Abstract
Carica papaya L. (family: Caricaceae), also known as ‘papaya,’ is a tropical American fruit tree. Due to the bioactive components (carpaines, BITC, benzyl glucosinolates, latex, papain, zeaxanthin, choline, etc.) in its seeds, leaves, and fruits, it is revered for its excellent antioxidant, digestive, and nutraceutical benefits. Papayas are high in vitamins A, B, C, E, and K, folate, pantothenic acid, zeaxanthin, lycopene, lutein, magnesium, copper, calcium, and potassium. Being rich in fiber, antioxidants, and vitamin C, it lowers the cholesterol in the arteries; prevents arthritis; reduces aging, cancer, macular degradation, risk of cardiovascular diseases, and stress; increases platelet count; controls dengue fever; facilitates digestion, and lowers body weight. Papaya leaf extract, with many in vitro and case studies in combination therapies with modern medicine, especially for cancers and many other viral diseases, has been found to be an efficient cure. Humans have cultivated papaya cultivars for millions of years because of their significant commercial, medicinal, and agronomic value. Several reports have been published on the genetic modification of papaya for resistance to abiotic (herbicide, Al toxicity, etc.) and biotic stressors (PRSV, mites, Phytophthora, etc.), delaying ripening, and improving shelf life. However, most of these traits have not been introduced globally to all commercial papaya varieties. Unraveling the genetics of papaya has shed light on various domestication impacts, evolutionary patterns, and sex determination in fruit tree crops. It also serves as a potential step toward developing new cultivars to fight climate-oriented stress. Furthermore, extensive research on the stability of the ‘transgene’ across generations, and the ‘yield-penalty’ caused by the transgene, is required. Thus, meticulous crop improvement research on commercial papaya cultivars is necessary for long-term food and health security. This review article encompasses information on the traditional and modern medicinal uses, nutritional properties, phytochemistry, diseases and etiology, post-harvest measures, genomics, biotechnological strategies (for papaya improvement), and value-added products of papaya for food and health security.
1. Introduction
The papaya tree (Carica papaya L.) is a tropical American fruit tree. It is grown worldwide in tropical and sub-tropical climates. According to a recent papaya production report (2020), India is the world’s largest producer of papaya, generating 13.9 million tonnes (mt) per year, or 43% of global papaya production. In contrast, the United States is the world’s largest consumer of papaya. Carica has been classified under various families, including Passifloraceae, Cucurbitaceae, Bixaceae, and Papayaceae, but it is now placed under the Caricaceae family, which includes 35 latex-producing species divided into four genera: Carica, Jarillaand, Jacaratia, and Cyclicomorpha [1]. Some species previously attributed to Carica have been reclassified and are now assigned to Vasconcella [2].
Carica papaya L. originated in southern Mexico, the Philippines, and Central America [3,4]. The Caribbean coast of Central America, Argentina, Chile, and southern Mexico were identified as the origins of papaya, resulting from natural hybridization between Carica peltata and other wild species [5]. Papaya is domesticated in tropical and sub-tropical regions of the world (Asia, Africa, Oceania, and North America). According to FAOSTAT 2020, the chief producers are India, Brazil, Mexico, Indonesia, and Nigeria. Figure 1 shows the origin, distribution, and cultivation of papaya. In 2011, researchers from the Philippines reported the development of PRSV-resistant papaya by crossing papaya with Vasconcella quercifolia. Papaya fruits are natural gifts, possessing a large proportion of vitamins, macro and micro minerals, bioactive substances, and secondary metabolites. In addition to fruits, the leaves, stems, seeds, and other plant parts are high in alkaloids and flavonoids, which have antimicrobial and medicinal properties. Their numerous medical benefits have been discovered over the years, making papaya a plant of considerable therapeutic relevance. Endopeptidases, papain, caricain, and proteinase enzymes found in papaya latex are important industrial enzymes used in many commercial applications.
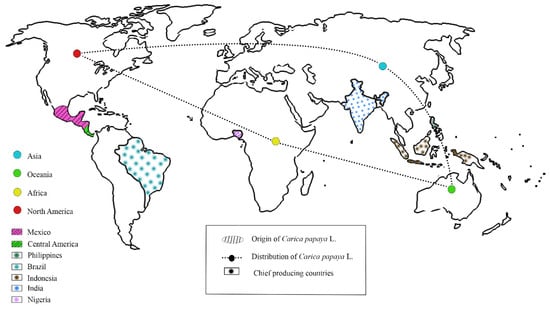
Figure 1.
Carica papaya: World map showing the origin, distribution, and the chief production countries.
The objective of this review article is to summarize the current state of knowledge on various aspects of papaya, such as nutrition, phytochemistry, use of papaya derivatives in traditional and modern medicine, disease pathologies and post-harvest control measures, genomics, and advancements in the genetic modification of papaya, and commercially available value-added products. The investigators in this work mainly intend to deliver an in-depth view of past studies and accomplishments in the broader conceptual aspects of Papaya, which could help promote and progress a new scope of research with reconciled information. All the topics covered focus on the integrated and aggregate data from several reliable databases that describe the accomplished milestones in the gradual evolution of the crop from its origin and contribution to biotechnology.
2. Botanical Description
Papaya is a tropical, semi-wood, rapid-growing herbaceous plant. The stem is usually hollow, solitary, and straight with conspicuous leaf scars. The plant shows apical dominance, branching is infrequent throughout the lower part of the stem unless the apical meristem is completely removed, and the top part bears a crown of large petiolate, deeply lobed leaves [6]. The leaves are large, palmately lobed, spirally organized, and clustered at the crown. In Malaysian cultivars, leaf arrangement and structure differences have been reported [7]. Papaya varieties can be distinguished based on their leaf structure, stomatal shape, number of central leaf veins, number of lobes at the leaf margins, wax coating on the leaf surface, and petiole color.
Papaya can grow in three sexes: female, male, and hermaphrodite (flowers with both male and female reproductive organs). The shape of the fruit also varies among different varieties. Some are oval to nearly round, elongated, club-shaped, pyriform, and have variable weights of up to 9 kg [8]. The unripe fruit has a lot of white latex, a hard skin, and is green in color, but the ripe fruit is light or deep yellow-orange, thick, succulent, with orange or red juicy flesh, and has a sweet aroma. Several gray to black colored seeds are partially connected to the flesh by white, soft, and fibrous tissue in the adult ripened fruits. Two forms of papaya cultivars are often grown. One is called ‘red papaya’, with orange or red flesh, and the other, ’ yellow papaw’, is a large fruit. ‘Caribbean Red’, ‘Sunrise’, and red-fleshed ‘Mardol’ papayas are commonly sold in U.S. markets in Mexico and Belize. Few of the most popular cultivated variety in India is ‘Red Lady’ (orange-red flesh), which is used for processing; ‘Washington’, a table variety ranging from 1.5–2 kg with yellow pulp; and the ‘Coorg honeydew’ cultivar (orange flesh, great flavor), which is valuable for processing and daily consumption.
‘Co-1’ is a dwarf cultivar that yields exquisite orange-yellow fruits with spherical flesh. ‘Co-2’ is obovate, medium-sized, and has firm red flesh. The ‘Co-3’ hybrid [‘Co-1’ (♀) × ‘Washington (♂)’] yielded medium-sized fruits (1.2–1.5 kg) with a yellow flesh and a purple hue, suitable for home gardening.
3. Nutritional Benefits
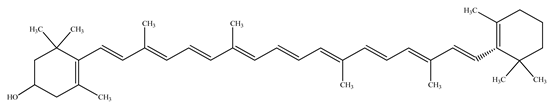
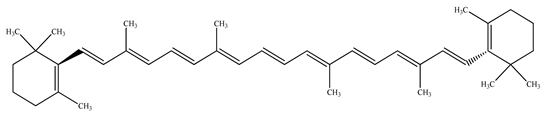
Papaya is known as a “common man’s fruit” because of its low cost and excellent nutritional value. Phytonutrients are present in papaya leaves, and unripe and ripe fruits [9]. Papaya is adored among all other fruits for its thiamine, folate, riboflavin, niacin, vitamins A, B1, B2, and C, and fiber content. It has been placed in the top five fruits (together with kiwi, watermelon, grapefruit, and guava) based on nutritional scores [10,11]. It does not have fat and protein content and is thus low in calories (energy value: 200 kJ/100 g). The principal sugars include glucose (29.8 g/100 g), sucrose (48.3 g/100 g), and fructose (21.9 g/100 g). Chemical assessment of papaya revealed the presence of essential elements, such as potassium (223 mg), sodium (8 mg), phosphorus (10 mg), calcium (20 mg), iron (0.25 mg), zinc (0.08 mg), copper (0.05 mg), manganese (0.04 mg), and magnesium (21 mg) per 100 g of fresh ripened fruit. Papaya fruits also contain folate (37 µg), thiamin (0.023 mg), riboflavin (0.027 mg), niacin (0.357 mg), pantothenic acid (0.191 mg), and macular carotenoids (1.2–6.4 mg), such as β-carotene and β-cryptoxanthin, along with major pigments such as lycopene (5.7 mg) per 100 g of fresh fruit.
4. Traditional and Modern Medicinal Uses
Papaya is known as a “quasi-drug,” owing to its pharmaceutical properties and has been utilized as a folk medicine to heal various disorders [12]. In Africa, papaya pulp is used in hospitals to cure burns and heal wounds [13]. Papain (EC: 3.4.22.2) is a crucial enzyme in drug development and has a variety of biological and commercial activities. The fruit latex of unripe papaya contains chymopapain, which is used to treat herniated lower lumbar vertebrae [14]. Another component, lycopene, prevents various types of cancer.
The fermented fruit of the papaya plant has potential nutraceutical significance as a source of antioxidative components, such as vitamin C, citric and malic acids, and glucose [15]. The efficacy of a mixture of papain and urea has been scientifically proven for enzyme wound debridement. Papaya latex helps treat dyspepsia and is applied topically to treat burns, scalds, diarrhea, and whooping cough [16]. Unripe papaya fruit is a valuable remedy for impotence and ulcers, and reduces menstrual unevenness, thereby assisting in menstrual flow [17]. Antioxidant and immunostimulant substances are thought to be present in fruits. By clearing out infection, mucus, and pus, papaya juice helps alleviate colon infections. The presence of vitamins A and C aids in the improvement of eyesight, prevention of early blindness in children, prevention of flu or cough, and strengthens the immune system of children suffering from colds regularly. Besides the benefits mentioned above, fresh green papaya leaves can also be used as antiseptics, whereas brown leaves are a great tonic for blood-purifying activity. Figure 2 depicts the health benefits of papaya latex, leaves, roots, flower seeds, and raw and ripe fruits.
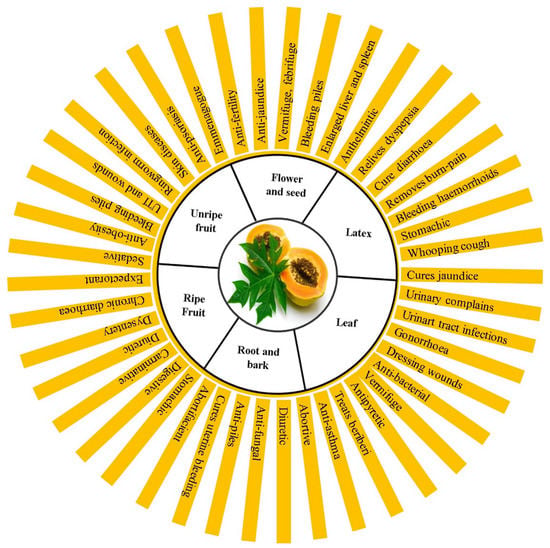
Figure 2.
Health benefits of papaya (Carica papaya L.) plant parts.
5. Papaya Leaf Extract for Disease Management
As discussed in the above sections on the modern medicinal uses and pharmacological properties of papaya, the papaya leaf extract plays a prominent role in managing human diseases compared to all other vital products and compounds obtained from the remaining plant parts. The bioactive components of the leaf extract are of nutritional, medicinal, and therapeutic importance against a wide variety of human diseases, including lethal viruses, which are currently a global threat to the future [18].
Since the early years of extraction of papaya leaf extract, many methods have been developed, including water, ethanol, methanol, and freeze-dried papaya leaf juice. The therapeutic properties of the leaf extract are mainly due to the presence of alkaloids, glycosides, tannins, saponins, steroids, and flavonoids in papaya leaves [19,20]. It may treat the most prevalent hair dandruff condition, has a synergistic effect on spleen and liver enlargement, and removes venom from snakebites. It is effective in a wide range of pathological conditions. The leaves are exceptionally high in iron and can be used to treat growth-related problems, tuberculosis, and anemia, as well as a cleanser in herbal medicine. The compounds present in the leaf extract interact with a wide range of cellular substrates, including activities such as suppression of DNA synthesis, downregulation of harmful gene expression, upregulation of anti-inflammatory genes, modification of cell signaling pathways, and cleavage of several proteins that have therapeutic activity in a wide range of disease treatments [21]. Many improved therapies are available in modern medicine for cancer treatment based on the location and intensity of phases, such as surgery, chemotherapy, radiotherapy, immunotherapy, and vaccination. However, Carica papaya leaf extract can be used in combination therapy to prevent and regulate the subsequent proliferation of malignant cells [22]. Several studies on the action of papaya leaf extract on in vitro cancer have shown that it decreases cancer cell proliferation [23,24,25].
However, Nisar et al. (2011) found that papaya leaf extracts could be used to treat dengue fever [26]. Papaya leaves can be used for malaria treatment owing to the presence of several alkaloids, including quinine, which has been proven to be an anti-malarial agent [27]. The ‘chikungunya’ vector is another causal agent that poses a health risk. The combination of papaya leaf extract with spinosad (a bacterial insecticide) has proven to be a helpful and environmentally friendly treatment. The larvicidal and pupicidal effects of this bacterial insecticide dominate the vector. Papaya methanolic leaf extract has a significant larval and pupal mortality rate [28]. Additionally, the Table 1 summarizes scientific reports on the pharmaceutical properties (using human and animal models) of papaya latex, fruit (ripe or unripe), leaf, root, flower, and seed extracts.

Table 1.
Pharmacological properties of Carica papaya.
6. Phytochemistry
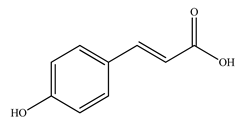
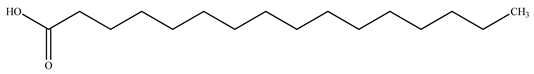
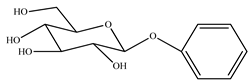
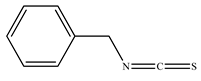
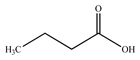
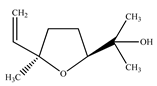
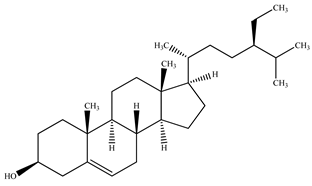
The presence of volatile compounds in papaya, such as alcohols, terpenes, aldehydes, organic acids, ketones, esters, and benzyl isothiocyanate (BITC), is responsible for its sensory characteristics, such as scent and flavor [52]. However, ethyl hexanoate, ethyl 2–methyl butanoate, and ethyl acetate are all linked to fruit scent [53]. Linalool is the most prevalent volatile component in 94% of solo papaya varieties [54]. Aromatic chemicals such as butanol, terpineol, and 3–methyl butanol are abundant during the mature stage [55], whereas methyl butyrate is the most abundant of the 103 esters in papaya [56].
Phytochemicals have been reported in different parts of the papaya plant, including the roots, shoots, heartwood, bark, leaves, fruits, fruit latex, juice, and seeds. In total, 166 volatile and non-volatile compounds belonging to the chemical classes of terpenoids, flavonoids, alkaloids, carotenoids, vitamins, and glycosides have been reported. The most important phytochemicals used are listed in Table 2.

Table 2.
Leading phytochemicals isolated and characterized in C. papaya plant.
LC-MS analysis of several papaya plant extracts revealed the presence of sinapic acid–O–hexoside, cyaniding–3–O–glucose, 5–hydroxy caffeic quinic acid, 5–hydroxy feruloyl quinic acid, acetyl p–coumaryl quinic acid, quercetin–3–O–rhamnoside, syringic acid hexoside, peonidin–3–O–glucoside, and methyl feruloyl glycoside [57]. It has been reported that an extract of papaya leaves processed with hexane possesses antiplasmodial effects [58]. Four of the nine chemicals were flavonols, whereas the other five were alkaloids. Papain was isolated and characterized from ripened fruits to investigate its catalytic capabilities and potential usage in wine stabilization [59].
Sancho et al. (2011) used HPLC–MS to determine the presence of vitamin C, phenols, and carotenoids in papaya fruit flesh and skin [65]. The human body is protected from oxidative stress by these substances, which lowers the risk of cardiovascular diseases and some types of cancer. Caprine, a significant alkaloid present in all green sections and seeds of C. papaya, was discovered by Burdick (1971) [60]. The anti-sickling substance ‘caricapinoside’ was found in phytochemicals extracted from the ethyl acetate fraction of an aqueous extract of C. papaya unripe fruit [66].
To investigate volatile chemicals, Canini et al. (2007) used quantitative phenolic acid analysis (GC–MS) [67]. Chlorogenic acid was recorded at trace levels, and 0.25 mg/g (dry leaf) caffeic acid, 0.33 mg/g p–coumaric acid, 0.11 mg/g protocatechuic acid, 0.03 mg/g kaempferol, and 0.04 mg/g of quercetin was also observed. GC–MS and NMR analyses of papaya seed oil extracted with supercritical fluid revealed the presence of BITC [64].
7. Papaya Diseases and Etiology
Papaya has always been a revenue-generating crop for farmers in tropical and sub-tropical regions, after commercial horticultural crops, such as bananas and mangoes. Unripe raw fruits are consumed as culinary vegetables, whereas ripe fruits are consumed as table fruits. The overall global production amounts to 13.89 million tonnes [68]. Bacteria, viruses, fungi, and nematodes incur losses in production every year, and post-harvest losses due to infections caused during storage and transport of this fruit tend to be a severe constraint [69] (Figure 3).
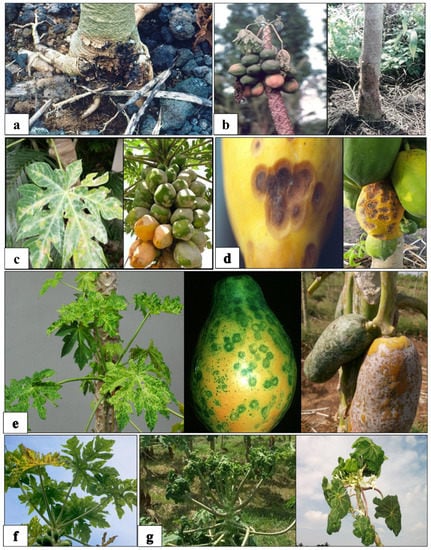
Figure 3.
Fungal and viral disease in papaya. (a) Stem rot in papaya due to Pythium aphanidermatum. (b) Infected stem and malformed apical portion caused by Phytophthora palmivora. (c) Infection in leaves and fruits caused by Oidium caricae. (d) Fruits affected by fungus Colletotrichum gloeosporioides. (e) Ring-shaped spots on leaves and fruits due to PRSV. (f) Leaves affected by PMV and (g) curly leaves symptoms due to PLCV.
7.1. Foot Rot/Stem Rot (Fungi: Pythium Aphanidermatum)
Soil-borne fungal pathogens of the oomycetes class are usually found in waterlogged and humid conditions. Water-soaked lesions form at the base of the stem, decaying the tissue and transforming it into a black necrotic area [70]. Because of an insufficient water supply and nutrients through the main stem, the fruits of diseased plants become deformed and shriveled. Pathogenicity occurs predominantly during the monsoon season when a seedling or plant encounters oospores, sporangia, or hyphae that can live in the soil and degrade organic materials.
Control measures: Avoid waterlogging and follow a ring irrigation system. During planting, apply 15 g of Trichoderma viride mixed with manure near each plant root. Drenching copper oxychloride at 3 g/L of water every 15 days from planting and applying the paste of copper oxychloride to the rotted area.
7.2. Collar Rot/Soft Fruit Rot (Fungi: Phytophthora Palmivora)
Soil-borne fungi of the oomycetes class cause widespread diseases in nations such as India, Sri Lanka, Indonesia, Australia, Malaysia, Hawaii, Taiwan, Brazil, and Spain. Lesions form at the base of the stem, which could be below or above the ground, resulting in yellow wilted leaves [71]. Higher soil temperatures and inadequate drainage aid in the spread of this disease. Lower leaves and fruits shrink and fall prematurely because of the white mycelial coat [72]. Fungi produce zoospores (infective and motile) and chlamydospores (dormant), which can become active at high temperatures and spread by wind and flowing water [73].
Control measures: Grow papayas where they have never been previously cultivated. Use disease-free seedlings, drenching copper oxychloride 3 g/L mixed with organic manure in the pit near each plant’s root.
7.3. Powdery Mildew (Fungi: Oidium Caricae)
Originally described in 1898 in Brazil, this fungal pathogen classified under Leotiomycetes, was later detected in all other subtropical climates, and papaya was the only host for this organism. The fungus has no saprophytic phase in its life cycle and can be detected only in living plants [74]. High humidity, moderate light, rainfall, and temperature promote disease spread, resulting in minor fuzzy spots on leaves and the spread of white powdery mycelia all over senescent leaves, leading to chlorosis and leaf fall [75]. The fruits of older plants become deformed and distorted; however, young plants suffer severe damage. The fungus develops asexual spores known as conidia and globose sexual spores known as ascospores [71].
Control measures: Spraying the trees with chemicals after disease incidence. 0.1% tridemorph, 0.25% wettable sulfur, or 0.05% dinocap.
7.4. Papaya Anthrocnose (Fungi: Colletotrichum Gloeosporioides)
Many early studies mentioned this fungus of Sordariomycetes class as an important disease-causing agent in citrus, mango, and several other hosts. This disease is distinguished by sunken patches of varied colors on plant parts such as leaves, stems, and fruits, which enlarge and eventually cause wilting and withering of the plant [76]. C. gloeosponoides has an asexual or anamorphic phase, in which conidia grow and proliferate anthracnose by rain or irrigation water in warm conditions. Conidia generate typical structures known as appressoria and acervuli, which aid fungal penetration into host plant cells [77].
Control measures: Chemical spraying of either 2 g/L cholothalonil at 10–15 day intervals with 2 g/L Mancozebat at 10-day intervals, or carbendazim 1 g/L at every 45-day interval.
7.5. Papaya Ring Spot (Virus: Papaya Ring Spot Virus, PRSV)
PRSV is a single-stranded positive-sense RNA virus that belongs to the Potyvirus genus and is classified under the Potyviridae family. PRSV is more catastrophic to Papayaceae, Chenopodiaceae, Cucurbitaceae, and Caricaceae family members [78]. It was first identified in 1949 as a destructive virus of the papaya crop in the Hawaiian Islands. By 1992, it had destroyed 100% of papaya production in East Asian countries and Hawaii [3]. Aphids serve as vectors for PSRV infection and induce symptoms, such as vein cleaning and young leaf yellowing. Moist and oily streaks on the stem, chlorosis on the root lamina, vein clearing, and mosaic patches on the leaf surface are the leading causes of yield loss. Fruits develop distinct ring-spot patterns with concentric rings, and these spots degrade the quality and taste [79].
Control measures: Use of healthy or disease-free seedlings. Identifying the symptoms at early stages and culling out the infected plants, using yellow sticky traps for aphids, crop rotation with maize or sorghum to evade the aphids, and avoiding planting the crops of the Cucurbitaceae family.
7.6. Papaya Mosaic (Virus: Papaya Mosaic Virus, PMV)
Papaya mosaic virus (PMV), a member of the genus Potexvirus and the family Flexiviridae, is a rod-shaped, single-stranded RNA plant virus that was initially detected in 1962 as a threat to papaya crops after PRSV [80]. Its morphological and structural properties resemble those of the tobacco mosaic virus [81]. Infected plants exhibit significant deformation and string-shaped mosaic patterns on their leaves, causing them to be stunted, as well as water-soaked sores on their stems. PMV has a high impact on the production of native varieties and rapidly spreads even in transgenically produced PRSV-resistant cultivars [82].
Control measures: Use healthy seedlings and exercise caution when handling plants during intercultural operations. Roughing out diseased plants and immediately burning them. All intercultural operating tools should be sterilized with sodium hypochlorite solution.
7.7. Papaya Leaf Curl (PLCV)
Papaya leaf curl virus (PLCV), a geminivirus belonging to the Geminiviridae family with single-stranded DNA (ssDNA) as its fundamental genetic component, was first discovered in India in 1939 [83]. The virus is transmitted by the whitefly (Bemisia tabaci), which was discovered and proven by Saxena et al., 1998 [84]. This viral disease significantly impacts commercial sectors, such as pharmaceutical firms that rely on papaya leaves. This disease is distinguished by the inward or downward curling of the leaves, which resembles an inverted cup shape. The leaf surface becomes hard and leathery textured with thick veins, resulting in stunted growth, defoliation, and failure to blossom and set fruits [85].
Control measures: Roughing out the infected plants immediately and using systemic insecticides against the vector. Cultivation of Solanaceae crops on the same land should be avoided.
8. Post-Harvest Diseases of Papaya
Post-harvest diseases of papaya cause significant revenue losses. Owing to improper handling and transportation, most disease outbreaks occur before they reach the market [86]. Fruits with a thin outer coat and a fleshy core are very susceptible to diseases such as Rhizopus rot, Fusarium rot, Anthracnose rot, Aspergillus rot, stem end rot, and black rot.
The fungus Mycosphaerella caricae causes dry rot and stem-end rot of papaya, mainly through damage or cuts on the fruit. A rigid layer of tissue with wrinkles was observed on the outer layer of the fruits, followed by translucent edges and brown lesions [87]. The fungus Phomopsis sp. induces wet rot in papaya, causing tissue softening and discoloration in the infected area during the early stages of infection. A spot is usually observed in the center of a wet lesion, which develops into a hollow cavity and white mycelial growth on the surface [88]. Charcoal rot is a common disease of pulse and oil crops such as soybean, sesame, peanut, cowpea, chickpea, green gram, and cluster bean, but it also affects crops such as cotton, maize, sorghum, and papaya. The causal organism is Macrophomina phaseolina (Tassi) goid, a fungal pathogen from the Ascomycetes class. Ripe fruits are particularly vulnerable to this fungus, with pycnidia entering through incisions, insect bites, or abrasions during transit and spreading infection. Sclerotia and pycnidia produced by the fungus result in black-to-brownish patches on the skin [89]. Alternaria alternata (Fr) Keissl is a fungal pathogen that causes papaya fruit rot and brown leaf spots. The infection is most visible on the outer skin of fruits, where black spores and circular ring patterns can be observed. Older leaves are a source of infection, forming brown lesions that spread to other fruits during transport and storage [90].
Stem end rot in papaya fruits is a common disease caused by the combined effects of numerous pathogens. This can differ among species of Phomopsis, Fusarium, Rhizopus, Botruodiplodia, Mycosphaerella, Ascochyta, Alternaria, and other organisms [91]. During harvest, the pathogen enters the fruit via cuts and wounds in the peduncle region and fruit. Abnormalities such as dark lesions, soaked lesions, rough black patches on the skin, wrinkles, yellowing, spore patterns, and a carpet of white mycelium can be noticed on the infected fruit [92]. During transportation and extended storage, it rapidly spreads to other fruits. Negligence in storage conditions may also result in infections in the fleshy portions of fruit, where Fusarium, Penicillium, Cladosporium, and bacteria Enterobacter cloacae and Erwinia herbicola have been found to cause purple streaks and yellowing of the mesocarp [93].
Control measures: The majority of post-harvest diseases are caused by harvesting activities, wherein the microbial inoculum is transferred from damaged plants to healthy fruits. To prevent further disease spread, diseased fruit should be removed and discarded as early as possible. Handling during harvest and post-harvest should be performed with caution to avoid physical damage to the fruits. The use of sterilized wares or inoculum-free packaging materials, as well as proper fruit arrangement, could decrease mutual stress on the fruits. Spraying healthy fruits with contact fungicides such as 2 g/L chlorothalonil or mancozeb before rainfall and 7–10 days after harvesting will reduce the spread of diseases. Pre-harvest treatment with 2% enhanced freshness formulations (EFF) using hexanal as an active ingredient resulted in delayed maturity, color change, ripening, and reduced post-harvest disease incidence [94].
Although pre-harvest chemical sprays offer protection, subsequent treatments with synthetic chemicals, such as post-harvest fungicidal spray containing 0.2% hexaconazole, propiconazole, copper oxychloride, zineb, benomyl, chlorothalonil, or 0.1% carbendazim, would aid in preventing post-harvest infections [95,96]. Using natural oils from lemongrass, mint, castor, cinnamon, and thyme as fruit-coating materials has reduced the incidence of Anthracnose and Rhizopus rot [97,98]. Hot water dips, dry heated air, and heat vapor treatment at 49 °C significantly reduced fruit ripening and increased the shelf life of papaya [99,100]. Papaya fruits were treated with chitosan, aloe, papaya leaf extract [101], or chitosan with carbonate salts of ammonium ((NH4)2CO3) and sodium (Na2CO3) to reduce post-harvest infections [102]. A hypoxic storage system (20 mm Hg at 10 °C, 90–98% relative humidity) of the fruits during lengthy shipments and freight resulted in reduced disease development, and no change in the texture or quality of fruits has been observed after this pressure or controlled atmospheric storage conditions [103].
Temperature and storage conditions significantly affect the spread of post-harvest diseases. Even temperatures during transit and storage are recommended to supply high-quality fruits. In contrast, temperature alterations may cause early ripening of fruits, soft flesh, color changes, and decay [93]. The summarized packages of various preharvest and post-harvest methods, together with environmentally friendly measures, will prevent infections and meet global nutritional demands through delicious papaya fruits.
9. Papaya Genomics
Papaya (Carica papaya L.) belongs to the flowering plant taxa classified under the order Brassicales and the family Caricaceae, with six genera and 35 species [104]. Carica was subsequently partitioned into two genera: Vasconcellea with 21 species and Carica with one species called papaya [105,106]. Vasconcellea and Carica have the same chromosome number, 2n = 2x = 18, that is, nine pairs of chromosomes with varied sizes, seven of which are metacentric and two of which are submetacentric, according to karyotypic studies [107]. Chromosome 1 is distinct and responsible for the determination of sex, thus categorized as a sex chromosome, and the remaining chromosomes are responsible for vegetative function confirmed based on studies performed with the help of fluorescent in situ hybridization (FISH) and bacterial artificial chromosomes (BAC) [108,109].
Papaya serves as an excellent model organism to study the evolutionary patterns of tree crops, their genetic resources, and variations in the genome structure, and proposes developmental strategies as its sequenced is comparably small, around 372 Mb [110], and has already been mapped [105]. The traditional whole-genome shotgun sequencing method was used to study the genetic arrangement of papaya, yielding 372 Mb of sequenced data, of which 271 Mb (nearly 70%) accounts for assembled contigs (contiguous sequences with overlapping data sets), and the remaining portion is scaffolds (including contigs and gaps) [111]. Genome annotation revealed the presence of 24,746 genes, which is proportionally less than that in other important crop genomes, such as rice (34%), Arabidopsis (20%), and grapes (19%) [112,113,114]. The advent of sequencing technology, primarily next-generation sequencing (NGS), has had a significant positive impact on the total genome sequencing of several crops, with papaya standing out as the fifth flowering crop after Arabidopsis, poplar, rice, and grape [115]. Single nucleotide polymorphism (SNP) markers were employed in conjunction with NGS to obtain 23,318 scaffolds (> 200 bp lengths) in two commercially established varieties (‘Eksotika’ and ‘Sekaki’) and previously mapped ‘Solo’ papaya from Hawaii. These SNP markers could be useful in developing novel breeding programs using marker-assisted selection approaches in the future [116]. Wu et al. (2017) presented evidence for the establishment of the recessive y gene and its selection in red-fleshed papaya following domestication [117]. Haplotyping analyses have shown that the recessive y allele originates in wild cultivars. Genetic variability studies have confirmed a considerable reduction in recessive y alleles compared to dominant Y alleles in yellow-fleshed cultivars (wild and cultivated) [117].
Papaya is recognized as a trioecious tree species with three plant types: male, female, and hermaphrodites, which is an attractive element for investigating the genetic makeup and evolutionary behaviors of the species. The morphological differences among the three sex types benefit the progression of offspring with ease of reproduction. Male and female plants follow cross-pollination, whereas hermaphroditic plants prefer self-pollination, which is a peculiar commercial cultivating feature. Any stress caused by water and high temperature immediately alters the inflorescence behavior and changes the bisexual flower to male flowers with pollen-bearing stamens, and aborted and degenerated pistils and no fruits [118,119]. Pistil abortion could be caused by improper hormone signal transduction, lack of expression of hormone-related genes, transcriptional factors, or variation in auxin-producing genes between flowers [120].
The use of BAC in gene sequencing studies has been beneficial in revealing information on the XY system of sex determination and in evolutionary studies on sex chromosomes in plants [121,122]. Sequencing of sex chromosomal DNA, when aligned with BAC physical chromosomal maps and comparing the divergence between X and Y genes, has shown homomorphic evidence. In contrast, Y (male-specific regions of Y) resulted in many alterations such as insertions, duplications, deletions, and translocations, and divergence studies between male-specific Y and hermaphroditic Y (Yh) regions demonstrated that the hermaphroditic trait in plants evolved at the species level in Papaya rather than at the family level in the course of evolution around 2.2 million years [121,122]. Wild varieties of Papaya and Vasconcellea relatives are dioecious, have significant genetic variability, and are inter-compatible, facilitating the creation of new hybrids with beneficial traits. Commercially grown papaya cultivars are gynodioecious, with two-thirds of the population being hermaphroditic and one-third female. However, they possess minimal genetic diversity due to their geographical isolation [123,124]. Such genetic dissimilarity prohibits hybridization, demonstrating a major need for trait introgression from wild varieties, embryo rescue to develop new cultivars with diversity, and overcoming compatibility issues [125].
Structural variations (SV) are complex genomic DNA mutations that include gene insertions, deletions, inversions, and transposable elements (TEs) at single genes or many places in the genome. Variations in copy number are also responsible for the size and species expressional behavior [126]. SVs are usually lengthy modifications of DNA and are random in every chromosome, rather than SNPs and indels (short insertions and deletions), which can have a proportional impact on the gene expression of various traits in the species, modifying or developing new biological processes, the association between specific variation and its impact on an individual’s phenotypic behavior, and evolutionary and genetic diversity studies.
In the papaya genome, 8083 SVs, 5260 deletions, 552 duplications, and 2271 insertions were found, where 1794 genes overlapped with SVs, with 1350 genes expressed in at least one tissue, highlighting the prominence of deletions during evolution and domestication [127]. The identified SVs showed a co-expressional relationship in several plant tissues and played a vital role in the growth and biotic stress response. SVs genes that influenced environmental tolerance, reproduction, and yield qualities during papaya domestication were identified and summarized using copy number varying gene (CNVs) correlation analysis. Such variability investigations will help to understand the evolution of male to hermaphroditic flowers in papaya.
This papaya genome study revealed many fundamental and interesting facts about papaya and will serve as a foundation for identifying and designing DNA markers for papaya enhancement. It would also aid in phylogenetic and evolutionary studies of other tropical fruit crops and Brassicaceae family members [125]. Many genetic markers have been developed to differentiate hermaphroditic and female plants. Hermaphroditic plants grow longer and larger fruits, whereas female plants yield round fruits. Owing to packaging and shipping limitations, the fruits of hermaphroditic plants are preferred over those of females. The use of genetic markers aided in the removal of females during seed production and tissue culture screening [128] and successfully developed two genetic markers for males in papaya using an 8396 bp insertion transposon sequence to identify sex reversal [129]. Morphological papaya sex determination is only viable six months after germination. The application of these modern genetic markers aids in the rapid determination of sex types over several hours. Using such markers in ecological surveys will be extremely valuable for estimating population dynamics based on population sex ratios [130]. Genomics as a whole, in conjunction with proteomics and transcriptomics, could provide a solution for developing new cultivars of tropical fruits to cope with high temperature and drought stress and combat the adverse effects of global warming [131,132].
Genetic diversity and phylogeny investigations, and their organization in wild ancestors, will represent genetic sources that can aid in developing breeding methods for climate-smart cultivars of all crops. Currently, papaya is considered a crop of high agronomic, therapeutic, and economic importance in many aspects that result from its domestication. Further research into the history, evolution, and domestication of these plants is required to counteract future concerns, such as overpopulation, global climate change, and food scarcity and security.
10. Papaya Biotechnology—Crop Improvement
Papaya is amenable to plant transformation; thus, foreign genes can be introduced into the papaya genome using various transgenic procedures such as particle bombardment (biolistic approach) and Agrobacterium-mediated transformations [133,134]. After PRSV was eliminated from Hawaiian papaya production in 1994, many PRSV-resistant varieties were developed using traditional methods, but they were not long-term remedies. The use of transgenics has paved the way for improving the production of commercial cultivars against this virus. Papaya was the world’s first crop to have transgenic cultivars released for commercial cultivation in Hawaii in 1998 by institutions in the USA [135]. The development of a PRSV-resistant papaya cultivar is based on pathogen-derived resistance (PDR), which includes the DNA sequence from a target pathogen to be engineered and incorporated into the host plant; thus, the host-pathogen interaction in the plant is disrupted by the transgenic protein produced during pathogen attack, which subsequently delays the resistance of plants against the disease [136,137,138]. Successful expression of the PSRV coat protein has led to the development and release of transgenic PSRV-resistant papaya [139]. The co-cultivation of embryogenic cultures with disarmed A. tumefaciens containing a vector with the gene of interest is required for papaya transformation mediated by A. tumefaciens. Acetosyringone is a phenolic substance frequently added to bacterial suspensions to increase the virulence of vir genes and to improve transformation efficiency [140]. In transgenic papaya, the GUS gene (β-glucuronidase gene from E. coli) and GFP (green fluorescent protein from jellyfish) have been used as reporter genes to distinguish between transgenic and non-transformed tissues [141,142]. The GUS gene encodes a β-glucuronidase enzyme that produces a vivid blue color when combined with a colorless substrate 5-Bromo 4-chloro-3-indolyl-glucuronic acid (X-Gluc). GFP has the additional benefit of being a non-destructive screening tool for identifying altered tissues. As an alternative selection system, phospho-mannose isomerase (PMI)/mannose (Man) has been developed to efficiently perform the transformation procedure [143]. Embryogenic cells transformed with the pmi gene consistently expressed the PMI enzyme and could use mannose added to the culture medium. Genetic modification of papaya has been performed for biotic and abiotic stress tolerance, qualitative traits, and production of plant-based vaccines. The chitinase gene from Manduca sexta has been reported to be helpful in the development of transgenic papaya resistant to mites [144]. In another report, transgenic papaya expressing the agglutin gene from Galanthus nivalis was developed by McCafferty et al. (2008) for resistance against spider mites [145]. The stilbene synthase gene (vst1) from grapevine was introduced into the papaya genome for resistance against biotic stress caused by Phytophthora palmivora [143]. Currently, efforts are being made to develop transgenic papaya resistant to fungal diseases. The esat-6 gene from M. tuberculosis has also been introduced into the papaya genome to produce vaccines against tuberculosis [146].
The transgenic approach also improved the shelf life of ripe papaya fruit. Papaya has a short shelf life and is highly prone to chilling injuries when stored at low temperatures during long transport [147]. Storage rot can result in significant losses of market value [148]. Fruit ripening in papaya is triggered by a rapid burst of ethylene production, which activates downstream genes responsible for fruit softening [149]. The selection of transgenic markers for sex determination in papaya seedlings is another example of genetic engineering [150]. The bright yellow protein (Eyfp) and a selectable bar gene conferring glufosinate resistance were introduced into embryogenic cells in this study. Transgenic plants with insertions near sex-linked genes are currently being evaluated to develop rapid assays for sex identification in papaya seedlings. Table 3 shows selected reports on papaya crop improvement under various abiotic and biotic stressors.

Table 3.
Reports on the development of transgenic papaya.
11. Value-Added Products of Papaya
Papaya can also be used to create value-added products. Products such as pickles, candy, papaya-mix juices, and face wash are already being sold in the market [155] (Figure 4).
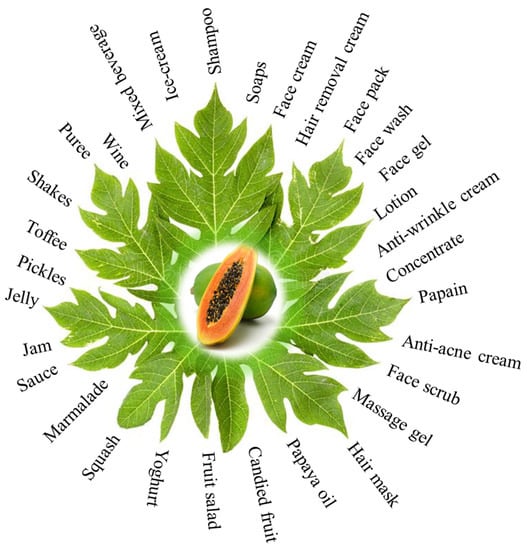
Figure 4.
Value-added commercial products of Carica papaya.
Papaya milkshake is regarded as one of the most important components of a decent breakfast menu. Thus, there is a continuous and growing demand for papaya in the food and pharmaceutical industries. In this section, we discuss the importance and use of the selected papaya products. They are (i) Papain: It is the dried milk of papaya that is collected, treated, packaged, and sold for various industrial uses, including softening meat, oil extraction from Tuna fish, in brewing industry, leather curing, preshrinking of wool, degumming of silk and rayon, in preparation of face cream, dental paste, and wound healing properties [156]. Papain-based proprietary medicinal products are available in the market. It is used in medicine to treat necrotic tissues, skin rashes, renal problems, digestive disorders, dyspepsia, and ring or roundworm infection; (ii) Papaya candy: Preparation of papaya candy is a unique strategy to prevent post-harvest loss of papaya fruit which accounts for a loss in millions per annum. Papaya candy is a value-added product that can reduce papaya fruit losses by up to 10%.
To prepare the papaya candy, mature unripe papaya was harvested and streaked to drain the papain. After that, they were peeled (seeds were removed), diced into pieces of uniform size (approx 2–3 cm in dimension), and dipped for one or half-hour in a cold solution containing salt (2 g/100 mL) and calcium chloride (1 g/100 mL). The solution was drained, and the pieces were washed with cold or fresh water. The pieces were then cooked for five minutes along with one-fourth of the sugar (w/w), color, and/or flavor (cardamom and/or tulsi and/or lemongrass powder), followed by cooling at room temperature for four hours. Thereafter, more sugar was added (Brix 70°) stepwise, along with a preservative (citric acid: 1–1.5 g/100 mL) and cooked for five minutes. Finally, the syrup was drained, and the candy was dried in the shade, followed by packaging in sterilized containers or polythene bags. These candies are medicinal foods that are very useful in digestion and bowel cleansing. They even lower cholesterol, boost immunity, and protect against arthritis; (iii) Papaya jelly and jam: Papaya jellies are relished by all age groups as they are tasty and digestive. To prepare papaya jelly, fully ripe papaya was peeled, and the seeds and inner skin were removed.
Papaya pieces were blended into a puree, which was sieved and poured into a pan kept on a flame. For 200 mL of papaya puree, 100 mL of milk was added, and 1.5 tablespoons of agar-agar powder were added with continuous stirring (to dissolve the agar-agar powder), followed by the addition of sugar and/or 2 tablespoons of condensed milk. The slurry was then poured into molds and maintained at 4 °C for setting. For the preparation of jam, to 200 mL of papaya puree, three-fourths of a cup of sugar was added and continuously stirred on a flame. Thereafter, 10 mL of lemon juice was mixed, and the thick puree (jam) was transferred into a glass container and cooled in a refrigerator; (iv) Papaya squash: The initial steps for squash preparation are similar to that of jelly preparation, but agar-agar powder is not added, water (1 kg/kg papaya pulp) is added instead of milk or condensed milk, and only mildly heated to dissolve the sugar (1.8 kg/kg papaya pulp) and citric acid (2.5 g/kg papaya pulp). After straining, potassium metabisulphite (2.5 KMS/lite of squash) was added as a preservative, and the syrup was stored in a bottle. It is diluted with water before serving; (v) Papaya pickle: The food item (fruits or vegetables) may be preserved through pickling in common salt and vinegar. Papaya pickles are tasty appetizers and improve the overall flavor of the dinner, similar to any other pickle. It stimulates the production of stomach juices, aiding digestion [157]. The ingredients required for papaya pickle are peeled mature green-papaya pieces, red chili powder, mustard seeds, fenugreek, salt to taste, asafoetida, and vinegar. The mature green papaya was peeled, seeds were removed, cut into pieces, washed with water, boiled, and drained. The pieces were mixed with salt and spices and then stored in vinegar in a jar; (vi) Papaya face cream: The common ingredients are papaya enzymes, wheat germ, almond, and sesame oil, which make the skin light, smooth, and youthful. Natural papaya extract in papaya face cream removes dead skin cells, clears blemishes, hydrates and smoothes, and makes the skin spot-free; (vii) Papaya toothpaste: Papaya contains the enzyme papain, which loosens the enamel stains and therefore finds application in toothpaste preparation along with mint, pineapple extracts, and other ingredients [158]. Thus, there is a long list of papaya products, some of which are commercialized, and several are routinely used or consumed.
12. Conclusions
Papaya is a widely consumed fruit that consists of several varieties of compounds with pharmacological properties. Papaya fruit is a boon for health-conscious people, particularly for weight management. Apart from fruits, the plant and its parts contain a substantial number of phytochemicals, particularly the leaves, which have been used for traditional medicinal purposes from ancient times to the current era of medicine. This could lead to various therapeutic combinations to address numerous catastrophic disease outbreaks involving multiple viruses and other parasites. Because of domestication, papaya is now widely recognized as a crop with significant agronomic, medicinal, and commercial potential. The history, evolution, and domestication of these plants must be studied in greater depth to address future issues, such as overcrowding, global climate change, and food scarcity and security. The development of new ecologically sustainable practices for the production, post-harvest, and processing of delectable papaya fruits will keep illnesses aside and meet the world’s nutritional needs. Therefore, papaya conservation and comprehensive investigations of its phytochemistry, pharmacodynamic characteristics, and kinetics, as well as proper standardization and clinical trials, are essential to fully harness its medicinal potential and effectiveness against diseases, such as dengue and malaria. It also provides the necessity to develop upgraded transgenic cultivars using various transformations with enhanced phytochemical contents in plants or plant parts to meet global needs. Moreover, crop improvement of papaya varieties through transgenic technologies is required to efficiently cope with various biotic stresses, especially PSRV infection.
Author Contributions
All authors contributed to writing and accepted the final version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Yeungnam University Research Fund (2022).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are thankful to the Lovely Professional University (LPU), Punjab, India; the Centre for Aromatic Plants (CAP), Dehradun, Uttarakhand, India; Amity University, Rajasthan, India; and the University of South Bohemia, Czech Republic.
Conflicts of Interest
There were no conflict of interest in the study, according to the authors.
References
- Kumar, L.; Srinivasan, V. Chromosome number of carica dodecaphylla vell fl. Flum. Curr. Sci. 1944, 13, 15. [Google Scholar]
- Badillo, V. Nota correctiva vasconcellea. St. Hill. Y No Vascon. (Caricaceae). Ernstia 2001, 11, 75–76. [Google Scholar]
- Gonsalves, C.; Lee, D.R.; Gonsalves, D. The adoption of genetically modified papaya in hawaii and its implications for developing countries. J. Dev. Stud. 2007, 43, 177–191. [Google Scholar] [CrossRef]
- Fuentes, G.; Santamaría, J.M. Papaya (Carica papaya L.): Origin, domestication, and production. In Genetics and Genomics of Papaya; Springer: Berlin/Heidelberg, Germany, 2014; pp. 3–15. [Google Scholar]
- Manshardt, R.M. Papaya. In Biotechnology of Perennial Fruit Crops; Hammerschlag, f.A., Litz, r.E., Eds.; Cambridge University Press: Oxford, UK, 1992; pp. 489–511. [Google Scholar]
- Silva, J.d.; Rashid, Z.; Nhut, D.T.; Sivakumar, D.; Gera, A.; Souza, M.T.; Tennant, P. Papaya (Carica papaya L.) biology and biotechnology. Tree For. Sci. Biotechnol. 2007, 1, 47–73. [Google Scholar]
- Chen, L.-F.; Bau, H.-J.; Yeh, S.-D. Identification of Viruses Capable of Breaking Transgenic Resistance of Papaya Conferred by the Coat Protein Gene of Papaya Ringspot Virus. Acta Hortic. 2002, 575, 465–474. [Google Scholar] [CrossRef]
- Morton, J.F.D.C.F. Fruits of Warm Climates; Morton, J.F., Ed.; Distributed by Creative Resources Systems: Miami, FL, USA; Winterville, NC, USA, 1987. [Google Scholar]
- Karunamoorthi, K.; Kim, H.-M.; Jegajeevanram, K.; Xavier, J.; Vijayalakshmi, J. Papaya: A gifted nutraceutical plant—A critical review of recent human health research. Cellmed 2014, 4, 2.1–2.17. [Google Scholar] [CrossRef][Green Version]
- Saeed, F.; Arshad, M.U.; Pasha, I.; Naz, R.; Batool, R.; Khan, A.A.; Nasir, M.A.; Shafique, B. Nutritional and phyto-therapeutic potential of papaya (Carica papaya Linn.): An overview. Int. J. Food Prop. 2014, 17, 1637–1653. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Alara, J.A. Carica papaya: Comprehensive overview of the nutritional values, phytochemicals and pharmacological activities. Adv. Tradit. Med. 2020, 22, 1–31. [Google Scholar] [CrossRef]
- De Oliveira, J.G.; Vitória, A.P. Papaya: Nutritional and pharmacological characterization, and quality loss due to physiological disorders. An overview. Food Res. Int. 2011, 44, 1306–1313. [Google Scholar] [CrossRef]
- Starley, I.F.; Mohammed, P.; Schneider, G.; Bickler, S.W. The treatment of paediatric burns using topical papaya. Burns 1999, 25, 636–639. [Google Scholar] [CrossRef]
- Maciunas, R.J.; Onofrio, B.M. The long-term results of chymopapain. Ten-year follow-up of 268 patients after chemonucleolysis. Clin. Orthop. Relat. Res. 1986, 206, 37–41. [Google Scholar] [CrossRef]
- Begum, M. Phytochemical and Pharmacological Investigation of Carica Papaya Leaf; East west University: Dhaka, Bangladesh, 2014. [Google Scholar]
- Reed, C.F. Information summaries on 1000 economic plants. Typescripts Submitt. USDA 1976, 102–103. [Google Scholar]
- Elizabeth, K. Immense help from nature’s workshop. Edn 1994, 1, 207–209. [Google Scholar]
- Sharma, A.; Sharma, R.; Sharma, M.; Kumar, M.; Barbhai, M.D.; Lorenzo, J.M.; Sharma, S.; Samota, M.K.; Atanassova, M.; Caruso, G. Carica papaya L. Leaves: Deciphering its antioxidant bioactives, biological activities, innovative products, and safety aspects. Oxid. Med. Cell. Longev. 2022, 2022, 1–20. [Google Scholar] [CrossRef]
- Hu, T.; Guo, Y.Y.; Zhou, Q.F.; Zhong, X.K.; Zhu, L.; Piao, J.H.; Chen, J.; Jiang, J.G. Optimization of ultrasonic-assisted extraction of total saponins from eclipta prostrasta l. Using response surface methodology. J. Food Sci. 2012, 77, C975–C982. [Google Scholar] [CrossRef]
- Patil, T.; Patil, S.; Patil, A.; Patil, S. Carica papaya leaf extracts—An ethnomedicinal boon. Int. J. Pharmacogn. Phytochem. 2014, 6, 260–265. [Google Scholar]
- Rumiyati, S. Effect of the protein fraction of Carica papaya L. Leaves on the expressions of p53 and bcl-2 in breast cancer cells line. Maj. Farm. Indones 2006, 17, 170–176. [Google Scholar]
- Singh, S.P.; Kumar, S.; Mathan, S.V.; Tomar, M.S.; Singh, R.K.; Verma, P.K.; Kumar, A.; Kumar, S.; Singh, R.P.; Acharya, A. Therapeutic application of carica papaya leaf extract in the management of human diseases. DARU J. Pharm. Sci. 2020, 28, 735–744. [Google Scholar] [CrossRef]
- Otsuki, N.; Dang, N.H.; Kumagai, E.; Kondo, A.; Iwata, S.; Morimoto, C. Aqueous extract of carica papaya leaves exhibits anti-tumor activity and immunomodulatory effects. J. Ethnopharmacol. 2010, 127, 760–767. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Parat, M.-O.; Hodson, M.P.; Pan, J.; Shaw, P.N.; Hewavitharana, A.K. Chemical characterization and in vitro cytotoxicity on squamous cell carcinoma cells of carica papaya leaf extracts. Toxins 2015, 8, 7. [Google Scholar] [CrossRef]
- Pandey, S.; Walpole, C.; Cabot, P.J.; Shaw, P.N.; Batra, J.; Hewavitharana, A.K. Selective anti-proliferative activities of carica papaya leaf juice extracts against prostate cancer. Biomed. Pharmacother. 2017, 89, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Fazal, H.; Ayaz, M.; Abbasi, B.H.; Mohammad, I.; Fazal, L. Dengue fever treatment with Carica papaya leaves extracts. Asian Pac. J. Trop Biomed. 2011, 1, 330–333. [Google Scholar] [CrossRef]
- Saotoing, P.; Tchuenguem, F.F.; Njan, N.A.; Messi, J. Medicinal plants used in traditional treatment of malaria in cameroon. J. Ecol. Nat. Environ. 2011, 3, 104–117. [Google Scholar]
- Kovendan, K.; Murugan, K.; Naresh Kumar, A.; Vincent, S.; Hwang, J.-S. Bioefficacy of larvicdial and pupicidal properties of carica papaya (caricaceae) leaf extract and bacterial insecticide, spinosad, against chikungunya vector, aedes aegypti (diptera: Culicidae). Parasitol. Res. 2012, 110, 669–678. [Google Scholar] [CrossRef]
- Morimoto, C.; Dang, N. Compositions for Cancer Prevention, Treatment, or Amelioration Comprising Papaya Extract. US Patent 20080069907, 20 March 2008. [Google Scholar]
- Fauziya, S.; Krishnamurthy, R. Papaya (carica papaya): Source material for anticancer. CIBTech. J. Pharm. Sci. 2013, 2, 25–34. [Google Scholar]
- Nguyen, T.T.; Shaw, P.N.; Parat, M.O.; Hewavitharana, A.K. Anticancer activity of c arica papaya: A review. Mol. Nutr. Food Res. 2013, 57, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Anjum, V.; Arora, P.; Ansari, S.H.; Najmi, A.K.; Ahmad, S. Antithrombocytopenic and immunomodulatory potential of metabolically characterized aqueous extract of carica papaya leaves. Pharm. Biol. 2017, 55, 2043–2056. [Google Scholar] [CrossRef] [PubMed]
- Imaga, N.A.; Gbenle, G.O.; Okochi, V.I.; Akanbi, S.; Edeoghon, S.O.; Oigbochie, V.E.; Kehinde, M.O.; Bamiro, S.B. Antisickling property of carica papaya leaf extract. Afr. J. Biochem. Res. 2009, 3, 102–106. [Google Scholar]
- Gurung, S.; Škalko-Basnet, N. Wound healing properties of carica papaya latex: In vivo evaluation in mice burn model. J. Ethnopharmacol. 2009, 121, 338–341. [Google Scholar] [CrossRef]
- Sagnia, B.; Fedeli, D.; Casetti, R.; Montesano, C.; Falcioni, G.; Colizzi, V. Antioxidant and anti-inflammatory activities of extracts from cassia alata, eleusine indica, eremomastax speciosa, carica papaya and polyscias fulva medicinal plants collected in cameroon. PLoS ONE 2014, 9, e103999. [Google Scholar] [CrossRef]
- Salim, E.; Kumolosasi, E.; Jantan, I. Inhibitory effect of selected medicinal plants on the release of pro-inflammatory cytokines in lipopolysaccharide-stimulated human peripheral blood mononuclear cells. J. Nat. Med. 2014, 68, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Sadek, K.M. Antioxidant and immunostimulant effect of carica papaya linn. Aqueous extract in acrylamide intoxicated rats. Acta Inform. Med. 2012, 20, 180. [Google Scholar] [CrossRef] [PubMed]
- Teh, B.P.; Ahmad, N.B.; Mohamad, S.B.; Tan, T.Y.C.; Mohd Abd Razak, M.R.B.; Afzan, A.B.; Syed Mohamed, A.F.B. Carica papaya Leaf Juice for Dengue: A Scoping Review. Nutrients 2022, 14, 1584. [Google Scholar] [CrossRef]
- Dharmarathna, S.L.C.A.; Wickramasinghe, S.; Waduge, R.N.; Rajapakse, R.P.V.J.; Kularatne, S.A.M. Does carica papaya leaf-extract increase the platelet count? An experimental study in a murine model. Asian Pac. J. Trop. Biomed. 2013, 3, 720–724. [Google Scholar] [CrossRef]
- Gammulle, A.; Ratnasooriya, W.; Jayakody, J.; Fernando, C.; Kanatiwela, C.; Udagama, P.V. Thrombocytosis and anti-inflammatory properties, and toxicological evaluation of carica papaya mature leaf concentrate in a murine model. Online Int. J. Med. Plant Res. 2012, 1, 21–30. [Google Scholar]
- Yunita, F.; Hanani, E.; Kristianto, J. The effect of Carica papaya L. Leaves extract capsules on platelets count and hematocrit level in dengue fever patient. Int. J. Med. Aromat. Plants 2012, 2, 573–578. [Google Scholar]
- Norahmad, N.A.; Mohd Abd Razak, M.R.; Mohmad Misnan, N.; Md Jelas, N.H.; Sastu, U.R.; Muhammad, A.; Ho, T.C.D.; Jusoh, B.; Zolkifli, N.A.; Thayan, R. Effect of freeze-dried carica papaya leaf juice on inflammatory cytokines production during dengue virus infection in ag129 mice. BMC Complement Altern. Med. 2019, 19, 44. [Google Scholar] [CrossRef]
- Ranasinghe, P.; Ranasinghe, P.; Abeysekera, W.K.M.; Premakumara, G.S.; Perera, Y.S.; Gurugama, P.; Gunatilake, S.B. In vitro erythrocyte membrane stabilization properties of Carica papaya L. Leaf extracts. Pharmacogn. Res. 2012, 4, 196. [Google Scholar] [CrossRef]
- Srikanth, B.K.; Reddy, L.; Biradar, S.; Shamanna, M.; Mariguddi, D.D.; Krishnakumar, M. An open-label, randomized prospective study to evaluate the efficacy and safety of carica papaya leaf extract for thrombocytopenia associated with dengue fever in pediatric subjects. Pediatric Health Med. Ther. 2019, 10, 5–11. [Google Scholar] [CrossRef]
- Isela, E.J.-R.; Juan, C.D.-Z.; Jorge, L.; Pedro, H.M.-O.; Andrés, E.C.-R.; Carlos, A.; Tovilla-Z, A.; Rodríguez-H, H.; Aguilar-M, T.; Ramón-F, D.Y.; et al. Hypoglycemic effect of carica papaya leaves in streptozotocin-induced diabetic rats. BMC Complement Alternat. Med. 2012, 12, 236. [Google Scholar]
- Aruoma, O.I.; Somanah, J.; Bourdon, E.; Rondeau, P.; Bahorun, T. Diabetes as a risk factor to cancer: Functional role of fermented papaya preparation as phytonutraceutical adjunct in the treatment of diabetes and cancer. Mutat. Res.-Fundam. Mol. Mech. Mutagen. 2014, 768, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Nurain, I.O.; Bewaji, C.O.; Johnson, J.S.; Davenport, R.D.; Zhang, Y. Potential of three ethnomedicinal plants as antisickling agents. Mol. Pharm. 2017, 14, 172–182. [Google Scholar] [CrossRef]
- Suresh, K. Antimicrobial and phytochemical investigation of the leaves of Carica papaya L., Cynodon dactylon (L.) pers., Euphorbia hirta L., Melia azedarach L. And Psidium guajava L. Ethnobot. Leafl. 2008, 2008, 157. [Google Scholar]
- Baskaran, C.; Ratha bai, V.; Velu, S.; Kumaran, K. The efficacy of carica papaya leaf extract on some bacterial and a fungal strain by well diffusion method. Asian Pac. J. Trop. Dis. 2012, 2, S658–S662. [Google Scholar] [CrossRef]
- Indran, M.; Mahmood, A.; Kuppusamy, U. Protective effect of carica papaya l leaf extract against alcohol induced acute gastric damage and blood oxidative stress in rats. West. Indian Med. J. 2008, 57, 323–326. [Google Scholar] [PubMed]
- Owoyele, B.V.; Adebukola, O.M.; Funmilayo, A.A.; Soladoye, A.O. Anti-inflammatory activities of ethanolic extract of carica papaya leaves. Inflammopharmacology 2008, 16, 168–173. [Google Scholar] [CrossRef]
- Fuggate, P.; Wongs-Aree, C.; Noichinda, S.; Kanlayanarat, S. Quality and volatile attributes of attached and detached ‘pluk mai lie’papaya during fruit ripening. Sci. Hortic. 2010, 126, 120–129. [Google Scholar] [CrossRef]
- Balbontín, C.; Gaete-Eastman, C.; Vergara, M.; Herrera, R.; Moya-León, M.A. Treatment with 1-mcp and the role of ethylene in aroma development of mountain papaya fruit. Postharvest Biol. Technol. 2007, 43, 67–77. [Google Scholar] [CrossRef]
- Flath, R.A.; Forrey, R.R. Volatile components of papaya (Carica papaya L., solo variety). J. Agric. Food Chem. 1977, 25, 103–109. [Google Scholar] [CrossRef]
- Almora, K.; Pino, J.A.; Hernández, M.; Duarte, C.; González, J.; Roncal, E. Evaluation of volatiles from ripening papaya (Carica papaya L., var. Maradol roja). Food Chem. 2004, 86, 127–130. [Google Scholar] [CrossRef]
- MacLeod, A.J.; Pieris, N.M. Volatile components of papaya (Carica papaya L.) with particular reference to glucosinolate products. J. Agric. Food Chem. 1983, 31, 1005–1008. [Google Scholar] [CrossRef]
- Zunjar, V.; Mammen, D.; Trivedi, B. Antioxidant activities and phenolics profiling of different parts of carica papaya by lcms-ms. Nat. Prod. Res. 2015, 29, 2097–2099. [Google Scholar] [CrossRef] [PubMed]
- Julianti, T.; De Mieri, M.; Zimmermann, S.; Ebrahimi, S.N.; Kaiser, M.; Neuburger, M.; Raith, M.; Brun, R.; Hamburger, M. Hplc-based activity profiling for antiplasmodial compounds in the traditional indonesian medicinal plant Carica papaya L. J. Ethnopharmacol. 2014, 155, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Esti, M.; Benucci, I.; Lombardelli, C.; Liburdi, K.; Garzillo, A.M.V. Papain from papaya (Carica papaya L.) fruit and latex: Preliminary characterization in alcoholic–acidic buffer for wine application. Food Bioprod. Process. 2013, 91, 595–598. [Google Scholar] [CrossRef]
- Burdick, E.M. Carpaine: An alkaloid of carica papaya: Its chemistry and pharmacology. Econ. Bot. 1971, 25, 363–365. [Google Scholar] [CrossRef]
- Krishna, K.; Paridhavi, M.; Patel, J.A. Review on nutritional, medicinal and pharmacological properties of papaya (carica papaya linn.). Nat. Prod. Rad. 2008, 7, 364–373. [Google Scholar]
- Yogiraj, V.; Goyal, P.K.; Chauhan, C.S.; Goyal, A.; Vyas, B. Carica papaya linn: An overview. Int. J. Herb. Med. 2014, 2, 1–8. [Google Scholar]
- Tang, C.-S. Localization of benzyl glucosinolate and thioglucosidase in carica papaya fruit. Phytochemistry 1973, 12, 769–773. [Google Scholar] [CrossRef]
- Barroso, P.T.; de Carvalho, P.P.; Rocha, T.B.; Pessoa, F.L.; Azevedo, D.A.; Mendes, M.F. Evaluation of the composition of Carica papaya L. Seed oil extracted with supercritical co2. Biotechnol. Rep. 2016, 11, 110–116. [Google Scholar] [CrossRef]
- Sancho, L.E.G.-G.; Yahia, E.M.; González-Aguilar, G.A. Identification and quantification of phenols, carotenoids, and vitamin c from papaya (Carica papaya L., cv. Maradol) fruit determined by hplc-dad-ms/ms-esi. Food Res. Int. 2011, 44, 1284–1291. [Google Scholar] [CrossRef]
- Oduola, T.; Adeniyi, F.; Ogunyemi, E.; Bello, I.; Idowu, T. Antisickling agent in an extract of unripe pawpaw (carica papaya): Is it real? Afr. J. Biotechnol. 2006, 5, 1947–1949. [Google Scholar]
- Canini, A.; Alesiani, D.; D’Arcangelo, G.; Tagliatesta, P. Gas chromatography–mass spectrometry analysis of phenolic compounds from Carica papaya L. Leaf. J. Food Compost. Anal. 2007, 20, 584–590. [Google Scholar] [CrossRef]
- FAOSTAT; FAO. Food and Agriculture Data. Crop Statistics. 2019. Available online: http://www.fao.org/faostat (accessed on 14 August 2020).
- Srivastava, J.; Singh, A. Diseases of Horticultural Crops: Diagnosis and Management: Volume 1: Fruit Crops; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar]
- Available online: http://agropedia.iitk.ac.in/content/papaya-diseases-its-control (accessed on 13 August 2012).
- Ventura, J.A.; Costa, H.; Tatagiba, J.d.S. Papaya Diseases and Integrated Control. In Diseases of Fruits and Vegetables: Volume II; Naqvi, S.A.M.H., Ed.; Springer: Dordrecht, Germany, 2004. [Google Scholar] [CrossRef]
- Narayanasamy, P. Crop Diseases Management: Principles and Practices; New India Publishing Agency: Delhi, India, 2011. [Google Scholar]
- Cannon, P.F.; Kirk, P.M. Fungal Families of the World; Cabi: Wallingford, UK, 2007. [Google Scholar]
- Hine, R.B.; Holtzmann, O.V.; Raabe, R.D. Diseases of Papaya (Carica papaya L.) in Hawaii; University of Hawaii: Honolulu, HI, USA, 1965; 26p, (Bulletin 136); Available online: http://hdl.handle.net/10125/14967 (accessed on 8 February 2010).
- Ooka, J. Oidium caricae. In Crop Knowledge Master; University of Hawai’i at Manoa, College of Tropical Agriculture and Human Resources: Honolulu, Hawaii, 1993. [Google Scholar]
- Snowdon, A.L. A Colour Atlas of Post-Harvest Diseases and Disorders of Fruits and Vegetables. Volume 1: General Introduction and Fruits; Wolfe Scientific Ltd.: London UK, 1990. [Google Scholar]
- Rana, S. Fungal Diseases of Papaya; Diseases of Fruit Crops; Oxford and IBH Publishing Co. Pvt. Ltd.: New Delhi, India, 2001. [Google Scholar]
- Umer, M.; Mubeen, M.; Iftikhar, Y.; Ali, H.; Zafar-ul-Hye, M.; Asghar, R.; Abbas, M.; Rehman, M.A.; Moya-Elizondo, E.A.; He, Y. Papaya Ring Spot Virus: An Understanding of a Severe Positive-Sense Single Stranded RNA Viral Disease and its Management. Phyton 2022, 91, 2099–2110. [Google Scholar] [CrossRef]
- Gonsalves, D. Control of papaya ringspot virus in papaya: A case study. Annu. Rev. Phytopathol. 1998, 36, 415–437. [Google Scholar] [CrossRef]
- Conover, R. Virus Diseases of Papaya in Florida; Amer Phytopathological Soc 3340 Pilot Knob Road, Phytopathology: St. Paul, MN, USA, 1962; p. 6. [Google Scholar]
- Bau, H.-J.; Kung, Y.-J.; Raja, J.; Chan, S.-J.; Chen, K.-C.; Chen, Y.-K.; Wu, H.-W.; Yeh, S.-D. Potential threat of a new pathotype of papaya leaf distortion mosaic virus infecting transgenic papaya resistant to papaya ringspot virus. Phytopathology 2008, 98, 848–856. [Google Scholar] [CrossRef]
- Shen, W.; Wang, Y.; Tuo, D.; Yan, P.; Yang, Y.; Li, X.; Zhou, P. Agroinoculation of carica papaya with infectious clones of papaya mosaic virus. Acta Virol. 2014, 58, 380–382. [Google Scholar] [CrossRef][Green Version]
- Thomas, K.; Krishnaswami, C. Leaf crinkle—A transmissible disease of papaya. Curr. Sci. 1939, 8, 316. [Google Scholar]
- Saxena, S.; Hallan, V.; Singh, B.; Sane, P. Evidence from nucleic acid hybridization tests for a geminivirus infectioncausing leaf curl disease of papaya in india. Indian J. Exp. Biol. 1998, 36, 229–232. [Google Scholar]
- Bananej, K.; Shafiq, M.; Shahid, M.S. Association of cotton leaf curl gezira virus with tomato leaf curl betasatellite infecting carica papaya in iran. Australas. Plant Dis. Notes 2021, 16, 1–4. [Google Scholar] [CrossRef]
- Fatima, S.; Javeed, Z.; Ade, A. Post-Harvest Rots of Fruits; Discovery Publishing House: Delhi, India, 2006. [Google Scholar]
- Rahman, M.; Mahmud, T.; Kadir, J.; Abdul Rahman, R.; Begum, M. Major postharvest fungal diseases of papaya cv.‘Sekaki’in selangor, malaysia. Pertanika J. Trop. Agric. Sci 2008, 31, 27–34. [Google Scholar]
- Hunter, J.E.; Buddenhagen, I. Incidence, epidemiology and control of fruit diseases of papaya in hawaii. Trop Agr. 1972, 49, 61–72. [Google Scholar]
- Gupta, G.K.; Sharma, S.K.; Ramteke, R. Biology, epidemiology and management of the pathogenic fungus macrophomina phaseolina (tassi) goid with special reference to charcoal rot of soybean (Glycine max (L.) merrill). J. Phytopathol. (1986) 2012, 160, 167–180. [Google Scholar] [CrossRef]
- Helal, R.; Hosen, S.; Shamsi, S. Mycoflora associated with post-harvest disease of papaya (Carica papaya L.) and their pathogenic potentiality. Bangladesh J. Bot. 2018, 47, 389–395. [Google Scholar] [CrossRef]
- Nishijima, K.; Miura, C.; Armstrong, J.; Brown, S.; Hu, B. Effect of Forced, Hot-Air Treatment of Papaya Fruit on Fruit Quality and Incidence of Postharvest Diseases. Plant Dis. 1992, 76, 723–727. [Google Scholar] [CrossRef]
- Amaral, D.D.; Monteiro, A.L.R.; Silva, E.I.D.; Lins, S.R.D.O.; OLIVEIRA, S. Frequency of quiescent fungi and post-harvest alternative management of stem end rot in papaya. Revista Caatinga 2017, 30, 786–793. [Google Scholar] [CrossRef]
- Palou, L.; Smilanick, J.L. Postharvest Pathology of Fresh Horticultural Produce; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Debysingh, N.; Wickham, L.D.; Mohammed, M.; Legall, G.; Paliyath, G.; Subramanian, J. Effects of pre-and post-harvest treatments with hexanal formulations on time to ripening and shelf life of papaya (Carica papaya L.) fruits. Trop. Agric. 2018, 95, 36–42. [Google Scholar]
- Bautista-Baños, S.; Sivakumar, D.; Bello-Pérez, A.; Villanueva-Arce, R.; Hernández-López, M. A review of the management alternatives for controlling fungi on papaya fruit during the postharvest supply chain. Crop Prot. 2013, 49, 8–20. [Google Scholar] [CrossRef]
- Suseela Bhai, R.; Ishwara Bhat, A.; Anandaraj, M. Premature yellowing and bean shedding in vanilla (vanilla planifolia andrews). In Proceedings of the Symposium on Resent Development in the Diagnosis and Management of Plant Diseases for Meeting Global Challenges, Dharwad, KA, India, 18–20 December 2003; pp. 88–89. [Google Scholar]
- Bosquez-Molina, E.; Ronquillo-de Jesús, E.; Bautista-Baños, S.; Verde-Calvo, J.; Morales-López, J. Inhibitory effect of essential oils against colletotrichum gloeosporioides and rhizopus stolonifer in stored papaya fruit and their possible application in coatings. Postharvest Biol. Technol. 2010, 57, 132–137. [Google Scholar] [CrossRef]
- Sarkhosh, A.; Schaffer, B.; Vargas, A.; Palmateer, A.; Lopez, P.; Soleymani, A.; Farzaneh, M. Antifungal activity of five plant-extracted essential oils against anthracnose in papaya fruit. Biol. Agric. Hortic. 2018, 34, 18–26. [Google Scholar] [CrossRef]
- Allong, R.; Wickham, L.; Majeed, M. Effect of cultivar, hot water treatment and storage conditions on quality of fresh-cut papaya (Carica papaya L.). J. Appl. Hortic. 2000, 2, 15–18. [Google Scholar] [CrossRef]
- Li, X.; Zhu, X.; Zhao, N.; Fu, D.; Li, J.; Chen, W.; Chen, W. Effects of hot water treatment on anthracnose disease in papaya fruit and its possible mechanism. Postharvest Biol. Technol. 2013, 86, 437–446. [Google Scholar] [CrossRef]
- Marpudi, S.; Abirami, L.S.S.; Pushkala, R.; Nagarajan, S. Enhancement of storage life and quality maintenance of papaya fruits using aloe vera based antimicrobial coating. Indian J. Biotechnol. 2011, 10, 83–89. [Google Scholar]
- Sivakumar, D.; Sultanbawa, Y.; Ranasingh, N.; Kumara, P.; Wijesundera, R. Effect of the combined application of chitosan and carbonate salts on the incidence of anthracnose and on the quality of papaya during storage. J. Hortic. Sci. Biotechnol. 2005, 80, 447–452. [Google Scholar] [CrossRef]
- Chau, K.; Alvarez, A.M. Postharvest fruit rot of papaya caused by stemphylium lycopersici. Plant Dis. 1983, 67, 1279–1281. [Google Scholar] [CrossRef]
- Ming, R.; van Droogenbroeck, B.; Moore, P.H.; Zee, F.T.; Kyndt, T.; Scheldeman, X.; Sekioka, T.; Gheysen, G. Molecular diversity of carica papaya and related species. Plant Genome Biodivers. Evol. 2005, 1, 229–254. [Google Scholar]
- Ming, R.; Hou, S.; Feng, Y.; Yu, Q.; Dionne-Laporte, A.; Saw, J.H.; Senin, P.; Wang, W.; Ly, B.V.; Lewis, K.L.; et al. The draft genome of the transgenic tropical fruit tree papaya (carica papaya linnaeus). Nature 2008, 452, 991–996. [Google Scholar] [CrossRef]
- Van Droogenbroeck, B.; Breyne, P.; Goetghebeur, P.; Romeijn-Peeters, E.; Kyndt, T.; Gheysen, G. Aflp analysis of genetic relationships among papaya and its wild relatives (caricaceae) from ecuador. Theor. Appl. Genet. 2002, 105, 289–297. [Google Scholar] [CrossRef]
- Wai, C.M.; Ming, R.; Moore, P.H.; Paull, R.E.; Yu, Q. Development of chromosome-specific cytogenetic markers and merging of linkage fragments in papaya. Trop. Plant Biol. 2010, 3, 171–181. [Google Scholar] [CrossRef]
- Zhang, W.; Wai, C.M.; Ming, R.; Yu, Q.; Jiang, J. Integration of genetic and cytological maps and development of a pachytene chromosome-based karyotype in papaya. Trop. Plant Biol. 2010, 3, 166–170. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, X.; Yu, Q.; Ming, R.; Jiang, J. DNA methylation and heterochromatinization in the male-specific region of the primitive y chromosome of papaya. Genome Res. 2008, 18, 1938–1943. [Google Scholar] [CrossRef]
- Arumuganathan, K.; Earle, E.D. Estimation of nuclear DNA content of plants by flow cytometry. Plant Mol. Biol. Rep. 1991, 9, 229–241. [Google Scholar] [CrossRef]
- Stokstad, E. Papaya Takes on Ringspot Virus and Wins; American Association for the Advancement of Science: Washington, DC, USA, 2008. [Google Scholar]
- Jaillon, O.; Aury, J.; Noel, B.; Policriti, A.; Clepet, C.; Casagrande, A.; Choisne, N.; Aubourg, S.; Vitulo, N.; Jubin, C. French-italian public the grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature 2007, 449, 463. [Google Scholar]
- Sasaki, T. The map-based sequence of the rice genome. Nature 2005, 436, 793–800. [Google Scholar] [CrossRef]
- Tuskan, G.A.; Difazio, S.; Jansson, S.; Bohlmann, J.; Grigoriev, I.; Hellsten, U.; Putnam, N.; Ralph, S.; Rombauts, S.; Salamov, A. The genome of black cottonwood, populus trichocarpa (torr. & gray). Science 2006, 313, 1596–1604. [Google Scholar]
- Kanchana-udomkan, C.; Ford, R.; Drew, R. Molecular markers in papayas. In Genetics and Genomics of Papaya; Springer: Berlin/Heidelberg, Germany, 2014; pp. 355–375. [Google Scholar]
- Adawiah, Z.R.; Norliza, A.; Fairuz, Y.M.; Norzihan, A.; Kalsom, A.U. Sequence information on single nucleotide polymorphism (snp) through genome sequencing analysis of carica papaya variety eksotika and sekaki. J. Trop. Agric. Fd. Sci. 2016, 44, 219–228. [Google Scholar]
- Wu, M.; Lewis, J.; Moore, R.C. A wild origin of the loss-of-function lycopene beta cyclase (cyc-b) allele in cultivated, red-fleshed papaya (carica papaya). Am. J. Bot. 2017, 104, 116–126. [Google Scholar] [CrossRef]
- Chia, C.L.; Manshardt, R.M. Why some Papaya Plants Fail to Fruit; (Fruits and Nuts; F&N-5); University of Hawaii: Honolulu, HI, USA, 2001; 2p, Available online: http://hdl.handle.net/10125/12186 (accessed on 1 July 2022).
- Ming, R.; Yu, Q.; Moore, P.H. Sex determination in papaya. Semin. Cell Dev. Biol. 2007, 18, 401–408. [Google Scholar] [CrossRef]
- Liao, Z.; Dong, F.; Liu, J.; Xu, L.; Marshall-Colon, A.; Ming, R. Gene regulation network analyses of pistil development in papaya. BMC Genom. 2022, 23, 8. [Google Scholar] [CrossRef]
- Liu, Z.; Moore, P.H.; Ma, H.; Ackerman, C.M.; Ragiba, M.; Yu, Q.; Pearl, H.M.; Kim, M.S.; Charlton, J.W.; Stiles, J.I.; et al. A primitive y chromosome in papaya marks incipient sex chromosome evolution. Nature 2004, 427, 348–352. [Google Scholar] [CrossRef]
- Yu, Q.; Hou, S.; Feltus, F.A.; Jones, M.R.; Murray, J.E.; Veatch, O.; Lemke, C.; Saw, J.H.; Moore, R.C.; Thimmapuram, J. Low x/y divergence in four pairs of papaya sex-linked genes. Plant J. 2008, 53, 124–132. [Google Scholar] [CrossRef]
- Aradhya, M.K.; Manshardt, R.M.; Zee, F.; Morden, C.W. A phylogenetic analysis of the genus carica l.(caricaceae) based on restriction fragment length variation in a cpdna intergenic spacer region. Genet. Resour. Crop Evol. 1999, 46, 579–586. [Google Scholar] [CrossRef]
- Kim, M.; Moore, P.; Zee, F.; Fitch, M.M.; Steiger, D.; Manshardt, R.; Paull, R.; Drew, R.; Sekioka, T.; Ming, R. Genetic diversity of carica papaya as revealed by aflp markers. Genome 2002, 45, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Ming, R.; Yu, Q.; Moore, P. Papaya Genome and Genomics; Springer: New York, NY, USA, 2012; pp. 241–259. [Google Scholar]
- Yang, N.; Liu, J.; Gao, Q.; Gui, S.; Chen, L.; Yang, L.; Huang, J.; Deng, T.; Luo, J.; He, L. Genome assembly of a tropical maize inbred line provides insights into structural variation and crop improvement. Nat. Genet. 2019, 51, 1052–1059. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Zhang, X.; Zhang, S.; Lin, Z.; Zhang, X.; Ming, R. Structural variations in papaya genomes. BMC Genom. 2021, 22, 335. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.; Castro, L.; Fermin, G.; Tennant, P. 10 chapter advances in papaya genomics. In Omics Technologies and Crop Improvement; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2014; pp. 219–252. [Google Scholar]
- Liao, Z.; Yu, Q.; Ming, R. Development of male-specific markers and identification of sex reversal mutants in papaya. Euphytica 2017, 213, 53. [Google Scholar] [CrossRef]
- Chávez-Pesqueira, M.; Núnez-Farfán, J. Genetic diversity and structure of wild populations of carica papaya in northern mesoamerica inferred by nuclear microsatellites and chloroplast markers. Ann. Bot. 2016, 118, 1293–1306. [Google Scholar] [CrossRef]
- Abberton, M.; Batley, J.; Bentley, A.; Bryant, J.; Cai, H.; Cockram, J.; Costa de Oliveira, A.; Cseke, L.J.; Dempewolf, H.; De Pace, C. Global agricultural intensification during climate change: A role for genomics. Plant Biotechnol. J. 2016, 14, 1095–1098. [Google Scholar] [CrossRef]
- Guerra-García, A.; Piñero, D. Current approaches and methods in plant domestication studies. Bot. Sci. 2017, 95, 345–362. [Google Scholar] [CrossRef][Green Version]
- Fitch, M.M.; Manshardt, R.M.; Gonsalves, D.; Slightom, J.L.; Sanford, J.C. Stable transformation of papaya via microprojectile bombardment. Plant Cell Rep. 1990, 9, 189–194. [Google Scholar] [CrossRef]
- Fitch, M.M.; Manshardt, R.M.; Gonsalves, D.; Slightom, J.L. Transgenic papaya plants from agrobacterium-mediated transformation of somatic embryos. Plant Cell Rep. 1993, 12, 245–249. [Google Scholar] [CrossRef]
- Manshardt, R. History and future of the solo papaya. In Genetics and Genomics of Papaya; Ming, R., Moore, P.H., Eds.; Springer: New York, NY, USA, 2014; pp. 95–113. [Google Scholar]
- Ye, C.; Li, H. 20 years of transgenic research in china for resistance to papaya ringspot virus. Transgenic Plant J. 2010, 4, 58–63. [Google Scholar]
- Randle, M.; Tennant, P. Transgenic papaya. In Genetically Modified Crops; Springer: Berlin/Heidelberg, Germany, 2021; pp. 129–160. [Google Scholar]
- Tecson Mendoza, E.M.; Antonio, C.L.; Botella, J.R. Recent advances in the development of transgenic papaya technology. Biotechnol. Annu. Rev. 2008, 14, 423–462. [Google Scholar] [CrossRef] [PubMed]
- Bau, H.-J.; Cheng, Y.-H.; Yu, T.-A.; Yang, J.-S.; Yeh, S.-D. Broad-spectrum resistance to different geographic strains of papaya ringspot virus in coat protein gene transgenic papaya. Phytopathology 2003, 93, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Ying, Z.; Yu, X.; Davis, M.J. New Method for Obtaining Transgenic Papaya Plants by Agrobacterium-Mediated Transformation of Somatic Embryos. Proc. Florida State Hortic Soc. 1999, 112, 201–205. [Google Scholar]
- Jefferson, R.A.; Kavanagh, T.A.; Bevan, M.W. Gus fusions: Beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987, 6, 3901–3907. [Google Scholar] [CrossRef]
- Stewart, C. The utility of green fluorescent protein in transgenic plants. Plant Cell Rep. 2001, 20, 376–382. [Google Scholar] [CrossRef]
- Zhu, Y.J.; Agbayani, R.; Jackson, M.C.; Tang, C.; Moore, P.H. Expression of the grapevine stilbene synthase gene vst1 in papaya provides increased resistance against diseases caused by phytophthora palmivora. Planta 2004, 220, 241–250. [Google Scholar] [CrossRef]
- McCafferty, H.R.K.; Moore, P.H.; Zhu, Y.J. Improved carica papaya tolerance to carmine spider mite by the expression of manduca sexta chitinase transgene. Transgenic Res. 2006, 15, 337–347. [Google Scholar] [CrossRef]
- McCafferty, H.R.K.; Moore, P.H.; Zhu, Y.J. Papaya transformed with the galanthus nivalis gna gene produces a biologically active lectin with spider mite control activity. Plant Sci. 2008, 175, 385–393. [Google Scholar] [CrossRef]
- Genglin, Z.; Peng, Z.; Anping, G.; Wentao, S.; Xiaoying, L. An initial study of transgenic carica papaya used as a kind of vaccine for anti tuberculosis. Acta Bot. Yunnanica 2003, 25, 223–229. [Google Scholar]
- CHEN, N.-M.; Paull, R. Development and prevention of chilling injury in papaya fruit. J. Am. Soc. Hortic. Sci. 1986, 111, 639–643. [Google Scholar] [CrossRef]
- Paull, R.E.; Nishijima, W.; Reyes, M.; Cavaletto, C. Postharvest handling and losses during marketing of papaya (Carica papaya L.). Postharvest Biol. Technol. 1997, 11, 165–179. [Google Scholar] [CrossRef]
- Lelièvre, J.M.; Latchè, A.; Jones, B.; Bouzayen, M.; Pech, J.C. Ethylene and fruit ripening. Physiol. Plant. 1997, 101, 727–739. [Google Scholar] [CrossRef]
- Trang, T.T.L.; Manshardt, R. A transgenic approach for determining sex of papaya seedlings. Acta Hortic. 2010, 851, 179–188. [Google Scholar] [CrossRef]
- Fitch, M.M.; Manshardt, R.M.; Gonsalves, D.; Slightom, J.L.; Sanford, J.C. Virus resistant papaya plants derived from tissues bombarded with the coat protein gene of papaya ringspot virus. Biotechnology 1992, 10, 1466–1472. [Google Scholar] [CrossRef]
- Yang, J.-S.; Yu, T.-A.; Cheng, Y.-H.; Yeh, S.-D. Transgenic papaya plants from agrobacterium-mediated transformation of petioles of in vitro propagated multishoots. Plant Cell Rep. 1996, 15, 459–464. [Google Scholar] [CrossRef]
- De la Fuente, J.M.; Ramírez-Rodríguez, V.; Cabrera-Ponce, J.L.; Herrera-Estrella, L. Aluminum tolerance in transgenic plants by alteration of citrate synthesis. Science 1997, 276, 1566–1568. [Google Scholar] [CrossRef]
- Zhu, Y.J.; Agbayani, R.; Moore, P.H. Ectopic expression of dahlia merckii defensin dmamp1 improves papaya resistance to phytophthora palmivora by reducing pathogen vigor. Planta 2007, 226, 87–97. [Google Scholar] [CrossRef]
- CS, D.; Samreen, F.; Prakash, J. A review on composition, processed products and medicinal uses of papaya (Carica papaya L.). Int. J. Food Sci. Nutr. 2015, 3, 99–117. [Google Scholar]
- Mahmood, A.; Sidik, K.; Salmah, I. Wound healing activity of Carica papaya L. Aqueous leaf extract in rats. Int. J. Mol. Med. Adv. Sci. 2005, 1, 398–401. [Google Scholar]
- Davison, J. Pickles: A Global History; Reaktion Books: London, UK, 2018. [Google Scholar]
- Saliasi, I.; Llodra, J.C.; Bravo, M.; Tramini, P.; Dussart, C.; Viennot, S.; Carrouel, F. Effect of a toothpaste/mouthwash containing carica papaya leaf extract on interdental gingival bleeding: A randomized controlled trial. Int. J. Environ. Res. Public Health 2018, 15, 2660. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).