Abstract
Wood distillate (WD) is an environmentally safe bio-based product stimulating plant growth and yield and allowed in Italy in organic farming. To the best of our knowledge, there are no studies on the effects of WD on spontaneous plants growing among crops, including their functional traits such as biomass. To test such effects, we carried out a lab experiment on artificially reconstructed arable plant communities composed of five species of conservation interest, which are specialist winter cereal crops: Bromus secalinus L., Centaurea cyanus L., Lathyrus aphaca L., Legousia speculum-veneris (L.) Chaix, and Scandix pecten-veneris L. After sowing 45 pots under controlled conditions, we applied WD at three concentrations (0%, 0.2%, and 0.5%) six times over 7 weeks. The number of emerged plants in each pot was counted every two weeks. Finally, we harvested all plants and measured the fresh and dry above-ground weight of each species in each pot. The resulting data were analyzed by Permutational Analysis of Variance. The application of 0.2% and 0.5% WD modified the community composition after two weeks, but such differences later disappeared. Both 0.2% and 0.5% WD had a positive effect on the dry weight of S. pecten-veneris and a negative effect on that of L. speculum-veneris. Moreover, 0.2% and 0.5% WD increased seedling emergence in L. aphaca, and 0.5% WD increased seedling emergence in S. pecten-veneris. Both 0.2% and 0.5% WD enhanced seedling emergence in the entire community. We suggest that the use of WD at low concentrations in winter cereals may be a sustainable agricultural practice that benefits crops without harming the associated plant diversity.
1. Introduction
Arable plant diversity is one of the most characteristic elements of agricultural landscapes [1]. It includes mostly annual plants that are adapted to live in arable land, under the recurrent disturbance of tillage [2]. Arable plants, which include many rare species, are of high importance for biodiversity in agroecosystems [3,4]. Some of them are completely dependent on arable habitats, into which they evolved in the last millennia, to the extent that their origins in some areas are hardly definable [5,6]. In the last decades, arable plant diversity underwent a noticeable decline all over Europe due to the intensification of agricultural practices. This caused a regression of many species that were once widespread in arable land, deeply modifying the structure and composition of arable plant communities [7,8,9]. For such reasons, several arable plants and one arable habitat are currently included in European Red Lists [10,11]. This increasing interest in arable plant diversity also led to the birth of specific biodiversity data repositories [12,13].
At middle latitudes, arable plant communities are usually divided in two broad groups based on their phenology, i.e., summer-annual or winter-annual [2,14]. Winter arable plants are the most specialized to live in their habitat and sometimes are dependent on one specific crop, while summer arable plants are usually adapted to many different disturbed environments [7,15]. For these reasons, the former are particularly vulnerable to agricultural intensification and to the disappearance of certain traditional crops such as spelt and flax, which makes many of such species in need of conservation [11]. The main pressures of intensive agriculture on arable plants come from the application of high amounts of herbicides and synthetic fertilizers [7,8]. In fact, herbicides tend to remove and modify arable plant communities, and many winter arable plants do not tolerate nutrient-rich soils [16]. Thus, in the context of a sustainable increase in food production [17], there is the need to find products benefiting crops without negative effects on farmland plant diversity.
Wood distillate (WD) is a bio-based product derived from the distillation of condensed gasses during the pyrolysis of woody biomass, for which its use has a long tradition in farming [18]. The products of wood pyrolysis include a solid component (biochar) and a liquid-gaseous component (WD). Several production methods can be used, but the most effective to maximize the quantity of resulting WD over biochar is fast pyrolysis at temperatures of 625–775 K. In this process, biomass is rapidly heated for less than 5 s and then rapidly cooled, resulting in the production of 60–75% of liquid component, 15–25% of solid component, and 10–20% of gaseous component. WD is composed of 80–90% water and 10–20% of over 200 water-soluble chemical compounds, including organic acids, alkane, phenolic, alcohol, and ester [18]. It is used in agriculture since such compounds have a stimulating action on plant growth and yield [19,20,21,22,23]. Moreover, it can protect plants exposed to high levels of ozone [24]. The varying chemical compositions of different plant species result in different compositional features and specific properties of WD, although its main effects are basically the same [18]. WD has recently been included in the list of products that can be used in Italian organic farming [25]. Adverse effects of WD have not been observed on sensitive bioindicators such as lichens, mosses, aquatic, and soil organisms [26,27,28,29]. Moreover, WD has also been found to be a safe product for humans [30].
While there is evidence that WD promotes growth and yield in cultivated plants [19,20,21,22,23], to the best of our knowledge, nothing is known about its possible effects on the spontaneous plant diversity of crop fields. In this study, we tested the hypothesis that WD does not have any negative effect on the plants spontaneously developing among crops during emergence and first-stage growth, which are the main critical stages of a plant’s life cycle [31,32]. For this purpose, we carried out a laboratory experiment on artificially reproduced arable plant communities made up of five specialist species of winter cereal crops that are of European conservation concern.
2. Materials and Methods
2.1. Species Selection and Propagule Retrievement
We selected the following five species from the list of the rarest/most threatened European arable plants [7]: Bromus secalinus L., Centaurea cyanus L., Lathyrus aphaca L., Legousia speculum-veneris (L.) Chaix, and Scandix pecten-veneris L. These species are all annuals and related to winter cereal crops or allied crop types, thus having a winter-spring life cycle [20]. We obtained the propagules (seeds for B. secalinus, L. aphaca, and L. speculum-veneris; achenes for C. cyanus and S. pecten-veneris) by requesting them via the “Botanic Gardens Conservation International” network [33]. C. cyanus and S. pecten-veneris achenes were provided by the Agro-Botanical Garden of the University of Cluj-Napoca (Romania). B. secalinus, L. aphaca, and L. speculum-veneris seeds were provided by the Botanical Garden of Ulm University (Germany). Seed viability was preliminary evaluated and was found to be >95% for all tested species. Plant nomenclature follows [34,35].
2.2. Wood Distillate
The WD used in this experiment derived from the pyrolysis of sweet chestnut (Castanea sativa Mill.) wood. It has a pH of 3.5–4.5 and a density of 1.05 kg/L. It contains 2–2.3% acetic acid, 2.9–3.02 g/kg of total phenols, and 23–26 g/kg of polyphenols. Its use is recommended for a large variety of crops and ornamental plants both in the open field and in greenhouses, and in Italy, it is allowed in organic farming [36].
2.3. Experimental Design
Eight propagules of each of the five previously listed species were sown in plastic pots (20 × 20 × 15 cm), filled using commercial potting soil as substrate, according to a systematic design (Figure 1).
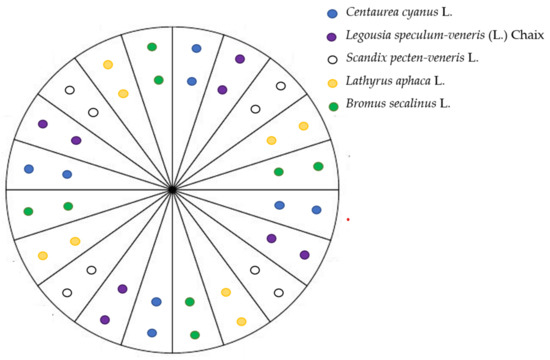
Figure 1.
Sowing design for the five selected species in each of the 45 pots, simulating an arable plant community.
We sowed a total of 45 pots (15 pots × 3 treatments). Then, the pots were placed in a growth chamber at 22 ± 2 °C, 70 ± 1% RH, and 250 μM s−1 m−2 PAR with a day/night cycle of 16–8 h. We applied 3 different treatments: (i) 0% WD (control-C); (ii) 0.2% (v/v) WD (WD1); (iii) 0.5% (v/v) WD (WD2). The 0.2% and 0.5% concentrations are those recommended by the producer to be used on any crop growing in the open field [36]. To minimize possible microclimatic effects, we organized the pots into 3 blocks, each one containing 5 replicates (pots) per treatment (Figure 2). We applied treatments once a week, spraying the solutions (1.5 L for each of the sets of 15 pots) on the entire pot’s surface, following the procedure described by Vannini et al. [37]. The applications were performed outside of the growth chamber to avoid any type of contamination between the sets. After the treatment, the pots were stored back in the growth chamber and randomly rotated every three days inside each block. The experiment lasted 7 weeks, for a total of 6 WD applications.
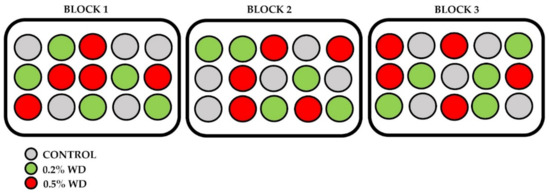
Figure 2.
Organization of the 45 pots into three blocks containing 5 replicates per treatment each, inside the climatic chamber. Within each block, pots were mixed every three days. WD = Wood Distillate.
2.4. Community Attributes
At every two weeks, we counted the number of emerged individuals per species in each pot. We made three total counts: at week 2 (W2), at week 4 (W4), and at week 6 (W6).
2.5. Morphological Traits
At seven weeks from sowing, we measured with a precision scale the above-ground fresh and dry weight of seedlings as a measure of plant growth and water content. To determine dry weight, the samples were placed in an oven at 70 °C for 56 h, following the method described by Perez-Harguindeguy et al. [38].
2.6. Statistical Analyses
To test for significant effects of WD application, we carried out Permutational Uni- and Multivariate Analyses of Variance (PERMANOVA) on the responses of single species and of communities, respectively. Univariate analyses were based on Euclidean distance matrices, while multivariate ones were based on Bray–Curtis dissimilarity matrices, calculated on untransformed data. The following settings were used for all tests: 999 unrestricted permutations of raw data, α = 0.05. Significant terms were then investigated using a posteriori pairwise comparisons with the PERMANOVA t statistic and 999 permutations to test for significant differences between the three treatments. All the analyses were performed using the PERMANOVA routine in the program PRIMER v.6 [39], including the add-on package PERMANOVA+ [40]. PERMANOVA correctly calculates an appropriate pseudo-F statistic for each term in the model for uni- and multivariate datasets. Moreover, the permutation approach is free from many of the assumptions of parametric statistics [40].
We assessed significant effects of WD addition in the following responses:
- Total number of individuals per pot (TNI) at W2, W4, and W6;
- Total dry weight per pot (TDW);
- Total fresh weight per pot (TFW);
- Mean dry weight (DW) of individuals per pot (TMDW = TDW/TNI);
- Mean fresh weight (FW) of individuals per pot (TMFW = TFW/TNI);
- TNI per species per pot;
- DW per species per pot;
- FW per species per pot;
- Mean DW of individuals per species per pot (MDW);
- Mean FW of individuals per species per pot (MFW);
- Community composition (species occurrence and abundance) using TNI as a measure of species abundance at W2, W4, and W6;
- Community composition using DW, FW, MDW, and MFW as measures of species abundance.
3. Results
3.1. Number of Individuals
PERMANOVA performed on community structure based on the number of individuals per species showed a significant effect of WD only at W2, where C was significantly different from WD1 and from WD2. WD had a significant effect also on TNI at W2 (Figure 3), on TNI of S. pecten-veneris at W6, where the TNI increased from C to WD2, and on TNI of L. aphaca at W2, where there was an increase from C to WD1 and from C to WD2 (Table 1 and Table 2 and Figure 4). All other species did not show any statistically significant response to the treatment.
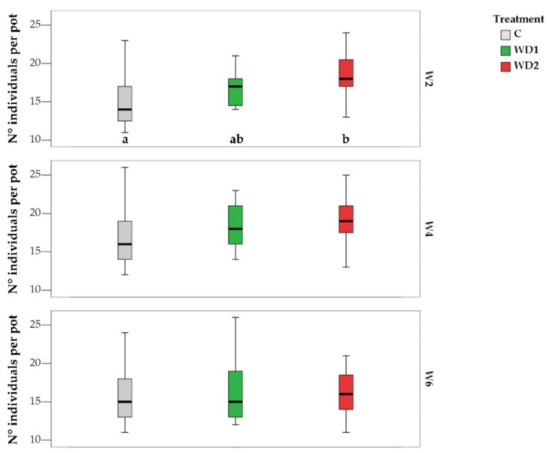
Figure 3.
Boxplots showing the total number of individuals per pot per treatment (C = 0% wood distillate; WD1 = 0.2% wood distillate; WD2 = 0.5% wood distillate) at week 2 (W2), week 4 (W4), and week 6 (W6). Different letters indicate statistically significant differences (α = 0.05).

Table 1.
Results of the PERMANOVA analyses on community composition with the number of individuals per species as an abundance measure, on the total number of individuals per pot (TNI), and on the total number of individuals of single species per pot at week 2 (W2), week 4 (W4), and week 6 (W6).

Table 2.
Results of PERMANOVA pairwise t-tests (number of individuals) between treatments; TNI = total number of individuals; C = 0% wood distillate; WD1 = 0.2% wood distillate; WD2 = 0.5% wood distillate; W2 = week 2; W6 = week 6.
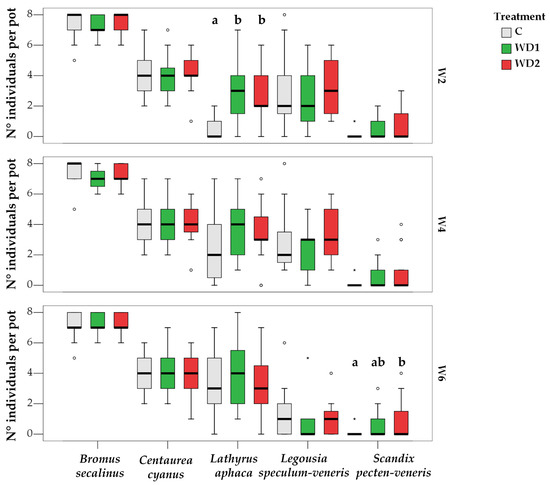
Figure 4.
Boxplots showing the total number of individuals of each species per pot per treatment (C = 0% wood distillate; WD1 = 0.2% wood distillate; WD2 = 0.5% wood distillate) at week 2 (W2), week 4 (W4), and week 6 (W6). Different letters indicate statistically significant differences between treatments for each species (α = 0.05). ° = outlier; * = extreme value.
3.2. DW and MDW
The PERMANOVA analysis on community composition using DW as an abundance measure did not reveal any statistically significant effect of WD. Conversely, WD had a significant effect on DW of S. pecten-veneris and L. speculum-veneris with contrasting results: the increase in DW from C to WD1 and WD2 and the decrease from C to WD1 and WD2, respectively (Table 3 and Table 4). TMDW significantly decreased from C and WD1 to WD2 (Table 5 and Table 6). All other dependent variables did not show any statistically significant responses to the treatment.

Table 3.
Results of the PERMANOVA analyses on community composition with the dry weight of each species as an abundance measure, on the total dry weight per pot (TDW), and on the dry weight of single species per pot.

Table 4.
Results of PERMANOVA pairwise t-tests (dry weight) between treatments; DW = Dry Weight; C = 0% wood distillate; WD1 = 0.2% wood distillate; WD2 = 0.5% wood distillate.

Table 5.
Results of the PERMANOVA analyses on community composition with the mean dry weight of each species as an abundance measure, on the total mean dry weight per pot (TMDW), and on the mean dry weight of individuals per species per pot.

Table 6.
Results of PERMANOVA pairwise t-tests (mean dry weight) between treatments; TMDW = total mean dry weight per pot; C = 0% wood distillate; WD1 = 0.2% wood distillate; WD2 = 0.5% wood distillate.
3.3. FW and MFW
WD had no statistically significant effects on FW and MFW for all investigated variables (Table 7 and Table 8).

Table 7.
Results of the PERMANOVA analyses on community composition with the fresh weight of each species as an abundance measure, on the total fresh weight per pot (TFW), and on the fresh weight of single species per pot.

Table 8.
Results of the PERMANOVA analyses on community composition with the mean fresh weight of each species as an abundance measure, on the total mean fresh weight per pot (TMFW), and on the mean fresh weight of single species per pot.
4. Discussion
Our study provided the first evidence that the application of WD is not harmful to the spontaneous plant diversity associated with cereal crops, when applied at the concentrations suggested to improve crop growing and yield [21,22]. Previous studies highlighted that WD is very effective as an herbicide on ruderal annual weeds such as Avena spp., Bromus spp., and Hordeum spp., but only at very high concentrations (25–100% v/v) [41]. Another study showed its effectiveness, when applied purely, in the control of annual and perennial broadleaved weeds such as Erigeron annuus (L.) Desf., Trifolium repens L., and Veronica persica Poir. in dormant turfgrasses of Zoysia japonica Steud. [42]. Conversely, studies on the effects of WD on non-target plants developing among crops are missing in literature.
In our research study, the effects of WD on community composition and single species were mainly detectable in the very first stages of plant development, i.e., seedling emergence. Treated pots showed a higher number of emerged individuals at W2, but such differences vanished at W4 and W6, suggesting that the application of WD only sped up seedling emergence, but it did not increase it with respect to control pots. This evidence can be relevant in light of promoting a sustainable maintenance of within-field plant diversity without increasing the competition ability of arable plants. Moreover, the application of WD could be used to promote seedling emergence in areas dedicated to the conservation of arable plants, such as conservation headlands, uncropped margins, wildflower strips, and arable reserves [43,44].
Overall, three out of five single species were insensitive to WD treatment. L. aphaca showed an increased emergence at W2, which was no more detectable at W4 and W6. S. pecten-veneris had higher TNI only at W6, probably determined by a possibly longer germination time required by this species. The seedling emergence of the other species was not influenced by the treatments. However, we detected a faster emergence in treated pots through the counts of all the individuals, meaning that WD effects on emergence were less species-specific.
Fresh weight was not influenced by any of the tested treatments. On the contrary, we observed a decrease in TMDW in both treatments with respect to the control. This means that treated communities were composed by individuals with a lower DW. Such evidence suggests that the application of WD could promote the growth of a sustainable arable plant community. In fact, the most effective trade-off between crop production and the conservation of arable plant diversity is the maintenance of species-rich, but low-biomass arable plant communities. The achievement of such a balance allows a reduction in crop yield losses, while still preserving adequate biodiversity levels in arable crops [45]. For some taxa, there was a species-specific effect of WD on DW. This was the case of S. pecten-veneris, for which its DW increased in treated pots, and of L. speculum-veneris, for which its DW decreased in the treatments with respect to the control.
Most rare arable plants are weak competitors in arable fields. From this perspective, crop cover and vigor may highly influence the biological performances of these species, which worsen under conditions of high competition [46,47]. This suggests that the improvement of crop performance by the application of WD might have a negative effect on arable plant diversity. This is particularly true in the context of our findings that WD decreases DW in some of the studied arable plant species, which might increase their susceptibility to crop competition. However, we did not observe any effects on FW, suggesting that WD may preserve the ability of the plants to efficiently use and store water.
In the future, open field experiments will be necessary to verify the overall effect of WD on crops, arable plants (both rare and common), and on the interactions between these two components of the agroecosystem under different treatments. In particular, observations conducted all along the growing season will be necessary to exclude negative effects on the crop species due to competition by the spontaneous flora in a real field, even though our results suggest the potentiality for the development of sustainable and harmless arable plant communities. Increased competition by generalist annual plants during seedling emergence could also result in negative effects on rare arable plants, since the growth of the former is inhibited only by applying high concentrations of WD [41]. The effects of WD should also be tested across different soil types, especially considering that the species composition of arable plant communities can be strongly influenced by soil features [2,14].
5. Conclusions
In this study, using simulated plant communities, we provided the first evidence that the use of WD at low concentrations is compatible with a sustainable management of plant diversity in arable crops. Further studies involving more species or run in the open field will help in elucidating the role of WD as a sustainable product not only boosting crop performances but also not having negative effects on arable plant diversity.
Author Contributions
Conceptualization, E.F., A.V., R.F. and S.M.; methodology, S.M., E.F. and R.F.; statistical analyses, S.M.; data curation, S.M., E.F., R.F., T.F. and L.d.S.; writing—original draft preparation, E.F., R.F. and S.M.; writing—review and editing, E.F., R.F., S.M., S.L., C.A., L.d.S., T.F. and A.V.; supervision, S.M., S.L. and C.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request to the corresponding author.
Acknowledgments
Thanks are due to the Agro-Botanical Garden of the University of Cluj-Napoca (Romania) and to the Botanical Garden of Ulm University (Germany) for providing the seed material used in this research. We also ought to thank Francesco Barbagli (BioDea and BioEsperia) for kindly providing the wood distillate and P. Jepkogei for helping with the measurements of DW.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Petit, S.; Boursault, A.; Le Guilloux, M.; Munier-Jolain, N.; Reboud, X. Weeds in agricultural landscapes. A review. Agron. Sustain. Dev. 2011, 31, 309–317. [Google Scholar] [CrossRef]
- Holzner, W. Weed species and weed communities. Vegetatio 1978, 38, 13–20. [Google Scholar] [CrossRef]
- Marshall, E.J.P.; Brown, V.K.; Boatman, N.D.; Lutman, P.J.W.; Squire, G.R.; Ward, L.K. The role of weeds in supporting biological diversity within crop fields. Weed Res. 2003, 43, 77–89. [Google Scholar] [CrossRef]
- Rosati, L.; Fascetti, S.; Romano, V.A.; Potenza, G.; Lapenna, M.R.; Capano, A.; Stinca, A. New chorological data for the Italian vascular flora. Diversity 2020, 12, 22. [Google Scholar] [CrossRef]
- Scholz, H. Questions about indigenous plants and anecophytes. Taxon 2007, 56, 1255–1260. [Google Scholar] [CrossRef]
- Stinca, A.; Musarella, C.M.; Rosati, L.; Laface, V.L.A.; Licht, W.; Fanfarillo, E.; Mei, G. Italian vascular flora: New findings, updates and exploration of floristic similarities between regions. Diversity 2021, 13, 600. [Google Scholar] [CrossRef]
- Storkey, J.; Meyer, S.; Still, K.S.; Leuschner, C. The impact of agricultural intensification and land-use change on the European arable flora. Proc. R. Soc. B 2012, 279, 1421–1429. [Google Scholar] [CrossRef]
- Richner, N.; Holderegger, R.; Linder, H.P.; Walter, T. Reviewing change in the arable flora of Europe: A meta-analysis. Weed Res. 2015, 55, 1–13. [Google Scholar] [CrossRef]
- Fanfarillo, E.; Kasperski, A.; Giuliani, A.; Abbate, G. Shifts of arable plant communities after agricultural intensification: A floristic and ecological diachronic analysis in maize fields of Latium (central Italy). Bot. Lett. 2019, 166, 356–365. [Google Scholar] [CrossRef]
- Janssen, J.A.M.; Rodwell, J.S.; Criado, M.G.; Gubbay, S.; Haynes, T.; Nieto, A.; Calix, M. European Red List of Habitats; Publications Office of the European Union: Luxembourg, 2016. [Google Scholar]
- Orsenigo, S.; Fenu, G.; Gargano, D.; Montagnani, C.; Abeli, T.; Alessandrini, A.; Rossi, G. Red list of threatened vascular plants in Italy. Plant Biosyst. 2021, 155, 310–335. [Google Scholar] [CrossRef]
- Fanfarillo, E.; Latini, M.; Iberite, M.; Abbate, G. The segetal flora of Italy: An occurrence dataset from relevés in winter cereals and allied crop types. PhytoKeys 2020, 161, 107. [Google Scholar] [CrossRef]
- Küzmič, F.; Šilc, U.; Lososová, Z.; Chytrý, M.; Knollová, I.; Mucina, L.; Tereshenko, S. European Weed Vegetation Database–a gap-focused vegetation-plot database. Phytocoenologia 2020, 50, 93–100. [Google Scholar] [CrossRef]
- Lososová, Z.; Chytrý, M.; Cimalová, S.; Kropáč, Z.; Otýpková, Z.; Pyšek, P.; Tichý, L. Weed vegetation of arable land in Central Europe: Gradients of diversity and species composition. J. Veg. Sci. 2004, 15, 415–422. [Google Scholar] [CrossRef]
- Fanfarillo, E.; Zangari, G.; Küzmič, F.; Fiaschi, T.; Bonari, G.; Angiolini, C. Summer roadside vegetation dominated by Sorghum halepense in peninsular Italy: Survey and classification. Rend. Lincei Sci. Fis. Nat. 2022, 33, 93–104. [Google Scholar] [CrossRef]
- Fanfarillo, E.; Latini, M.; Iberite, M.; Bonari, G.; Nicolella, G.; Rosati, L.; Abbate, G. The segetal flora of winter cereals and allied crops in Italy: Species inventory with chorological, structural and ecological features. Plant Biosyst. 2020, 154, 935–946. [Google Scholar] [CrossRef]
- United Nations. Progress towards the Sustainable Development Goals. Report of the Secretary-General. 2022. Available online: https://sustainabledevelopment.un.org/content/documents/29858SG_SDG_Progress_Report_2022.pdf (accessed on 9 April 2022).
- Grewal, A.; Abbey, L.; Gunupuru, L.R. Production, prospects and potential application of pyroligneous acid in agriculture. J. Anal. Appl. Pyrol. 2018, 135, 152–159. [Google Scholar] [CrossRef]
- Zulkarami, B.; Ashrafuzzaman, M.; Husni, M.O.; Ismail, M.R. Effect of pyroligneous acid on growth, yield and quality improvement of rockmelon in soilless culture. Aust. J. Crop Sci. 2011, 5, 1508–1514. [Google Scholar]
- Mu, J.; Yu, Z.M.; Wu, W.Q.; Wu, Q.L. Preliminary study of application effect of bamboo vinegar on vegetable growth. For. Stud. China 2006, 8, 43–47. [Google Scholar] [CrossRef]
- Fedeli, R.; Vannini, A.; Guarnieri, M.; Monaci, F.; Loppi, S. Bio-Based Solutions for Agriculture: Foliar Application of WoodDistillate Alone and in Combination with Other Plant-Derived Corroborants Results in Different Effects on Lettuce (Lactuca Sativa L.). Biology 2022, 11, 404. [Google Scholar] [CrossRef]
- Fedeli, R.; Vannini, A.; Celletti, S.; Maresca, V.; Munzi, S.; Cruz, C.; Alexandrov, D.; Guarnieri, M.; Loppi, S. Foliar application of wood distillate boosts plant yield and nutritional parameters of chickpea. Ann. Appl. Biol. 2022, 1–9. [Google Scholar] [CrossRef]
- Berahim, Z.; Panhwar, Q.A.; Ismail, M.R.; Saud, H.M.; Mondal, A.; Naher, U.A.; Islam, R. Rice yield improvement by foliarapplication of phytohormone. J. Food Agric. Environ. 2018, 12, 399–404. [Google Scholar]
- Vannini, A.; Fedeli, R.; Guarnieri, M.; Loppi, S. Foliar Application of Wood Distillate Alleviates Ozone-Induced Damage in Lettuce (Lactuca sativa L.). Toxics 2022, 10, 178. [Google Scholar] [CrossRef]
- Italian Ministerial Decree 6793. 18 July 2018. Available online: https://www.gazzettaufficiale.it/eli/id/2018/09/05/18A05693/sg (accessed on 20 June 2022).
- Fackovcová, Z.; Vannini, A.; Monaci, F.; Grattacaso, M.; Paoli, L.; Loppi, S. Effects of wood distillate (pyroligneous acid) on sensitive bioindicators (lichen and moss). Ecotoxicol. Environ. Saf. 2020, 204, 111117. [Google Scholar] [CrossRef]
- Fackovcová, Z.; Vannini, A.; Monaci, F.; Grattacaso, M.; Paoli, L.; Loppi, S. Uptake of trace elements in the water fern Azolla filiculoides after short-term application of chestnut wood distillate (Pyroligneous Acid). Plants 2020, 9, 1179. [Google Scholar] [CrossRef]
- Hagner, M.; Pasanen, T.; Lindqvist, B. Effects of birch tar oils on soil organisms and plants. Agric. Food Sci. 2010, 19, 13–23. [Google Scholar] [CrossRef]
- Hagner, M.; Penttinen, O.P.; Pasanen, T.; Tiilikkala, K.; Setälä, H. Acute toxicity of birch tar oil on aquatic organisms. Agric. Food Sci. 2010, 19, 24–33. [Google Scholar] [CrossRef]
- Filippelli, A.; Ciccone, V.; Loppi, S.; Morbidelli, L. Characterization of the safety profile of sweet chestnut wood distillate employed in agriculture. Safety 2021, 7, 79. [Google Scholar] [CrossRef]
- Pessarakli, M. (Ed.) Handbook of Plant and Crop Stress; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Gulías, J.; Traveset, A.; Riera, N.; Mus, M. Critical stages in the recruitment process of Rhamnus alaternus L. Ann. Bot. 2004, 93, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Botanic Gardens Conservation International. 2022. Available online: https://www.bgci.org/ (accessed on 10 April 2022).
- Bartolucci, F.; Peruzzi, L.; Galasso, G.; Albano, A.; Alessandrini, A.N.M.G.; Ardenghi, N.M.G.; Conti, F. An updated checklist of the vascular flora native to Italy. Plant Biosyst. 2018, 152, 179–303. [Google Scholar] [CrossRef]
- Galasso, G.; Conti, F.; Peruzzi, L.; Ardenghi, N.M.G.; Banfi, E.; Celesti-Grapow, L.; Bartolucci, F. An updated checklist of the vascular flora alien to Italy. Plant Biosyst 2018, 152, 556–592. [Google Scholar] [CrossRef]
- BioDea. Available online: https://biodea.bio/bio-wood-distillate/?lang=en (accessed on 6 August 2022).
- Vannini, A.; Moratelli, F.; Monaci, F.; Loppi, S. Effects of wood distillate and soy lecithin on the photosynthetic performance and growth of lettuce (Lactuca sativa L.). SN Appl. Sci. 2021, 3, 113. [Google Scholar] [CrossRef]
- Perez-Harguindeguy, N.; Diaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Cornelissen, J.H.C. Corrigendum to: New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 2016, 64, 715–716. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N. PRIMER v6: USersManual/Tutorial PRIMER-E; Plymouth Marine Laboratory: Plymouth, UK, 2006. [Google Scholar]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods; PRIMER-E: Plymouth, UK, 2008. [Google Scholar]
- Aguirre, J.L.; Baena, J.; Martín, M.T.; González, S.; Manjón, J.L.; Peinado, M. Herbicidal effects of wood vinegar on nitrophilous plant communities. Food Energy Secur. 2020, 9, e253. [Google Scholar] [CrossRef]
- Hao, Z.; Bagavathiannan, M.; Li, Y.; Qu, M.; Wang, Z.; Yu, J. Wood vinegar for control of broadleaf weeds in dormant turfgrass. Weed Technol. 2021, 35, 901–907. [Google Scholar] [CrossRef]
- Albrecht, H.; Cambecèdes, J.; Lang, M.; Wagner, M. Management options for the conservation of rare arable plants in Europe. Bot. Lett. 2016, 163, 389–415. [Google Scholar] [CrossRef]
- Wietzke, A.; Albert, K.; Bergmeier, E.; Sutcliffe, L.M.; van Waveren, C.S.; Leuschner, C. Flower strips, conservation field margins and fallows promote the arable flora in intensively farmed landscapes: Results of a 4-year study. Agric. Ecosyst. Environ. 2020, 304, 107142. [Google Scholar] [CrossRef]
- Adeux, G.; Vieren, E.; Carlesi, S.; Bàrberi, P.; Munier-Jolain, N.; Cordeau, S. Mitigating crop yield losses through weed diversity. Nat. Sustain. 2019, 2, 1018–1026. [Google Scholar] [CrossRef]
- Meyer, S.; Wesche, K.; Leuschner, C.; van Elsen, T.; Metzner, J. A new conservation strategy for arable weed vegetation in Germany: The project ‘100 fields for biodiversity’. Plant Breed. Seed Sci. 2010, 61, 25–34. [Google Scholar]
- Epperlein, L.R.; Prestele, J.W.; Albrecht, H.; Kollmann, J. Reintroduction of a rare arable weed: Competition effects on weed fitness and crop yield. Agric. Ecosyst. Environ. 2014, 188, 57–62. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).