Pore Water Chemical Variability and Its Effect on Phenological Production in Three Mangrove Species under Drought Conditions in Southeastern Mexico
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Analysis of Drought
2.3. Wind
2.4. Forest Attributes and Physiognomic Type
2.5. Reproductive Phenology Based on Litterfall
2.6. Pore Water Chemistry
2.7. Statistical Analysis
3. Results
3.1. Drought Analysis
3.2. Wind Analysis
3.3. Forest Attributes and Physiognomy of Mangroves
3.4. Temporal and Spatial Variation of Litterfall
3.5. Reproductive Phenology Based on Litterfall
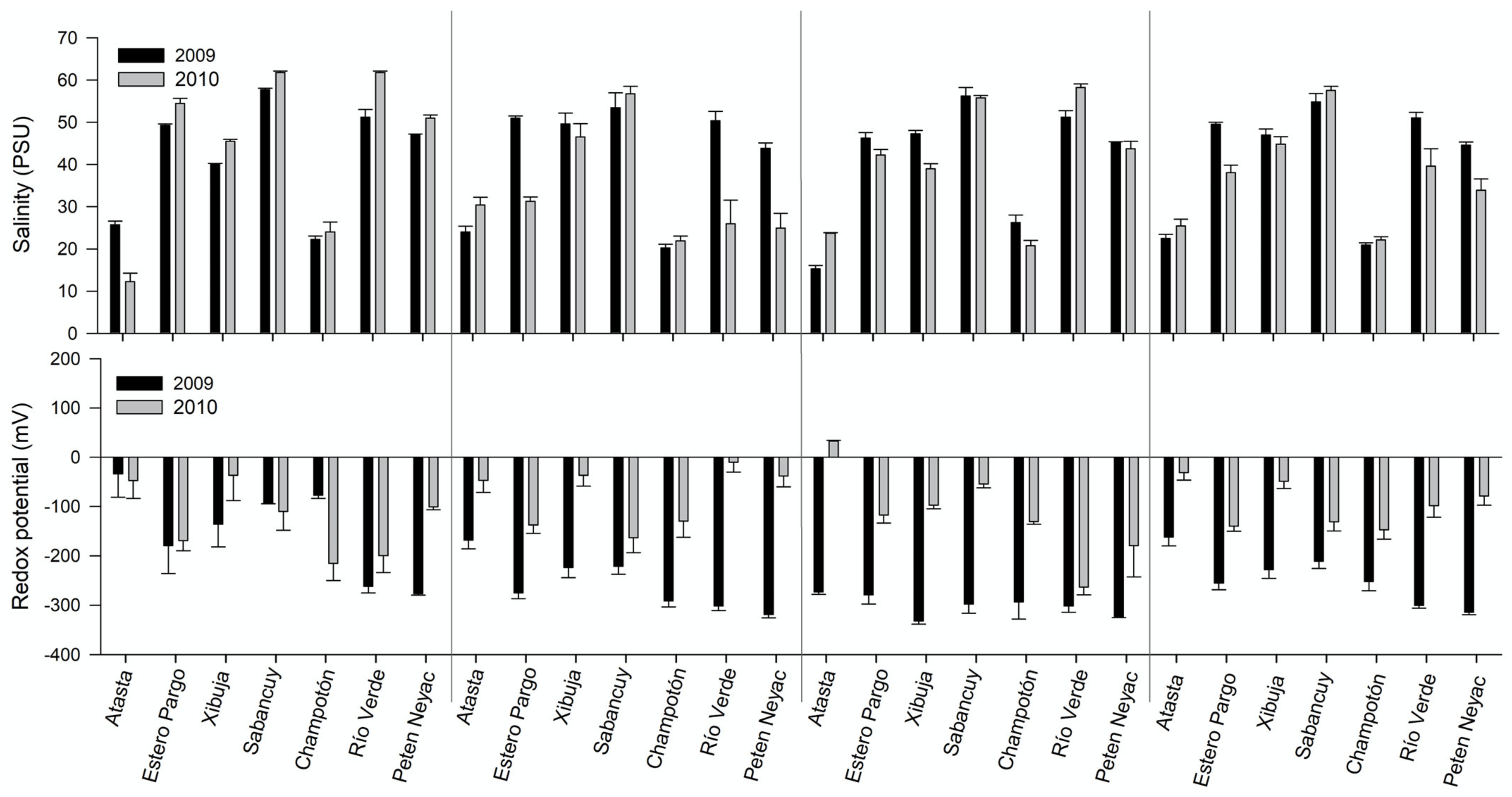
3.6. Pore-Water Chemistry
3.7. Correlation between Pore-Water Chemistry and Reproductive Phenology by Species
4. Discussion
4.1. Temporal and Spatial Variations in Pore Water Chemistry along the Coast of Campeche
4.2. Responses of Mangroves to Variability in Pore-Water Chemistry at the Inter- and Intraspecific Levels
4.3. Species Show the Greatest Adaptation under Severe Drought
5. Conclusions
6. Implications for Conservation
7. Recommendations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agraz-Hernández, C.M.; Osti-Sáenz, J.; Expósito-Díaz, G.; Martínez-Reyes, A.A.; Chan-Keb, G.; Martínez-Muñiz, G.; Chan-Canul, E. Estudio del Posible Impacto Ambiental en el Ecosistema del Manglar Debido a la Operación del Proyecto Hidroeléctrico las Cruces; Informe final; Universidad Autónoma de Campeche, Comisión Federal de Electricidad: Juárez, México, 2015. [Google Scholar]
- Cuellar-Martinez, T.; Ruiz-Fernández, A.C.; Sanchez-Cabeza, J.-A.; Pérez-Bernal, L.; López-Mendoza, P.G.; Carnero-Bravo, V.; Agraz-Hernández, C.M.; van Tussenbroek, B.I.; Sandoval-Gil, J.; Cardoso-Mohedano, J.G.; et al. Temporal Records of Organic Carbon Stocks and Burial Rates in Mexican Blue Carbon Coastal Ecosystems throughout the Anthropocene. Glob. Planet. Chang. 2020, 192, 103215. [Google Scholar] [CrossRef]
- Zeng, Y.; Friess, D.A.; Sarira, T.V.; Siman, K.; Koh, L.P. Global Potential and Limits of Mangrove Blue Carbon for Climate Change Mitigation. Curr. Biol. 2021, 31, 1737–1743.e3. [Google Scholar] [CrossRef] [PubMed]
- Adame, M.F.; Kauffman, J.B.; Medina, I.; Gamboa, J.N.; Torres, O.; Caamal, J.P.; Reza, M.; Herrera-Silveira, J.A. Carbon Stocks of Tropical Coastal Wetlands within the Karstic Landscape of the Mexican Caribbean. PLoS ONE 2013, 8, e56569. [Google Scholar] [CrossRef] [PubMed]
- Donato, D.C.; Kauffman, J.B.; Murdiyarso, D.; Kurnianto, S.; Stidham, M.; Kanninen, M. Mangroves among the Most Carbon-Rich Forests in the Tropics. Nat. Geosci. 2011, 4, 293–297. [Google Scholar] [CrossRef]
- Lloret, F.; Escudero, A.; Iriondo, J.M.; Martínez-Vilalta, J.; Valladares, F. Extreme Climatic Events and Vegetation: The Role of Stabilizing Processes. Glob. Chang. Biol. 2012, 18, 797–805. [Google Scholar] [CrossRef]
- De Lima Nadia, T.; Morellato, L.P.C.; Machado, I.C. Reproductive Phenology of a Northeast Brazilian Mangrove Community: Environmental and Biotic Constraints. Flora-Morphol. Distrib. Funct. Ecol. Plants 2012, 207, 682–692. [Google Scholar] [CrossRef]
- Agraz-Hernández, C.M.; Chan-Keb, C.A.; Chávez-Barrera, J.; Osti-Sáenz, J.; Expósito-Díaz, G.; Alonso-Campos, V.A.; Muñiz-Salazar, R.; Ruiz-Fernández, A.C.; Pérez-Bernal, L.H.; Sánchez-Cabeza, J.A.; et al. Reserva de Carbón en un Ecosistema de Manglar al Norte de México: Cambios Ambientales Durante 35 Años. Rev. Mex. Biodiv. 2020, 91, 912910. [Google Scholar] [CrossRef]
- Drexler, J.Z.; Ewel, K.C. Effect of the 1997–1998 ENSO-Related Drought on Hydrology and Salinity in a Micronesian Wetland Complex. Estuaries 2001, 24, 347. [Google Scholar] [CrossRef]
- Ward, R.D.; Friess, D.A.; Day, R.H.; Mackenzie, R.A. Impacts of Climate Change on Mangrove Ecosystems: A Region by Region Overview. Ecosyst. Health Sustain. 2016, 2, e01211. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Vegetation Stress: An Introduction to the Stress Concept in Plants. J. Plant Physiol. 1996, 148, 4–14. [Google Scholar] [CrossRef]
- Saravanakumar, A.; Mayalagu, R.; Serebiah, S.; Thivakaran, A. Seasonal Variations in Physico-Chemical Characteristics of Water, Sediment and Soil Texture in Arid Zone Mangroves of Kachchh-Gujarat. J. Environ. Biol. Acad. Environ. Biol. India 2008, 29, 725–732. [Google Scholar]
- Smithson, P.A. Climate Change 2001: The Scientific Basis: Contribution of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change; Houghton, J.T., Griggs, D.J., Noguer, M., Van der Linden, P.J., Dai, X., Johnson, C.A., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2001; ISBN 978-0-521-80767-8. [Google Scholar]
- Chan-Keb, C.; Agraz-Hernández, C.; Muñiz-Salazar, R.; Posada-Vanegas, G.; Osti-Sáenz, J.; Reyes Castellano, J.; Conde-Medina, K.; Vega-Serratos, B. Ecophysiological Response of Rhizophora mangle to the Variation in Hydrochemistry during Five Years along the Coast of Campeche, México. Diversity 2018, 10, 9. [Google Scholar] [CrossRef]
- Méndez, M.; Magaña, V. Regional Aspects of Prolonged Meteorological Droughts over Mexico and Central America. J. Clim. 2010, 23, 1175–1188. [Google Scholar] [CrossRef]
- Colorado-Ruiz, G.; Cavazos, T.; Salinas, J.A.; De Grau, P.; Ayala, R. Climate Change Projections from Coupled Model Intercomparison Project Phase 5 Multi-Model Weighted Ensembles for Mexico, the North American Monsoon, and the Mid-Summer Drought Region. Int. J. Climatol. 2018, 38, 5699–5716. [Google Scholar] [CrossRef]
- De la Barreda, B.; Metcalfe, S.E.; Boyd, D.S. Precipitation Regionalization, Anomalies and Drought Occurrence in the Yucatan Peninsula, Mexico. Int. J. Climatol. 2020, 40, 4541–4555. [Google Scholar] [CrossRef]
- Toledo-Ocampo, A. Marco Conceptual: Caracterización Ambiental del Golfo de México. In Golfo de México Contaminación e Impacto Ambiental: Diagnóstico y Tendencias; Botello, A.V., Rendón von Osten, J., Gold-Bouchot, G., Agraz-Hernández, C., Eds.; Univ. Auton. de Campeche, Univ. Nal. Auton. de México, Instituto Nacional de Ecología: Campeche, México, 2005; pp. 25–52. [Google Scholar]
- Day, J.W.; Conner, W.H.; Ley-Lou, F.; Day, R.H.; Navarro, A.M. The Productivity and Composition of Mangrove Forests, Laguna de Términos, Mexico. Aquat. Bot. 1987, 27, 267–284. [Google Scholar] [CrossRef]
- Yáñez-Arancibia, A.; Day, J.; Jacob, J.; Arroyo, A.M.; Alonso, S.M.; Martínez, A.T.; Welsh, C.; Edwards, A.C. Conclusiones 2do Panel Internacional Sobre Cambio Climático: La Zona Costera y Su Impacto Ecológico, Económico y Social; Instituto de Ecología AC, Texas Sea Grant Program, INESEMARNAT: Xalapa, Mexico, 2008; Volume 16. [Google Scholar]
- Agraz-Hernández, M.; Chan-Keb, C.; Iriarte-Vivar, S.; Posada Vanegas, G.; Vega Serratos, B.; Osti-Sáenz, J. Phenological Variation of Rhizophora mangle and Ground Water Chemistry Associated to Changes of the Precipitation. Hidrobiológica Rev. Dep. Hidrobiol. 2015, 25, 61–73. [Google Scholar]
- Orellana, R.; Espadas, C.; Conde, C.; Gray, C. Atlas. Escenarios de Cambio Climático En La Península de Yucatán; Centro de Investigación Científica de Yucatán, UNAM: Merida, Mexico, 2009. [Google Scholar]
- Barreiro-Güemes, M.T. Aporte de Hojarasca y Renovación Foliar del Manglar en un Sistema Estuarino del Sureste de México. RBT 1999, 47, 729–737. [Google Scholar] [CrossRef]
- INEGI Instituto Nacional de Estadística y Geografía. 2017. Available online: https://www.inegi.org.mx/ (accessed on 13 February 2022).
- CONABIO. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad; CONABIO: Mexico City, Mexico, 2020.
- Valderrama-Landeros, L.H.; Rodríguez-Zúñiga, M.T.; Troche-Souza, C.; Velázquez-Salazar, S.; Villeda-Chávez, E.; Alcántara-Maya, J.A.; Ressl, R. Manglares de México: Actualización y Exploración de los Datos del Sistema de Monitoreo 1970/1980–2015; CONABIO: Mexico City, Mexico, 2017.
- Khan, M.A.; Weber, D.J. Ecophysiology of High Salinity Tolerant Plants; Tasks for Vegetation Science; Springer: Dordrecht, The Netherlands, 2006; ISBN 978-1-4020-4018-4. [Google Scholar]
- Bhuiyan, C.; Singh, R.; Kogan, F. Monitoring Drought Dynamics in the Aravalli Region (India) Using Different Indices Based on Ground and Remote Sensing Data. Int. J. Appl. Earth Obs. Geoinf. 2006, 8, 289–302. [Google Scholar] [CrossRef]
- Mckee, T.B.; Doesken, N.J.; Kleist, J. The Relationship of Drought Frequency and Duration to Time Scales. In Manuscripts of the 8th Conference on Applied Climatology; American Meteorological Society: Boston, MA, USA, 1993; pp. 179–184. [Google Scholar]
- Paulo, A.; Ferreira, E.; Coelho, C.; Pereira, L. Drought Class Transition Analysis through Markov and Loglinear Models, an Approach to Early Warning. Agric. Water Manag. 2005, 77, 59–81. [Google Scholar] [CrossRef]
- Copernicus. Copernicus Climate Data Store. 2021. Available online: https://cds.climate.copernicus.eu/cdsapp#!/home (accessed on 2 July 2022).
- Dahdouh-Guebas, F.; Koedam, N. Empirical Estimate of the Reliability of the Use of the Point-Centred Quarter Method (PCQM): Solutions to Ambiguous Field Situations and Description of the PCQM+ Protocol. For. Ecol. Manag. 2006, 228, 1–18. [Google Scholar] [CrossRef]
- Cintrón, G.; Schaeffer-Novelli, Y. Methods for Studying Mangrove Structure. Mangrove Ecosyst. Res. Methods Monogr. Oceanogr. Methodol. 1984, 8, 91–113. [Google Scholar]
- Akodekou, D.A. Ecophysiological Response of Avicennia germinans (L.) L. and Rhizophora racemosa (G. Mey.) to the Variation in Groundwater Salinity in Ouidah, Benín. Master’s Thesis, School of Environment Management, University of Abomey-Calavi, Godomey, Benin, 2021. [Google Scholar]
- Flores-Verdugo, F.; Gonzalez-Farias, F.; Zamorano, D.S.; Ramirez-Garcia, P. Mangrove Ecosystems of the Pacific Coast of Mexico: Distribution, Structure, Litterfall, and Detritus Dynamics. In Coastal Plant Communities of Latin America; Elsevier: Amsterdam, The Netherlands, 1992; pp. 269–288. ISBN 978-0-08-092567-7. [Google Scholar]
- Agraz Hernández, C.M.; García Zaragoza, C.; Iriarte-Vivar, S.; Flores-Verdugo, F.J.; Moreno Casasola, P. Forest Structure, Productivity and Species Phenology of Mangroves in the la Mancha Lagoon in the Atlantic Coast of Mexico. Wetl. Ecol. Manag. 2011, 19, 273–293. [Google Scholar] [CrossRef]
- Kamruzzaman, M.D.; Sharma, S.; Hagihara, A. Vegetative and Reproductive Phenology of the Mangrove Kandelia Obovata: Phenology of Kandelia obovata. Plant Species Biol. 2012, 28, 118–129. [Google Scholar] [CrossRef]
- Tomlinson, P.B. The Botany of Mangroves; Cambridge University Press: Cambridge, UK, 1986. [Google Scholar]
- Hermosilla, Z.; Jorge, C.; Romero, I.; Martí, E.; Cabanero, M.D. Diferencias Espaciales y Estacionales en el Contenido de Nutrientes, Demanda de Oxígeno y Potencial Redox en Sedimentos Bajo una Instalación de Producción Acuícola en Jaulas. Boletín Inst. Español Oceanográfia 2005, 21, 29–35. [Google Scholar]
- Agraz-Hernández, C.M.; Expósito-Díaz, C.; Márquez, E.; Osti-Sáenz, J.; Chan-Keb, C.A.; Chan-Canul, E.; Acosta, J. Los Ecosistemas de Manglar en el Estado de Campeche: Diagnóstico de la Conservación e Identificación de Áreas Potenciales para la Restauración (Etapa I); Universidad Autónoma de Campeche: Campeche, Mexico, 2012. [Google Scholar]
- Flores-Verdugo, F.; Moreno-Casasola, P.; Agraz-Hernández, C.M.; López-Rosas, H.; Benítez-Pardo, D.; Travieso-Bello, A.C. La Topografía y el Hidroperíodo: Dos Factores Que Condicionan la Restauración de los Humedales Costeros. Bot. Sci. 2007, 80S, 33–47. [Google Scholar] [CrossRef]
- Cronk, J.K.; Fennessy, M.S. Wetland Plants: Biology and Ecology, 1st ed.; CRC Press: Boca Raton, FL, USA, 2001; p. 482. ISBN 978-04-291-4901-6. [Google Scholar]
- Zar, J.H. Biostatistical Analysis, Books a la Carte, 5th ed.; Prentice-Hall/Pearson: Upper Saddle River, NJ, USA, 2010; Volume 13. [Google Scholar]
- Ribeiro, R.d.A.; Rovai, A.S.; Twilley, R.R.; Castañeda-Moya, E. Spatial Variability of Mangrove Primary Productivity in the Neotropics. Ecosphere 2019, 10, e02841. [Google Scholar] [CrossRef]
- Sippo, J.Z.; Lovelock, C.E.; Santos, I.R.; Sanders, C.J.; Maher, D.T. Mangrove Mortality in a Changing Climate: An Overview. Estuar. Coast. Shelf Sci. 2018, 215, 241–249. [Google Scholar] [CrossRef]
- Comisión Nacional del Agua (CONAGUA). Estadísticas del Agua en México; D.R. Secretaría de Medio Ambiente y Recursos Naturales: Rioverde, México, 2014; p. 242.
- Saenger, P. Mangrove Ecology, Silviculture and Conservation; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Eisenreich, S.J. Climate Change and the European Water Dimension. A Report to the European Water Directors; Eisenreich, S.J., Ed.; Institute for Environment and Sustainaibility: Ispra, Italy, 2005. [Google Scholar]
- Vega, M.C.; Miranda, S. Efectos de la Sequia en la Produccion Sembrada a Nivel Nacional en 2009. Rev. Económica Actual. 2009, 4, 29–31. [Google Scholar]
- NOAA National Centers for Environmental Information. State of the Climate: Monthly Global Climate Report for Annual 2018. 2019. Available online: https://www.ncei.noaa.gov/access/monitoring/monthly-report/global/201913 (accessed on 16 November 2016).
- Ahmed, S.; Kamruzzaman, M. Species-Specific Biomass and Carbon Flux in Sundarbans Mangrove Forest, Bangladesh: Response to Stand and Weather Variables. Biomass Bioenergy 2021, 153, 106215. [Google Scholar] [CrossRef]
- Villalobos-Zapata, G.J.; Yañez-Arancibia, A.; Day, J.W.; Lara-Domínguez, A.L. Ecología y Manejo de los Manglares en la Laguna de Términos, Campeche, México; Yañez-Arancibia, A., Lara-Domínguez, A.L., Eds.; Instituto de Ecología A.C.: Xalapa, México; UICN/ORMA: Kuridabat, Costa Rica; NOAA/NMFS Silver Spring MDUSA: Washington, DC, USA, 1999; pp. 263–274. [Google Scholar]
- Coronado-Molina, C.; Alvarez-Guillen, H.; Day, J.W.; Reyes, E.; Perez, B.C.; Vera-Herrera, F.; Twilley, R. Litterfall Dynamics in Carbonate and Deltaic Mangrove Ecosystems in the Gulf of Mexico. Wetl. Ecol. Manag. 2012, 20, 123–136. [Google Scholar] [CrossRef]
- Lopez-Portillo, J.; Ezcurra, E. Litter Fall of Avicennia germinans L. in a One-Year Cycle in a Mudflat at the Laguna de Mecoacan, Tabasco, Mexico. Biotropica 1985, 17, 186. [Google Scholar] [CrossRef]
- Moreno-Casasola, P.; López-Rosas, H. Muestreo y análisis de la vegetación de humedales. In Breviario para Describir, Observar y Manejar Humedales; Moreno-Casasola, P., Warner, B.G., Eds.; RAMSAR, Instituto de Ecología, A.C.: Xalapa, Mexico, 2009; p. 406. ISBN 978-607-7579-12-0. [Google Scholar]
- Kamruzzaman, M.D.; Ahmed, S.; Osawa, A. Biomass and Net Primary Productivity of Mangrove Communities along the Oligohaline Zone of Sundarbans, Bangladesh. For. Ecosyst. 2017, 4, 16. [Google Scholar] [CrossRef]
- Agraz-Hernández, C.M.; Osti-Sáenz, J.; Reyes-Castellanos, J.; Chan-Keb, C.; Arriaga-Martinez, V.; Conde-Medina, K.P.; Martinez-Kumul, J. Caracterización y Estado de Conservación de los Manglares del Estado de Campeche: Elementos para Establecer una Política Ambiental para Su Preservación, México. In Golfo de México. Contaminación e Impacto Ambiental: Diagnóstico y Tendencias; Botello, J., Rendon-von Osten, J., Benitez, A., Gold-Bouchot, G., Eds.; UAC; UNAM-ICMYL; CINVESTAV-Unidad: Merida, Mexico, 2014; pp. 1035–1052. [Google Scholar]
- Osland, M.J.; Enwright, N.M.; Day, R.H.; Gabler, C.A.; Stagg, C.L.; Grace, J.B. Beyond Just Sea-Level Rise: Considering Macroclimatic Drivers within Coastal Wetland Vulnerability Assessments to Climate Change. Glob. Chang. Biol. 2016, 22, 1–11. [Google Scholar] [CrossRef]
- Feller, I.C.; Lovelock, C.E.; Berger, U.; McKee, K.L.; Joye, S.B.; Ball, M.C. Biocomplexity in Mangrove Ecosystems. Annu. Rev. Mar. Sci. 2010, 2, 395–417. [Google Scholar] [CrossRef]
- Hogarth, P.J. The Biology of Mangroves and Seagrasses; Oxford University Press: Oxford, UK, 2015; ISBN 978-0-19-871654-9. [Google Scholar]
- Biber, P. Measuring the Effects of Salinity Stress in the Red Mangrove, Rhizophora mangle L. Afr. J. Agric. Res. 2006, 1, 1–4. [Google Scholar]
- Ball, M. Mangrove Species Richness in Relation to Salinity and Waterlogging: A Case Study along the Adelaide River Floodplain, Northern Australia. Glob. Ecol. Biogeogr. Lett. 1998, 7, 73–82. [Google Scholar] [CrossRef]
- Lugo, A.E.; Snedaker, S.C. The Ecology of Mangroves. Annu. Rev. Ecol. Syst. 1974, 5, 39–64. [Google Scholar] [CrossRef]
- Naidoo, G. The Mangroves of South Africa: An Ecophysiological Review. S. Afr. J. Bot. 2016, 107, 101–113. [Google Scholar] [CrossRef]
- Youssef, T.; Saenger, P. Anatomical Adaptive Strategies to Flooding and Rhizosphere Oxidation in Mangrove Seedlings. Aust. J. Bot. 1996, 44, 297. [Google Scholar] [CrossRef]
- Sobrado, M.A. Effect of High External NaCl Concentration on the Osmolality of Xylem Sap, Leaf Tissue and Leaf Glands Secretion of the Mangrove Avicennia germinans (L.) L. Flora 2001, 196, 63–70. [Google Scholar] [CrossRef]
- Parida, A.K.; Jha, B. Salt Tolerance Mechanisms in Mangroves: A Review. Trees 2010, 24, 199–217. [Google Scholar] [CrossRef]
- Stuart, S.A.; Choat, B.; Martin, K.C.; Holbrook, N.M.; Ball, M.C. The Role of Freezing in Setting the Latitudinal Limits of Mangrove Forests. New Phytol. 2007, 173, 576–583. [Google Scholar] [CrossRef]
- Pezeshki, S.R.; DeLaune, R.D.; Patrick, W.H., Jr. Differential Response of Selected Mangroves to Soil Flooding and Salinity: Gas Exchange and Biomass Partitioning. Can. J. For. Res. 1990, 20, 869–874. [Google Scholar] [CrossRef]
- Krauss, K.W.; Doyle, T.W.; Twilley, R.R.; Rivera-Monroy, V.H.; Sullivan, J.K. Evaluating the Relative Contributions of Hydroperiod and Soil Fertility on Growth of South Florida Mangroves. Hydrobiologia 2006, 569, 311–324. [Google Scholar] [CrossRef]
- Lugo, A.E.; Snedaker, S.C. Properties of a Mangrove Forest in Southern Florida. In Proceedings of the International Symposium on Biology and Management of Mangroves, Honolulu, HI, USA, 8–11 October 1974; Walsh, G.E., Snedaker, S.C., Teas, H.J., Eds.; University of Florida: Gainesville, FL, USA, 1975; Volume 1, pp. 170–212. [Google Scholar]
- Psuty, N.P.; Martínez, M.L.; López-Portillo, J.; Silveira, T.M.; García-Franco, J.G.; Rodríguez, N.A. Interaction of Alongshore Sediment Transport and Habitat Conditions at Laguna la Mancha, Veracruz, Mexico. J. Coast. Conserv. 2009, 13, 77–87. [Google Scholar] [CrossRef][Green Version]
- Oudejans, L. Environmental Protection Agency (EPA) International Decontamination Research and Development Conference; U.S. Environmental Protection Agency: Washington, DC, USA, 2016.
- Agraz-Hernández, C.M. Reforestación Experimental de Manglares en Ecosistemas Lagunares Estuarinos de la Costa Noroccidental de México. Ph.D. Thesis, Universidad Autónoma de Nuevo León, San Nicolas de los Garza, Mexico, 1999. [Google Scholar]
- Friess, D.A.; Adame, M.F.; Adams, J.B.; Lovelock, C.E. Mangrove Forests under Climate Change in a 2 °C World. WIREs Clim. Chang. 2022, 13, e792. [Google Scholar] [CrossRef]
- Rodríguez Ramírez, A.; Nivia Ruíz, J.; Garzón Ferreira, J. Características estructurales y Funcionales del Manglar de Avicennia germinans en la Bahía de Chengue (Caribe Colombiano). BIM 2004, 33, 223–244. [Google Scholar] [CrossRef]
- Lüttge, U. Mangroves. In Physiological Ecology of Tropical Plants; Springer: Berlin/Heidelberg, Germany, 1997; pp. 197–223. ISBN 978-3-662-03342-5. [Google Scholar]
- Wang’ondu, V.W.; Kairo, J.G.; Kinyamario, J.I.; Mwaura, F.B.; Bosire, J.O.; Dahdouh-Guebas, F.; Koedam, N. Vegetative and Reproductive Phenological Traits of Rhizophora mucronata Lamk. and Sonneratia alba Sm. Flora-Morphol. Distrib. Funct. Ecol. Plants 2013, 208, 522–531. [Google Scholar] [CrossRef]
- Aké-Castillo, J.A.; Vázquez, G.; López-Portillo, J. Litterfall and Decomposition of Rhizophora mangle L. in a Coastal Lagoon in the Southern Gulf of Mexico. Hydrobiologia 2006, 559, 101–111. [Google Scholar] [CrossRef]
- Koshita, Y.; Takahara, T. Effect of Water Stress on Flower-Bud Formation and Plant Hormone Content of Satsuma Mandarin (Citrus unshiu Marc.). Sci. Hortic. 2004, 99, 301–307. [Google Scholar] [CrossRef]
- Sharp, R.G.; Else, M.A.; Cameron, R.W.; Davies, W.J. Water Deficits Promote Flowering in Rhododendron via Regulation of Pre and Post Initiation Development. Sci. Hortic. 2009, 120, 511–517. [Google Scholar] [CrossRef]
- Lovelock, C.E.; Feller, I.C.; Reef, R.; Hickey, S.; Ball, M.C. Mangrove Dieback during Fluctuating Sea Levels. Sci. Rep. 2017, 7, 1680. [Google Scholar] [CrossRef]
- Imbert, D.; Menard, S. Structure de la Vegetation et Production Primaire Dans la Mangrove de la Baie de Fort-de-France, Martinique (F.W.I.). Biotropica 1997, 29, 413–426. [Google Scholar] [CrossRef]
- Das, S.K.; Patra, J.K.; Thatoi, H. Antioxidative Response to Abiotic and Biotic Stresses in Mangrove Plants: A Review: Antioxidative Response to Abiotic and Biotic Stresses. Int. Rev. Hydrobiol. 2016, 101, 3–19. [Google Scholar] [CrossRef]
- Srikanth, S.; Lum, S.K.Y.; Chen, Z. Mangrove Root: Adaptations and Ecological Importance. Trees 2016, 30, 451–465. [Google Scholar] [CrossRef]
- Ellison, J.C. How South Pacific Mangroves May Respond to Predicted Climate Change and Sea-Level Rise. In Climate Change in the South Pacific: Impacts and Responses in Australia, New Zealand, and Small Island States; Advances in Global Change Research; Gillespie, A., Burns, W.C.G., Eds.; Springer: Dordrecht, The Netherlands, 2000; Volume 2, pp. 289–300. ISBN 978-90-481-5365-7. [Google Scholar]
- Erwin, K.L. Wetlands and Global Climate Change: The Role of Wetland Restoration in a Changing World. Wetl. Ecol. Manag. 2009, 17, 71–84. [Google Scholar] [CrossRef]
- Mitsch, W.; Gosselink, J. Mangrove Wetlands. Wetlands 1993, 2, 293–328. [Google Scholar]
- Kauffman, J.B.; Donato, D.C.; Adame, M.F. Protocolo para la Medición, Monitoreo y Reporte de la Estructura, Biomasa y Reservas de Carbono de los Manglares; CIFOR: Bogor, Indonesia, 2013; Volume 117. [Google Scholar]
- Brandt, M.; Tappan, G.; Diouf, A.; Beye, G.; Mbow, C.; Fensholt, R. Woody Vegetation Die off and Regeneration in Response to Rainfall Variability in the West African Sahel. Remote Sens. 2017, 9, 39. [Google Scholar] [CrossRef]
- Djebou, D.C.S.; Singh, V.P.; Frauenfeld, O.W. Vegetation Response to Precipitation across the Aridity Gradient of the Southwestern United States. J. Arid Environ. 2015, 115, 35–43. [Google Scholar] [CrossRef]







| Category | SPI |
|---|---|
| Extreme wet | ≥2.0 |
| Severe wet | 1.50–1.99 |
| Moderate wet | 1.00–1.49 |
| Humidity | 0.50–0.99 |
| Normal prone to wet | 0.00–0.49 |
| Normal prone to dry | −0.00 to −0.49 |
| Drought | −0.50 to −0.99 |
| Moderate Drought | −1 to −1.49 |
| Severe Drought | −1.50 to −1.99 |
| Extreme Drought | ≤2.0 |
| Factor | Precipitation | ||
|---|---|---|---|
| df | F | p | |
| A (Site) | 6 | 11.25 | <0.0001 ** |
| B (Year) | 1 | 8.86 | <0.003 * |
| C (Season) | 2 | 290.21 | <0.0001 ** |
| A × B | 6 | 0.61 | 0.725 |
| A × C | 12 | 1.68 | 0.069 |
| B × C | 2 | 32.31 | <0.0001 ** |
| A × B × C | 12 | 0.60 | 0.839 |
| Error | 360 | ||
| Ecosystem | Sites | Total Density Trees (Stems ha−1) | Total Basal Area (m2 ha−1) | Stand Height (m) | Species | Physonomic Type |
|---|---|---|---|---|---|---|
| Laguna de Terminos | Atasta | 1914.4 | 65.0 | 12.0 | Ag *, Rm, Lr | R |
| Estero Pargo | 2562.7 | 20.1 | 6.1 | Rm *, Ag, Lr | R-F | |
| Sabancuy | 4784.2 | 15.5 | 6.0 | Ag *, Rm, Lr | R-F | |
| Xi buja | 3470.9 | 39.6 | 6.0 | Ag * | R-F | |
| Rio Champoton | Champoton | 1090.0 | 5.8 | 7.9 | Rm *, Ag, Lr | B-F |
| Los Petenes Biosphere Reserve | Peten Neyac | 2930.8 | 25.3 | 7.3 | Rm *, Ag, Lr | F |
| Río Verde | 2672.4 | 10.2 | 10.3 | Rm *, Ag, Lr | F |
| Litter Fall | |||
|---|---|---|---|
| Factor | df | F | p |
| A (Sites) | 6 | 6.15 | <0.0001 ** |
| B (Year) | 1 | 41.31 | <0.0001 ** |
| C (Season) | 2 | 67.87 | <0.0001 ** |
| D (Species) | 2 | 87.34 | <0.0001 ** |
| A × B | 6 | 0.29 | 0.943 |
| A × C | 12 | 0.879 | 0.576 |
| A × D | 12 | 15.32 | <0.0001 ** |
| B × C | 2 | 33.19 | <0.0001 ** |
| B × D | 2 | 2.50 | <0.043 * |
| C × D | 4 | 1.44 | 0.239 |
| A × B × C | 12 | 1.08 | 0.379 |
| A × B × D | 12 | 0.41 | 0.995 |
| A × C × D | 24 | 1.70 | 0.150 |
| B × C × D | 4 | 0.38 | 0.971 |
| A × B × C × D | 24 | 0.69 | 0.865 |
| Error | 293 | ||
| Factor | Leaves | Flowers | Propagules | ||||
|---|---|---|---|---|---|---|---|
| df | F | p | F | p | F | p | |
| A (Sites) | 6 | 7.30 | <0.0001 | 7.36 | <0.0001 ** | 6.26 | <0.0001 ** |
| B (Year) | 1 | 1.30 | 0.256 | 13.06 | <0.0001 ** | 0.01 | 0.926 |
| C (Season) | 2 | 23.97 | <0.0001 | 31.04 | <0.0001 ** | 32.74 | <0.0001 ** |
| D (Species) | 2 | 91.52 | <0.0001 | 199.18 | <0.0001 ** | 36.39 | <0.0001 ** |
| A × B | 6 | 0.98 | 0.438 | 1.26 | 0.28 | 0.46 | 0.835 |
| A × C | 12 | 1.48 | 0.129 | 1.89 | <0.035 * | 1.18 | 0.298 |
| A × D | 12 | 17.73 | <0.0001 | 9.28 | <0.0001 ** | 10.64 | <0.0001 ** |
| B × C | 2 | 0.38 | 0.681 | 0.05 | 0.95 | 0.85 | 0.429 |
| B × D | 2 | 1.71 | 0.148 | 6.75 | <0.0001 ** | 4.80 | <0.001 * |
| C × D | 4 | 0.75 | 0.472 | 1.30 | 0.27 | 3.67 | <0.027 * |
| A × B × C | 12 | 0.85 | 0.604 | 0.67 | 0.79 | 1.08 | 0.376 |
| A × B × D | 12 | 0.43 | 0.992 | 0.97 | 0.51 | 0.57 | 0.952 |
| A × C × D | 24 | 0.78 | 0.539 | 2.27 | <0.049 * | 1.05 | <0.383 |
| B × C × D | 4 | 0.34 | 0.981 | 1.16 | 0.31 | 1.91 | 0.033 * |
| A × B × C × D | 24 | 0.46 | 0.988 | 0.84 | 0.68 | 0.71 | 0.838 |
| Error | 293 | ||||||
| Factor | Salinity (PSU) | Redox Potential (mV) | |||
|---|---|---|---|---|---|
| df | F | p | F | p | |
| A (Site) | 6 | 113.75 | <0.0001 ** | 17.89 | <0.0001 ** |
| B (Year) | 1 | 4.24 | <0.040 * | 217.34 | <0.0001 ** |
| C (Season) | 2 | 13.47 | <0.0001 ** | 18.02 | <0.0001 ** |
| A × B | 6 | 1.66 | 0.131 | 3.21 | <0.004 * |
| A × C | 12 | 8.61 | <0.0001 ** | 4.98 | <0.0001 ** |
| B × C | 2 | 12.95 | <0.0001 ** | 36.74 | <0.0001 ** |
| A × B × C | 12 | 6.76 | <0.0001 ** | 7.09 | <0.0001 ** |
| Error | 360 | ||||
| Canonical Variables | F1 | F2 | |
|---|---|---|---|
| Canonical Correlation | 0.754 | 0.544 | |
| Production Phenology | |||
| Leaves—Ag | −0.461 | −0.503 | |
| Flowers—Ag | 0.276 | 0.433 | |
| Propagules—Ag | −0.211 | 0.897 | |
| Litterfall—Ag | 0.386 | 0.050 | |
| Leaves—Rm | −0.634 | −0.369 | |
| Flowers—Rm | −0.279 | 0.237 | |
| Propagules—Rm | −0.711 | 0.194 | |
| Litterfall—Rm | 0.499 | −0.204 | |
| Leaves—Lr | 0.211 | 0.036 | |
| Flowers—Lr | 0.234 | 0.266 | |
| Propagules—Lr | 0.315 | 0.041 | |
| Litterfall—Lr | −0.761 | 0.097 | |
| Physicochemical parameters | |||
| Salinity (‰) | 0.994 | 0.287 | |
| Redox potential (mV) | 0.221 | −0.504 | |
| Precipitation (mm) | −0.093 | 0.909 |
| Canonical Variables | F1 | F2 | |
|---|---|---|---|
| Canonical Correlation | 0.683 | 0.511 | |
| Production Phenology | |||
| Leaves—Ag | −0.260 | 0.121 | |
| Flowers—Ag | −0.447 | 0.200 | |
| Propagules—Ag | −0.132 | 0.152 | |
| Litterfall—Ag | −0.192 | 0.080 | |
| Leaves—Rm | 4.771 | 6.671 | |
| Flowers—Rm | −5.849 | −1.671 | |
| Propagules—Rm | 1.796 | −4.609 | |
| Litterfall—Rm | −0.089 | −0.229 | |
| Leaves—Lr | −0.785 | −0.527 | |
| Flowers—Lr | −0.106 | −0.190 | |
| Propagules—Lr | −0.319 | −0.631 | |
| Litterfall—Lr | 0.534 | 0.150 | |
| Physicochemical parameters | |||
| Salinity (‰) | 0.231 | 0.883 | |
| Redox potential (mV) | −0.567 | 0.622 | |
| Precipitation (mm) | −0.789 | 0.015 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agraz-Hernández, C.M.; Chan-Keb, C.A.; Muñiz-Salazar, R.; Pérez-Balan, R.A.; Vanegas, G.P.; Manzanilla, H.G.; Osti-Sáenz, J.; del Río Rodríguez, R. Pore Water Chemical Variability and Its Effect on Phenological Production in Three Mangrove Species under Drought Conditions in Southeastern Mexico. Diversity 2022, 14, 668. https://doi.org/10.3390/d14080668
Agraz-Hernández CM, Chan-Keb CA, Muñiz-Salazar R, Pérez-Balan RA, Vanegas GP, Manzanilla HG, Osti-Sáenz J, del Río Rodríguez R. Pore Water Chemical Variability and Its Effect on Phenological Production in Three Mangrove Species under Drought Conditions in Southeastern Mexico. Diversity. 2022; 14(8):668. https://doi.org/10.3390/d14080668
Chicago/Turabian StyleAgraz-Hernández, Claudia M., Carlos A. Chan-Keb, Raquel Muñiz-Salazar, Román A. Pérez-Balan, Gregorio Posada Vanegas, Hector G. Manzanilla, Juan Osti-Sáenz, and Rodolfo del Río Rodríguez. 2022. "Pore Water Chemical Variability and Its Effect on Phenological Production in Three Mangrove Species under Drought Conditions in Southeastern Mexico" Diversity 14, no. 8: 668. https://doi.org/10.3390/d14080668
APA StyleAgraz-Hernández, C. M., Chan-Keb, C. A., Muñiz-Salazar, R., Pérez-Balan, R. A., Vanegas, G. P., Manzanilla, H. G., Osti-Sáenz, J., & del Río Rodríguez, R. (2022). Pore Water Chemical Variability and Its Effect on Phenological Production in Three Mangrove Species under Drought Conditions in Southeastern Mexico. Diversity, 14(8), 668. https://doi.org/10.3390/d14080668







