Ericaceous Plants: A Review for the Bioprospecting of Ericoid Mycorrhizae from Ecuador
Abstract
1. Introduction
2. Mycorrhizal Symbiosis of Ericaceous Plants
2.1. Ectomycorrhizae
2.2. Endomycorrhizae
2.3. Ectendomycorrhizae
3. Associative Mechanism of Plant–Fungus Interaction
4. Considerations before the Investigation of Ericoid Mycorrhizae
5. Distribution of Ericaceous Plants in Ecuador
6. Ericaceae Studied in Ecuador
7. Future Research Perspectives
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Botero, D.O.; Bustamante, M.J.; Cerón, M.A.; Guevara, J.D. Anatomía y morfología de Cavendishia cordifolia (Ericaceae). In Morfoanatomía Reproductiva De Plantas Vasculares: Teoría y Estudio de Casos; Universidad Nacional de Colombia: Bogotá, Colombia, 2010; pp. 62–73. ISBN 9789587017953. [Google Scholar]
- Caranqui, J.; Ortíz, M. Diversity and Floristic Composition in the Analogous Vegetation of Indiviso, Baquerizo Moreno, Tungurahua. ESPOCH Congr. Ecuad. J. STEAM 2021, 1, 1120–1128. [Google Scholar] [CrossRef]
- Iturralde, R.B. Diversidad y distribución de Ericaceae en las Antillas Mayores. Rev. Del Jardín Botánico Nac. 2006, 27, 65–73. [Google Scholar]
- Urgiles, N.; Cofre, D.; Loján, P.; Maita, J.; Albarez, P.; Báez, S.; Tamargo, E.; Eguiguren, P.; Ojeda, T.; Aguirre, N. Plant diversity, community structure, and aereal biomass in a paramo ecosystem of Southern Ecuador. Bosques Latid. Cero 2018, 8, 13. [Google Scholar]
- Setaro, S.; Weiß, M.; Oberwinkler, F.; Kottke, I. Sebacinales form ectendomycorrhizas with Cavendishia nobilis, a member of the Andean clade of Ericaceae, in the mountain rain forest of southern Ecuador. New Phytol. 2006, 169, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Castro Zaruma, E.; Moreno Lalama, C. Variación de Caracteres Funcionales de Morfos de Flores de Bejaria resinosa con Relación a la Comunidad de Visitantes Florales. Bachelor’s Thesis, Universidad del Azuay, Cuenca, Ecuador, 2020. [Google Scholar]
- Meléndez-Jácome, M.R.; Flor-Romero, L.E.; Sandoval-Pacheco, M.E.; Vasquez-Castillo, W.A.; Racines-Oliva, M.A. Vaccinium spp.: Karyotypic and phylogenetic characteristics, nutritional composition, edaphoclimatic conditions, biotic factors and beneficial microorganisms in the rhizosphere. Sci. Agropecu. 2021, 12, 109–120. [Google Scholar] [CrossRef]
- Gui, L.X.; Lu, S.S.; Chen, Q.; Yang, L.; Xiao, J.X. iTRAQ-based proteomic analysis reveals positive impacts of arbuscular mycorrhizal fungi inoculation on photosynthesis and drought tolerance in blueberry. Trees—Struct. Funct. 2021, 35, 81–92. [Google Scholar] [CrossRef]
- Vohník, M.; Sadowsky, J.J.; Kohout, P.; Lhotáková, Z.; Nestby, R.; Kolařík, M. Novel root-fungus symbiosis in Ericaceae: Sheathed ericoid mycorrhiza formed by a hitherto undescribed basidiomycete with affinities to trechisporales. PLoS ONE 2012, 7, e39524. [Google Scholar] [CrossRef]
- Bermudes, D.; Benzing, D.H. Fungi in neotropical epiphyte roots. BioSystems 1989, 23, 65–73. [Google Scholar] [CrossRef]
- Vega, A.M.; Muñoz, C.S. Presencia de micorrizas en Ericaceas en Chile. Aricultura Tec. 1994, 54, 332–339. [Google Scholar]
- Camargo-Ricalde, S.; Montaño, N.; De La Rosa-Mera, C.; Montaño-Arias, A. Micorrizas: Una gran unión debajo del suelo. Rev. Digit. Univ. 2012, 13, 19. [Google Scholar]
- Cullings, K.W. Single phylogenetic origin of ericoid mycorrhizae within the Ericaceae. Can. J. Bot. 1996, 74, 1896–1909. [Google Scholar] [CrossRef]
- Buscardo, E.; Rodríguez-Echeverría, S.; Angelis, P.; Freitas, H. Comunidades de hongos ectomicorrícicos en ambientes propensos al fuego: Compañeros esenciales para el reestablecimiento de pinares mediterráneos. Ecosistemas 2009, 18, 55–63. [Google Scholar] [CrossRef]
- Cáceres, Y.; Rada, F. ¿Cómo responde la especie leñosa Vaccinum meridionale a la temperatura en su límite altitudinal de distribución en los Andes tropicales? Ecotrópicos 2011, 24, 80–91. [Google Scholar]
- Vohník, M. Ericoid mycorrhizal symbiosis: Theoretical background and methods for its comprehensive investigation. Mycorrhiza 2020, 30, 671–695. [Google Scholar] [CrossRef] [PubMed]
- Luna., C.N.; Ruiz, L.V. Simbiosis micorrícica: Un análisis. In Interacciones Ecológicas; Universidad de Guadalajara: Guadalajara, Mexico, 2014; Volume 1, pp. 37–61. [Google Scholar] [CrossRef]
- Urgiles, N.; Haug, I.; Setaro, S.; Aguirre, N. Introduction to Mycorrhizas in the Tropics with Emphasis on the Montane Forest in Southern Ecuador. EDILOJA: Loja, Ecuador, 2016; Volume 348977215, ISBN 9789978355329. [Google Scholar]
- Noboa Silva, V. Efecto de seis tipos de sustratos y tres dosis de ácido a naftalenacético en la propagación vegetativa de Mortiño (Vaccinium floribundum Kunth). Eur. Sci. J. ESJ 2019, 15, 359. [Google Scholar] [CrossRef]
- Bautista, J.M.; Posadas, L.; Urbina, J.; Larsen, J.; Segura, S. Colonization by mycorrhizae in the production of cranberry seedlings in nursery (Vaccinium spp.) cv Biloxi. Rev. Mex. Cienc. Agrícolas 2017, 8, 695–703. [Google Scholar] [CrossRef]
- Català, S.; Garrido, I.; Tejedor, F. Dna barcoding de especies mediterráneas críticas (i): Primeros datos moleculares de gomphidius tyrrhenicus d. Antonini & m. Antonini y sus implicaciones evolutivas en el ambiente mediterráneo. Butlletí Soc. Micológica Valencia 2011, 16, 239. [Google Scholar]
- Pedraza-Peñalosa, P.; Valencia, R.; Montúfar, R.; Santiana, J.; Tye, A. Ericaceae. In Libro Rojo de Las Plantas Endémicas del Ecuador; Pontificia Universidad Católica del Ecuador: Quito, Ecuador, 2017. [Google Scholar]
- Camarena-Gutierrez, G. Interaccion planta-hongos micorrizicos arbusculares. Rev. Chapingo Ser. Cienc. For. Del Ambiente 2012, 18, 409–421. [Google Scholar] [CrossRef]
- Cullings, K. Molecular phylogeny of the Monotropoideae (Ericaceae) with a note on the placement of the Pyroloideae. J. Evol. Biol. 1994, 7, 501–516. [Google Scholar] [CrossRef]
- Read, D.J.; Perez-Moreno, J. Mycorrhizas and nutrient cycling in ecosystems—A journey towards relevance? New Phytol. 2003, 157, 475–492. [Google Scholar] [CrossRef]
- Figueiredo, A.F.; Boy, J.; Guggenberger, G. Common mycorrhizae network: A review of the theories and mechanisms behind underground interactions. Front. Fungal Biol. 2021, 2, 1–13. [Google Scholar] [CrossRef]
- Hughes, E.; Mitchell, D.T. Utilization of sucrose by Hymenoscyphus ericae (an ericoid endomycorrhizal fungus) and ectomycorrhizal fungi. Mycol. Res. 1995, 99, 1233–1238. [Google Scholar] [CrossRef]
- Bonfante-Fasolo, P.; Perotto, S. Visualization of surface sugar residues in mycorrhizal ericoid fungi by fluorescein conjugated lectins. Symbiosis 1986, 1, 269–288. [Google Scholar]
- Correia, M.; Heleno, R.; da Silva, L.; Costa, J.; Rodríguez-Echeverría, S. First evidence for the joint dispersal of mycorrhizal fungi and plant diaspores by birds. New Phytol. 2019, 222, 1054–1060. [Google Scholar] [CrossRef] [PubMed]
- Correia, M.; Heleno, R.; Vargas, P.; Rodríguez-Echeverría, S. Should I stay or should I go? Mycorrhizal plants are more likely to invest in long-distance seed dispersal than non-mycorrhizal plants. Ecol. Lett. 2018, 21, 683–691. [Google Scholar] [CrossRef]
- Naranjo-Morán, J.; Vera-Morales, M.; Barcos-Arias, M.; Oviedo-Anchundia, R.; Sánchez-Rendón, V.; Pino-Acosta, A. Dispersión y transporte de propágulos micorrícicos en el bosque seco tropical. Ecosistemas 2021, 30, 2062. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Zobel, M. How mycorrhizal associations drive plant population and community biology. Science 2020, 367, eaba1223. [Google Scholar] [CrossRef]
- Vega, A.R.; Garciga, M.; Rodriguez, A.; Prat, L.; Mella, J. Blueberries mycorrhizal symbiosis outside of the boundaries of natural dispersion for ericaceous plants in Chile. Acta Hortic. 2009, 810, 665–672. [Google Scholar] [CrossRef]
- Garibay-Orijel, R.; Martínez-Ramos, M.; Cifuentes, J. Disponibilidad de esporomas de hongos comestibles en los bosques de pino-encino de Ixtlán de Juárez, Oaxaca. Rev. Mex. Biodivers. 2009, 80, 521–534. [Google Scholar] [CrossRef]
- Llivisaca-Contreras, S.A.; Manzano-Santana, P.; Ruales, J.; Naranjo-Morán, J.; Serrano-Mena, L.; Chica-Mart, E.; Cevallos-Cevallos, J.M. Mortiño (Vaccinium floribundum Kunth): An Underutilized superplant from the Andes. Horticulturae 2022, 8, 358. [Google Scholar] [CrossRef]
- Coba Santamaría, P.; Coronel, D.; Verdugo, K.; Paredes, M.; Yugsi, E.; Huachi, L. Estudio etnobotánico del mortiño (Vaccinium floribundum) como alimento ancestral y potencial alimento funcional. Granja 2012, 16, 5. [Google Scholar] [CrossRef]
- Aguirre Mendoza, Z.; Aguirre Mendoza, N.; Muñoz Ch, J. Biodiversidad de la provincia de Loja, Ecuador. Arnaldoa 2017, 24, 523–542. [Google Scholar] [CrossRef]
- Mostacedo, B.; Fredericjsen, T. Manual de Métodos Básicos de Muestreo y Análisis en Ecología Vegetal; Proyecto de Manejo Froestal Sostenible (BOLFOR): Santa Cruz de la Sierra, Bolivia, 2000. [Google Scholar]
- Kottke, I.; Haug, I. The significance of mycorrhizal diversity of trees in the tropical mountain forest of southern Ecuador. Iyonia 2004, 7, 49–56. [Google Scholar]
- Kottke, I.; Haug, I.; Setaro, S.; Suárez, J.P.; Weiß, M.; Preußing, M.; Nebel, M.; Oberwinkler, F. Guilds of mycorrhizal fungi and their relation to trees, ericads, orchids and liverworts in a neotropical mountain rain forest. Basic Appl. Ecol. 2008, 9, 13–23. [Google Scholar] [CrossRef]
- Luteyn, J.L. Ericacea del Ecuador. New York Botanical Garden; Institute of Systematic Botany: New York, NY, USA, 2007; pp. 1–20. [Google Scholar]
- Luteyn, J. The plant family Ericaceae (“Blueberries”) in Ecuador: Ecology, diversity, economic importance, and conservation. Rev. Ecuat. Med. Cienc. Biol. 2021, 42, 79–98. [Google Scholar] [CrossRef]
- Uehara, Y.; Sugiura, N. Cockroach-mediated seed dispersal in Monotropastrum humile (Ericaceae): A new mutualistic mechanism. Bot. J. Linn. Soc. 2017, 185, 113–118. [Google Scholar] [CrossRef]
- Luteyn, J.L. Diversity, adaptation, and endemism in neotropical ericaceae: Biogeographical patterns in the vaccinieae. Bot. Rev. 2002, 68, 55–87. [Google Scholar] [CrossRef]
- Setaro, S.; Kottke, I.; Oberwinkler, F. Anatomy and ultrastructure of mycorrhizal associations of neotropical Ericaceae. Mycol. Prog. 2006, 5, 243–254. [Google Scholar] [CrossRef]
- Selosse, M.; Setaro, S.; Glatard, F.; Richard, F.; Urcelay, C.; Weiß, M. Sebacinales are common mycorrhizal associates of Ericaceae. New Phytol. 2007, 174, 864–878. [Google Scholar] [CrossRef]
- Setaro, S.D.; Kron, K. Neotropical and north american vaccinioideae (ericaceae) share their mycorrhizal sebacinales-an indication for concerted migration? PLoS Curr. 2011, 3, RRN1227. [Google Scholar] [CrossRef]
- Setaro, S.D.; Garnica, S.; Herrera, P.I.; Suárez, J.P.; Göker, M. A clustering optimization strategy to estimate species richness of Sebacinales in the tropical Andes based on molecular sequences from distinct DNA regions. Biodivers. Conserv. 2012, 21, 2269–2285. [Google Scholar] [CrossRef]
- Setaro, S.; Suárez, J.P.; Herrera, P.; Cruz, D.; Kottke, I. Distinct but closely related Sebacinales form mycorrhizae with coexisting Ericaceae and Orchidaceae in a Neotropical Mountain Area. In Piriformospora Indica; Springer: Berlin/Heidelberg, Germany, 2013; pp. 81–105. [Google Scholar] [CrossRef]
- Kottke, I.; Setaro, S.; Haug, I.; Herrera, P. Mycorrhiza networks promote biodiversity and stabilize the tropical mountain rain forest ecosystem: Perspectives for understanding complex communities. In Ecosystem Services, Biodiversity and Environmental Change in a Tropical Mountain Ecosystem of South Ecuador; Springer: Berlin/Heidelberg, Germany, 2013; pp. 187–203. [Google Scholar] [CrossRef]
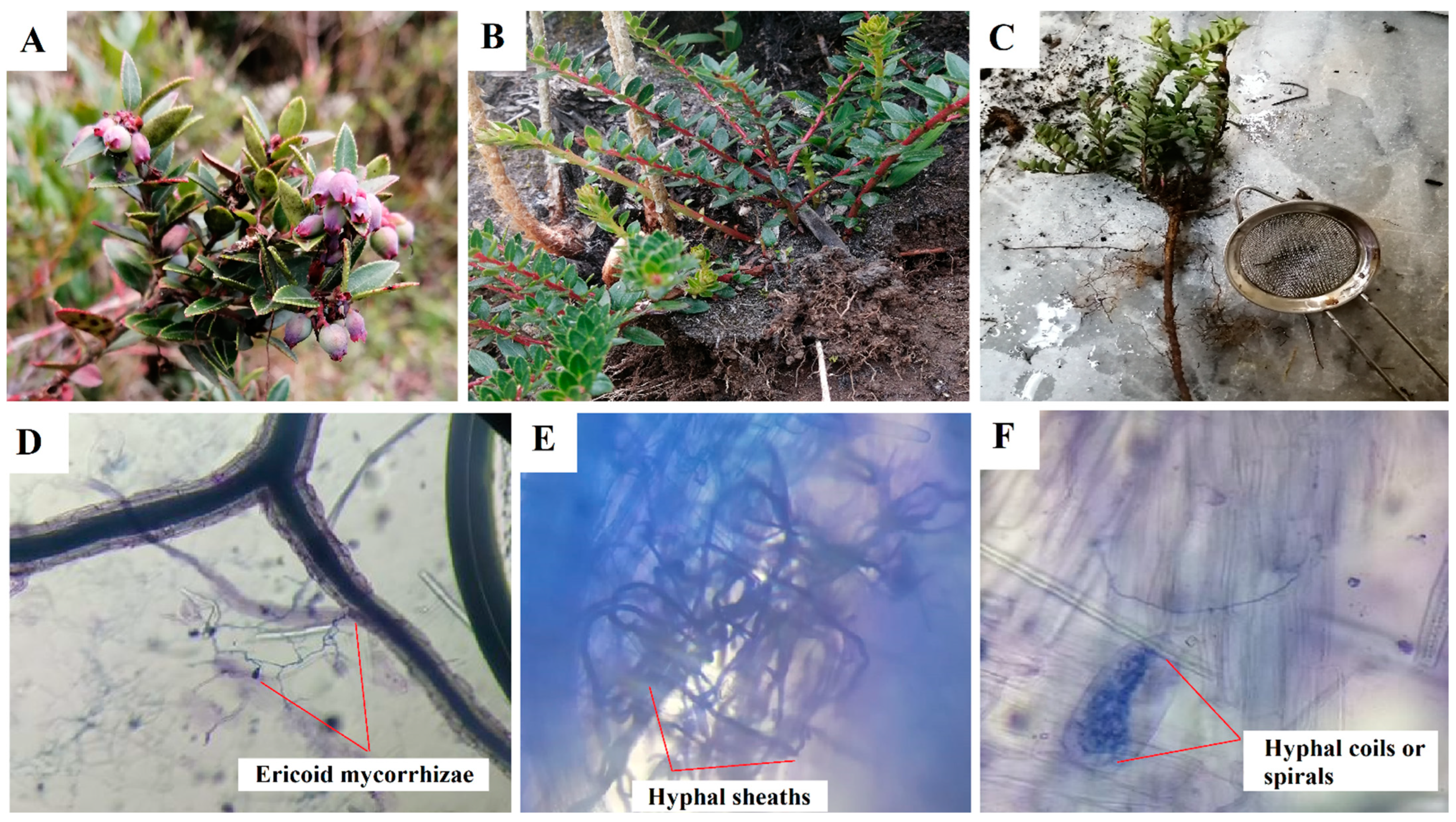
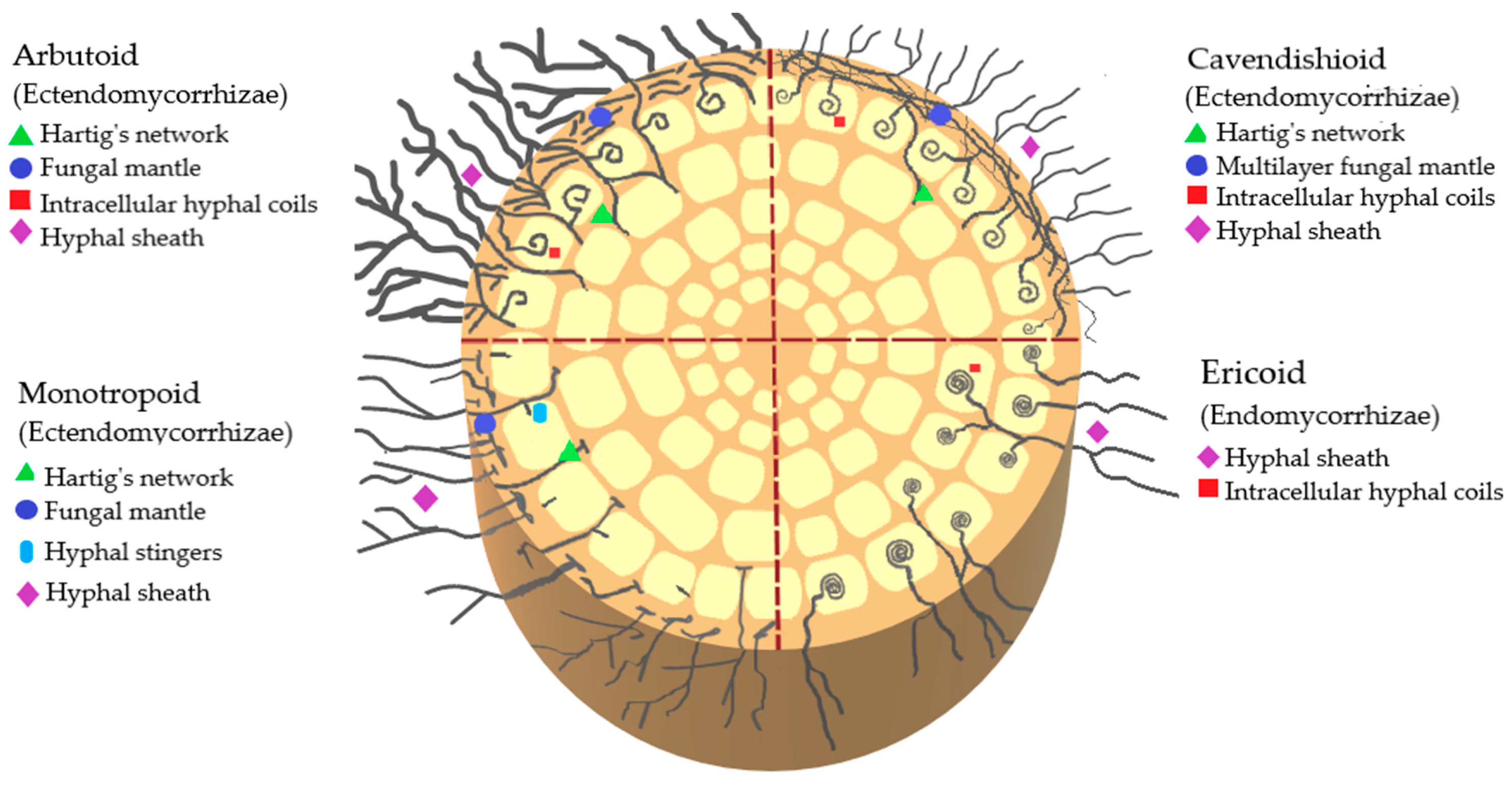
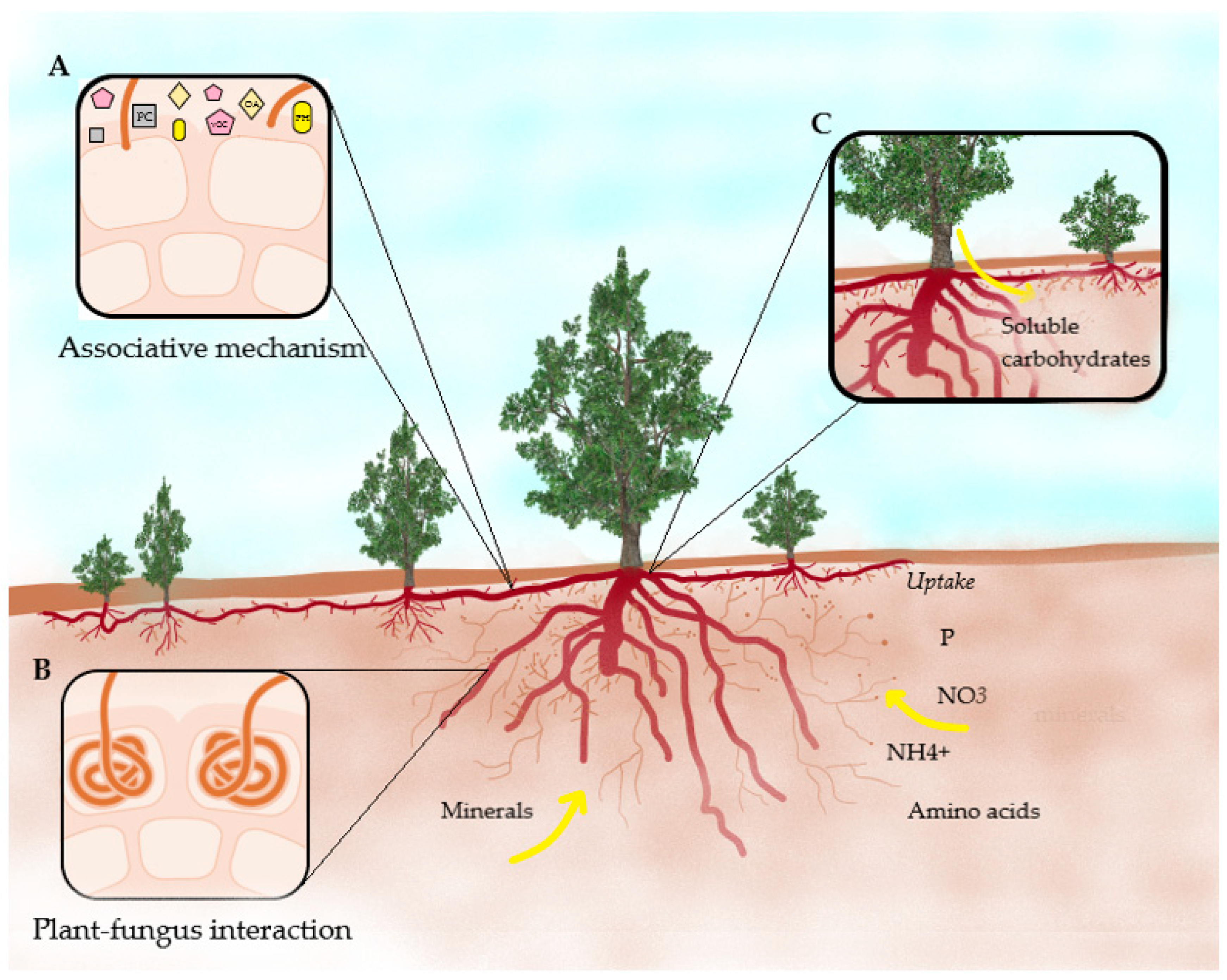
| Title | Ericaceae | Study Site | Aim | Findings | References |
|---|---|---|---|---|---|
| Fungi in neotropical epiphyte roots | Cavendishia sp. Macleania cordifolia Macleania sp. Psamisia sp. | Los Ríos, Santo Domingo de los Colorados, and Imbabura. | To examine roots of Ecuadorian vascular epiphytes for the presence of symbiotic microorganisms. | In Ericaceae and Campanulaceae, a fungal association similar to the dematiaceous surface fungi described for alpine and grassland plants was present. | [10] |
| Diversity, Adaptation, and Endemism in Neotropical Ericaceae | Genera Vaccinium, Gaultheria, Cavendishia, Macleania, Orthaea, Anthopterus, Psammisia, Sphyrospermum, Themistoclesia, Oreanthes, Ceratostema and Thibaudia. | The Andean Chocó, Ecuadorian Andes. | Botanical Review | High diversity and uniqueness of the Neotropical Ericaceae, high levels of habitat alteration. The protection of high-Andean ecosystems should be a priority for conservation efforts in the Neotropics. | [44] |
| Sebacinales form ectendomycorrhizas with Cavendishia nobilis, a member of the Andean clade of Ericaceae, in the mountain rainforest of southern Ecuador | Species Cavendishia nobilis var. capitata | San Francisco River between the Podocarpus National Park, Loja and Zamora-Chinchipe. | To investigate mycorrhizal structures in detail using transmission electron microscopy and identify associated fungi using molecular phylogenetics. | This is the first study to obtain evidence of ectendomycorrhizae in the Vaccinioideae. They propose the term ‘mycorrhizae cavendishioides’. | [5] |
| Anatomy and ultrastructure of mycorrhizal associations of neotropical Ericaceae | Genera Cavendishia Ceratostema, Diogenesia, Disterigma, Macleania, Orthaea, Psammisia, Semiramisia, Sphyrospermum, Thibaudia, Bejaria, Pernettya, Gaultheria and Vaccinium. | The border of the Podocarpus National Park. | To determine if Ericaceae of the Andean clade form cavendishioid mycorrhizae in contrast to those found in the same area but which do not belong to the Andean clade. | The Ericaceae of the Andean clade showed a hyphal sheath and inter- and intracellular colonization by hyphae, justifying the new term “cavendishioid mycorrhiza”. | [45] |
| Sebacinales are common mycorrhizal associates of Ericaceae | Genera Vaccinium, Semiramisia, Gaultheria, Cavendishia, Disterigma Macleania, Ceratostema, Diogenesia, Psammisia, and Sphyrospermum, | - | To answer questions about the identity, diversity, and structural interactions of sebacinoids associated with Ericaceae. | The ectendomycorrhizae of Ericaceae involve ectomycorrhizal fungal taxa. | [46] |
| Guilds of mycorrhizal fungi and their relation to trees, ericads, orchids, and liverworts in a neotropical mountain rainforest | Genera Cavendishia, Ceratostema, Gaultheria, Sphyrospermum, Psammisia, Diogenesia, Semiramisia, Pleurothallis, Stelis, Disterigma, Macleania. | Mountain tropical forest area of Cordillera El Consuelo and in the shrubby “páramo” nearby. | To discover whether symbiotic fungi with a wide host range create shared guilds or even fungal networks between different plant species and plant families. | Sebacinales and Tulasnellales were only shared between Ericaceae and orchids, or between hemiepiphytes and orchids, respectively. | [40] |
| Neotropical and North American Vaccinioideae (Ericaceae) share their mycorrhizal Sebacinales—an indication for concerted migration? | Vaccinieae and Gaultherieae plant. | South of Ecuador—San Francisco Biological Reserve | To investigate Sebacinales in Vaccinioideae from Ecuador, Panama, and North America to examine whether the mycobionts in each region are closely or distantly related. | North American neotropical and temperate Vaccinioideae share their Sebacinal communities and plants and fungi migrated together. | [47] |
| Ethnobotanical study of mortiño (Vaccinium Floribundum) as an ancestral food and potential functional food. | SpeciesVaccinium floribundum | The provinces of Carchi, Imbabura, Pichincha, Cotopaxi, Tungurahua, Bolivar, Chimborazo, Cañar, Azuay and Loja. | To know the botanical aspects and their applications within popular culture, such as their current use in the medical, industrial, and culinary fields. | Mortiño have a large number of polyphenols assisted by the content of sugars, fiber, lipids, minerals and vitamins, proanthocyanidins, anthocyanidins, and flavonoids. | [36] |
| A clustering optimization strategy to estimate species richness of Sebacinales in the tropical Andes based on molecular sequences from distinct DNA regions. | 39 individuals of Ericaceae. | San Francisco Biological Reserve and its surroundings. | To investigate the Sebacinal mycorrhizae of individuals of Ericaceae and Orchidaceae in a tropical montane ecosystem in southern Ecuador to provide a first estimate of whether these fungi are diverse in the Northern Andes. | Around 8–9% of observed Sebacinales are present in southern Ecuador and most of these molecular operational taxonomic units are endemic. | [48] |
| Distinct but Closely Related Sebacinales form Mycorrhizae with Coexisting Ericaceae and Orchidaceae in a Neotropical Mountain Area | Genera Vaccinium, Gaultheria, Cavendishia, Ceratostema, Psammisia, Sphyrospermum, Rhododendron, Disterigma, Ceratostema and Pyrola. | San Francisco Biological Reserve and El Condor Biosphere Reserve. | To present a study of Sebacinales associated with coexisting Ericaceae and Orchidaceae from two different habitats: pristine forest and regenerating landslides in a montane rain forest in southern Ecuador. | The structural distinction of mycorrhizal associations in Ericaceae and Orchidaceae formed by Sebacinales is presented, and evidence is provided that different guilds are associated with both plant families. | [49] |
| Mycorrhiza Networks Promote Biodiversity and Stabilize the Tropical Mountain Rain Forest Ecosystem: Perspectives for Understanding Complex Communities. | 31 species in 14 genera of epiphytic Ericaceae. | San Francisco Biological Reserve, Zamora-Chinchipe Province, Southern Ecuador. | To understand the mechanisms behind the maintenance of the extraordinary diversity of plants and fungi in tropical montane forests. | The preferential attachment of new members to existing linkages integrates and maintains rare species and stabilizes our species-rich assemblages. | [50] |
| Introduction to Mycorrhizas in the Tropics with Emphasis on the Montane Forest in Southern Ecuador | Genera Cavendishia, Ceratostema, Diogenesia, Macleania, Orthaea, Psammisia, Sphyrospermum, Thibaudia. Vaccinium, Bejaria, Gaultheria, Pyrola, and Orthilia. | Tropical forests of Ecuador, San Francisco Biological Reserve | To help interested readers to access the world of mycorrhizae, especially the mycorrhizal associations of the tropical forests of Ecuador. In particular, we hope that this new knowledge will be applied in projects focused on afforestation, reforestation, and ecological restoration. | How to identify the different types of mycorrhizae, the relationship between mycorrhizal fungi and their host plants, and deals with the characteristics of mycorrhizal fungi. | [18] |
| Vaccinium spp.: Karyotypic and phylogenetic characteristics, nutritional composition, edaphoclimatic conditions, biotic factors, and beneficial microorganisms in the rhizosphere. | Species Vaccinium distichum, Vaccinium crenatum, and Vaccinium floribundum | Quito-Santo Domingo, Tandayapa-Quito and on the Quito-Nono-Mindo road. From the province of Carchi to Cañar, Loja and Azuay. | To know the ecology of the crop, the chemical composition of the fruit, the effect of endophytic microorganisms, and those associated with the rhizosphere on the growth and development of Vaccinium sp. | The relationships of mutualism and symbiosis of endophytic microorganisms with Vaccinium. | [7] |
| The plant family Ericaceae (“mortiños”) in Ecuador: Ecology, Diversity, Economic Importance, and Conservation | Ericaceae family | Andean East from Sucumbíos to Loja; the high Andean “páramo” of Ecuador; and “Chocó biogeographic region”, from Carchi to Pichincha. | To discuss and characterize the diversity, economic importance, conservation, and ecology of the species of the plant family Ericaceae from Ecuador. | Ericaceous mycorrhizae in tropical regions. | [42] |
| Mortiño (Vaccinium floribundum Kunth): An Underutilized Superplant from the Andes | Species Vaccinium floribundum Kunth | The tropical Andes of Ecuador | To review aspects related to the ecology, horticulture, composition, and possible biotechnological applications of the blueberry to help researchers better target its potential. | Review of aspects related to the ecology, horticulture, composition, and possible biotechnological applications of the blueberry. | [35] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pacheco Flores de Valgaz, A.; Barcos-Arias, M.; Naranjo-Morán, J.; Peña Tapia, D.; Moreira-Gómez, R. Ericaceous Plants: A Review for the Bioprospecting of Ericoid Mycorrhizae from Ecuador. Diversity 2022, 14, 648. https://doi.org/10.3390/d14080648
Pacheco Flores de Valgaz A, Barcos-Arias M, Naranjo-Morán J, Peña Tapia D, Moreira-Gómez R. Ericaceous Plants: A Review for the Bioprospecting of Ericoid Mycorrhizae from Ecuador. Diversity. 2022; 14(8):648. https://doi.org/10.3390/d14080648
Chicago/Turabian StylePacheco Flores de Valgaz, Angela, Milton Barcos-Arias, Jaime Naranjo-Morán, Denisse Peña Tapia, and Rebeca Moreira-Gómez. 2022. "Ericaceous Plants: A Review for the Bioprospecting of Ericoid Mycorrhizae from Ecuador" Diversity 14, no. 8: 648. https://doi.org/10.3390/d14080648
APA StylePacheco Flores de Valgaz, A., Barcos-Arias, M., Naranjo-Morán, J., Peña Tapia, D., & Moreira-Gómez, R. (2022). Ericaceous Plants: A Review for the Bioprospecting of Ericoid Mycorrhizae from Ecuador. Diversity, 14(8), 648. https://doi.org/10.3390/d14080648






