Abstract
In our study the presence of bacteria, yeast, and microscopic fungi was evaluated. Three forms of corn silage were made including silage without additive, silage with microbial additive (lactic acid bacteria), and silage with nutritional additive (urea). Silage additives were applied to the matter within the recommended dosage, then the matter was ensiled into plastic bags and stored at a constant temperature. After 5.5 months of storage, average samples for microbial and mycotoxins analysis were taken. From microbiological points, the plate count agar method for enumeration of total count of bacteria, lactic acid bacteria, enterococci, yeasts, and microscopic fungi and mass spectrometry for microbiota identification were used. In total, 43 species of bacteria and yeasts and 6 genera of microscopic fungi were identified from all samples of corn silages. The most isolated species were Lentilactobacillus buchneri and Kazachstania exigua from bacteria resp. yeasts and Aspergillus and Penicillium from microscopic fungi. Mycotoxins were determined by HPLC-MS/MS and divided into two groups as regulated and emerging. In the corn silages only Fusarium mycotoxins were observed. All corn silages, regardless of the addition of the additive, were the highest in nivalenol content. Deoxynivalenol and beauvericin with the highest concentrations were present in silage with urea. Although the mycotoxins content of the variants changed, these changes were not statistically significant. In general, addition of lactic acid bacteria Lentilactobacillus buchneri and Lacticaseibacillus casei and urea as silage additives affect the microbial diversity; however, the hygienic quality of whole crop corn silage was not negatively changed.
1. Introduction
The silage microbiota contains beneficial microorganisms such as lactic acid bacteria (LAB) and spoilage microorganisms such as mold and yeast [1]. Therefore, the abundance and species of epiphytic bacteria in harvested matter (before silaging) are crucial for the spontaneous fermentation process and microbial succession in silage production [2]. The members of the epiphytic microbiota and their variations are a critical factor in determining whether LAB inoculation was necessary for silage production [3].
The presence of mycotoxins has been confirmed worldwide in a variety of forages of plant origin, and several mycotoxins may be present in a single forage [4]. As discussed by Juan et al. [5], mycotoxins pose a serious threat to feed safety due to their negative impact on consumer’s health. Additionally, due to climate change, which could affect the degree of contamination of feedstuffs, concerns about feed safety and health are increasing. The potential of using preharvest models to predict risk from deoxynivalenol (DON) in wheat, fumonisin B1 in maize, and aflatoxins in maize in different continents are considered in the context of potential for adaptation to include climate-change scenarios. In addition, changes in post-weather conditions may lead to the development and growth of molds that have not been observed in the area and may become more important as warmer climatic conditions would be conducive to Penicillium and Aspergillus, respectively [6].
Dzuman et al. [7] argue that feed can be contaminated with a wide range of mycotoxins and in addition to the classical regulated mycotoxins such as aflatoxins, deoxynivalenol, ochratoxins, fumonisins, and zearalenone, these can also be emerging mycotoxins (enniatins, beauvericin, and moniliformin). Numerous analogues of enniatin, including types A, A1, B, B1, B2, B3, B4, D, E, F, and G, are known, according to Santini et al. [8]. Of these modified toxins, enniatins A, A1, B, and B1 are the most found in Europe, specifically in cereals [9], silages, and inoculated corn [10]. The maximum levels for these mycotoxins are currently not regulated by any legislation [11], as they are considered to be of minor importance in terms of their concentration in feed and food, as well as their toxic effect on humans and animals [12]. Although no limits have yet been set for enniatins, for other mycotoxins of the genus Fusarium such as deoxynivalenol (DON), T-2 toxinHT-2 toxin, fumonisins (FUM), and zearalenone (ZEA), the concentrations are regulated by the authorities [13].
Since the formation and development of mycotoxins is influenced by many factors (temperature, water activity, pH value, fungal strain), researchers are trying to figure out effective ways to improve the hygienic parameters of silages [14]. Generally, the most used are biological silage additives (inoculants), which in most cases have been able to reduce the mycotoxin content, but in some cases also to increase it [15,16]. Fabiszewska et al. [2] mentioned that mycotoxins such as deoxynivalenol, fumonisins, fusarium toxins, zearalenone, and ochratoxins can be eliminated by using strains of lactic acid bacteria. Additionally, mycotoxins content can be reduced by adding chemical additives [17], and some nutrients such as urea [16,17]. Moreover, during the silage fermentation process, urea is partially degraded to ammonia (acts as a buffer–alkaline environment), which has an antifungal effect [18,19]. Urea as a silage additive is mainly used for forages with carbohydrate character, is effective at inhibiting growth of molds and yeasts [20,21]. Furthermore, the addition of urea affects the nutritional value (by increasing NH3-N, crude protein), but also the fermentation quality of silages (by boosting acetic, propionic and butyric acid, and by slowing pH decline) with decreasing nutrient losses and improved aerobic stability of silages [22].
Another option to decrease yeast and mold populations in silages is addition of facultatively and obligately heterofermentative bacteria, which are produced in addition to lactic acid, also acetic acid [18]. Inoculation by L. plantarum and L. buchneri would mitigate potential negative effects arising from fungal infestation by production of main fermentation compounds (acetic, propionic, and lactic acid) and bacteriocins [23]. Moreover, many species of bacteria and some specific fungi have been shown to enzymatically degrade mycotoxins, which are potentially promising candidates used to detoxify mycotoxins in feed and foods [24].
The hypothesis is regarding which types of silage additives are more effective against molds and mycotoxins formation and beneficial for silage microbiota during the fermentation process in whole crop corn silage. The aim of this study was to (a) determine the presence of microbiota and concentration of different mycotoxins in corn silage and (b) to determine the effect of silage additives on mycotoxin content for the improvement in silage hygienic quality and production of safe feeds for animals with the sustainable production of quality food for the human population
2. Materials and Methods
2.1. Ensilage of Corn Matter
In cooperation with the University Farm in Oponice, whole corn matter (hybrid FAO 480, dent grain type) was ensiled. The corn matter with a dry matter content of 39% at the milky-wax stage of the grain was harvested (harvested after 140 days after the sowing) with a self-propelled forage harvester (CLAAS, Omaha, Nebrasca, USA) and chopped to 2 cm of chopped length. The corn matter was ensiled in 3 variants and 3 repetitions: control (CONT /CONT1,2,3/, ALAB (additive on the base of lactic acid bacteria)/ALAB1,2,3/ and NAUR (nutritional additive: urea)/NAUR1,2,3/. The commercial additive in water-soluble powder on the base of lactic acid bacteria (Lentilactobacillus buchneri LN40177: obligately heterofermentative, Lacticaseibacillus casei LC32909: facultatively heterofermentative; min. lactic acid bacteria 1.1 × 1011 CFU/g) was applied using an applicator (Appli Pro Super Low Volume-Pioneer) directly on the forage harvester in a liquid state (10 mL/t) at a dose of 1 g/t of ensilaged matter. In the NAUR variant, a nutritional additive, urea, was applied to the untreated matter at a dose of 5000 g/t of ensilaged matter (applied manually in a solid state and subsequently homogenized by mixing with the matter). In the CONT control variant, the matter was ensiled without the addition of additives. The corn matter in all variants was ensiled into plastic bags (in one bag approx. 1.2 kg of ensilaged matter) in 3 repetitions using a vacuum packing machine MSW Motor Technics (Expendo Polska, Zielona Góra, Poland) and subsequently stored in the laboratory at a constant temperature (22 ± 2 °C). The plastic bags were opened, and average samples (n = 3) were taken for microbiological analysis after 5.5 months of storage. After pre-drying (at 60 °C), the dry matter content of the gravimetric method was determined at 103 ± 2 °C in corn silages. Laboratory samples of corn silages (n = 3) were subjected to mycotoxin analysis.
2.2. Microbiological Analyses
In the primary dilution of silage, 0.87% sterile saline with the quantity of 45 mL was used, to which 5 g of sample was added. Subsequently, serial dilutions (10–2 to 10–4) were prepared, and 100 μL of them were applied to Tryptic Soya agar plates (TSA, Sigma-Aldrich®, St. Louis, USA) to determine the total number of bacteria. The presence of bacterial colonies was examined in the inoculated plates after the incubation period of 48–72 h at 30 °C.
Typical colonies of coliform bacteria were enumerated after 24–48 h (37 °C) of incubation on inoculated McConkey agar (MC, Sigma-Aldrich®, St. Louis, MO, USA) plates. Formation of typical colonies for enterococci was examined with the use of Enterococcus selective agar (ESA, Sigma-Aldrich®, St. Louis, MO, USA), whereas incubation time and temperature were the same as for coliform bacteria. Lactic acid bacteria were cultivated with the use of three different agars, specifically MRS (De Man, Rogosa and Sharpe agar), MSE (Mayeux, Sandine and Elliker), and APT (All Purpose TWEEN® agar, Sigma-Aldrich®, St. Louis, MO, USA). Inoculated plates were incubated under the anaerobic conditions for 72 h at 37 °C. For microscopic fungi and yeast identification, malt extract agar (MEA, Sigma-Aldrich®, St. Louis, MO, USA) and acid base indicator bromocresol green (Sigma-Aldrich®, St. Louis, MO, USA) (0.020 g/L) were used. The growth on inoculated plates was evaluated after 5 days of aerobic exposure and an incubation temperature of 25 °C. Because of the macroscopic morphological differences between the growing colonies, recultivation on TSA (Tryptic Soya agar, Oxoid, Basingstoke, UK) was completed. The cultivation of inoculated plates took place for 24 h at 30 or 25 °C for bacteria and yeasts, respectively. After the cultivation, the protein extraction was undertaken. One colony of each bacterial isolate was transferred into an Eppendorf tube and mixed with 300 μL of sterile water. After addition of ethanol (900 μL), the suspension was mixed and centrifuged (13,000× g, 2 min). After removal of supernatant, the pellets were dried at room temperature at least for 5 min. The bacterial pellets were resuspended in 20–50 μL of formic acid (70%) and the same amount of acetonitrile. After centrifugation (2 min at 13,000× g), 1 μL of supernatant was spotted onto a sample position of a polished steel MALDI target plate and dried at room temperature. Then, 1 μL of MALDI matrix (solution of α-cyano-4-hydroxycinnamic acid (HCCA) in 50% acetonitrile/2.5% trifluoro-acetic acid) was added to the spot and dried.
The MALDI target plate was introduced into the MALDI-TOF mass spectrometer for automated measurement and data interpretation. MALDI-TOF profile mass spectra were imported into the MALDI Biotyper 3.0 software (Bruker Daltonics, Bremen, Germany) and processed automatically after measurement. The logarithm of the score (log/score) was displayed as the matching result. The MALDI Biotyper output was a log(score) between 0 and 3.0, which was calculated from a comparison of the peak list from an unknown isolate with the reference MSP in the database. A log(score) ≥ 1.7 indicated identification at the genus level, log(score) ≥ 2.0 was set as the threshold for a match at the species level. Isolates with ≥2.0 were accepted as a correct identification. Further confirmation of microorganisms (the colonies from total microbial count, coliform bacteria, enterococci, lactic acid bacteria, fungi, and yeasts) was performed using MALDI-TOF MS Biotyper. Identification of selected colonies was examined after aerobic or anaerobic subculture on TSA agar overnight. The preparation of microbial isolates for MALDI-TOF MS analysis was previously published by Kačániová et al. [25] and realized according to the manufacturer´s extraction procedure (Bruker Daltonik, Bremen, Germany). Additionally, Singh et al. [26] published identification for fungal isolates. Identification was completed using MALDI-TOF MS Biotyper (Bruker Daltonics, Bremen, Germany) with Flex Control 3.4 software and Biotyper Realtime Classification 3.1 with BC specific software (Bruker Daltonics, Bremen, Germany).
2.3. Mycotoxin Analysis
Multimycotoxin analysis by HPLC-MS/MS (AT-SOP 31) based on EN 17280 in cooperation with the Romer Labs Diagnostic GmbH Austria was used for mycotoxin analysis. Samples (10 g) for mycotoxin analysis were extracted using 30 mL of extraction solution (mixture of acetonitrile/water 7:3). The sample was then centrifugated. The aliquot amount was diluted with eluent A of HPLC by factor 10. Before dilution, an internal standard of most mycotoxins was added. The diluted sample was injected to an HPLC system (Agilent -Agilent Technologies, Santa Clara, California, USA - 1260 infinity II binary pump with integrated degasser, column oven, and multisampler) with a column (Phenomenex Gemini C18/4.6.0 × 150 mm; 5 µm/). Data were acquired using LC-MS/MS at two mass transitions by using a C18 column and gradient program for eluents. Used eluents were: eluent A: 88.5% water, 10% MeOH, 1% acetic acid, 0.5% 1 m ammonium acetate; eluent B: 1.5% water, 97% MeOH, 1% acetic acid, 0.5% 1 m ammonium acetate. The method of internal standard for quantification was used. The calibration curve was used as the dependency of the concentration of analyte to ratio of the relevant analyte and the corresponding internal standard. The calibration was completed via external solvent calibration standards. The internal standards were 13C isotopic labeled standards, in which all 12C carbon atoms were replaced by 13C carbon. The internal standards were added to the injection sample in the autosampler prior to analysis. Identification followed SANTE 12089/2016 [27] (guidance document on identification of mycotoxins in food and feed) involving retention time and two product ions. In each batch, two internal control samples were running; results of the internal controls are recorded in a control chart.
The mycotoxin content was determined in the dry matter of the laboratory samples and subsequently converted to 88% dry matter. The mycotoxin content is presented in μg/kg. The determined mycotoxin profile is shown in Table 1.

Table 1.
Mycotoxin groups and analytes analyzed by HPLC-MS/MS.
2.4. Statistical Evaluation of Results
The results were statistically evaluated using IBM SPSS 26.0 (Armonk, New York, NY, USA). The description statistics and differences between the variables were compared using a one-way ANOVA (Tukey test, p ˂ 0.05). The correlation relationships between the molds and yeasts and determined mycotoxins were calculated using the Pearson correlation coefficient (r). The coefficient of determination was recalculated as the powered Pearson correlation coefficient (r2).
3. Results and Discussion
3.1. Microbiota of Corn Silage
In the control samples, lactic acid bacteria ranged from 2.69 log cfu/g on APT to 5.11 log cfu/g on MRS, the numbers of coliform bacteria and enterococci were under the detection limit, the total number of microorganisms was 3.54 ± 0.43 log cfu/g and microscopic filamentous fungi was 2.73 ± 0.33 log cfu/g. In the silage with the additive on the base of lactic acid bacteria, lactic acid bacteria ranged from 2.83 log cfu/g on MSE to 5.05 log cfu/g on MRS, the numbers of coliform bacteria and enterococci were under the detection limit, total counts of microorganisms ranged from 3.23 to 3.51 log cfu/g and microscopic filamentous fungi ranged from 2.13 to 2.21 log cfu/g. In the silage with the nutritional addition, urea, lactic acid bacteria ranged from 4.14 to 4.21 log cfu/g, numbera of coliform bacteria and enterococci were under the detection limit, total number of microorganisms was 3.26 ± 0.22 log cfu/g, and microscopic filamentous fungi was 3.38 ± 0.45 log cfu/g (Table 2). Generally, the fermentation quality is largely influenced by the characteristic of the raw material and the epiphytic microorganisms on its surface [28]. Final feed quality is largely influenced by the species and numbers of dominant microorganisms in the fermentation process [29].

Table 2.
Number of isolated groups of microorganisms in log cfu/g.
In our study, a total of 43 species of bacteria and yeast were identified (Table 3). The 17 families included Bacillaceae, Burkholderiaceae, Clostridiaceae, Cryptococcaceae, Enterobacteriaceae, Lachnospiraceae, Lactobacillaceae, Moraxellaceae, Micrococaceae, Paenibacillaceae, Promicromonosporaceae, Pseudomonadaceae, Rhizobiaceae, Saccharomycetaceae, Shewanellaceae, Sphingomonadaceae, and Staphylococcaceae, and 25 genera included Acinetobacter, Arthrobacter, Alkalihalobacillus, Priestia, Bacillus, Blastomonas, Cellulosimicrobium, Citrobacter, Clostridium, Lacrimispora, Cryptococcus, Kazachstania, Lactobacillus, Levilactobacillus, Lentilactobacillus, Companilactobacillus, Secundilactobacillus, Lacticaseibacillus, Paenibacillus, Pseudomonas, Ralstonia, Rhizobium, Shewanella, Sphingomonas, and Staphylococcus that were isolated from all samples of corn silage (Table 4). The most isolated family from all corn silage was Lactobacillaceae. The most isolated species was Lentilactobacillus buchneri (17%) (Figure 1). Bacteria, yeast, and fungi with detoxification abilities were isolated from different sources, and LAB are the preferred candidates for eliminating mycotoxins in silages because they play a critical role in the ensiling fermentation. Lactiplantibacillus plantarum and Lentilactobacillus buchneri are known to enhance lactic acid (LA) fermentation and acetic acid (ACA) production to improve fermentation quality and inhibit aerobic spoilage [23].

Table 3.
The number of isolated species of bacteria and yeasts from corn silage.

Table 4.
Isolated species, genera, and families from corn silage samples.
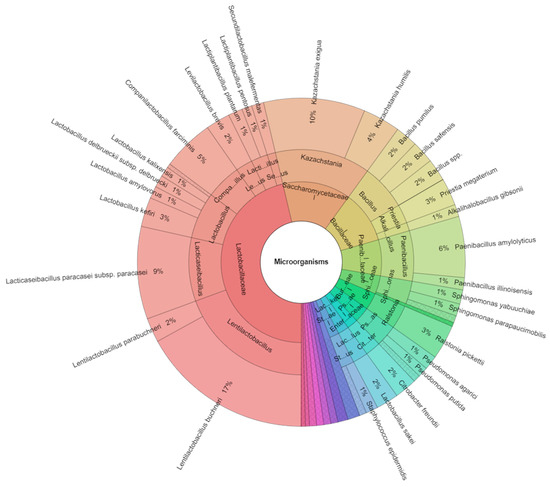
Figure 1.
Krona chart of isolated species of bacteria and yeasts from all samples of corn silage.
In the study of Wang et al. [30] they examined the effects of lactic acid bacteria (LAB) Lactiplantibacillus plantarum subsp. ZA3 and Artemisia argyi (AA) on the fermentation characteristics, microbial community, and mycotoxins. The results showed that corn silage has microbial communities, Acetobacter and Enterobacter, which were inhibited in all AA groups, while a higher abundance of lactobacilli was maintained; moreover, Candida, Pichia, and Kazachstania abundances were decreased in both groups. In our study, different results were found, the most isolated species were from the family Lactobacillaceae and yeast Kazachastania exigua (10%). Our results did not confirm previous research. We can assume that the increased number of LAB increases the production of lactic acid, which affects the growth of yeast. The relatively lower pH values in inoculated silages, combining activities of acidification and antagonistic activity towards other bacteria, promotes the reduction in bacterial and fungi diversities, and ultimately improves feed quality. This observation indicated that the fungi community can change when the environment changes from anaerobic to aerobic. It is possible that acid-tolerant bacteria still dominate the bacterial community in the early period of aerobic exposure, and the variation in microbial community.
In our study, microscopic fungi in each group of samples were isolated and they were of the genera Alternaria, Aspergillus, Fusarium, Mucor, Rhizopus, and Penicillium. The most isolated genera 25% resp. 25% in control samples were Aspergillus and Penicillium. Similar results were found in samples treated with the addition of LAB with an incidence of Aspergillus of 12.5% and Penicillium of 18%, and in samples with nutritional additive urea with an incidence of Aspergillus of 15% and Penicillium of 20%.
In the study of Krustev and Khristov [31], eight species of microscopic fungi were demonstrated in the sampled corn silage: Mucor, Penicillium, Aspergillus, Alternaria, and Trichoderma, similar as in our study. It was established that the number of species in the surface layer of the ensilaged mass was the highest.
Isolation of novel LAB strains for application in silage has been a common practice over the years but it is still an activity with current importance around the globe [32,33,34], due to the interest in collecting diverse strains for future applications not only as silage inoculants but also in other plant-based food for animal and human uses [35].
Lentilactobacillus buchneri is presently the gold standard to promote aerobic stability in corn silage [36,37]. Acetic acid is one of the main organic acids produced by heterofermentative LAB and it has the capacity of promoting aerobic stability when silos are opened [38].
All isolated species in control samples were 27 species and the most isolated species was from the family Lactobacillaceae (9 species, Figure 2). The most isolated species from control samples were Lentilactobacillus buchneri (12%) and Kazachstania exigua (11%), following with Paenibacillus amylolyticus (8%), Citrobacter freundii, and Ralstonia picketii (6%). L. buchneri Ls141 and 463 were used as external reference strains. L. buchneri Ls141 had been isolated from corn silage in a previous study [39]. Candida ethanolica, Saccharomyces bulderi, Pichia anomala, Kazachstania unispora, and Saccharomyces cerevisiae were the predominant yeasts. Pichia anomala, Issatchenkia orientalis, S. cerevisiae, and Pichia fermentans were the prevalent species in high moisture corn [36].
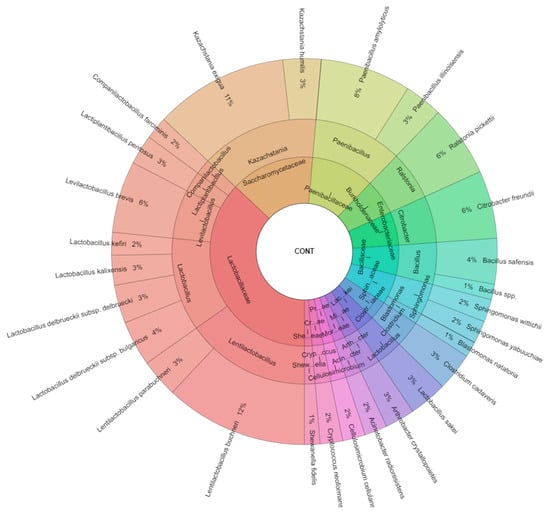
Figure 2.
Krona chart of isolated species of bacteria and yeasts from control samples of corn silage.
All isolated species in corn samples with the addition of LAB were 19 species and the most isolated species were from the families Lactibacillaceae (5 species) and Bacillaceae (4 species, Figure 3). The most isolated species from corn samples with the addition of LAB were Lentilactobacillus buchneri (18%) and Lacticaseibacillus paracasei subsp. paracasei (16%), following with Kazachstania exiqua (10%), Paenibacillus amylolyticus (9%), and Priestia megaterium (8%). Driehuis et al. [40] observed that strains of Lentilactobacillus buchneri (Lactobacillus buchneri) [41] were able to degrade lactic acid into acetic acid and 1,2-propanediol [42], which could then be metabolized into propionic acid [43]. Since both acetate and propionate are strong yeast inhibitors [44,45], these modifications positively improve the aerobic stability of silage. More recently, co-inoculation with L. buchneri NCIMB 40788 and Lentilactobacillus hilgardii CNCM-I-4785 (Lactobacillus hilgardii) was reported to increase the stability of different silages [46,47]. While microbial dynamics during fermentation were recently characterized in corn silage inoculated with these two microorganisms [48], little research has been undertaken to characterize microbial succession and mycotoxin production in inoculated vs. uninoculated silages during the feed-out phase [49,50]. Kazachstania exiqua was isolated from corn silage in the study of Santos et al. [36].
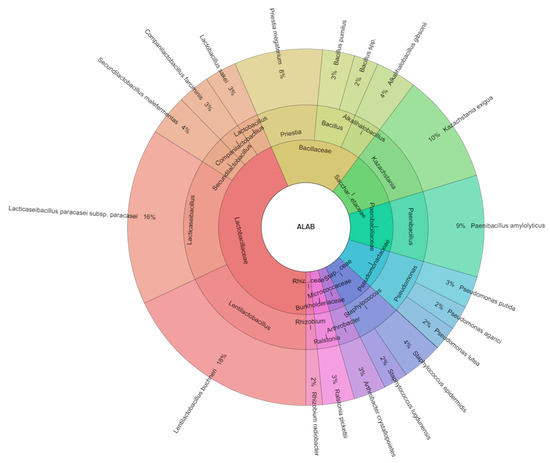
Figure 3.
Krona chart of isolated species of bacteria and yeasts from samples of corn silage with additive on the base of lactic acid bacteria.
Ruminal microorganisms are capable of transforming nitrogen from NPN compounds into protein of high nutritional value. However, if the release of ammonia promoted by NPN exceeds the use capacity by ruminal microbiota, there will be an excretion of this excess with a consequent loss of energy. If the ammonia concentration extrapolates the excretion capacity, the intoxication of the animal may occur [51]. Nitrogen from NPN compounds can be converted by ruminal microbes into protein with a high nutritional value [52]. However, there will be the expulsion of this surplus and a resulting loss of energy if the release of ammonia encouraged by NPN exceeds the capacity for utilization by ruminal bacteria [53].
All isolated species from samples with nutritional additive urea were 16 species and most isolated species were from the Lactibacillaceae family similar to 7 species (Figure 4). The most isolated species from the treated samples with nutritional additive urea were Lentilactobacillus buchneri (22%) and Lacticaseibacillus paracasei subsp. paracasei (12%), following with Companilactobacillus farciminis (11%) and Lactobacillus kefiri (8%). Pang et al. [54] reported that most of the bacterial community in silage belonged to the phylum Firmicutes and the genera Lactobacillus, Pedicoccus, and Weissella. These results showed that the dominant phyla in the measured samples were Proteobacteria and Firmicutes. This result was different from that of other researchers [54,55], who found that most bacteria involved in lactic acid fermentation of silage belonged to the genera Lactobacillus, Pedicoccus, Weissella, and Leuconostoc. Metagenomic analysis revealed that urea addition in the sheep diet significantly increased the relative abundance of genera involved in nitrogen metabolism especially. Increasing nitrogen sources by urea addition may be beneficial to microbial protein production. This could improve microbial utilization of additional N sources during ruminal fermentation. Therefore, the synchronization between ruminal ammonia nitrogen release and carbohydrate availability resulted in greater microbial protein synthesis [56]. The bacterial composition was also altered by lysine supplementation to support energy metabolism, in which the microbial diversity was unchanged [57].
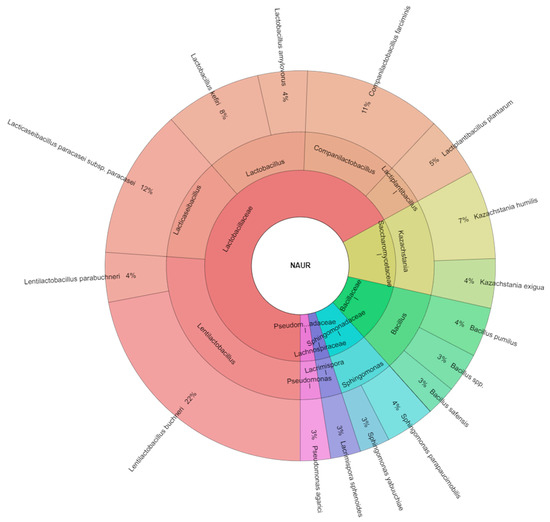
Figure 4.
Krona chart of isolated species of bacteria and yeasts from samples of corn silage with nutritional additive: urea.
3.2. Mycotoxin Composition of Corn Silage
Several mycotoxin species were identified in corn silage samples. Table 5 and Table 6 provide an overview on detected mycotoxin concentrations as well as on the level of significance. In this study, there were no statistically significant differences between variants of each mycotoxin species. Penagos-Tabares et al. [58] confirmed the occurrence of the same mycotoxins as detected in this experiment. When mycotoxins are present, there are few ways to avoid unwanted problems and therefore prevention is essential. Some silage additives can reduce growth of fungi and hence mycotoxin formation [14,59]. Whitlow and Hagler [59] found that these can be additives such as ammonia, propionic acid, sorbic acid, and bacterial or enzymatic additives. Dong et al. [60] examined the interactions between the harvest stage and the dose of inoculant in corn silages and found that inoculant lowered (p > 0.05) concentrations of deoxynivalenol at the milk stage. Furthermore, the inoculant significantly decreased or increased deoxynivalenol content with its different dosing at the dough stage [60]. Aflatoxins and ochratoxins were not identified neither in control variant nor in experimental variants. In contrast, Kalúzová et al. [16] determined these types of mycotoxins and decreased them with urea and inoculant addition. The effect of silage additives was manifested in reducing zearalenone content in experimental variants (Table 5). Corn silage with urea achieved a significantly (p < 0.05) higher concentration of zearalenone but its concentration after inoculant addition was even higher [16]. An increased concentration of zearalenone was also reported by Drouin et al. [61] when inoculant was added to the corn silage. The application of inoculant to corn silage resulted in increased fumonisin production, and Bakri [62] registered the same effect in his experiment. Gallo et al. [63] also detected fumonisins and found a greater level of fumonisin B1 (p > 0.05) and fumonisin B2 (p < 0.05) in inoculated corn. Corn silage NAUR resulted in a more than two times higher mean value of fumonisin B1 compared to CONT. Kalúzová et al. [16] found a similar result with this nutritional additive but the differences were not significant. Teller et al. [64] studied the effect of various additives on mycotoxin concentration in corn silage. A higher content of deoxynivalenol and fumonisin B1 was observed after inoculant treatment. As for zearalenone, the microbial additive lowered its content in silage samples [64]. The highest value of all mycotoxins reached nivalenol in both CONT and ALAB variants. In comparison with the control, a higher content of nivalenol was observed in variants with inoculant addition. Wang et al. [65] found a significant tendency in differences in nivalenol content depending on storage temperature and type/use of lactic acid bacteria. Eckard et al. [66] detected nivalenol in 8 of 19 samples of corn silage between a range of 190 and 760 µg/kg. A lowering trend (p > 0.05) of this mycotoxin in inoculated corn silage was noticed by Bakri [62]. Variant NAUR was higher in nivalenol content than CONT, but lower compared to variant ALAB. Although type A trichothecenes and ergot alkaloids are present in cereal crops, they were not detected by the HPLC-MS/MS method in this experiment. Type A trichothecenes include, for instance, mycotoxins such as T-2 toxin and HT-2 toxin, which are quite often found in silage. Some authors [16,65,67] confirmed a change in T-2 toxin concentration by using urea and various microbial additives in corn silages. Contamination by ergot alkaloids is mostly seen in forages such as tall fescue, sorghum, and ryegrass, but their presence in other forages is not refuted [68,69]. Zhang et al. [69] noted that ergot alkaloids are rarely present in corn silage in China, but still, they occurred in one of their samples of corn silage at a concentration of 15.3 µg/kg.

Table 5.
The content of regulated mycotoxins in corn silage variants in μg/kg.

Table 6.
The content of emerging mycotoxins in corn silage variants in μg/kg.
Besides free Fusarium mycotoxins (deoxynivalenol, fumonisin B1, nivalenol, and zearalenone), the presence of some other Fusarium contaminants known as emerging mycotoxins (beauvericin, enniatins, and moniliformin) were also found. Beauvericin was detected only in corn silage with the addition of urea. An average concentration of 47 µg/kg of beauvericin by Zachariasova et al. [70] was determined in corn silage samples. Reisinger et al. [71] found 120 samples of corn silage positive on beauvericin, and this mycotoxin belonged to five most frequently detected mycotoxins in their study. In corn silage samples, Sørensen et al. [72] did not detect the presence of enniatin A and A1 but confirmed the presence of enniatin B and B1. In contrast to Sørensen et al. [72], the mean value of enniatin B was 28.01 µg/kg lower and that of enniatin B1 was 9.50 µg/kg lower in our corn silage samples without additive. On the other hand, neither enniatin A nor B1 were confirmed in the corn silage from Rasmussen et al. [73], but the mean value of enniatin B (44.00 µg/kg) was lower compared to our study of silage without additive. Similarly, Storm et al. [74] only reported the occurrence of enniatin B with an average value higher (53.00 µg/kg) than that reported in Table 6 for the silage without additive. In whole-plant corn silage, enniatins did not appear in any of the samples or were detected at very low concentrations [75]. However, Shimshoni et al. [76] detected the occurrence of enniatin A (0.3 µg/kg), enniatin A1 (0.8 µg/kg), enniatin B (0.2 µg/kg), as well as enniatin B1 (0.9 µg/kg). Identical to the previous author, McElhinney et al. [77] also found the presence of these mycotoxins, but in grass silage. Moreover, the presence of enniatin B was confirmed by Wambacq et al. [78] in 82 corn silage samples with concentrations up to 5000 µg/kg. As in the present study, these values were not statistically significant in studies, which all the authors mentioned. Moniliformin was present in all samples; however, lower concentrations were obtained in both treated variants. Zhang et al. [69] reported relatively low concentrations of moniliformin, and the highest concentration of 116 µg/kg was found. On the contrary, Gräfenhan et al. [79] did not detect moniliformin in red clover silage (control and treated variant), but after addition of soil, moniliformin concentration jumped up to 222 µg/kg. As Kalúzová et al. [14] mentioned, the effect of urea on mycotoxin concentrations has not been widely monitored so far and the effect on chemical composition and fermentation parameters was more closely monitored in this case. Some studies confirmed a positive suppression effect of silage additives on mycotoxin concentrations [23,61,62,63]. However, other studies reveal the increasing mycotoxin content after the application of silage additives [17,65]. Contradictory results are caused probably by many factors such as temperature, water activity, and pH value [80,81,82], which are affecting the environment of microscopic fungi producing the mycotoxins in stressful conditions; thus other experiments are necessary in this research field.
3.3. Relationship between the Mycotoxin Concentrations and Microscopic Fungi and Yeasts, Harmful Effects, and Mycotoxin Limits in Feeds
The main correlation characteristics between the mold and yeast populations and detected mycotoxins in corn silages were not statistically significant; however, the coefficient of determination between the May and FUMB1 (r2) was relatively high (0.79%) (Table 7). This was also confirmed by Barug et al. [83], where a direct relationship between the microscopic fungi and yeast and certain mycotoxins in silage was not observed. Similarly, no correlations were found between fungal DNA and mycotoxin concentrations [84]. According to Schenck et al. [85], a correlation between the presence of Fusarium toxins (NIV, DON, 3-ACDON, HT-2, T-2, BEAU, and ENNB) and the presence of Fusarium culmorum, F. equiseti, F. graminearum, or F. poae could not be proved. However, there were negative significant correlations between the nivalenol and enniatin A1 (p < 0.01) and moniliformin (p < 0.05). On the other side, the positive correlation between the enniatin A1 and moniliformin (p < 0.05) was observed. Aspergillus, Fusarium, and Penicillium species comprise a well-known group of microscopic filamentous fungi that are infamous for their ability to make many potent mycotoxins. Mycotoxins play a significant role in the defensive strategies of mycotoxigenic fungi. The fungal species more frequently identified in this work have been previously reported in silage [86]. The presence of mycotoxins, which are produced by Aspergillus and Penicilium species, was not evaluated in corn silages in this study.

Table 7.
Mold and yeast population and mycotoxin Pearson’s correlation relationship.
Aflatoxins are mainly produced by toxigenic strains of Aspergillus molds. In ruminants, reduced milk production in dairy cows, decreased milk quality and safety due to carry-over of toxins from contaminated feed, liver malfunctions, decreased feed efficiency and rate of gain in beef cows, and compromised immune and ruminal functions were observed [86]. The maximum content of aflatoxin B1 for complete foodstuffs for cattle, sheep, and goats is 0.02 mg/kg with the exception of complete foodstuffs for dairy animals (0.005 mg/kg) and for calves and lambs (0.01 mg/kg). The maximum content of aflatoxin B1 for complementary foodstuffs for cattle, sheep, and goats is 0.02 mg/kg (except complementary foodstuffs for dairy animals, calves, and lambs: 0.005 mg/kg) [87].
Ochratoxins are produced by several Penicilium and Aspergillus species, have hepatotoxic and nephrotoxic effects, causing poor feed conversion and limiting weight gains in ruminants [88]. The guidance limit of ochratoxin A for feed materials (cereals and cereal products) is 0.25 mg/kg, and for complementary and complete foodstuffs are limited only for poultry and pigs [89].
In analyzed corn silages, only fusarium mycotoxins were found. Deoxynivalenol causes feed refusal and lower weight gains, diarrhea, lower milk production, hepatotoxicity (in young preruminants), and immune alterations [86,88,90,91]. The regulatory level (guidance value) of deoxynivalenol for complementary and complete foodstuffs for ruminants is 5 mg/kg. For feed materials, guidance values are 8 mg/kg (for cereals and cereal products) and 12 mg/kg (for maize by-products) [89].
Clinical signs in ruminants caused by fumonisins are decreased feed intake, milk production, and mild liver diseases [86,92]. The results of Roberts et al. [93] revealed that exposure to deoxynivalenol and fumonisins was detrimental to the welfare of finishing steers and may compromise their ability to withstand other stressors such as disease, heat stress, or other toxins. The regulatory level (guidance value) of fumonisins (B1 + B2) for complementary and complete foodstuffs for adult ruminants (>4 months) is 50 mg/kg, and for feed materials (maize and maize products) is 60 mg/kg [89].
Higher zearalenone contamination is linked with a risk of vaginal and rectal prolapses, infertility, hyperestrogenism, swelling of mammary glands, and milk production reduction in dairy cows [88,94]. The regulatory level (guidance value) of zearalenone for complementary and complete foodstuffs for calves, dairy cattle, sheep (including lambs), and goats (including kids) is 0.5 mg/kg. For feed materials, guidance values are 2 mg/kg (for cereals and cereal products) and 3 mg/kg (for maize by-products) [89].
Nivalenol belongs to the B group of trichothecene mycotoxins along with deoxynivalenol, 3-acetyl-deoxynivalenol, 15-acetyl-deoxynivalenol, and fusarenon-X [95]. Generally, the toxicity of deoxynivalenol and nivalenol is similar [88,96] and effects of nivalenol include immunotoxicity and hematotoxicity. With the exception of forage maize (and maize silage), levels of nivalenol in forages are generally low. For lactating dairy cows and beef cattle, the estimated lower-bound and upper-bound exposures to nivalenol are between 0.077 and 0.69 µg/kg of live body weight per day, except for maize-silage-based diets (1.9 and 4.6 µg/kg body weight per day) [95]. The concentrations of regulated mycotoxins in our experiment did not exceed the maximum permitted, guidance, and daily tolerable intake according to the limits [87,89,95,97].
Animal exposure to enniatins and beauvericin is primarily from feed intake of cereal grains and cereal by-products [97]. The primary toxic action of beauvericin and enniatins is related to their ability to form ion channels and transport NH4+ or K+ ions across the cell membrane, resulting in disturbance of the ion homeostasis and eventually cell death [98,99].
Enniatins are mutagenic and embryotoxic for animals [100,101]. For the sum of enniatins, the calculated lower-bound and upper-bound (UB) chronic exposures for ruminants ranged from 3.30 to 8.26 μg/kg body weight per day and estimated acute UB exposure is 32.6 μg/kg body weight per day, for ruminants [97].
Moniliformin is mainly detected in cereal grains and cereal-based feed [102]. The main pathological change observed in sheep was the degeneration of the proximal tubules of the kidneys after moniliformin intake [103]. No toxicity data suitable for hazard characterization of moniliformin were identified for ruminants, farmed rabbits, horses, farmed fish, dogs, and cats. Therefore, the EFSA Panel on Contaminants in the Food Chain (CONTAM) [104] considered of 0.20 mg moniliformin/kg body weight identified for pigs as an indicative reference point [102].
4. Conclusions
The number of microbiota varied with the control to nutritional additives. The most isolated group of bacteria was lactic acid bacteria in all groups of corn silages. The most isolated species of LAB were similar as bacterial additives added to silages. The application of additives did not affect the number of lactic acid bacteria, in both groups with additives. The total numbers of microorganisms in both groups with additives were affected; however, the number of microscopic filamentous fungi only in the group with the addition of lactic acid bacteria compared to the control group was lower. In both groups with the additive treatment, there was a lower diversity of isolated species of microorganisms, and a higher proportion of main species of lactic acid bacteria compared to the control group. The following Fusarium mycotoxins have been recorded in corn silages: deoxynivalenol, zearalenone, fumonisin B1, nivalenol (regulated mycotoxins), beauvericin, enniatin A1, B, B1, and moniliformin (emerging mycotoxins). Nivalenol reached the highest total mean value of regulated mycotoxins in silage samples. The highest prevalence of deoxynivalenol and beauvericin in silage with urea addition was observed. While beauvericin was found only in silage with urea, other emerging mycotoxins (enniatin A1, B, B1, and moniliformin) were present in all variants. However, the mycotoxin content after the addition of silage additives was not statistically significant, hence, their effect in corn silage was not confirmed. On the other side, it can be stated that monitored mycotoxin did not affect the hygienic quality and safety of analyzed corn silage. More studies for investigation of the effect of silage additives on mycotoxin concentration in silages are needed.
Author Contributions
Visualization, writing—original draft M.K. (Mária Kalúzová); data curation, methodology, validation, writing—original draft M.K. (Miroslava Kačániová); supervision D.B.; conceptualization M.Š.; funding acquisition, project administration B.G.; investigation M.R., software, writing—review and editing O.H.; methodology, validation S.F.; writing—original draft, writing—review and editing M.J. All authors have read and agreed to the published version of the manuscript.
Funding
This publication was supported by the Scientific Grant Agency of the Ministry of Education, Science, Research and Sport of the Slovak Republic and the Slovak Academy of Sciences, project no. 1/0474/19 (The application of additives in animal nutrition for nutrients transformation improve with the accent on quality, safety and sustainability of animal production) and by the Operational program Integrated Infrastructure within the project: Sustainable smart farming systems taking into account the future challenges 313011W112, cofinanced by the European Regional Development Fund.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
Many thanks to Jaroslav Langer and the University farm in Oponice, and Romer Labs Diagnostic GmbH Austria for cooperation.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Kim, D.; Lee, K.D.; Choi, K.C. Role of LAB in silage fermentation: Effect on nutritional quality and organic acid production-an overview. AIMS Agric. Food 2021, 6, 216–234. [Google Scholar] [CrossRef]
- Fabiszewska, A.U.; Zielińska, K.J.; Wróbel, B. Trends in designing microbial silage quality by biotechnological methods using lactic acid bacteria inoculants: A minireview. World J. Microbiol. Biotechnol. 2019, 35, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jaipolsaen, N.; Sangsritavong, S.; Uengwetwanit, T.; Angthong, P.; Plengvidhya, V.; Rungrassamee, W.; Yammuenart, S. Comparison of the effects of microbial inoculants on fermentation quality and microbiota in napier grass (Pennisetum purpureum) and Corn (Zea mays L.) Silage. Front. Microbiol. 2022, 12, 784535. [Google Scholar] [CrossRef] [PubMed]
- Bryła, M.; Waśkiewicz, A.; Podolska, G.; Szymczyk, K.; Jędrzejczak, R.; Damaziak, K.; Sułek, A. Occurrence of 26 mycotoxins in the grain of cereals cultivated in Poland. Toxins 2016, 8, 160. [Google Scholar] [CrossRef]
- Juan, C.; Covarelli, L.; Beccari, G.; Colasante, V.; Mañes, J. Simultaneous analysis of twenty-six mycotoxins in durum wheat grain from Italy. Food Control. 2016, 62, 322–329. [Google Scholar] [CrossRef]
- Magan, N.; Medina, A.; Aldred, D. Possible climate-change effects on mycotoxin contamination of food crops pre-and postharvest. Plant Pathol. 2011, 60, 150–163. [Google Scholar] [CrossRef]
- Dzuman, Z.; Zachariasova, M.; Lacina, O.; Veprikova, Z.; Slavikova, P.; Hajslova, J. A rugged high-throughput analytical approach for the determination and quantification of multiple mycotoxins in complex feed matrices. Talanta 2014, 121, 263–272. [Google Scholar] [CrossRef]
- Santini, A.; Meca, G.; Uhlig, S.; Ritieni, A. Fusaproliferin, beauvericin and enniatins: Occurrence in food—A review. World Mycotoxin J. 2012, 5, 71–81. [Google Scholar] [CrossRef]
- Ivanova, L.; Egge-Jacobsen, W.M.; Solhaug, A.; Thoen, E.; Fæste, C.K. Lysosomes as a possible target of enniatin B-induced toxicity in Caco-2 cells. Chem. Res. Toxicol. 2012, 25, 1662–1674. [Google Scholar] [CrossRef]
- Renaud, J.B.; Kelman, M.J.; McMullin, D.R.; Yeung, K.K.C.; Sumarah, M.W. Application of C8 liquid chromatography-tandem mass spectrometry for the analysis of enniatins and bassianolides. J. Chromatogr. A 2017, 1508, 65–72. [Google Scholar] [CrossRef]
- Jajić, I.; Dudaš, T.; Krstović, S.; Krska, R.; Sulyok, M.; Bagi, F.; Savić, Z.; Guljaš, D.; Stankov, A. Emerging Fusarium mycotoxins fusaproliferin, beauvericin, enniatins, and moniliformin in Serbian maize. Toxins 2019, 11, 357. [Google Scholar] [CrossRef] [PubMed]
- Kolawole, O.; Graham, A.; Donaldson, C.; Owens, B.; Abia, W.A.; Meneely, J.; Alcorn, M.J.; Connolly, L.; Elliott, C.T. Low doses of mycotoxin mixtures below EU regulatory limits can negatively affect the performance of broiler chickens: A longitudinal study. Toxins 2020, 12, 433. [Google Scholar] [CrossRef] [PubMed]
- Prosperini, A.; Berrada, H.; Ruiz, M.J.; Caloni, F.; Coccini, T.; Spicer, L.J.; Perego, M.C.; Lafranconi, A. A review of the mycotoxin enniatin B. Front. Public Health 2017, 5, 304. [Google Scholar] [CrossRef] [PubMed]
- Kalúzová, M.; Juráček, M.; Bíro, D.; Šimko, M.; Gálik, B.; Rolinec, M.; Hanušovský, O.; Mixtajová, E.; Kolláthová, R.; Brek, P. Mycotoxic contamination of silages: A review. In Proceedings of the NutriNET 2021, Košice, Slovakia, 9 September 2021; University of Veterinary Medicine and Pharmacy in Košice: Košice, Slovakia. [Google Scholar]
- Juráček, M.; Felšöciová, S.; Bíro, D.; Šimko, M.; Gálik, B.; Rolinec, M.; Hanušovský, O.; Kolláthová, R.; Kalúzová, M.; Kačániová, M. The effect of lactic acid bacteria addition on occurrence of microbiota and mycotoxins in rye silages. J. Cent. Eur. Agric. 2022, 23, 342–350. [Google Scholar] [CrossRef]
- Kalúzová, M.; Juráček, M.; Bíro, D.; Gálik, B.; Šimko, M.; Rolinec, M.; Hanušovský, O.; Mixtajová, E.; Kolláthová, R.; Drotárová, S. The effect of additives on mycotoxic contamination of maize silages. J. Cent. Eur. Agric. 2022, 23, 299–304. [Google Scholar] [CrossRef]
- Bíro, D.; Juráček, M.; Kačániová, M.; Šimko, M.; Gálik, B.; Michálková, J.; Gyongyova, E. Occurrence of microscopic fungi and mycotoxins in conserved high moisture corn from Slovakia. Ann. Agric. Environ. Med. 2009, 16, 227–232. [Google Scholar]
- Kung, L., Jr.; Stokes, M.R.; Lin, C. Silage Additives. Silage Sci. Technol. 2003, 42, 305–360. [Google Scholar]
- Schmidt, P.; Mari, L.J.; Nussio, L.G.; de Faria Pedroso, A.; de Fátima Paziani, S.; Wechsler, F.S. Aditivos químicos e biológicos na ensilagem de cana-de-açúcar: Composição química das silagens, ingestão, digestibilidade e comportamento ingestivo. Rev. Bras. de Zootec. 2007, 36, 1666–1675. [Google Scholar] [CrossRef]
- Araki, H.M.C.; De Oliveira, E.R.; Gandra, J.R.; De Goes, R.H.T.B.; Takiya, C.S.; Jacaúna, A.G.; De Oliveira, K.M.P.; Vasques, D.N.; Brandão Cônsolo, N.R.; Del Valle, T.A.; et al. Association of biological and chemical additives on nutrient composition, total losses, microbiological and fermentative profile of sugarcane silage. Iran. J. Appl. Anim. Sci. 2017, 7, 577–584. [Google Scholar]
- Santos, A.P.M.D.; Santos, E.M.; Oliveira, J.S.D.; Ribeiro, O.L.; Perazzo, A.F.; Pinho, R.M.A.; Macêdo, A.J.D.S.; Pereira, G.A. Effects of urea addition on the fermentation of sorghum (Sorghum bicolor) silage. Afr. J. Range Forage Sci. 2018, 35, 55–62. [Google Scholar] [CrossRef]
- Kaewpila, C.; Khota, W.; Gunun, P.; Kesorn, P.; Cherdthong, A. Strategic addition of different additives to improve silage fermentation, aerobic stability and in vitro digestibility of napier grasses at late maturity stage. Agriculture 2020, 10, 262. [Google Scholar] [CrossRef]
- Li, J.; Wang, W.; Chen, S.; Shao, T.; Tao, X.; Yuan, X. Effect of lactic acid bacteria on the fermentation quality and mycotoxins concentrations of corn silage infested with mycotoxigenic fungi. Toxins 2021, 13, 699. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Wang, W.; Wang, Z.; Tan, Z.; Qin, G.; Wang, Y.; Pang, H. Microbial population succession and community diversity and its correlation with fermentation quality in soybean meal treated with Enterococcus faecalis during fermentation and aerobic exposure. Microorganisms 2022, 10, 530. [Google Scholar] [CrossRef]
- Kačániová, M.; Kunová, S.; Horská, E.; Nagyová, Ľ.; Puchalski, C.; Haščík, P.; Terentjeva, M. Diversity of microorganisms in the traditional Slovak cheese. Potr. S.J.F.Sci. 2019, 13, 532–537. [Google Scholar] [CrossRef][Green Version]
- Singh, A.; Singh, P.K.; Kumar, A.; Chander, J.; Khanna, G.; Roy, P.; Meis, J.F.; Chowdhary, A. Molecular and matrix-assisted laser desorption ionization–time of flight mass spectrometry-based characterization of clinically significant melanized fungi in India. J. Clin. Microbiol. 2017, 55, 1090–1103. [Google Scholar] [CrossRef] [PubMed]
- SANTE. European Commission. European Commission Health & Consumer Protection Directorate-General. Document No. SANTE 12089/2016. Guidance Document on Identification of Mycotoxins in Food and Feed. Available online: https://ec.europa.eu/food/document/download/f16cac78-9318-4f1f-b2fa-efb25d2f1880_en.pdf (accessed on 20 June 2022).
- Wilkins, R.J. The biochemistry of silage. Anim. Feed Sci. Technol. 1982, 7, 317–318. [Google Scholar] [CrossRef]
- Namihira, T.; Shinzato, N.; Akamine, H.; Maekawa, H.; Matsui, T. Influence of nitrogen fertilization on tropical-grass silage assessed by ensiling process monitoring using chemical and microbial community analyses. J. Appl. Microbiol. 2010, 108, 1954–1965. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Tan, Z.; Gu, L.; Ma, H.; Wang, Z.; Wang, L.; Wu, G.; Qin, G.; Wang, Y.; Pang, H. Variation of microbial community and fermentation quality in corn silage treated with lactic acid bacteria and Artemisia argyi during aerobic exposure. Toxins 2022, 14, 349. [Google Scholar] [CrossRef]
- Krustev, E.; Khristov, B. Mikroflora v tsarevichen silazh Microflora in corn silage. Vet. Med. Nauki. 1981, 18, 88–91. [Google Scholar]
- dos Santos Leandro, E.; Ginani, V.C.; de Alencar, E.R.; Pereira, O.G.; Rose, E.C.P.; do Vale, H.M.M.; Pratesi, R.; Hecht, M.M.; Cavalcanti, M.H.; Tavares, C.S.O. Isolation, identification, and screening of lactic acid bacteria with probiotic potential in silage of different species of forage plants, Cocoa beans, and artisanal salami. Probiot. Antimicrob. Proteins 2020, 13, 173–186. [Google Scholar] [CrossRef]
- Paradhipta, D.H.V.; Lee, S.S.; Kang, B.; Joo, Y.H.; Lee, H.J.; Lee, Y.; Kim, J.; Kim, S.C. Dual-purpose inoculants and their effects on corn silage. Microorganisms 2020, 8, 765. [Google Scholar] [CrossRef] [PubMed]
- Tanizawa, Y.; Kobayashi, H.; Nomura, M.; Sakamoto, M.; Arita, M.; Nakamura, Y.; Ohkuma, M.; Tohno, M. Lactobacillus buchneri subsp. silagei subsp. nov., isolated from rice grain silage. Int. J. Systemat. Evolution. Microbiol 2020, 70, 3111–3116. [Google Scholar] [CrossRef] [PubMed]
- Wuyts, S.; Van Beeck, W.; Allonsius, C.N.; van den Broek, M.F.; Lebeer, S. Applications of plant-based fermented foods and their microbes. Curr. Opin. 2020, 61, 45–52. [Google Scholar] [CrossRef]
- Santos, A.O.; Ávila, C.L.S.; Schwan, R.F. Selection of tropical lactic acid bacteria for enhancing the quality of maize silage. J. Dairy Sci. 2013, 96, 7777–7789. [Google Scholar] [CrossRef]
- Da Silva, N.C.; Dos Santos, J.P.; Ávila, C.L.S.; Evangelista, A.R.; Casagrande, D.R.; Bernardes, T.F. Evaluation of the effects of two Lactobacillus buchneri strains and sodium benzoate on the characteristics of corn silage in a hot-climate environment. Grassl. Sci. 2014, 60, 169–177. [Google Scholar] [CrossRef]
- Danner, H.; Holzer, M.; Mayrhuber, E.; Braun, R. Acetic acid increases stability of silage under aerobic conditions. Appl. Environ. Microbiol. 2003, 69, 562–567. [Google Scholar] [CrossRef]
- Burns, P.; Borgo M., F.; Binetti, A.; Puntillo, M.; Bergamini, C.; Páez, R.; Mazzoni, R.; Reinheimer, J.; Vinderola, G. Isolation, characterization and performance of autochthonous spray dried lactic acid bacteria in maize micro and bucket-silos. Front. Microbiol. 2018, 9, 2861. [Google Scholar] [CrossRef]
- Driehuis, F.; Oude Elferink, S.J.W.H.; Spoelstra, S.F. Anaerobic lactic acid degradation during ensilage of whole crop maize inocu-lated with Lactobacillus buchneri inhibits yeast growth and improves aerobic stability. J. Appl. Microbiol. 1999, 87, 583–594. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Oude Elferink, S.J.W.H.; Krooneman, J.; Gottschal, J.C.; Spoelstra, S.F.; Faber, F.; Driehuis, F. Anaerobic conversion of lactic acid to acetic acid and 1, 2-propanediol by Lactobacillus buchneri. Appl. Environ. Microbiol. 2001, 67, 125–132. [Google Scholar] [CrossRef]
- Krooneman, J.; Faber, F.; Alderkamp, A.C.; Oude Elferink, S.J.W.H.; Driehuis, F.; Cleenwerck, I.; Swings, J.; Gottschal, J.C.; Vancanneyt, M. Lactobacillus diolivorans sp. nov., a 1, 2-propanediol-degrading bacterium isolated from aerobically stable maize silage. Int. J. Syst. Evol. Microbiol. 2002, 52, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Guaragnella, N.; Bobba, A.; Passarella, S.; Marra, E.; Giannattasio, S. Yeast acetic acid-induced programmed cell death can occur without cytochrome c release which requires metacaspase YCA1. FEBS Lett. 2010, 584, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Lourenco, A.B.; Ascenso, J.R.; Sá-Correia, I. Metabolic insights into the yeast response to propionic acid based on high resolution 1H NMR spectroscopy. Metabolomics 2011, 7, 457–468. [Google Scholar] [CrossRef]
- Ferrero, F.; Piano, S.; Tabacco, E.; Borreani, G. Effects of conservation period and Lactobacillus hilgardii inoculum on the fermentation profile and aerobic stability of whole corn and sorghum silages. J. Sci. Food Agric. 2019, 99, 2530–2540. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.B.; Smith, M.L.; Savage, R.M.; Polukis, S.A.; Drouin, P.; Kung, L. Effects of Lactobacillus hilgardii 4785 and Lactobacillus buchneri 40788 on the bacterial community, fermentation and aerobic stability of high-moisture corn silage. J. Appl. Microbiol. 2020, 130, 1481–1493. [Google Scholar] [CrossRef]
- Drouin, P.; Tremblay, J.; Chaucheyras-Durand, F. Dynamic succession of microbiota during ensiling of whole plant corn following inoculation with Lactobacillus buchneri and Lactobacillus hilgardii alone or in combination. Microorganisms 2019, 7, 595. [Google Scholar] [CrossRef]
- Hu, Z.; Chang, J.; Yu, J.; Li, S.; Niu, H. Diversity of bacterial community during ensiling and subsequent exposure to air in wholeplant maize silage. Asian-australas. J. Anim. 2018, 31, 1464–1473. [Google Scholar] [CrossRef]
- Liu, B.; Huan, H.; Gu, H.; Xu, N.; Shen, Q.; Ding, C. Dynamics of a microbial community during ensiling and upon aerobic exposure in lactic acid bacteria inoculation-treated and untreated barley silages. Bioresour. Technol. 2019, 273, 212–219. [Google Scholar] [CrossRef]
- Bull, L.; Chalupa, W.; Owens, F.; Satter, L.D.; Sniffen, C.J.; Trenkle, A.H.; Waldo, D.R. National Research Council-NRC. Ruminant Nitrogen Usage; The National Academies Press: Washington, DC, USA, 1985; p. 148. [Google Scholar]
- Sumadong, P.; Cherdthong, A.; So, S.; Wanapat, M. Sulfur, fresh cassava root, and urea independently enhanced gas production, ruminal characteristics, and in vitro degradability. BMC Vet. Res. 2021, 17, 304. [Google Scholar] [CrossRef]
- Cherdthong, A.; Wanapat, M. Development of urea products as rumen slow-release feed for ruminant production: A review. Aust. J. Basic Appl. Sci. 2010, 4, 2232–2241. [Google Scholar]
- Pang, H.; Qin, G.; Tan, Z.; Li, Z.; Wang, Y.; Cai, Y. Natural populations of lactic acid bacteria associated with silage fermentation as determined by phenotype, 16S ribosomal RNA and recA gene analysis. Syst. Appl. Microbiol. 2011, 34, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Ni, K.; Zhao, J.; Zhu, B.; Su, R.; Pan, Y.; Ma, J.; Zhou, G.; Tao, Y.; Liu, X.; Zhong, J. Assessing the fermentation quality and microbial community of the mixed silage of forage soybean with crop corn or sorghum. Bioresour. Technol. 2018, 265, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Liu, Y.; Wang, S.; Zhang, Y.; Wang, W.; Yang, H.; Lu, N.; Li, S. Nutrient digestibility, microbial fermentation, and response in bacterial composition to methionine dipeptide: An in vitro study. Biology 2022, 11, 93. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Gao, Y.; Tang, M.; Fu, T.; Diao, Q.; Bi, Y.; Tu, Y. Effects of dietary rumen-protected Lys levels on rumen fermentation and bacterial community composition in Holstein heifers. Appl. Microbiol. Biotechnol. 2020, 104, 6623–6634. [Google Scholar] [CrossRef] [PubMed]
- Penagos-Tabares, F.; Khiaosa-Ard, R.; Schmidt, M.; Pacífico, C.; Faas, J.; Jenkins, T.; Nagl, V.; Sulyok, M.; Labuda, R.; Zebeli, Q. Fungal species and mycotoxins in mouldy spots of grass and maize silages in Austria. Mycotoxin Res. 2022, 38, 117–136. [Google Scholar] [CrossRef] [PubMed]
- Whitlow, L.W.; Hagler, W.M. Mycotoxins in dairy cattle: Occurrence, toxicity, prevention and treatment. In Proceedings of the Southwest Nutrition Conference, Tempe, AZ, USA, 24–25 February 2005. [Google Scholar]
- Dong, J.; Li, S.; Chen, X.; Sun, Z.; Sun, Y.; Zhen, Y.; Qin, G.; Wang, T.; Demelash, N.; Zhang, X. Effects of Lactobacillus plantarum inoculation on the quality and bacterial community of whole-crop corn silage at different harvest stages. Res. Sq. preprint. 2022. [Google Scholar] [CrossRef]
- Drouin, P.; Tremblay, J.; Renaud, J.; Apper, E. Microbiota succession during aerobic stability of maize silage inoculated with Lentilactobacillus buchneri NCIMB 40788 and Lentilactobacillus hilgardii CNCM-I-4785. Microbiologyopen 2021, 10, e1153. [Google Scholar] [CrossRef]
- Bakri, M.M. Evaluating the effects of cellulolytic enzymes and Lactobacillus bulgaricus on mycotoxins production and the quality of maize silage. BioResources 2021, 16, 8366–8378. [Google Scholar] [CrossRef]
- Gallo, A.; Bernardes, T.F.; Copani, G.; Fortunati, P.; Giuberti, G.; Bruschi, S.; Bryan, K.A.; Nielsen, N.G.; Witt, K.L.; Masoero, F. Effect of inoculation with Lactobacillus buchneri LB1819 and Lactococcus lactis O224 on fermentation and mycotoxin production in maize silage compacted at different densities. Anim. Feed Sci. Technol. 2018, 246, 36–45. [Google Scholar] [CrossRef]
- Teller, R.S.; Schmidt, R.J.; Whitlow, L.W.; Kung, L., Jr. Effect of physical damage to ears of corn before harvest and treatment with various additives on the concentration of mycotoxins, silage fermentation, and aerobic stability of corn silage. J. Dairy Sci. 2012, 95, 1428–1436. [Google Scholar] [CrossRef]
- Wang, M.; Xu, S.; Wang, T.; Jia, T.; Xu, Z.; Wang, X.; Yu, Z. Effect of inoculants and storage temperature on the microbial, chemical and mycotoxin composition of corn silage. Asian-australas. J. Anim. Sci. 2018, 31, 1903–1912. [Google Scholar] [CrossRef] [PubMed]
- Eckard, S.; Wettstein, F.E.; Forrer, H.R.; Vogelgsang, S. Incidence of Fusarium species and mycotoxins in silage maize. Toxins 2011, 3, 949–967. [Google Scholar] [CrossRef] [PubMed]
- Latorre, A.; Dagnac, T.; Lorenzo, B.F.; Llompart, M. Occurrence and stability of masked fumonisins in corn silage samples. Food Chem. 2015, 189, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Driehuis, F.; Wilkinson, J.M.; Jiang, Y.; Ogunade, I.; Adesogan, A.T. Silage review: Animal and human health risks from silage. J. Dairy Sci. 2018, 101, 4093–4110. [Google Scholar] [CrossRef]
- Zhang, D.; Zhao, L.; Chen, Y.; Gao, H.; Hua, Y.; Yuan, X.; Yang, H. Mycotoxins in maize silage from China in 2019. Toxins 2022, 14, 241. [Google Scholar] [CrossRef]
- Zachariasova, M.; Dzuman, Z.; Veprikova, Z.; Hajkova, K.; Jiru, M.; Vaclavikova, M.; Zachariasova, A.; Pospichalova, M.; Hajslova, J. Occurrence of multiple mycotoxins in European feedingstuffs, assessment of dietary intake by farm animals. Anim. Feed Sci. Technol. 2014, 193, 124–140. [Google Scholar] [CrossRef]
- Reisinger, N.; Schürer-Waldheim, S.; Mayer, E.; Debevere, S.; Antonissen, G.; Sulyok, M.; Nagl, V. Mycotoxin occurrence in maize silage—A neglected risk for bovine gut health? Toxins 2019, 11, 577. [Google Scholar] [CrossRef]
- Sørensen, J.L.; Nielsen, K.F.; Rasmussen, P.H.; Thrane, U. Development of a LC-MS/MS method for the analysis of enniatins and beauvericin in whole fresh and ensiled maize. J. Agric. Food Chem. 2008, 56, 10439–10443. [Google Scholar] [CrossRef]
- Rasmussen, R.R.; Storm, I.M.L.D.; Rasmussen, P.H.; Smedsgaard, J.; Nielsen, K.F. Multi-mycotoxin analysis of maize silage by LC-MS/MS. Anal. Bioanal. Chem. 2010, 397, 765–776. [Google Scholar] [CrossRef]
- Storm, I.M.; Rasmussen, R.R.; Rasmussen, P.H. Occurrence of pre-and post-harvest mycotoxins and other secondary metabolites in Danish maize silage. Toxins 2014, 6, 2256–2269. [Google Scholar] [CrossRef]
- Van Pamel, E.; Verbeken, A.; Vlaemynck, G.; De Boever, J.; Daeseleire, E. Ultrahigh-performance liquid chromatographic–tandem mass spectrometric multimycotoxin method for quantitating 26 mycotoxins in maize silage. J. Agric. Food Chem. 2011, 59, 9747–9755. [Google Scholar] [CrossRef] [PubMed]
- Shimshoni, J.A.; Cuneah, O.; Sulyok, M.; Krska, R.; Galon, N.; Sharir, B.; Shlosberg, A. Mycotoxins in corn and wheat silage in Israel. Food Addit. Contam. Part A Chem. Anal. 2013, 30, 1614–1625. [Google Scholar] [CrossRef] [PubMed]
- McElhinney, C.; Danaher, M.; Elliott, C.T.; O′Kiely, P. Mycotoxins in farm silages–a 2-year I rish national survey. Grass Forage Sci. 2016, 72, 339–352. [Google Scholar] [CrossRef]
- Wambacq, E.; Vanhoutte, I.; Audenaert, K.; De Gelder, L.; Haesaert, G. Occurrence, prevention and remediation of toxigenic fungi and mycotoxins in silage: A review. J. Sci. Food Agric. 2016, 96, 2284–2302. [Google Scholar] [CrossRef]
- Gräfenhan, T.; Patrick, S.K.; Roscoe, M.; Trelka, R.; Gaba, D.; Chan, J.M.; McKendry, T.; Clear, R.M.; Tittlemier, S.A. Fusarium damage in cereal grains from Western Canada. 1. Phylogenetic analysis of moniliformin-producing Fusarium species and their natural occurrence in mycotoxin-contaminated wheat, oats, and rye. J. Agric. Food Chem. 2013, 61, 5425–5437. [Google Scholar] [CrossRef]
- Bazin, I.; Faucet-Marquis, V.; Monje, M.-C.; El Khoury, M.; Marty, J.-L.; Pfohl-Leszkowicz, A. Impact of pH on the stability and the cross-reactivity of ochratoxin A and citrinin. Toxins 2013, 5, 2324–2340. [Google Scholar] [CrossRef]
- Mannaa, M.; Kim, K.D. Influence of temperature and water activity on deleterious fungi and mycotoxin production during grain storage. Mycobiology 2017, 45, 240–254. [Google Scholar] [CrossRef]
- Garcia-Cela, E.; Kiaitsi, E.; Sulyok, M.; Krska, R.; Medina, A.; Petit Damico, I.; Magan, N. Influence of storage environment on maize grain: CO2 production, dry matter losses and aflatoxins contamination. Food Addit. Contam. Part A 2019, 36, 175–185. [Google Scholar] [CrossRef]
- Barug, D.; Bhatnagar, D.; van Egmond, H.; Van Der Kamp, J.; Van Osenbruggen, W.; Visconti, A. The Mycotoxin Factbook: Food & Feed Topics; Wageningen Academic Publishers: Wageningen, The Netherlands, 2006. [Google Scholar]
- Vandicke, J.; Visschere, K.D.; Ameye, M.; Croubels, S.; Saeger, S.D.; Audenaert, K.; Haesaert, G. Multi-mycotoxin contamination of maize silages in Flanders, Belgium: Monitoring mycotoxin levels from seed to feed. Toxins 2021, 13, 202. [Google Scholar] [CrossRef]
- Schenck, J.; Müller, C.; Djurle, A.; Jensen, D.F.; O’Brien, M.; Johansen, A.; Rasmussen, P.H.; Spörndly, R. Occurrence of filamentous fungi and mycotoxins in wrapped forages in Sweden and Norway and their relation to chemical composition and management. Grass Forage Sci. 2019, 74, 613–625. [Google Scholar] [CrossRef]
- Ogunade, I.; Martinez-Tuppia, C.; Queiroz, O.; Jiang, Y.; Drouin, P.; Wu, F.; Vyas, D.; Adesogan, A. Silage review: Mycotoxins in silage: Occurrence, effects, prevention, and mitigation. J. Dairy Sci. 2018, 101, 4034–4059. [Google Scholar] [CrossRef] [PubMed]
- Commission Directive 2003/100/EC of 31 October 2003 Amending Annex I to Directive 2002/32/EC of the European Parliament and of the Council on Undesirable Substances in Animal Feed. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32003L0100&rid=2 (accessed on 20 June 2022).
- Mostrom, M.S.; Jacobsen, B.J. Ruminant mycotoxicosis: An update. Vet. Clin. Food Anim. Pract. 2020, 36, 745–774. [Google Scholar] [CrossRef] [PubMed]
- Commission recommendation 2006/576/EC of 17 august 2006 on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 and fumonisins in products intended for animal feeding. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2006:229:0007:0009:EN:PDF (accessed on 20 June 2022).
- Gallo, A.; Giuberti, G.; Frisvad, J.C.; Bertuzzi, T.; Nielsen, K.F. Review on mycotoxin issues in ruminants: Occurrence in forages, effects of mycotoxin ingestion on health status and animal performance and practical strategies to counteract their negative effects. Toxins 2015, 7, 3057–3111. [Google Scholar] [CrossRef] [PubMed]
- Valgaeren, B.; Théron, L.; Croubels, S.; Devreese, M.; Baere, S.D.; Pamel, E.V.; Daeseleire, E.; Boevre, M.D.; Saeger, S.D.; Vidal, A.; et al. The role of roughage provision on the absorption and disposition of the mycotoxin deoxynivalenol and its acetylated derivatives in calves: From field observations to toxicokinetics. Arch. Toxicol. 2018, 93, 293–310. [Google Scholar] [CrossRef] [PubMed]
- Diaz, D.; Hopkins, B.A.; Leonard, L.M.; Hagler, W.M., Jr.; Whitlow, L.W. Effect of fumonisin on lactating dairy cattle. J. Dairy Sci. 2000, 83, 1171. [Google Scholar]
- Roberts, H.L.; Bionaz, M.; Jiang, D.; Doupovec, B.; Faas, J.; Estill, C.T.; Schatzmayr, D.; Duringer, J.M. Effects of deoxynivalenol and fumonisins fed in combination to beef cattle: Immunotoxicity and gene expression. Toxins 2021, 13, 714. [Google Scholar] [CrossRef]
- Vardon, P.; McLaughlin, C.; Nardinelli, C. CAST (Council for Agricultural Science and Technology) Technology Mycotoxins: Risks in Plant, Animal, and Human Systems; Task Force Report, No. 139; Council for Agricultural Science and Technology: Ames, IA, USA, 2003; ISBN 978-1-887383-22-6. [Google Scholar]
- Benford, D.; Ceccatelli, S.; Cottrill, B.; DiNovi, M.; Dogliotti, E.; Edler, L.; Farmer, P.; Fürst, P.; Hoogenboom, L.; Knutsen, H.K.; et al. Scientific opinion on risks for animal and public health related to the presence of nivalenol in food and feed. EFSA J. 2013, 11, 3262. [Google Scholar] [CrossRef]
- Eriksen, G.S.; Pettersson, H.; Lundh, T. Comparative cytotoxicity of deoxynivalenol, nivalenol, their acetylated derivatives and de-epoxy metabolites. Food Chem. Toxicol. 2004, 42, 619–624. [Google Scholar] [CrossRef]
- Benford, D.; Ceccatelli, S.; Cottrill, B.; DiNovi, M.; Dogliotti, E.; Edler, L.; Farmer, P.; Fürst, P.; Hoogenboom, L.; Knutsen, H.K.; et al. EFSA Panel on contaminants in the food chain (CONTAM). Scientific opinion on the risks for human and animal health related to the presence of modified forms of certain mycotoxins in food and feed. EFSA J. 2014, 12, 3916. [Google Scholar] [CrossRef]
- Kouri, K.; Lemmens, M.; Lemmens-Gruber, R. Beauvericin-induced channels in ventricular myocytes and liposomes. Biochim. Et Biophys. Acta (BBA)-Biomembr. 2003, 1609, 203–210. [Google Scholar] [CrossRef]
- Ornelis, V.; Rajkovic, A.; Decleer, M.; Sas, B.; Saeger, S.D.; Madder, A. Counteracting in vitro toxicity of the ionophoric mycotoxin beauvericin—synthetic receptors to the rescue. J. Org. Chem. 2019, 84, 10422–10435. [Google Scholar] [CrossRef] [PubMed]
- Maranghi, F.; Tassinari, R.; Narciso, L.; Tait, S.; Rocca, C.L.; Felice, G.D.; Butteroni, C.; Corinti, S.; Barletta, B.; Cordelli, E.; et al. In vivo toxicity and genotoxicity of beauvericin and enniatins. Combined approach to study in vivo toxicity and genotoxicity of mycotoxins geauvericin (BEA) and enniatin B (ENNB). EFSA Support. Publ. 2018, 15, 1406E. [Google Scholar] [CrossRef]
- Huang, C.-H.; Wang, F.-T.; Chan, W.-H. Enniatin B1 exerts embryotoxic effects on mouse blastocysts and induces oxidative stress and immunotoxicity during embryo development. Environ. Toxicol. 2019, 34, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; DiNovi, M.; Grasl-Kraupp, B.; Hogstrand, C.; et al. Risks to human and animal health related to the presence of moniliformin in food and feed. EFSA J. 2018, 16, 5082. [Google Scholar] [CrossRef]
- Lamprecht, S.C. Incidence and toxigenicity of seedborne Fusarium species from annual Medicago species in South Africa. Phytopathology 1986, 76, 1040–1042. [Google Scholar] [CrossRef]
- Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Grasl-Kraupp, B.; Hogstrand, C.; et al. Risks to human and animal health related to the presence of deoxyniva-lenol and its acetylated and modified forms in food and feed. EFSA J. 2017, 15, e04718. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).