Abstract
Secondary metabolites, in addition to playing an essential role in the adaptation of plants to the environment and phytochemical responses in recovery processes to stress conditions, are the base material of the healing effect of medicinal plants. In this study, the effect of growth conditions and localities of origin of Ageratina petiolaris on phenolic compounds content and antioxidant activity was evaluated; the plant is used for medicinal purposes in Oaxaca, Mexico. Samples of stem and young leaves were collected from plants growing naturally (in situ) in two locations in Oaxaca and from propagules collected in situ; plants were grown ex situ to obtain a set of equivalent samples to the first sampling (in situ). In both sets of samples, total polyphenol and flavonoid contents and antioxidant activity were evaluated by spectrometry, and later the phenolic acids and flavonoids were also identified and quantified by high-performance liquid chromatography with diode-array detection (HPLC-DAD). The growth conditions of A. petiolaris significantly influenced its phenolic composition and antioxidant activity, where samples collected in situ had a higher phenolic compounds content than did plants grown ex situ. Five phenolic acids and five flavonoids were identified, eight of which had not been reported in A. petiolaris: caffeic acid, ρ-coumaric acid, robinin, rutin, luteolin-7-glucoside, apigenin-7-glucoside, rosmarinic acid and kaempferol, in terms of HPLC-DAD analysis.
1. Introduction
Medicinal plants have been used since ancient times to cure different diseases, such as gastrointestinal disorders treatment [1]. In general, the plant kingdom is the source of a wide group of compounds or drugs for pharmacological use that contribute to human health. In recent decades, the potential biological use of compounds resulting from secondary metabolism has been explored for both direct use in the pharmacological industry and as phytodrugs in traditional and/or alternative medicine. Phenolic compounds are metabolites or molecules of high interest in alternative medicine and represent a response of plants to counteract the effects of biotic and abiotic stressors [2,3,4]. In general, the intrinsic and extrinsic factors affect the biosynthetic pathways of secondary metabolites in plants and mainly the phenolic compounds.
Phenolic compounds in medicinal plants are of great interest from multiple perspectives, e.g., to elucidate changes in biosynthesis pathways due to physiological stress effects, to identify products with pharmacological potential and/or antioxidant activity, and to identify products with potential use against cardiovascular and chronic degenerative diseases. The species or cultivars, growth and development stages, plant organs and tissues, growth conditions (environment) and plant–environment interactions affect the composition and concentration of phenolic compounds such as phenolic acids, flavonoids, lignans, stilbenes and total polyphenols, and these complex interactions determine some of the medicinal properties of plants [5,6,7,8].
Verma and Shukla [9] point out that genetic, ontogenic and morphogenetic factors of plants and environmental factors are responsible for the fluctuations in the synthesis of secondary metabolites in medicinal plants. In this sense, Li et al. [8] documented that the contents of phenols, flavonoids and terpenoids changed with the age of the plant, flowering or fruiting stage; for example, in some cases, the contents increased flowering and/or fruiting or were stable in the vegetative stage. Yang et al. [7] emphasized that ecological-environmental conditions alter the biosynthesis of secondary metabolites; for example, the photoperiod and short days regularly generate a decrease in phenols content and, on the contrary, they increase with the high intensity of light or shade, soil salinity, low or high temperatures, high concentrations of nitrogen fertilization or phosphates and also under water deficit or drought. However, the response is different depending on the species, the wild or cultivated forms, and the ontogenic characteristics of the plant and the group of compounds evaluated. In this sense, little is known about the response of wild medicinal plants to different growth conditions.
Ageratina petiolaris (Moc. and Sessé ex DC.) RM King & H. Rob. (syn. Eupatorium petiolare Moc. ex DC.) is a plant species widely used in traditional medicine [10] and distributed in the template regions from Mexico. A. petiolaris is also integrated in the medicinal bioculture of indigenous groups such as Purepechas in Michoacan, Otomi and Mazahua from Hidalgo and Estado de Mexico, Nahuatl of Veracruz, Zapotecos, Mixes, Mixtecos and Triqui of Oaxaca, Tzeltal and Tzotzil from Chiapas, and others. Main medicinal uses are against digestive disorders, stomach pain, ‘bilis’ (bile) or ‘corajes’ (courages), hepatic problems, dysentery, gastritis, indigestion, kidney problems, purgatives, ‘susto’ (scare), postpartum baths, rheumatism, against the nerves and more. A common way is by cooking or infusions of leaves and young stems as hot or semi-hot drinks such as tea, as alcoholic extract, aqueous extract or poultice, in function of the discomfort or problem [11,12]. In addition, this species is sporadically sold in the local markets and is unknown in urban centers.
The phytochemical analyses of A. petiolaris confer different current and potential properties, such as antibacterial activity, hepatic glucose inhibitor activity for type 2 diabetes treatment, hypoglycemic activity, anti-Helicobacter pylori activity and high antioxidant activity [10,11,12,13]. The main groups of compounds that have been identified in A. petiolaris include chlorogenic acid, L-chiro-inositol, 2α-iso-valeroyloxyeperuic acid, benzyl 2-hydroxy-6-methoxybenzoate, benzyl 2,6-dimethoxybenzoate, 3-methoxybenzyl, 2,6-dimethoxybenzoate, benzyl 2-hydroxy-3,6-dimethoxybenzoate and 2α-tigloyloxyeperuic acid [11]. These and other secondary metabolites tend to change in terms of biosynthesis and concentration in response to the environmental conditions that affect plant growth. Thus, the objective of this research was to evaluate the variation in the concentrations of total polyphenols, phenolic acids, flavonoids, and antioxidant activity in A. petiolaris as a function of growth conditions in situ (wild condition) and ex situ (cultivation) from populations of plants growing in their natural conditions in Oaxaca, Mexico.
2. Materials and Methods
2.1. Plant Material
Ageratina petiolaris (syn. Eupatorium petiolare) is a perennial shrub plant widely used in traditional medicine and is distributed in Mexico (classification corrected by a taxonomist, MEXU herbal); it has been used to treat gastrointestinal disorders and diabetes. In Spanish, it is known as ‘amargocilla’, ’angel grass’, ‘yolochíchitl’, ‘cahual’ or ‘cahual de burro’, and it is found from temperate to cold climates in altitudinal ranges from 900 to 3900 m [14,15]. In the municipal territory of San Martin Huamelulpam, Oaxaca, Mexico, it grows in spaces near cultivation plots, fences, backyards or on the banks of tributaries and is used for the treatment of diarrhea, pain, inflammation of the stomach, ‘empacho’ (indigestion) and ‘frialdad del estomago’ (stomach coldness) by topical applications on the stomach and lower back.
2.2. Approach and Experimental Design
The growth conditions of A. petiolaris were considered as factor A: in situ or natural growth conditions in SM Huamelulpam, Oaxaca, and ex situ or cultivated in Santa Cruz Xoxocotlan, Oaxaca, but from propagules of the same plants identified in the field and were assumed to be clones of the original plants. A second level or factor B included the sites or localities of ecogeographic origin of the populations in their natural locations: Primera Seccion and La Union within the municipal territory of SM Huamelulpam. In the context of this approach, we assumed a bifactorial design where factor A represented the growth conditions and factor B represented the locations where plants were obtained in situ, and all were established under a completely randomized experimental design. Table 1 describes the ecogeographic conditions of the in situ and ex situ growth conditions of A. petiolaris plants. In addition, samples were taken for subsequent soil chemical analysis in each locality of origin of the plants (in situ) and of soil substrate used for the growth of the ex situ clones, using the work of Borges et al. [16] as reference.

Table 1.
Geographic and environmental growing conditions of A. petiolaris evaluated from Oaxaca, Mexico.
Sampling of Plants for Analysis
In the first phase, three samples of stems and leaves of branches of plants at the vegetative stage (before flowering), which sections of leaves and young stems of 10–15 individuals were collected to integrate 1 kg of fresh tissue per sample from three different sites of the population or from a ‘patch’ of plants in the locality of the Primera Seccion, and three other samples of similar nature were collected from La Union, San Martin Huamelulpam, Oaxaca, between September and October 2020, which are the months following the intense rainy season. In the same places where the samples for phytochemical analysis were obtained from each locality, sections of plants were collected for clonal propagation or ex situ cultivation in pots with previously prepared soil substrates in Santa Cruz Xoxocotlán, Oaxaca, from September 2020 to May 2021 inside a space covered with shade mesh. The plant sections or propagules were transplanted to 20 L pots with an integrated substrate of organic matter, soil, and sand in a 1:1:1 ratio with subsequent chemical analysis of the substrate, and the daily temperatures and relative humidity were recorded (Table 1). Three pots from the locality La Union and three from Primera Seccion, SM Huamelulpam, were integrated, and from each pot, three samples of plant tissue equivalent to in situ sampling were collected using the same criteria. A central point in this design of in situ and ex situ sampling was to compare the same plant populations, in the first case growing in natural conditions, and later the same plants propagated clonally or vegetatively growing under cultivation.
2.3. Determination of Phenolic Compounds and Antioxidant Activity by Spectrophotometry
2.3.1. Sample Preparation
The in situ and ex situ plant material was washed, cut into small pieces, dried at 40 °C in a dehydrator (L’Equipe model 528) and pulverized using a mill (Krups® model GX4100, Mexico). Subsequently, samples were stored at −20 °C in an airtight container until evaluation. For spectrophotometric determinations of phenolic compounds and antioxidant activity, methanolic extract was obtained from 0.1 g of dry milled sample and 25 mL of methanol 60% (v/v), which was homogenized for 60 s in intervals of 30 (Ultra Turrax T 25 Digital, IKA, Staufen, Germany) and centrifuged (Eppendorf AG, Mod. 5811F, Hamburg, Germany) at 11,000 rpm for 15 min at 4 °C, and the supernatant was used for the respective analyses. All determinations were carried out in triplicate.
2.3.2. Total Polyphenols
The analysis of total polyphenols was performed according to the Folin–Ciocalteu method described by Singleton and Rossi [19]. Absorption was measured at 750 nm using a spectrophotometer (VELAB, Mod. VE-5600UV PC, TX, USA). The concentration was determined with reference to a calibration curve of a gallic acid standard of (from 0.021 to 0.165 mg mL−1, r2 = 0.9998), and results were expressed as milligrams equivalent to gallic acid per gram of dry weight (mg GAE g−1 dw).
2.3.3. Flavonoids
They were determined using two colorimetric methods. The first method followed the methodology of Lin and Tang [20] based on the reaction of aluminum chloride (AlCl3) in the presence of flavonoids, which helped to estimate the flavonols and flavones (luteolin) content [21] based on the absorbance at 415 nm in a spectrophotometer, and the concentration was estimated with reference to a standard quercetin fitted curve (from 0.01 to 0.17 mg mL−1, r2 = 0.9993) and expressed as mg quercetin equivalents per gram of dry weight (mg QE g−1 dw). The second estimation was performed with sodium nitrite (NaNO2) in an alkaline medium [22] to evaluate the contents of rutin, luteolin and catechins [21]. The absorbance at 510 nm was recorded in a spectrophotometer and quantified based on a standard catechin calibration curve (from 0.01 to 0.5 mg mL−1, r2 = 0.9988). Results were expressed as mg catechin equivalents per gram of dry weight (mg CE g−1 dw).
2.3.4. Antioxidant Activity by DPPH and FRAP
The DPPH (2,2-diphenyl-1-picrylhydrazyl, Sigma–Aldrich) method described by Brand-Williams et al. [23] was used. The absorbance was measured in a spectrophotometer at 517 nm, and the content was quantified with reference to a Trolox calibration curve (from 0.13 to 1.33 μmol mL−1, r2 = 0.9997). The antioxidant activity of the FRAP method (iron reducing power) was evaluated by the method described by Benzie and Strain [24]. The absorbance at 593 nm was recorded in a spectrophotometer and quantified with reference in a Trolox calibration curve of 0.05–1 μmol mL−1, r2 = 0.9994. The results of both determinations were expressed as equivalent micromoles of Trolox per gram of dry weight (μmol TE g−1 dw).
2.4. Determination of Phenolic Acids and Flavonoids by HPLC-DAD
2.4.1. Sample Preparation
A methanolic extract was obtained from 0.3 g of dry milled sample (Section 2.3.1) and 15 mL of methanol 60% (v/v), which was homogenized for 60 s in intervals of 30 s intervals (Ultra Turrax T 25 Digital, IKA, Staufen, Germany) and sonicated in an ultrasonic bath (Cole-Parmer, Mod. 08895-43, IL, USA) for 1 h. Subsequently, it was centrifuged at 11,000 rpm (refrigerated Eppendorf 5810R centrifuge) at 4 °C for 15 min, and the supernatant was used for the HPLC-DAD analysis. Then, a 2 mL aliquot of methanolic extract (60%) was filtered by a 0.2-μm PTFE syringe (Agilent Technologies®, Part. No. 5190-5086, Waldbronn, Germany) and placed in amber vials.
2.4.2. HPLC-DAD Analysis
The analysis of phenolic acids and flavonoids was performed using an HPLC-DAD following the method described by Pająk et al. [25] with some modifications. The instrument was an Agilent model Infinity II 1260 LC system equipped with a solvent degasser, quaternary pump, temperature-controlled autosampler, column oven and DAD (Agilent Technologies, Santa Clara, CA, USA). A reversed-phase column (Agilent® Hypersil 5 ODS, 250 × 4.6 mm, 5 μm) was used. The column temperature was maintained at 30 °C. The mobile phase consisted of 1% acetic acid in water (solvent A) and acetonitrile (solvent B). The gradient used was from 10 to 100% B from 0 to 60 min. The flow was 1 mL min−1, and the injection volume was 15 µL. The monitoring wavelengths for phenolic acids and flavonoids were 260 nm (luteolin-7-glucoside, rutin and robinin), 280 nm (gallic acid), 320 nm (apigenin-7-glucoside, caffeic acid, ρ-coumaric acid, chlorogenic acid and rosmarinic acid) and 370 nm (kaempferol). The identification of each compound was made with reference to the retention times and UV spectra of commercial standards (Phyproof®, Phytolab Gmb H & Co. KG, Vestenbergsgreuth, Germany, and Sigma-aldrich®, St. Louise, MO, USA), and the quantification by calibration curves of reference standards: gallic acid (from 0.04 to 6.4 µg mL−1, r2 = 0.999), chlorogenic acid (from 1.27 to 203 µg mL−1, r2 = 0.998); caffeic acid (from 0.04 to 12 µg mL−1, r2 = 0.999), ρ-coumaric acid (from 0.06 to 18 µg mL−1, r2 = 0.999), robinin (from 0.07 to 20 µg mL−1, r2 = 0.996), rutin (from 0.9 to 256 µg mL−1, r2 = 0.999), luteolin-7 -glucoside (from 0.2 to 70 µg mL−1, r2 = 0.996), apigenin-7-glucoside (from 0.03 to 64 µg mL−1, r2 = 0.999), rosmarinic acid (from 0.9 to 533 µg mL−1, r2 = 0.999) and kaempferol (from 0.12 to 18 µg mL−1, r2 = 0.999). The amount of each compound was expressed as micrograms per gram of dry weight (µg−1 dw).
2.5. Statistical Analysis
Based on the information from the phytochemical analysis of the samples, a database was established, and an analysis of variance was performed using a completely random linear model. To evaluate the specific differences between localities of sample origins and between plant growth environments (in situ and ex situ) and locality–environment interactions, comparisons of means were made by the Tukey method (p < 0.05). In addition, Pearson correlation analysis was performed between total polyphenols and flavonoids versus antioxidant activity, and principal component analysis was performed with average values per sample. All statistical analyses were performed with the statistical package SAS Inc. (SAS Institute Inc., Cary, NC, USA) [26].
3. Results
3.1. Total Phenolic Compounds and Antioxidant Activity
In the analysis of variance, significant differences (p < 0.01) were determined between environmental growth conditions and total polyphenols, quercetin equivalent flavonoids and antioxidant activity. Between localities of sample origin (populations of A. petiolaris), no significant differences were determined (p > 0.05) in phenolic compounds or antioxidant activity; and in growth environment–population interactions, significant differences were recorded (p < 0.01) in all the compounds evaluated and in antioxidant activity (Table 2). In this study, sampling in two localities of origin of A. petiolaris indicated that, at this level of phytochemical analysis, independent populations could not be considered. The greater magnitude of the variance (= mean square) due to the interaction between environments and local populations of A. petiolaris was an indicator that the phenolic compounds and antioxidant activity evaluated were affected or modified by the plant growth conditions.

Table 2.
Significance of square means of the analysis of variance of phenolic compounds and antioxidant activity of A. petiolaris from Oaxaca, Mexico.
The absence of significant differences between the samples of A. petiolaris carried out in the locality of the Primera Seccion and La Union, municipality of SM Huamelulpam, in terms of total polyphenols, flavonoids and antioxidant activity (Table 3), was an indicator of similar effect of growth locations in the phenolic compounds during the sampling season. The distance between sampling sites of the plant patches was not greater than 5 km. The growth conditions in situ or natural distribution place of A. petiolaris generated a higher concentration of total polyphenols, flavonoid quercetin equivalents and antioxidant activity than in ex situ conditions, where there were better conditions for the growth and development of the clonally propagated plants in situ (see Table 1).

Table 3.
Average content of phenolic compounds and antioxidant activity of A. petiolaris plants sampled in situ (natural conditions) and cultivated ex situ at Oaxaca, Mexico.
The interaction conditions of growth-locality of origin (E × Po) were significant and had a different pattern according to the origin. For example, the plants of the Primera Seccion sampled in situ showed higher contents of total polyphenols and flavonoids and higher antioxidant activity than their ex situ counterparts. In contrast, the plants cultivated and sampled ex situ from La Union showed higher contents of total polyphenols and flavonoid catechin equivalents than their counterparts in situ, but both had similar contents of flavonoid quercetin equivalents and antioxidant activity (Table 3).
In the correlation analysis between phenolic compounds and antioxidant activity, positive and significant correlations were determined between the content of total polyphenols and flavonoid quercetin and catechin equivalents and antioxidant activity evaluated by the DPPH and FRAP methods, both in ex situ cultivated plants (0.97 < r ≤ 0.99; p < 0.001) and in those sampled in situ (0.73 < r < 0.95, p < 0.001). In this case, regardless of the growth conditions of A. petiolaris, part of its antioxidant potential was due to the biosynthesis of phenolic compounds.
3.2. Profile of Phenolic Acids and Flavonoids
Through the analysis of phenolic acids and flavonoids by HPLC-DAD, in methanolic extracts of leaves and young stems of A. petiolaris, five phenolic acids were identified and quantified: gallic acid, chlorogenic acid, caffeic acid, ρ-coumaric acid and rosmarinic acid; additionally, five glycosylated flavonoids were identified and quantified: robinin or kaempferol-3-O-robinoside-7-O-rhamnoside, rutin or quercetin 3-rutinoside, luteolin-7-glucoside, apigenin-7-glucoside and flavonol kaempferol (Figure 1). This identification was based on reference standard spectra, which should be confirmed by such as mass spectrometry.
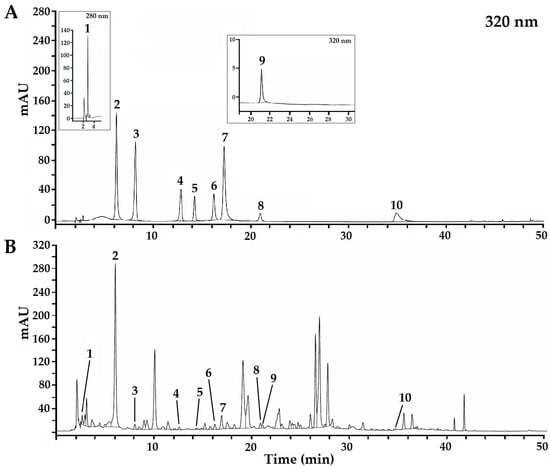
Figure 1.
Chromatographic profile based on HPLC-DAD analysis of standard compounds identified (A) and methanolic extracts from A. petiolaris (B): (1) gallic acid, (2) chlorogenic acid, (3) caffeic acid, (4) ρ-coumaric acid, (5) robinin, (6) rutin, (7) luteolin-7-glucoside, (8) apigenin-7-glucoside, (9) rosmarinic acid and (10) kaempferol.
Among localities of origin of the sampled plants (Po), significant differences (p < 0.01) in the contents of gallic acid, robinin, rutin, luteolin-7-glucoside and kaempferol were determined. Between environmental conditions of growth (E), the differences were significant in all evaluated phenolic acids and flavonoids, except chlorogenic acid and caffeic acid, and in the locality-environment interaction (Po × E), significant differences (p < 0.05, 0.01) were recorded in all compounds evaluated by HPLC-DAD (Table 4) in A. petiolaris. Regarding the effect’s magnitude of localities, growth environments and interaction between localities and environments, as estimated from the value of the variances or mean square, the contents of gallic acid, robinin, rutin, luteolin-7-glucoside, apigenin-7-glucoside and rosmarinic acid were strongly influenced by the growth conditions (E), with the exception of kaempferol, which had a strong effect on the localities of origin of the plant samples or the population effect (Po) of A. petiolaris.

Table 4.
Significance of square means of analysis of variance regarding phenolic acids and flavonoids evaluated in A. petiolaris by HPLC-DAD.
Among localities of origin of the evaluated plants, i.e., Primera Seccion and La Union de SM Huamelulpam, Mexico, higher concentrations of ρ-coumaric acid, robinin, rutin, luteolin-7-glucoside, apigenin-7-glucoside and rosmarinic acid were observed in Primera Seccion than in La Union, and in gallic acid and kaempferol, the pattern was reversed (Table 5).

Table 5.
Phenolic acid and flavonoid contents in A. petiolaris plants grown under natural (in situ) and cultivated (ex situ) conditions by HPLC-DAD from SM Huamelulpam, Oaxaca, Mexico.
In the comparison between plant growth environments, it was recorded that in the in situ growth conditions or in their natural place, the plants synthesized higher contents of the same compounds that were observed with higher concentrations in the Primera Seccion locality, and the exception was kaempferol, which had a higher concentration in plants cultivated ex situ (Table 5). In addition, the presence of gallic acid and rutin was not detected in ex situ conditions. In the interaction of localities–plant growth environments, no detection of gallic acid and rutin was recorded in plants sampled from both localities of origin and in those cultivated ex situ. Another observed pattern was that the plants sampled in situ in the Primera Seccion locality recorded higher concentrations of ρ-coumaric acid, robinin, rutin, luteolin-7-glucoside, apigenin-7-glucoside and rosmarinic acid, and only kaempferol was present at higher values in plants cultivated ex situ and in plants from La Union. In addition, the Primera Seccion plants showed significant decreases (> double) in all the evaluated compounds when they were cultivated ex situ, a pattern that was repeated, although to different magnitudes, in the plants from La Union.
In the multivariate descriptive analysis of principal components, two significantly different groups were distinguished in terms of composition, which corresponded to in situ and ex situ sampling, even when there were differences between localities of origin of the plants, where the highest concentrations of total polyphenols, phenolic acids, flavonols and flavonoids were recorded in the samples from plants growing in their natural place of distribution (in situ) and the concentration decreased when the plants were cultured ex situ (Figure 2). In addition, the differences between the localities of origin of the plants were noticeable; that is, the plants collected in the Primera Seccion exceeded the composition of total polyphenols, flavonoids, gallic acid, rosmarinic acid and chlorogenic acid compared with those of La Union. The intragroup patterns are shown in Figure 2, and both sites were located within the municipal territory of San Martin Huamelulpam, Oaxaca, Mexico.
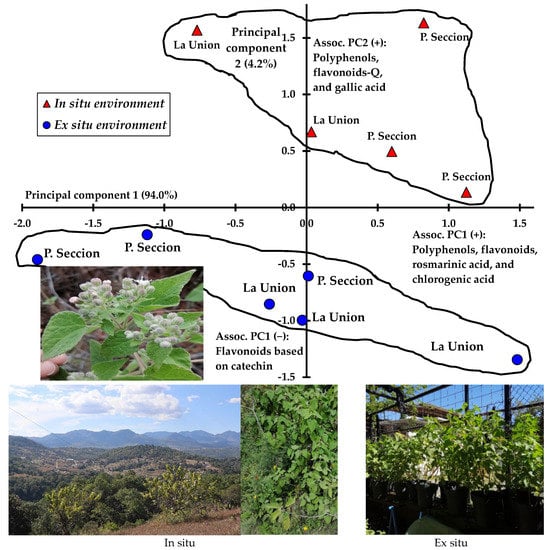
Figure 2.
Scatterplot of samples collected in situ and cultivated ex situ of A. petiolaris and based on principal component analysis of total polyphenols, flavonoids and phenolic acids.
4. Discussion
Ageratina petiolaris is a shrub plant endemic to the temperate zones of Mexico; it is distributed naturally in areas above 1000 m altitude in Oaxaca and has wide medicinal use among indigenous communities. It has healing properties that are commonly conferred in San Martin Huamelulpam, and it is associated with the bitter flavors of the infusions or decoctions that are prepared to counteract gastrointestinal disorders, digestive disorders, liver diseases, stomach pains and ‘bile’, among other uses. The spectrophotometric analyses of samples collected in the localities of Primera Seccion and La Union showed that there were no statistically significant differences in total polyphenols, flavonoids and antioxidant activity and indicated that the differences in the soil and ecological micro niche characteristics did not significantly influence their general composition (Table 2 and Table 3). The samples came from population patches because they were gathered in highly disturbed areas, and perhaps they were originally the same population since the distances between sites did not exceed 5 km. This approach was consistent with that of Gui et al. [27], who studied the structure and population diversity of Ageratina adenophora in China using ISSR markers, indicating that the populations collected from the same region or nearby regions did not differ genetically and had integrated genetic groups by geographic region.
The results showed that the conditions or place of growth and development of A. petiolaris significantly affected its contents of total polyphenols and quercetin equivalent flavonoids and its antioxidant activity. Specifically, under in situ conditions or natural growth conditions, A. petiolaris had higher concentrations of total polyphenols and flavonoids (Table 3) than ex situ plants (cultivated). In cultivation, water was provided at field capacity, with higher average temperatures (18–20 °C) and higher soil fertility (e.g., N, P, K, Ca, Mg, Na, Fe, Zn, Mn and Cu) than those under in situ conditions for the growth of A. petiolaris. These conditions contrast with the ecological-environmental conditions in situ where plants are exposed to more than one condition of abiotic stress such as drought, rain seasonality or water stress, low soil fertility or lower average temperature, e.g., 14–18 °C (Table 1). This result is consistent with what has been stated and documented by Arbona et al. [28], Bautista et al. [6], Yang et al. [7], and Banothu and Uma [4], who found that conditions of abiotic stress generated changes in metabolic processes (e.g., the shikimate pathway and phenylalanine) and induced an increase in the concentration of phenolic compounds. These authors also noted that under natural conditions, there were usually combinations of different stress factors that induced response mechanisms, such as physiological tolerance (biosynthesis of compounds) or evasion before death.
The interaction was significant between plant growth conditions and locality of origin of the evaluated samples of A. petiolaris (population × environment). That is, the content of phenolic compounds and antioxidant activity of A. petiolaris collected in the Primera Seccion or La Union, Huamelulpam, Mexico, was modified from its natural in situ condition when grown under controlled conditions of moisture and soil fertility (ex situ). The plants sampled in situ in the Primera Seccion showed a higher concentration of compounds and antioxidant activity than did those sampled ex situ, but in contrast, the ex situ cultivation of La Unión plants generated a higher content of total polyphenols and flavonoids than those in situ (Table 3). The plants of the Primera Seccion indicated that the in situ stress conditions favored an increase in compounds, as has been observed in other wild plants [2,6,29], but in plants of La Union, the increase may be due to differences in the ages of the plants collected in situ to be cultivated ex situ, as documented. Li et al. [8], in different medicinal plants, pointed out that older plants (from 10 to 13 years) tended to have increased concentrations of phenols and flavonoids.
Another consideration between in situ and ex situ conditions was slight differences in maximum solar irradiation, which could affect the compound phenolic biosynthesis. For example, in the collect locations (in situ), a variation was reported from 1155 to 1308 W m−2 and ex situ from 1297 to 1349 W m−2 (Table 1), which indicates the incident radiation or sunny days for each growth environment where ex situ was slight major.
Quantifying antioxidant activity is a methodological strategy to evaluate the biological (e.g., antimicrobial) and/or pharmacological (e.g., hypoglycemic, antiulcer, wound healing) potential of bioactive compounds in medicinal plants [30,31,32]. The positive correlation between total polyphenols and flavonoids and antioxidant activity evaluated by DPPH and FRAP in A. petiolaris indicates the species’ pharmacological and beneficial potential for human health, e.g., the use against gastrointestinal disorders recommended by people from San Martin Huamelulpam, Mexico, where the plant samples used in this study were collected. In addition to total polyphenols and flavonoids, A. petiolaris contains other compounds associated with antioxidant potential that confer hypoglycemic effects [11] or inhibit hepatic glucose production [12].
In previous studies on the composition of phenolic compounds in the genus Ageratina (syn. Eupatorium), different compounds have been identified. For example, in A. espinosarum, Díaz-Peralta et al. [33] recorded up to 20 compounds among the most relevant neo-clerodane diterpenes, flavonols, flavanones and flavones: taxifolin, naringenin, sakuranetin, persicogenin, apigenin, acacetin and kaempferol. In A. pichinchensis, Romero-Cerecero et al. [34] determined chlorogenic acid and glycosylated flavonoids. In A. petiolaris, Bustos-Brito et al. [11] isolated chlorogenic acid, L-chiro-inositol, benzyl 2-hydroxy-6-methoxybenzoate, benzyl 2-hydroxy-3,6-dimethoxybenzoate and 2α-tigloyloxyeperuic acid for the first time. In this work, out of a total of 10 compounds identified, eight have not been previously reported in A. petiolaris: caffeic acid, ρ-coumaric acid, robinin, rutin, luteolin-7-glucoside, apigenin-7-glucoside, rosmarinic acid, and kaempferol (Figure 1).
In A. petiolaris, there were no significant differences between localities of origin of the samples (Primera Seccion and La Union) in the contents of chlorogenic acid, caffeic acid, ρ-coumaric acid, apigenin-7-glucoside and rosmarinic acid; thus, these compounds do not help to differentiate populations or subpopulations within the same species when the distances between sampling sites are not greater than 5 km in temperate climate conditions (Table 1 and Table 5). However, the robinin, rutin and luteolin-7-glucoside contents were higher in the Primera Seccion than in La Union, and the inverse pattern was recorded for gallic acid and kaempferol. It should be noted that differences in soil chemical characteristics were recorded between localities; for example, higher contents of P, Mg, Fe, Zn, Mn, Cu and N inorganic and organic matter were recorded in La Unión than in the Primera Seccion (Table 1), which can confer a state of stress due to low soil fertility in the Primera Seccion and may affect the growth and development of plants as well as the phytochemical composition of the aerial parts. In the case of cultivated species, Yang et al. [7] documented that the contents of quercetin, kaempferol and isorhamnetin increased with low concentrations and excesses of N and P. However, the specific responses in A. petiolaris exposed to stress conditions have not been evaluated thus far.
In the profile of compounds identified in A. petiolaris, it was recorded, in general, that the growth conditions of the plants had a significant effect on the content of phenolic acids, except chlorogenic acid and caffeic acid (Table 4). In plants sampled under natural conditions (in situ), higher contents of ρ-coumaric acid, robinin, rutin, luteolin-7-glucoside, apigenin-7-glucoside and rosmarinic acid were found than in those cultivated ex situ (Table 5); these physiological and phytochemical responses are, in part, due to the combined effect of biotic and abiotic factors [4,28]. Even when it is not possible to establish a direct relationship between stress conditions and increased phenolic acids, in situ plants were exposed to rain seasonality, lower temperatures (14–18 °C) and lower soil fertility (N, P, K, Mg, Fe Zn, Cu and Mn) than ex situ plants where water was provided at field capacity, the average temperatures were 18–20 °C and the substrate had a better balance of fertility and organic matter (Table 1). In this sense, Yang et al. [7] indicated that, under natural or uncontrolled conditions, environmental factors acted simultaneously in the biosynthesis of secondary metabolites. In nineteen wild species from four Mediterranean ecosystems, Bautista et al. [6] determined a positive correlation between phenolic compounds and environmental parameters of plant growth, such as water stress, temperature, evapotranspiration and water deficit in the soil.
In the interaction of localities of origin of the evaluated plants and growth conditions, it was recorded that the production of gallic acid and rutin was not synthesized in sufficient quantity to be detected in the samples evaluated by HPLC-DAD when they came from plants grown ex situ, but production was detected in samples from in situ conditions (Table 5). This pattern indicates a possible plant response mechanism in modifying the biosynthetic processes associated with stress conditions in the growth environment [35]. The results coincide with the documentation carried out by Li et al. [8] in different medicinal plants, where they indicated that the concentration of rutin increased in the face of water stress. A similar pattern of increase was described by Bettaieb et al. [36] in the gallic acid content in Salvia officinalis plants subjected to moderate and severe water deficit conditions. In this work, it was determined that the natural conditions (in situ) prevailing in the locality of the Primera Seccion, with a temperate climate and lower soil fertility, significantly influenced the synthesis of higher concentrations of ρ-coumaric acid, robinin, rutin, luteolin-7-glucoside, apigenin-7-glucoside and rosmarinic acid (Table 5, Figure 2), and a similar effect was recorded. Bettaieb et al. [36] studied the contents of rosmarinic acid, ρ-coumaric acid and chlorogenic acid in S. officinalis plants subjected to water stress, but Singh et al. [37] noted that in medicinal plants, similar responses were not always obtained after identifying twelve phenolic compounds in twelve wild medicinal species of India by HPLC-DAD.
In Figure 2 is represented in a graphic way the contribution of polyphenols, flavonoids, phenolic acids, glycosylated flavonoids and one flavonol in the differentiation of origin locations as well as growth environments offering a complementarity of results, which are detailed in Table 3 and Table 5. In this work, it was noted that general composition allowed to guide the second phase on specific composition mainly in phenolic compounds related to gastrointestinal disorders based on the traditional use of A. petiolaris.
Phenolic compound biosynthesis in plants in natural conditions reflects different and complex regulatory processes. The comparison here described (in situ versus ex situ) was conditioned by seasonal climatic conditions, soil characteristics (e.g., organic matter, interchange cationic capacity, etc.), age of plants evaluated, probable genetic relationships, and other intrinsic and extrinsic factors. Consequently, it is necessary to evaluate under strict experimental control the specific effect of each factor in the composition, for example, temperature, light UV, humidity, pH, electric conductivity, and micro- and macronutrients.
5. Conclusions
The results of the phytochemical evaluation in samples of stem and young leaves of Ageratina petiolaris collected in their natural environment (in situ) and from cultivated plants (ex situ) showed that the differences in the composition of total polyphenols, flavonoids, phenolic acids, glycosylated flavonoids, flavonols and antioxidant activity were modified by the effect of growth conditions and interacted significantly with localities of sampling origin, and specifically, in in situ conditions where there were limiting edaphic-environmental factors, there were increases in phenolic compounds, flavonoids and antioxidant activity. Ten phenolic compounds were identified in terms of HPLC-DAD: five phenolic acids and five flavonoids, eight of which had not been previously reported in A. petiolaris: caffeic acid, ρ-coumaric acid, robinin, rutin, luteolin-7-glucoside, apigenin-7-glucoside, rosmarinic acid and kaempferol. In the composition of A. petiolaris identified and described, different compounds associated with antimicrobial, antifungal, antioxidant and anti-inflammatory activity were recognized that explained part of the medicinal properties conferred on this species by the communities of Oaxaca, Mexico.
Author Contributions
Conceptualization and methodology, M.L.P.-O., A.M.V.-G., D.M.M.-C., S.S.-T., J.C.C.-R. and J.L.C.-S.; investigation and writing, M.L.P.-O., A.M.V.-G. and J.L.C.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Instituto Politecnico Nacional-Mexico by projects no. SIP-20220980 and SIP-20220820.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge all families from the municipalities of San Martin Huamelulpam, Oaxaca, Mexico, who shared their experiences with medicinal plants. In addition, we appreciate the correction of the taxonomic classification of the plant species to Rosalinda Medina-Lemos of the Instituto de Biologia, Universidad Nacional Autonoma de Mexico.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pérez-Ochoa, M.L.; Chávez-Servia, J.L.; Vera-Guzmán, A.M.; Aquino-Bolaños, E.N.; Carrillo-Rodríguez, J.C. Medicinal plants used by indigenous communities of Oaxaca, Mexico, to treat gastrointestinal disorders. In Pharmacognosy—Medicinal Plants; Perveen, S., Al-Taweel, A., Eds.; IntechOpen: London, UK, 2019; pp. 1–37. [Google Scholar]
- Ramakrishna, A.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant. Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef] [PubMed]
- Nisar, A. Medicinal plants and phenolic compounds. In Phenolic Compounds—Chemistry, Synthesis, Diversity, Non-Conventional Industrial Pharmaceutical and Therapeutic Applications; Badria, F.A., Ed.; IntechOpen: London, UK, 2022; pp. 1–11. [Google Scholar] [CrossRef]
- Banothu, V.; Uma, A. Effect of biotic and abiotic stresses on plant metabolic pathways. In Phenolic Compounds—Chemistry, Synthesis, Diversity, Non-Conventional Industrial Pharmaceutical and Therapeutic Applications; Badria, F.A., Ed.; IntechOpen: London, UK, 2022; pp. 1–11. [Google Scholar] [CrossRef]
- Sampaio, B.L.; Edrada-Ebel, R.; Batista-Da Costa, F. Effect of the environment on the secondary metabolic profile of Tithonia diversifolia: A model for environmental metabolomics of plants. Sci. Rep. 2016, 6, 29265. [Google Scholar] [CrossRef] [Green Version]
- Bautista, I.; Boscaiu, M.; Lidón, A.; Linares, J.V.; Lull, C.; Donat, M.P.; Mayoral, O.; Vicente, O. Environmentally induced changes in antioxidant phenolic compounds levels in wild plants. Acta Physiol. Plant. 2016, 38, 9. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.-S.; Ruan, X.; Zhao, Y.-Y.; Wei, F.; Wang, Q. Response of plant secondary metabolites to environmental factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef]
- Verma, N.; Shukla, S. Impact of various factors responsible for fluctuation in plant secondary metabolites. J. Appl. Res. Med. Aromat. Plants 2015, 2, 105–113. [Google Scholar] [CrossRef]
- Sharma, A.; Flores-Vallejo, R.C.; Cardoso-Taketa, A.; Villarreal, M.L. Antibacterial activities of medicinal plants used in Mexican traditional medicine. J. Ethnopharmacol. 2017, 208, 264–329. [Google Scholar] [CrossRef] [PubMed]
- Bustos-Brito, C.; Andrade-Cetto, A.; Giraldo-Aguirre, J.D.; Moreno-Vargas, A.D.; Quijano, L. Acute hypoglycemic effect and phytochemical composition of Ageratina petiolaris. J. Ethnopharmacol. 2016, 185, 341–346. [Google Scholar] [CrossRef]
- Mata-Torres, G.; Andrade-Cetto, A.; Espinoza-Hernández, F.A.; Cárdenas-Vázquez, R. Hepatic glucose output inhibition by Mexican plants used in the treatment of type 2 diabetes. Front. Pharmacol. 2020, 11, 215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castillo-Juárez, I.; González, V.; Jaime-Aguilar, H.; Martínez, G.; Linares, E.; Bye, R.; Romero, I. Anti-Helicobacter pylori activity of plants used in Mexican traditional medicine for gastrointestinal disorders. J. Ethnopharmacol. 2009, 122, 402–405. [Google Scholar] [CrossRef]
- De Rzedowski, G.C.; Rzedowski, J. Flora Fanerogámica del Valle de México, 2nd ed.; Instituto de Ecología, A.C., Comisión Nacional para el Conocimiento, Uso de la Biodiversidad: Pátzcuaro, México, 2005; pp. 786–800. [Google Scholar]
- UNAM. Biblioteca Digital de la Medicina Tradicional Mexicana; Universidad Nacional Autónoma de México: Ciudad de México, Mexico, 2009; Available online: http://www.medicinatradicionalmexicana.unam.mx/index.php (accessed on 25 January 2022).
- Borges, L.L.; Alves, S.F.; Sampaio, B.L.; Conceição, E.C.; Bara, M.T.F.; Paula, J.R. Environmental factors affecting the concentration of phenolic compounds in Myrcia tomentosa leaves. Rev. Bras. Farmacogn.-Braz. J. Pharmacogn. 2013, 23, 230–238. [Google Scholar] [CrossRef] [Green Version]
- INEGI (Instituto Nacional de Estadística y Geografía). Compendio de Información Geográfica Municipal 2010. San Martín Huamelúlpam, Oaxaca; INEGI: Aguascalientes, Mexico, 2005.
- NOM (Norma Oficial Mexicana). NOM-021-RECNAT-2000; Especificaciones de Fertilidad, Salinidad y Clasificación de Suelos. Estudios, Muestreo y Análisis. Diario Oficial, 31 December 2002; 73. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Lin, J.-Y.; Tang, C.-Y. Determination of total phenolic and flavonoid contents in selected fruits and vegetables, as well as their stimulatory effects on mouse splenocyte proliferation. Food Chem. 2007, 101, 140–147. [Google Scholar] [CrossRef]
- Pękal, A.; Pyrzynska, K. Evaluation of aluminium complexation reaction for flavonoid content assay. Food Anal. Methods 2014, 7, 1776–1782. [Google Scholar] [CrossRef] [Green Version]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Pająk, P.; Socha, R.; Broniek, J.; Królikowska, K.; Fortuna, T. Antioxidant properties, phenolic and mineral composition of germinated chia, golden flax, evening primrose, phacelia and fenugreek. Food Chem. 2019, 275, 69–76. [Google Scholar] [CrossRef] [PubMed]
- SAS Institute Inc. (SAS). Base SAS® 9.1.3 Procedures Guide, 2nd ed.; SAS Institute Inc.: Cary, NC, USA, 2006; Volumes 1–4. [Google Scholar]
- Gui, F.-R.; Wan, F.-H.; Guo, J.-Y. Population genetics of Ageratina adenophora using inter-simple sequences repeat (ISSR) molecular markers in China. Plant Biosyst. 2008, 142, 255–263. [Google Scholar] [CrossRef]
- Arbona, V.; Manzi, M.; de Ollas, C.; Gómez-Cárdenas, A. Metabolomics as a tool to investigate abiotic stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 4885–4911. [Google Scholar] [CrossRef]
- Nurzyńska-Wierdak, R. Does mineral fertilization modify essential oil content and chemical composition in medicinal plants? Acta Sci. Pol. 2013, 12, 3–16. [Google Scholar]
- Krishnaiah, D.; Sarbatly, R.; Mithyanandam, R. A review of the antioxidant potential of medicinal plant species. Food Bioprod. Process. 2011, 89, 217–233. [Google Scholar] [CrossRef]
- Arciniegas, A.; Pérez-Castorena, A.L.; Meléndez-Aguirre, M.; Ávila, J.G.; García-Bores, A.M.; Villaseñor, J.L.; de Vivar, A.R. Chemical composition and antimicrobial activity of Ageratina deltoidea. Chem. Biodivers. 2018, 15, e1700529. [Google Scholar] [CrossRef]
- Sánchez-Ramos, M.; Marquina-Bahena, S.; Alvarez, L.; Román-Guerrero, A.; Bernabé-Antonio, A.; Cruz-Sosa, F. Phytochemical, Pharmacological, and Biotechnological Study of Ageratina pichinchensis: A Native Species of Mexico. Plants 2021, 10, 2225. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Peralta, L.; Ocampo-Acuña, Y.D.; Rios, M.Y. Secondary metabolites from two varieties of Ageratina espinosarum and their chemophenetic significance. Biochem. Syst. Ecol. 2022, 102, 104409. [Google Scholar] [CrossRef]
- Romero-Cerecero, O.; Zamilpa, A.; González-Cortazar, M.; Alonso-Cortés, D.; Jiménez-Ferrer, E.; Nicasio-Torres, P.; Aguilar-Santamaría, L.; Tortoriello, J. Pharmacological and chemical study to identify wound-healing active compounds in Ageratina pichinchensis. Planta Med. 2013, 79, 622–627. [Google Scholar] [CrossRef]
- Hodaei, M.; Rahimmalek, M.; Arzani, A.; Talebi, M. The effect of water stress on phytochemical accumulation, bioactive compounds and expression of key genes involved in flavonoid biosynthesis in Chrysanthemum morifolium L. Ind. Crops. Prod. 2018, 120, 295–304. [Google Scholar] [CrossRef]
- Bettaieb, I.; Hamrouni-Sellami, I.; Bourgou, S.; Limam, F.; Marzouk, B. Drought effects on polyphenol composition and antioxidant activities in aerial parts of Salvia officinalis L. Acta Physiol. Plant. 2011, 33, 1103–1111. [Google Scholar] [CrossRef]
- Singh, G.; Passsari, A.K.; Leo, V.V.; Mishra, V.K.; Subbarayan, S.; Singh, B.P.; Kumar, B.; Kumar, S.; Gupta, V.K.; Lalhlenmawia, H.; et al. Evaluation of phenolic content variability along with antioxidant, antimicrobial, and cytotoxic potential of selected traditional medicinal plants from India. Front. Plant Sci. 2016, 7, 407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).