Molecular Variation and Phylogeny within Fusarium avenaceum and Related Species
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Molecular Characterisation of Strains
3.2. Mycotoxin Production of Strains
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nalim, F.A.; Elmer, W.H.; McGovern, R.J.; Geiser, D.M. Multilocus phylogenetic diversity of Fusarium avenaceum pathogenic on lisianthus. Phytopathology 2009, 99, 462–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gräfenhan, T.; Patrick, S.K.; Roscoe, M.; Trelka, R.; Gaba, D.; Chan, J.M.; McKendry, T.; Clear, R.M.; Tittlemier, S.A. Fusarium damage in cereal grains from Western Canada. 1. Phylogenetic analysis of moniliformin-producing Fusarium species and their natural occurrence in mycotoxin-contaminated wheat, oats, and rye. J. Agric. Food Chem. 2013, 61, 5425–5437. [Google Scholar] [CrossRef] [PubMed]
- Jacobs-Venter, A.; Laraba, I.; Geiser, D.M.; Busman, M.; Vaughan, M.M.; Proctor, R.H.; McCormick, S.P.; O’Donnell, K. Molecular systematics of two sister clades, the Fusarium concolor and F. babinda species complexes, and the discovery of a novel microcycle macroconidium–producing species from South Africa. Mycologia 2018, 110, 1189–1204. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, K.; McCormick, S.P.; Busman, M.; Proctor, R.H.; Ward, T.J.; Doehring, G.; Geiser, D.M.; Alberts, J.F.; Rheeder, J.P. 1984 “Toxigenic Fusarium Species: Identity and Mycotoxicology” revisited. Mycologia 2018, 110, 1058–1080. [Google Scholar] [CrossRef] [PubMed]
- Yli-Mattila, T.; Hussien, T.; Gavrilova, O.; Gagkaeva, T. Morphological and molecular variation between Fusarium avenaceum, Fusarium arthrosporioides and Fusarium anguioides strains. Pathogens 2018, 7, 94. [Google Scholar] [CrossRef] [Green Version]
- Pollard, A.T.; Okubara, P.A. Real-time PCR quantification of Fusarium avenaceum in soil and seeds. J. Microbiol. Methods 2019, 157, 21–30. [Google Scholar] [CrossRef]
- Ponts, N.; Gautier, C.; Gouzy, J.; Pinson-Gadais, L.; Foulongne-Oriol, M.; Ducos, C.; Richard-Forget, F.; Savoie, J.-M.; Zhao, C.; Barroso, G. Evolution of Fusarium tricinctum and Fusarium avenaceum mitochondrial genomes is driven by mobility of introns and of a new type of palindromic microsatellite repeats. BMC Genom. 2020, 21, 358. [Google Scholar] [CrossRef]
- Cowger, C.; Ward, T.J.; Nilsson, K.; Arellano, C.; McCormick, S.P.; Busman, M. Regional and field-specific differences in Fusarium species and mycotoxins associated with blighted North Carolina wheat. Int. J. Food Microbiol. 2020, 323, 108594. [Google Scholar] [CrossRef]
- Geiser, D.M.; Al-Hatmi, A.M.S.; Aoki, T.; Arie, T.; Balmas, V.; Barnes, I.; Bergstrom, G.C.; Bhattacharyya, M.K.K.; Blomquist, C.L.; Bowden, R.; et al. Phylogenomic analysis of a 55.1-kb 19-gene dataset resolves a monophyletic fusarium that includes the Fusarium solani species complex. Phytopathology 2021, 111, 1064–1079. [Google Scholar] [CrossRef]
- Senatore, M.T.; Ward, T.J.; Cappellett, E.; Beccari, G.; McCormick, S.P.; Busman, M.; Laraba, I.; O’Donnell, K.; Prodi, A. Species diversity and mycotoxin production by members of the Fusarium tricinctum species complex associated with Fusarium head blight of wheat and barley in Italy. Int. J. Food Microbiol. 2021, 358, 109298. [Google Scholar] [CrossRef]
- Laraba, I.; Busman, M.; Geiser, D.M.; O’Donnell, K. Phylogenetic diversity and mycotoxin potential of emergent phytopathogens within the Fusarium tricinctum species complex. Phytopathology 2022, 112, 1284–1298. [Google Scholar] [CrossRef] [PubMed]
- Gerlach, W.; Nirenberg, H. The Genus Fusarium—A Pictorial Atlas; Mitteilungen aus der Biologischen Bundesanstalt fur Land- und Forstwirtschaft Berlin-Dahlem: Berlin, Germany, 1982; 406p. [Google Scholar]
- Nelson, P.E.; Toussoun, T.A.; Marasas, W.F.O. Fusarium Species: An Illustrated Manual for Identification; Pennsylvania State University Press: University Park, PA, USA, 1983; 206p. [Google Scholar]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual; Blackwell Publishing: Hoboken, NJ, USA, 2006; 388p. [Google Scholar]
- Yli-Mattila, T.; Mironenko, N.V.; Alekhina, I.A.; Hannukkala, A.; Bulat, S.A. Universally primed polymerase chain reaction analysis of Fusarium avenaceum isolated from wheat and barley in Finland. Agric. Food Sci. 1997, 6, 25–36. [Google Scholar] [CrossRef]
- Yli-Mattila, T.; Paavanen-Huhtala, S.; Bulat, S.A.; Alekhina, I.A.; Nirenberg, H.I. Molecular, morphological and phylogenetic analysis of Fusarium avenaceum/F. arthrosporioides/F. tricinctum species complex—A polyphasic approach. Mycol. Res. 2002, 106, 655–669. [Google Scholar] [CrossRef]
- Yli-Mattila, T.; Paavanen-Huhtala, S.; Parikka, P.A.; Jestoi, M.; Klemsdal, S.; Rizzo, A.A. Genetic variation, real-time PCR, metabolites and mycotoxins of Fusarium avenaceum and related species. Mycotoxin Res. 2006, 22, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Jestoi, M.; Paavanen-Huhtala, S.; Parikka, P.; Yli-Mattila, T. In vitro and in vivo mycotoxin production of Fusarium isolated from Finnish grains. Arch. Phytopathol. Plant Prot. 2008, 41, 545–558. [Google Scholar] [CrossRef]
- Yli-Mattila, T.; Paavanen-Huhtala, S.; Parikka, P.; Konstantinova, P.; Gagkaeva, T. Molecular and morphological diversity of Fusarium species in Finland and North-Western Russia. Eur. J. Plant Pathol. 2004, 110, 573–585. [Google Scholar] [CrossRef]
- Peters, J.C.; Lees, A.K.; Cullen, D.W.; Sullivan, L.; Stroud, G.P.; Cunnington, A.C. Characterization of Fusarium spp. responsible for causing dry rot of potato in Great Britain. Plant Pathol. 2008, 57, 262–271. [Google Scholar] [CrossRef]
- Sørensen, J.L.; Phipps, R.K.; Nielsen, K.F.; Schroers, H.J.; Frank, J.; Thrane, U. Analysis of Fusarium avenaceum metabolites produced during wet apple core rot. J. Agric. Food Chem. 2009, 57, 1632–1639. [Google Scholar] [CrossRef]
- Sakoda, T.; Yamasaki, N.; Abe, Y.; Yanagisawa, H.; Koike, M. Bulb rot of Sandersonia aurantiaca caused by Fusarium anguioides and Fusarium sp. intercepted at plant quarantine in Japan. Res. Bull. Plant Prot. Jpn. 2011, 47, 41–47. [Google Scholar]
- Hietaniemi, V.; Rämö, S.; Yli-Mattila, T.; Jestoi, M.; Peltonen, S.; Kartio, M.; Sieviläinen, E.; Koivisto, T.; Parikka, P. Updated survey of Fusarium species and toxins in Finnish cereal grains. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2016, 33, 831–848. [Google Scholar] [CrossRef]
- Moreira, G.M.; Machado, F.J.; Pereiera, C.B.; Neves, D.L.; Tessmann, D.J.; Ward, T.J.; Del Ponte, E.M. First report of the Fusarium tricinctum species complex causing Fusarium Head Blight of wheat in Brazil. Plant Dis. 2019, 104, 586. [Google Scholar] [CrossRef]
- Gavrilova, O.P.; Gagkaeva, T.Y.; Orina, A.S.; Gogina, N.N. Diversity of Fusarium species and their mycotoxins in cereals grain from the Asian territory of Russia. Mikol. I Fitopatol. 2022, 56, 194–206. [Google Scholar] [CrossRef]
- Parikka, P.; Hakala, K.; Tiilikkala, K. Expected shifts in Fusarium species’ composition on cereal grain in Northern Europe due to climate change. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2012, 29, 1543–1555. [Google Scholar] [CrossRef] [PubMed]
- Jestoi, M.; Rokka, M.; Yli-Mattila, T.; Parikka, P.; Rizzo, A.; Peltonen, K. The presence and concentrations of the Fusarium-related mycotoxins beauvericin, enniatins and moniliformin in Finnish grain samples. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2004, 21, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Uhlig, S.; Jestoi, M.; Parikka, P. Fusarium avenaceum—The North European situation. Int. J. Food Microbiol. 2007, 119, 17–24. [Google Scholar] [CrossRef]
- Vogelgsang, S.; Sulyok, M.; Bänziger, I.; Krska, R.; Schuhmacher, R.; Forrer, H.-R. Effect of fungal strain and cereal substrate on in vitro mycotoxin production by Fusarium poae and Fusarium avenaceum. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2008, 25, 745–757. [Google Scholar] [CrossRef]
- Stępień, Ł.; Waśkiewicz, A. Sequence divergence of the enniatin synthase gene in relation to production of beauvericin and enniatins in Fusarium species. Toxins 2013, 5, 537–555. [Google Scholar] [CrossRef]
- Covarelli, L.; Beccari, G.; Prodi, A.; Generotti, S.; Etruschi, F.; Meca, G.; Juan, C.; Mañes, J. Biosynthesis of beauvericin and enniatins in vitro by wheat Fusarium species and natural grain contamination in an area of central Italy. Food Microbiol. 2015, 46, 618–626. [Google Scholar] [CrossRef]
- Kulik, T.; Pszczółkowska, A.; Łojko, M. Multilocus phylogenetics show high intraspecific variability within Fusarium avenaceum. Int. J. Mol. Sci. 2011, 12, 5626–5640. [Google Scholar] [CrossRef] [Green Version]
- Eranthodi, A.; Schneiderman, D.; Harris, L.J.; Witte, T.E.; Sproule, A.; Hermans, A.; Overy, D.P.; Chatterton, S.; Liu, J.; Li, T.; et al. Enniatin production influences Fusarium avenaceum virulence on potato tubers, but not on durum wheat or peas. Pathogens 2020, 9, 75. [Google Scholar] [CrossRef] [Green Version]
- Hofgaard, I.S.; Aamot, H.U.; Torp, T.; Jestoi, M.; Lattanzio, V.M.T.; Klemsdal, S.S.; Waalwijk, C.; van der Lee, T.; Brodal, G. Associations between Fusarium species and mycotoxins in oats and spring wheat from farmers’ fields in Norway over a six-year period. World Mycotoxin J. 2016, 9, 365–378. [Google Scholar] [CrossRef]
- Gagkaeva, T.Y.; Gavrilova, O.P. Fusarium infection and mycotoxins contamination in grain of spring barley cultivars. Plant Prot. News 2017, 3, 39–43. [Google Scholar]
- Nelson, P.E.; Toussoun, T.A.; Marasas, W.F.O. Neotypification and emended description of Fusarium anguioides. Mycologia 1995, 8, 543–546. [Google Scholar] [CrossRef]
- O’Donnell, K.; Rooney, A.P.; Proctor, R.H.; Brown, D.W.; McCormick, S.P.; Ward, T.J.; Frandsen, R.J.N.; Lysøe, E.; Rehner, S.A.; Aoki, T.; et al. Phylogenetic analyses of RPB1 and RPB2 support a middle Cretaceous origin for a clade comprising all agriculturally and medically important fusaria. Fungal Genet. Biol. 2013, 52, 20–31. [Google Scholar] [CrossRef]
- Crous, P.W.; Lombard, L.; Sandoval-Denis, M.; Seifert, K.-A.; Schroers, H.-J.; Chaverri, P.; Gené, J.; Guarro, J.; Hirooka, Y.; Bensch, K.; et al. Fusarium: More than a node or a foot-shaped basal cell. Stud. Mycol. 2021, 98, 100116. [Google Scholar] [CrossRef]
- Sherbakoff, C.D. Fusaria of potatoes. In Memoirs of the Cornell University Agricultural Experimental Station; Cornell University, Agricultural Experiment Station: Ithaca, NY, USA, 1915; pp. 87–270. [Google Scholar]
- Stakheev, A.A.; Khairulina, D.R.; Zavriev, S.K. Four-locus phylogeny of Fusarium avenaceum and related species and their species-specific identification based on partial phosphate permease gene sequences. Int. J. Food Microbiol. 2016, 225, 27–37. [Google Scholar] [CrossRef]
- Ausubel, F.M.; Brent, R.; Kingston, R.E.; Moore, D.D.; Seidman, J.G.; Smith, J.A.; Struhl, K. Short Protocols in Molecular Biology, 5th ed.; John Wiley & Sons: New York, NY, USA, 2002; 1504p. [Google Scholar]
- Yli-Mattila, T.; Paavanen-Huhtala, S.; Jestoi, M.; Parikka, P.; Hietaniemi, V.; Gagkaeva, T.; Sarlin, T. Real-time PCR detection and quantification of Fusarium poae, F. graminearum, F. sporotrichioides and F. langsethiae in cereal grains in Finland and Russia. Arch. Phytopathol. Plant Prot. 2008, 41, 243–260. [Google Scholar] [CrossRef]
- O’Donnell, K.; Kistler, H.C.; Cigelnik, E.; Ploetz, R.C. Multiple evolutionary origins of the fungus causing Panama disease of banana: Concordant evidence from nuclear and mitochondrial gene genealogies. Proc. Natl. Acad. Sci. USA 1998, 95, 2044–2049. [Google Scholar] [CrossRef] [Green Version]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [Green Version]
- Tamura, K.; Stcher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Niessen, L.; Gräfenhan, T.; Vogel, R.F. ATP citrate lyase 1 (asc1) gene-based loop-mediated amplification assay for the detection of the Fusarium tricinctum species complex in pure cultures and in cereal samples. Int. J. Food Microbiol. 2012, 158, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Yli-Mattila, T.; Paavanen, S.; Hannukkala, A.; Parikka, P.; Tahvonen, R.; Karjalainen, R. Isozyme and RAPD-PCR analyses of Fusarium avenaceum strains in Finland. Plant Pathol. 1996, 45, 126–134. [Google Scholar] [CrossRef]
- Cerón-Bustamante, M.; Ward, T.J.; Kelly, A.; Vaughan, M.M.; McCormick, S.P.; Cowger, C.; Leyva-Mir, S.G.; Villaseñor-Mir, H.E.; Ayala-Escobar, V.; Nava-Díaz, C. Regional differences in the composition of Fusarium Head Blight pathogens and mycotoxins associated with wheat in Mexico. Int. J. Food Microbiol. 2018, 273, 11–19. [Google Scholar] [CrossRef]
- Lysøe, E.; Harris, L.J.; Walkowiak, S.; Subramaniam, R.; Divon, H.H.; Riiser, E.S.; Llorens, C.; Gabaldón, T.; Kistler, H.C.; Jonkers, W.; et al. The genome of the generalist plant pathogen Fusarium avenaceum is enriched with genes involved in redox, signaling and secondary metabolism. PLoS ONE 2014, 9, e112703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Logrieco, A.; Rizzo, A.; Ferracane, R.; Ritieni, A. Occurrence of beauvericin and enniatins in wheat affected by Fusarium avenaceum head blight. Appl. Environ. Microbiol. 2002, 68, 82–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrison, E.; Kosiak, B.; Ritieni, A.; Aastveit, A.; Uhlig, S.; Bernhoft, A. Mycotoxin production by Fusarium avenaceum strains isolated from Norwegian grain and the cytotoxicity of rice culture extracts to porcine kidney epithelial cells. J. Agric. Food Chem. 2002, 50, 3070–3075. [Google Scholar] [CrossRef]
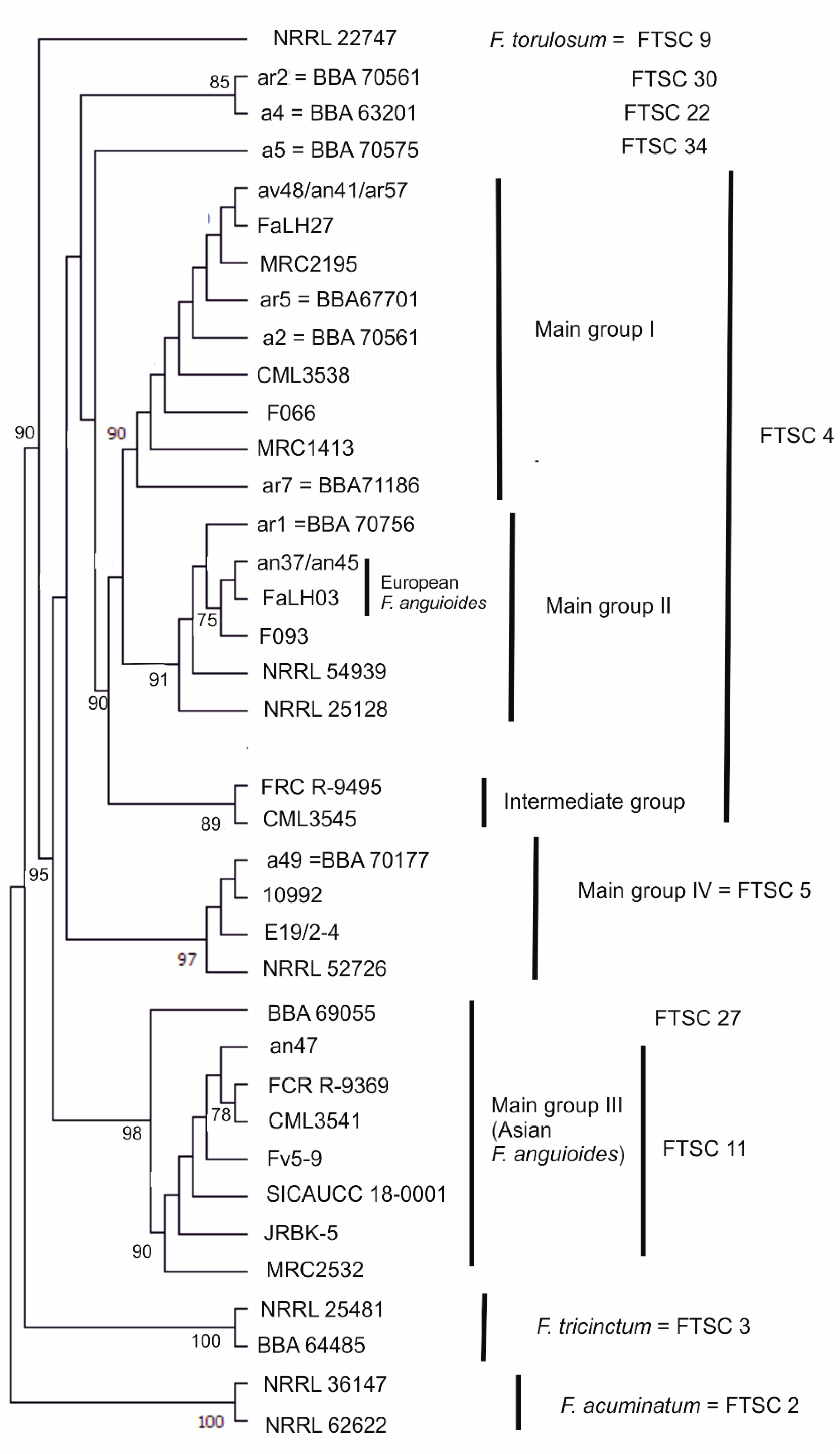

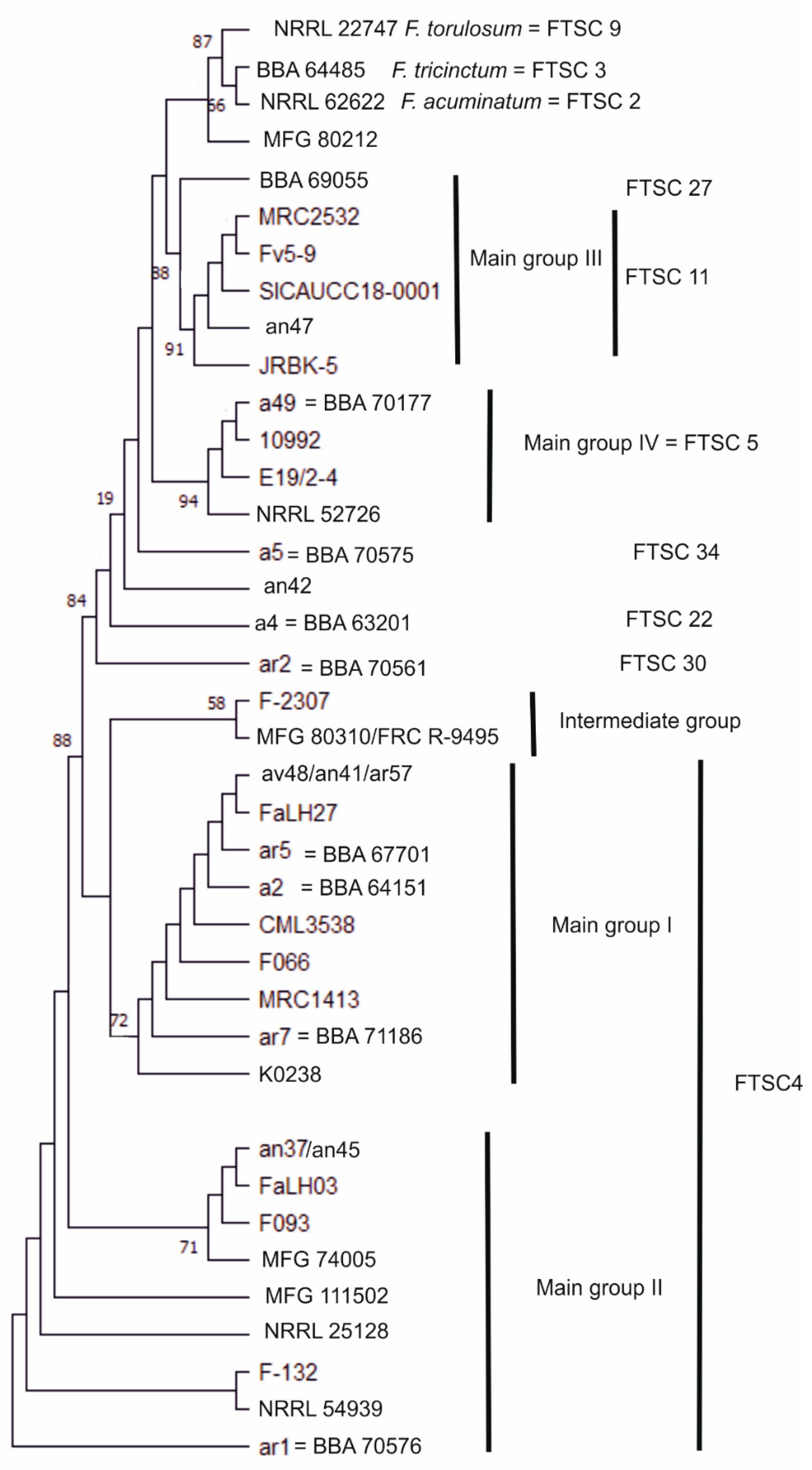


| Group | FTSC * | Morphological Species | Strain No. | Alternative Strain No. ** | Origin | Substrate | Year |
|---|---|---|---|---|---|---|---|
| Main Group I | 4 | F. avenaceum | MFG 118702 | av48 c | Russia, Pskov | barley, grain | 2009 |
| F. arthrosporioides | MFG 58655 | ar57 c | Russia, Leningrad | oat, grain | 2013 | ||
| F. avenaceum | BBA 64151 | a2 b | Germany | Solanum | 1980 | ||
| F. arthrosporioides | BBA 71186 | ar7 b | Germany | Bellis | 1999 | ||
| F. anguioides | MFG 114605 | an41 c | Russia, Kaliningrad | barley, grain | 2008 | ||
| F. anguioides | MFG 119913 | an42 c | Russia, Kirov | oat, grain | 2008 | ||
| F. anguioides | MFG 108904 | an45 c | Russia, Pskov | barley, grain | 2008 | ||
| F. arthrosporioides | BBA 67701 | ar5 b | France | cereals | 1993 | ||
| F. avenaceum | DDPP 04401 | NA *** | Poland | wheat | NA | ||
| F. avenaceum | KK12 | NA | Hungary | wheat | NA | ||
| F. avenaceum | F1 | NA | Hungary | wheat | NA | ||
| F. avenaceum | 18 | NA | Hungary | wheat | NA | ||
| F. avenaceum | DDPP 042836 | NA | Poland | wheat | NA | ||
| F. avenaceum | DDPP 04032 | NA | Poland | wheat | NA | ||
| F. avenaceum | K-0238 | NA | Russia | wheat | NA | ||
| F. avenaceum | FCR R6679 | NA | Australia | soil | NA | ||
| F. avenaceum | NRRL 26890 | NA | Finland | wheat | NA | ||
| F. avenaceum | FRC R-671 | NA | USA, New York | alfalfa | NA | ||
| F. avenaceum | FaLH27 | NA d | Canada | winter wheat | 2001 | ||
| Main Group II | 4 | F. anguioides | MFG 112804 | an37 c | Russia, Novgorod | barley, grain | 2008 |
| F. arthrosporioides | BBA 70576 | ar1 b | Finland | barley | 1986 | ||
| F. avenaceum | FaLH03 | NA d | Canada | spring wheat | 2001 | ||
| F. avenaceum | NRRL 54939 | Fa05001 d | Finland | barley | 2005 | ||
| F. avenaceum | F093 | NA | Canada | lupin | NA | ||
| F. avenaceum | NRRL 25128 | NA | Poland | Ichneumonidae | NA | ||
| F. avenaceum | CBS 15957 | NA | Italy | Fagus sylvatica | NA | ||
| F. avenaceum | DDPP 09a1 | NA | Poland | wheat | NA | ||
| F. anguioides | MFG 74005 | NA | Russia | wheat | NA | ||
| F. avenaceum | F-132 | NA | Russia | wheat | NA | ||
| F. anguioides | MFG 111502 | NA | Russia | barley | NA | ||
| Main Group V | 4 | F. avenaceum | NRRL 36069 | CBS 101627 | UK | carnation | NA |
| F. avenaceum | NRRL 53589 | CBS 386.62 | Netherlands | winter wheat | NA | ||
| F. avenaceum | NRRL 53729 | CBS 121289 | Switzerland | winter wheat | NA | ||
| F. avenaceum | CBS 409.86 | NA | USA | barley | NA | ||
| F. avenaceum | 05-003 | NA | Switzerland | wheat | NA | ||
| F. avenaceum | 376 | NA | Switzerland | wheat | NA | ||
| Intermediate | 4 | F. avenaceum | IBT 40026 | NA | Denmark | wheat | NA |
| Group | F. avenaceum | CBS 409.86 | NA | USA | barley | NA | |
| Between Main Groups I and II | F. avenaceum | DDPP 04401 | NA | Poland | wheat | NA | |
| F. avenaceum | 379 | NA | Switzerland | wheat | NA | ||
| F. avenaceum | CML 3545 | NA | Brazil | wheat | NA | ||
| F. avenaceum | FCR R-9495 | NA | USA, California | lisianthus | NA | ||
| F. avenaceum | MFG 80310 | NA | Russia | oat | NA | ||
| F. avenaceum | F-2307 | NA | Germany | wheat | NA | ||
| F. avenaceum | NRRL 36374 | CBS 239.94 | Netherlands | carnation | NA | ||
| Gibberella tricincta | NRRL 39591 | ICMP 5244 | New Zealand | garden pea | NA | ||
| F. avenaceum | NRRL 53733 | CBS 121294 | Switzerland | winter wheat | NA | ||
| Unknown Group I | 22 | F. avenaceum | FRC R-6739 | NA | Germany | codling moth | NA |
| F. avenaceum | BBA 63201 | a4 b | Austria | Ulmus scabra | 1974 | ||
| Main Group III | 11 | F. anguioides | MFG 58314 | an47 c | Russia, Vladivostok | rudbeckia, leaves | 2010 |
| Fusarium sp. | CML 3541 | NA | Brazil | wheat | NA | ||
| F. avenaceum | FRC-R9369 | NA | Canada | lisianthus | NA | ||
| F. avenaceum | JRBK-5 | NA | China, Hubei | walnut | NA | ||
| F. avenaceum | SICAUCC 18 | NA | China | Polygonatum cyrtonema | NA | ||
| F. sp. nov-9 | MRC 2532 | NA | Japan | soybean | NA | ||
| F. avenaceum | Fv5-9 | NA | China | Fragaria ananassa | NA | ||
| 27 | F. anguioides | BBA 69055 | an3 b | Japan | wheat, grain | 1994 | |
| Main Group IV | 5 | F. avenaceum | BBA 70177 | a49 a, NRRL 26893 | Finland | apple | NA |
| Fusarium sp. | NRRL 52726 | NA | Turkey | NA | NA | ||
| Fusarium sp. | KOD 810 | NA | USA, New Hampshire | fir | NA | ||
| F. avenaceum | 10992 | NA | New Zealand | Pinus radiata | NA | ||
| F. avenaceum | E19/2-4 | NA | Slovenia | Acer pseudoplatanus | NA | ||
| Unknown Group II | 30 | F. arthrosporioides | BBA 70561 b,e | ar2 b | Finland | barley | 1986 |
| Unknown Group III | ? | F. avenaceum | MFG 80212 | NA | Russia | oat | NA |
| Unknown Group IV | 34 | F. avenaceum | BBA 70575 b,e | a5 b | Germany | Cytisus | 1997 |
| 9 | F. torulosum | NRRL 22747 | NA | Hungary | barley | NA | |
| 3 | F. tricinctum | NRRL 25481 | NA | NA | NA | NA | |
| F. tricinctum | BBA 64485 | t6 b | Germany | wheat | 1986 | ||
| 2 | F. acuminatum | NRRL 36147 | NA | NA | NA | NA | |
| F. acuminatum | NRRL 62622 | CS4907 | NA | NA | NA |
| Main Group/FTSC * | Morphological Species | Strain No. ** | Mycotoxin Amount (ng/mL) | |||||
|---|---|---|---|---|---|---|---|---|
| BEA *** | ENN B | ENN B1 | ENN A | ENN A1 | AOD | |||
| −/4 | F. avenaceum | NRRL 13826 | 1.0 | 20.0 | 5.2 | 0 | LOQ | LOQ |
| I/4 | F. arthrosporioides | BBA 71186 a | 0 | 22.7 | 5.8 | 0 | LOQ | 41.5 |
| I/4 | F. anguioides | BBA 63598 a | 0 | 0 | 0 | 0 | 0 | LOQ |
| I/4 | F. avenaceum | MFG 151200 b | 0 | 10.5 | 1.5 | 0 | LOQ | 20.1 |
| II/4 | F. arthrosporioides | BBA 64134 a | 0 | 5.2 | LOQ | 0 | LOQ | 19.6 |
| II/4 | F. arthrosporioides | BBA 64215 a | 0 | 0 | 0 | 0 | 0 | LOQ |
| II/4 | F. anguioides | MFG 103100 b | 0 | 8.4 | 2.2 | 0 | LOQ | 31.5 |
| II/4 | F. anguioides | MFG 108904 b | 0 | LOQ | LOQ | 0 | LOQ | 13.9 |
| II/4 | F. anguioides | MFG 112804 b | 0 | 2.0 | LOQ | 0 | LOQ | 11.2 |
| III/11 | F. anguioides | MFG 58314 b | 0 | 1.6 | LOQ | 0 | 0 | 125.8 |
| III/27 | F. anguioides | BBA 69055 a,b | 0 | 41.2 | 21.2 | 0 | 3.9 | LOQ |
| –/22 | F. avenaceum | BBA 63201 a | 0 | 4.8 | LOQ | 0 | LOQ | LOQ |
| −/30 | F. arthrosporioides | BBA 70561 a | 0 | LOQ | LOQ | 0 | 1.7 | 1.5 |
| −/3 | F. tricinctum | BBA 64485 b | 0 | LOQ | LOQ | 0 | LOQ | 4.3 |
| −/2 | F. acuminatum | MFG 42305 | 0 | LOQ | LOQ | 0 | 0 | 1.2 |
| −/2 | F. acuminatum | BBA 67800 a | 0 | 36.0 | 38.5 | LOQ | 19.8 | 27.6 |
| −/2 | F. acuminatum | MFG 58674 | 0 | 25.1 | 22.5 | LOQ | 10.3 | 21.8 |
| −/2 | F. acuminatum | MFG 60360 | 0 | 22.1 | 20.4 | LOQ | 8.1 | 12.3 |
| −/− | F. graminum | BBA 65242 a | 0 | 0 | 0 | 0 | 0 | LOQ |
| −/− | F. graminum | MFG 60500 | 0 | LOQ | LOQ | 0 | LOQ | LOQ |
| −/− | F. reticulatum | MFG 58549 | 0 | 4 | 2.8 | 0 | 1.1 | 170.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yli-Mattila, T.; Abbas, A.; Gavrilova, O.; Gagkaeva, T. Molecular Variation and Phylogeny within Fusarium avenaceum and Related Species. Diversity 2022, 14, 574. https://doi.org/10.3390/d14070574
Yli-Mattila T, Abbas A, Gavrilova O, Gagkaeva T. Molecular Variation and Phylogeny within Fusarium avenaceum and Related Species. Diversity. 2022; 14(7):574. https://doi.org/10.3390/d14070574
Chicago/Turabian StyleYli-Mattila, Tapani, Asmaa Abbas, Olga Gavrilova, and Tatiana Gagkaeva. 2022. "Molecular Variation and Phylogeny within Fusarium avenaceum and Related Species" Diversity 14, no. 7: 574. https://doi.org/10.3390/d14070574
APA StyleYli-Mattila, T., Abbas, A., Gavrilova, O., & Gagkaeva, T. (2022). Molecular Variation and Phylogeny within Fusarium avenaceum and Related Species. Diversity, 14(7), 574. https://doi.org/10.3390/d14070574







