090701
APA StyleBrigić, A., Medak, K., Rebrina, F., Jelić, M., Alegro, A., & Kerovec, M. (2022). Ecology and Biology of the Rare Endemic Land Leech Xerobdella anulata (Xerobdellidae). Diversity, 14(9), 701. https://doi.org/10.3390/d14090701





