Fusarium casha sp. nov. and F. curculicola sp. nov. in the Fusarium fujikuroi Species Complex Isolated from Amaranthuscruentus and Three Weevil Species in South Africa
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Isolates
2.2. DNA Sequence Comparisons
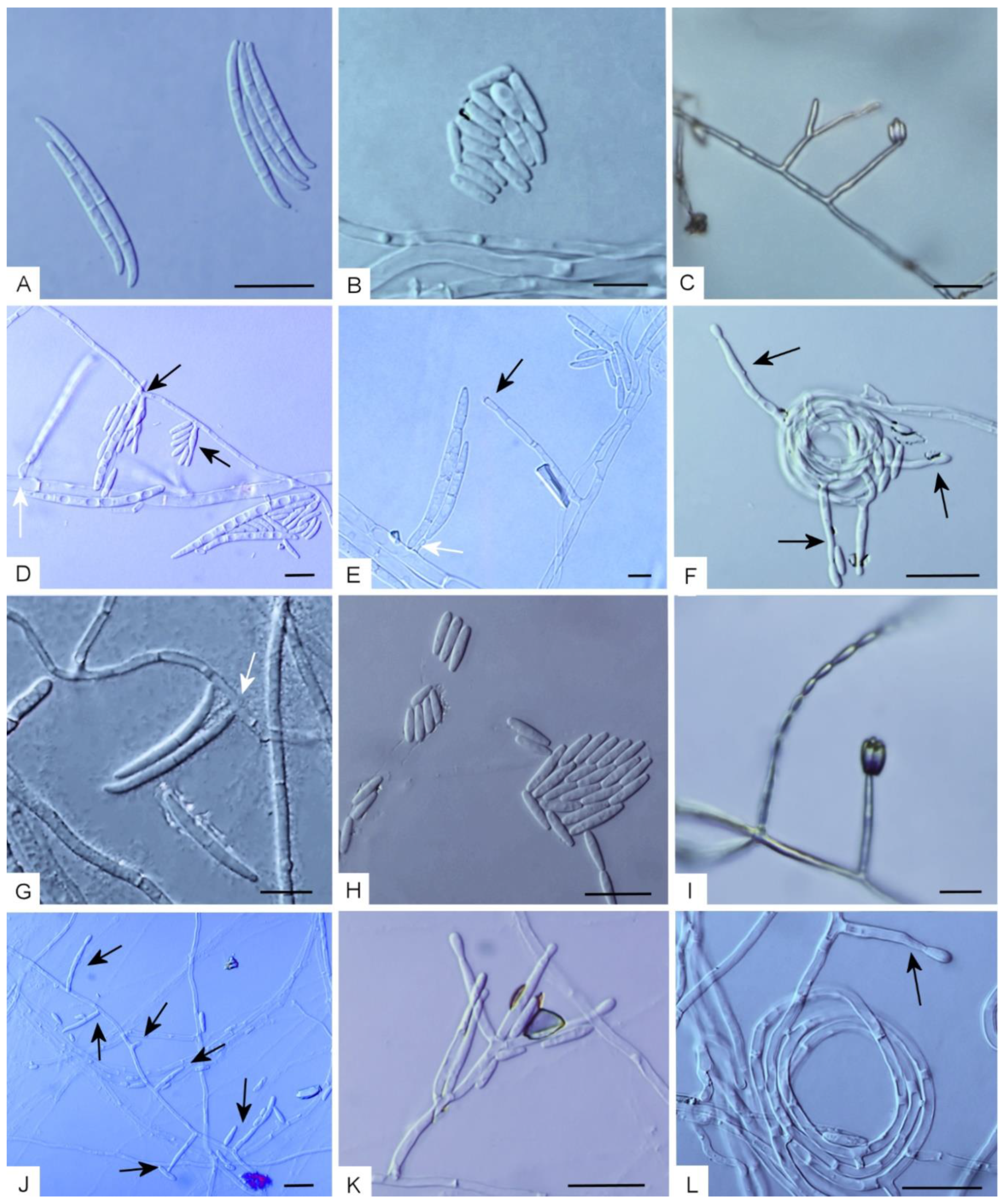
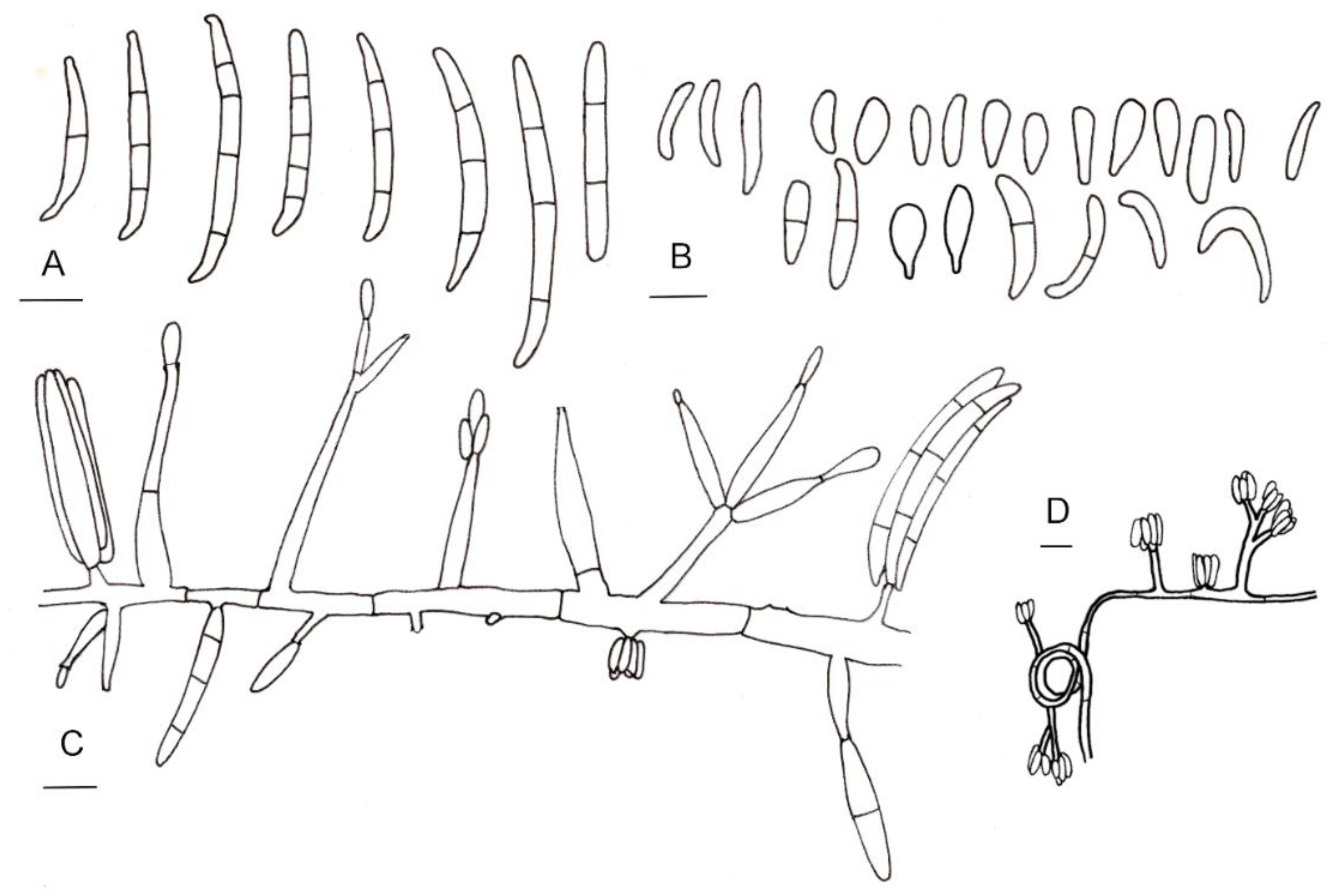
2.3. Morphology
2.4. Pathogenicity
3. Results
3.1. Fungal Isolates
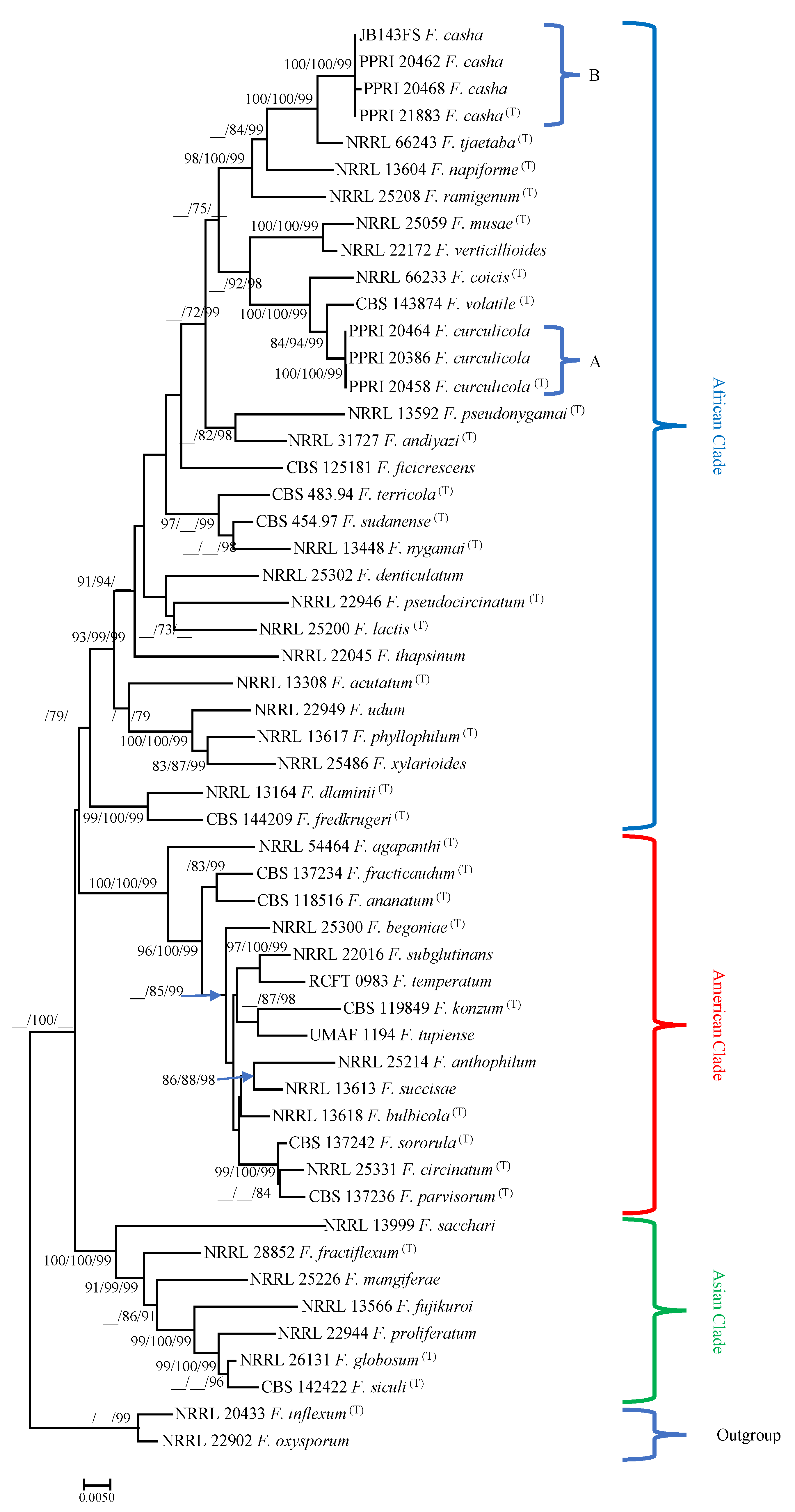
3.2. DNA Sequence Comparisons
3.3. Taxonomy
Taxonomic Notes
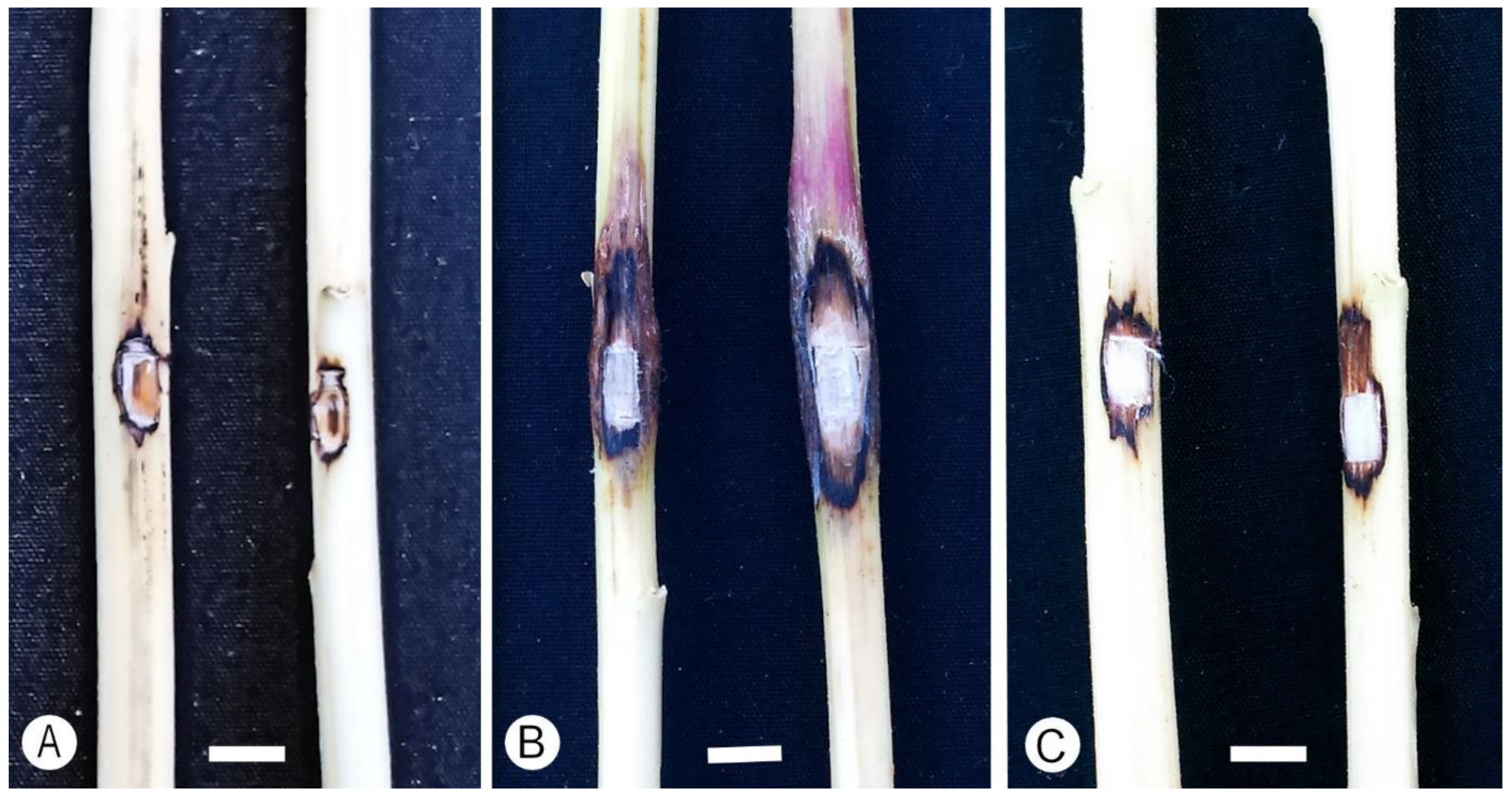
3.4. Pathogenicity
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Organism | Isolate | Host | Isolation Source | TEF1α GenBank Accession/ | Number of Isolates |
|---|---|---|---|---|---|
| Fusarium casha | D22 | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | MN217126 | 1 |
| Fusarium casha | D24 | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | MN217127 | 2 |
| Fusarium casha | D21 | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | MN217130 | 3 |
| Fusarium casha | D104 | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | MN217131 | 4 |
| Fusarium casha | D118 | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | MN217132 | 5 |
| Fusarium casha | D172 | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | MN217133 | 6 |
| Fusarium casha | D179 | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | MN217138 | 7 |
| Fusarium casha | D166 | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | MN217141 | 8 |
| Fusarium casha | D4 | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | MN217143 | 9 |
| Fusarium casha | D140 | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | MN217145 | 10 |
| Fusarium casha | D23 | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | MN217146 | 11 |
| Fusarium casha | D28 | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | MN217148 | 12 |
| Fusarium casha | D33 | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | MN217149 | 13 |
| Fusarium casha | D183 | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | MN217150 | 14 |
| Fusarium casha | D113 | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | MN217151 | 15 |
| Fusarium casha | D32 | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | MN217152 | 16 |
| Fusarium casha | D167 | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | MN217153 | 17 |
| Fusarium casha | D39 | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | MN217159 | 18 |
| Fusarium casha | D30 | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | MN217160 | 19 |
| Fusarium casha | D111 | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | MN217161 | 20 |
| Fusarium casha | D13 | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | MN217166 | 21 |
| Fusarium casha | D5 | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | MN217167 | 22 |
| Fusarium casha | D100 | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | MN217168 | 23 |
| Fusarium casha | D112 | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | MN217175 | 24 |
| Fusarium casha | D86 | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | MN217176 | 25 |
| Fusarium casha | D119 | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | MN217177 | 26 |
| Fusarium casha | D110 | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | MN217178 | 27 |
| Fusarium casha | D78 | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | MN217179 | 28 |
| Fusarium casha | D203 | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | MN217181 | 29 |
| Fusarium casha | D65 | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | MN217182 | 30 |
| Fusarium casha | D76 | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | MN217183 | 31 |
| Fusarium casha | D97 | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | MN217184 | 32 |
| Fusarium casha | D99 | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | MN217186 | 33 |
| Fusarium casha | D92 | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | MN217187 | 34 |
| Fusarium casha | D164 | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | MN217189 | 35 |
| Fusarium casha | D191 | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | MN217190 | 36 |
| Fusarium casha | D187 | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | MN217195 | 37 |
| Fusarium casha | D156 | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | MN217196 | 38 |
| Fusarium casha | D200 | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | MN217201 | 39 |
| Fusarium casha | D82 | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | MN217202 | 40 |
| Fusarium casha | 32B | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | MN217208 | 41 |
| Fusarium casha | 27B | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | MN217209 | 42 |
| Fusarium casha | PPRI 21883/PREM 61342 | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | MF787261 | 43 |
| Fusarium casha | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | Subtotal | 43 | |
| Fusarium curculicola | D175 | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | MN202618 | 1 |
| Fusarium curculicola | D74 | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | MN202619 | 2 |
| Fusarium curculicola | D10 | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | MN202621 | 3 |
| Fusarium curculicola | D12 | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | MN202622 | 4 |
| Fusarium curculicola | D129 | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | MN202624 | 5 |
| Fusarium curculicola | D199 | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | MN202627 | 6 |
| Fusarium curculicola | D176 | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | MN202629 | 7 |
| Fusarium curculicola | D14 | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | MN202630 | 8 |
| Fusarium curculicola | D154 | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | MN202632 | 9 |
| Fusarium curculicola | D6 | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | MN202635 | 10 |
| Fusarium curculicola | D91 | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | MN202636 | 11 |
| Fusarium curculicola | D115 | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | MN202643 | 12 |
| Fusarium curculicola | D107 | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | MN202644 | 13 |
| Fusarium curculicola | D106 | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | MN202645 | 14 |
| Fusarium curculicola | D102 | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | MN202646 | 15 |
| Fusarium curculicola | D98 | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | MN202647 | 16 |
| Fusarium curculicola | D67 | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | MN202648 | 17 |
| Fusarium curculicola | D36 | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | MN202649 | 18 |
| Fusarium curculicola | 14A | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | MN202653 | 19 |
| Fusarium curculicola | D103 | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | MN202654 | 20 |
| Fusarium curculicola | D88 | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | MN202656 | 21 |
| Fusarium curculicola | D87 | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | MN202657 | 22 |
| Fusarium curculicola | PPRI 20386/PREM 61347 | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | MF787268 | 23 |
| Fusarium curculicola | Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | Subtotal | 23 | |
| Amaranthus cruentus | Emergence holes and lesions associated with Athesapeuta dodonis and Baris amaranti weevils | Total | 66 | ||
| Fusarium casha | D216 | Amaranthus cruentus | Larval gallery of Hypolixus haerens | MN217142 | 1 |
| Fusarium casha | D144 | Amaranthus cruentus | Larval gallery of Hypolixus haerens | MN217144 | 2 |
| Fusarium casha | D128 | Amaranthus cruentus | Larval gallery of Hypolixus haerens | MN217147 | 3 |
| Fusarium casha | D158 | Amaranthus cruentus | Larval gallery of Hypolixus haerens | MN217158 | 4 |
| Fusarium casha | D57 | Amaranthus cruentus | Larval gallery of Hypolixus haerens | MN217171 | 5 |
| Fusarium casha | D81 | Amaranthus cruentus | Larval gallery of Hypolixus haerens | MN217172 | 6 |
| Fusarium casha | D62 | Amaranthus cruentus | Larval gallery of Hypolixus haerens | MN217173 | 7 |
| Fusarium casha | D59 | Amaranthus cruentus | Larval gallery of Hypolixus haerens | MN217174 | 8 |
| Fusarium casha | D209 | Amaranthus cruentus | Larval gallery of Hypolixus haerens | MN217199 | 9 |
| Fusarium casha | D121 | Amaranthus cruentus | Larval gallery of Hypolixus haerens | MN217200 | 10 |
| Fusarium casha | Amaranthus cruentus | Larval gallery of Hypolixus haerens | Subtotal | 10 | |
| Fusarium curculicola | D131 | Amaranthus cruentus | Larval gallery of Hypolixus haerens | MN202617 | 1 |
| Fusarium curculicola | D68 | Amaranthus cruentus | Larval gallery of Hypolixus haerens | MN202650 | 2 |
| Fusarium curculicola | Amaranthus cruentus | Larval gallery of Hypolixus haerens | Subtotal | 2 | |
| Fusarium curculicola | Amaranthus cruentus | Larval gallery of Hypolixus haerens | Total | 12 | |
| Fusarium casha | JB341FS | Amaranthus hybridus | Cankered stems of Amaranthus hybridus | MN217170 | 1 |
| Fusarium casha | Amaranthus hybridus | Cankered stems of Amaranthus hybridus | Subtotal | 1 | |
| Fusarium casha | JB143FS | Amaranthus hybridus | Larval gallery of Hypolixus haerens | MF787264 | 1 |
| Fusarium casha | Amaranthus hybridus | Larval gallery of Hypolixus haerens | Subtotal | 1 | |
| Fusarium casha | T82B | Athesapeuta dodonis | MN217125 | 1 | |
| Fusarium casha | P148 | Athesapeuta dodonis | MN217134 | 2 | |
| Fusarium casha | T22A | Athesapeuta dodonis | MN217136 | 3 | |
| Fusarium casha | P91A | Athesapeuta dodonis | MN217137 | 4 | |
| Fusarium casha | T73A | Athesapeuta dodonis | MN217139 | 5 | |
| Fusarium casha | P147A | Athesapeuta dodonis | MN217154 | 6 | |
| Fusarium casha | P70 | Athesapeuta dodonis | MN217162 | 7 | |
| Fusarium casha | P145A | Athesapeuta dodonis | MN217163 | 8 | |
| Fusarium casha | T62A | Athesapeuta dodonis | MN217164 | 9 | |
| Fusarium casha | P133A | Athesapeuta dodonis | MN217165 | 10 | |
| Fusarium casha | T142A | Athesapeuta dodonis | MN217169 | 11 | |
| Fusarium casha | P143A | Athesapeuta dodonis | MN217180 | 12 | |
| Fusarium casha | T27B | Athesapeuta dodonis | MN217185 | 13 | |
| Fusarium casha | P97A | Athesapeuta dodonis | MN217191 | 14 | |
| Fusarium casha | T20A | Athesapeuta dodonis | MN217192 | 15 | |
| Fusarium casha | P26A | Athesapeuta dodonis | MN217193 | 16 | |
| Fusarium casha | T49A | Athesapeuta dodonis | MN217194 | 17 | |
| Fusarium casha | P65B | Athesapeuta dodonis | MN217197 | 18 | |
| Fusarium casha | T41A | Athesapeuta dodonis | MN217198 | 19 | |
| Fusarium casha | T56A | Athesapeuta dodonis | MN217203 | 20 | |
| Fusarium casha | T71A | Athesapeuta dodonis | MN217204 | 21 | |
| Fusarium casha | T31A | Athesapeuta dodonis | MN217205 | 22 | |
| Fusarium casha | P69A | Athesapeuta dodonis | MN217206 | 23 | |
| Fusarium casha | P37A | Athesapeuta dodonis | MN217207 | 24 | |
| Fusarium casha | P135A | Athesapeuta dodonis | MN217210 | 25 | |
| Fusarium casha | P117B | Athesapeuta dodonis | MN217211 | 26 | |
| Fusarium casha | T28A | Athesapeuta dodonis | MN217212 | 27 | |
| Fusarium casha | P63A | Athesapeuta dodonis | MN217213 | 28 | |
| Fusarium casha | T137B | Athesapeuta dodonis | MN217214 | 29 | |
| Fusarium casha | PPRI 20462/PREM 61343 | Athesapeuta dodonis | MF787262 | 30 | |
| Fusarium casha | PPRI 20468/PREM 61344 | Athesapeuta dodonis | MF787263 | 31 | |
| Fusarium casha | Athesapeuta dodonis | Total | 31 | ||
| Fusarium curculicola | P120B | Athesapeuta dodonis | MN202631 | 1 | |
| Fusarium curculicola | T150B | Athesapeuta dodonis | MN202634 | 2 | |
| Fusarium curculicola | T113 | Athesapeuta dodonis | MN202638 | 3 | |
| Fusarium curculicola | T66 | Athesapeuta dodonis | MN202639 | 4 | |
| Fusarium curculicola | T11 | Athesapeuta dodonis | MN202640 | 5 | |
| Fusarium curculicola | P92B | Athesapeuta dodonis | MN202641 | 6 | |
| Fusarium curculicola | P141B | Athesapeuta dodonis | MN202642 | 7 | |
| Fusarium curculicola | T97A | Athesapeuta dodonis | MN202651 | 8 | |
| Fusarium curculicola | T43 | Athesapeuta dodonis | MN202652 | 9 | |
| Fusarium curculicola | T90A | Athesapeuta dodonis | MN202658 | 10 | |
| Fusarium curculicola | PPRI 20458/PREM 61345 | Athesapeuta dodonis | MF787266 | 11 | |
| Fusarium curculicola | PPRI 20464/PREM 61346 | Athesapeuta dodonis | MF787267 | 12 | |
| Fusarium curculicola | Athesapeuta dodonis | Subtotal | 12 | ||
| Athesapeuta dodonis | Total | 43 | |||
| Fusarium casha | D206 | Baris amaranti | MN217129 | 1 | |
| Fusarium casha | D157 | Baris amaranti | MN217155 | 2 | |
| Fusarium casha | Baris amaranti | Subtotal | 2 | ||
| Fusarium curculicola | P88A | Baris amaranti | MN202625 | 1 | |
| Fusarium curculicola | Baris amaranti | Subtotal | 1 | ||
| Baris amaranti | Total | 3 | |||
| Fusarium casha | D152 | Larvae of Hypolixus haerens | MN217157 | 1 | |
| Fusarium casha | D218 | Larvae of Hypolixus haerens | MN217188 | 2 | |
| Fusarium casha | Larvae of Hypolixus haerens | Subtotal | 2 | ||
| Fusarium curculicola | D37 | Larvae of Hypolixus haerens | MN202637 | 1 | |
| Fusarium curculicola | Larvae of Hypolixus haerens | Subtotal | 1 | ||
| Larvae of Hypolixus haerens | Subtotal | 3 | |||
| Sum total | 129 |
References
- Pusz, W. Fungi from seeds of Amaranthus spp. Phytopathologia 2009, 54, 15–21. [Google Scholar]
- Walsh, J.L.; Laurence, M.H.; Liew, E.C.Y.; Sangalang, A.E.; Burgess, L.W.; Summerell, B.A.; Petrovic, T. Fusarium: Two endophytic novel species from tropical grasses of northern Australia. Fungal Divers. 2010, 44, 149–159. [Google Scholar] [CrossRef]
- Herron, D.A.; Wingfield, M.J.; Wingfield, B.D.; Rodas, C.A.; Marincowitz, S.; Steenkamp, E.T. Novel taxa in the Fusarium fujikuroi species complex from Pinus spp. Stud. Mycol. 2015, 80, 131–150. [Google Scholar] [CrossRef] [PubMed]
- Al-Hatmi, A.M.S.; Mirabolfathy, M.; Hagen, F.; Normand, A.C.; Stielow, B.; Karami-Osbo, R.; van Diepeningen, A.D.; Meis, J.F.; De Hoog, S. DNA barcoding, MALDI-TOF, and AFLP data support Fusarium ficicrescens as a distinct species within the Fusarium fujikuroi species complex. Fungal Biol. 2016, 120, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.; Auer, D.; de Alwis, S.-K.; Summerell, B.; Aoki, T.; Proctor, R.H.; Busmann, M.; O’Donnell, K. Fusarium agapanthi sp. nov., a novel bikaverin and fusarubin-producing leaf and stem spot pathogen of Agapanthus praecox (African lily) from Australia and Italy. Mycologia 2016, 108, 981–992. [Google Scholar] [CrossRef]
- Laurence, M.H.; Walsh, L.A.; Shuttleworth, L.A.; Robinson, D.M.; Johansen, R.M.; Petrovic, T.; Vu, T.T.H.; Burgess, L.W.; Summerell, B.A.; Liew, E.C.Y. Six novel species of Fusarium from natural ecosystems in Australia. Fungal Divers. 2016, 77, 349–366. [Google Scholar] [CrossRef]
- Moussa, T.A.A.; Al-Zahrani, H.S.; Kadasa, N.M.S.; Ahmed, S.A.; De Hoog, G.S.; Al-Hatmi, A.M.S. Two new species of the Fusarium fujikuroi species complex isolated from the natural environment. Antonie Van Leeuwenhoek 2017, 110, 819–832. [Google Scholar] [CrossRef]
- Sandoval-Denis, M.; Swart, W.J.; Crous, P.W. New Fusarium species from the Kruger National Park, South Africa. MycoKeys 2018, 34, 63–92. [Google Scholar] [CrossRef] [PubMed]
- Al-Hatmi, A.M.S.; Sandoval-Denis, M.; Nabet, C.; Ahmed, S.A.; Demar, M.; Normand, A.-C.; De Hoog, G.S. Fusarium volatile, a new pathogen from human respiratory samples. Fungal Syst. Evol. 2019, 4, 171–181. [Google Scholar] [CrossRef]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual; Blackwell Professional: Ames, IA, USA, 2006. [Google Scholar]
- Kvas, M.; Marasas, W.F.O.; Wingfield, B.D.; Wingfield, M.J.; Steenkamp, E.T. Diversity and evolution of Fusarium species in the Gibberella fujikuroi complex. Fungal Divers. 2009, 34, 1–21. [Google Scholar]
- Proctor, R.H.; Van Hove, F.; Susca, A.; Stea, G.; Busman, M.; van der Lee, T.; Waalwijk, C.; Moretti, A.; Ward, T.J. Birth, death and horizontal transfer of the fumonisin biosynthetic gene cluster during the evolutionary diversification of Fusarium. Mol. Microbiol. 2013, 90, 290–306. [Google Scholar]
- Al-Hatmi, A.M.S.; Van Den Ende, A.H.G.; Stielow, B.; Van Diepeningen, A.D.; Seifert, K.A.; Mccormick, W.; Assabgui, R.; Gräfenhan, T.; De Hoog, G.S.; Levesque, C.A. Evaluation of two novel barcodes for species recognition of opportunistic pathogens in Fusarium. Fungal Biol. 2015, 120, 231–245. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, K.; Cigelnik, E.; Nirenberg, H.I. Molecular systematics and phylogeography of the Gibberella fujikuroi species complex. Mycologia 1998, 90, 465–493. [Google Scholar] [CrossRef]
- O’Donnell, K.; Nirenberg, H.I.; Aoki, T.; Cigelnik, E. A multigene phylogeny of the Gibberella fujikuroi species complex: Detection of additionally phylogenetically distinct species. Mycoscience 2000, 41, 61–78. [Google Scholar] [CrossRef]
- Geiser, D.M.; Ivey, M.L.; Hakiza, G.; Juba, J.H.; Miller, S.A. Gibberella xylarioides (anamorph: Fusarium xylarioides), a causative agent of coffee wilt disease in Africa, is a previously unrecognized member of the G. fujikuroi species complex. Mycologia 2005, 97, 191–201. [Google Scholar] [CrossRef]
- Moroti, R.V.; Gheorghita, V.; Al-Hatmi, A.M.S.; De Hoog, G.S.; Meis, J.F.; Netea, M.G. Fusarium ramigenum, a novel human opportunist in a patient with common variable immunodeficiency and cellular immune defects: Case report. BMC Infect. Dis. 2016, 16, 79. [Google Scholar] [CrossRef]
- Sauer, J.D. Historical Geography of Crop Plants: A Select Roster; CRC Press: Boca Raton, FL, USA, 1993. [Google Scholar]
- Bresler, G.; Brizzio, S.B.; Vaamonde, G. Mycotoxin-producing potential of fungi isolated from amaranth seeds in Argentina. Internat. J. Food Microbiol. 1995, 25, 101–108. [Google Scholar] [CrossRef]
- Blodgett, J.T.; Swart, W.J.; Chen, W. First report of Alternaria tenuissima as leaf pathogen of Amaranthus hybridus. Plant Dis. 1999, 83, 878. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.Q.; Swart, W.J. Fusarium oxysporum and F. sambucinum associated with root rot of Amaranthus hybridus in South Africa. Plant Dis. 2000, 84, 101. [Google Scholar] [CrossRef]
- Vermeulen, M.; Marais, G.J.; Louw, S.; van der, M.; Weeks, W.; Swart, W.J.; Gryzenhout, M. Fungi associated with disease and weevil damage of Amaranthus cruentus in South Africa. Afr. Entomol. 2017, 26, 174–188. [Google Scholar] [CrossRef]
- Blodgett, J.T.; Swart, W.J.; Louw, S.; van der, M. Identification of fungi and fungal pathogens associated with Hypolixus haerens and decayed and cankered stem of Amaranthus hybridus. Plant Dis. 2004, 88, 333–337. [Google Scholar] [CrossRef][Green Version]
- Möller, E.M.; Bahnweg, G.; Sandermann, H.; Geiger, H.H. A simple and efficient protocol for isolation of high molecular weight DNA from filamentous fungi, fruit bodies and infected plant tissues. Nucleic Acids Res. 1992, 20, 6115–6116. [Google Scholar] [CrossRef]
- O’Donnell, K.; Sutton, D.A.; Fothergill, A.; McCarthy, D.; Rinaldi, M.G.; Brandt, M.E.; Zhang, N.; Geiser, D.M. Molecular phylogenetic diversity, multilocus haplotype nomenclature, and in vitro antifungal resistance within the Fusarium solani species complex. J. Clin. Microbiol. 2008, 46, 2477–2490. [Google Scholar] [CrossRef]
- O’Donnell, K.; Cigelnik, E. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Mol. Phylogenetics Evol. 1997, 7, 103–116. [Google Scholar] [CrossRef]
- Liu, Y.J.; Whelen, S.; Hall, B.D. Phylogenetic relationships among Ascomycetes: Evidence from an RNA polymerase II subunit. Mol. Biol. Evol. 1999, 16, 1799–1808. [Google Scholar] [CrossRef] [PubMed]
- Sung, G.-H.; Sung, J.-M.; Hywel-Jones, N.L.; Spatafora, J.W. A multi-gene phylogeny of Clavicipitaceae (Ascomycota, Fungi): Identification of localized incongruence using a combinational bootstrap approach. Mol. Phylogenetics Evol. 2007, 44, 1204–1223. [Google Scholar] [CrossRef]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Kuma, K.; Toh, H.; Miyata, T. MAFFT version 5: Improvement in accuracy of multiple sequence alignment. Nucleic Acids Res. 2005, 33, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Katoh, K.; Toh, H. Recent developments in the MAFFT multiple sequence alignment program. Brief. Bioinform. 2008, 9, 286–298. [Google Scholar] [CrossRef]
- O’Donnell, K.; Kistler, H.C.; Cigelnik, E.; Ploetz, R.C. Multiple evolutionary origins of the fungus causing Panama disease of banana: Concordant evidence from nuclear and mitochondrial gene genealogies. Proc. Natl. Acad. Sci. USA 1998, 95, 2044–2049. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Posada, D. jModelTest: Phylogenetic Model Averaging. Mol. Biol. Evol. 2008, 25, 1253. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar] [PubMed]
- Tavare, S. Some probabilistic and statistical problems in the analysis of DNA sequences. In Some Mathematical Questions in Biology-DNA Sequence Analysis; Miura, R.M., Ed.; American Mathematical Society: Providence, RI, USA, 1986; pp. 57–86. [Google Scholar]
- Nirenberg, H.I. Untersuchungen über die morphologische und biologische Differenzierung in der Fusarium-sektion Liseola. Mitt. Biol. Für Land-Und Forstwirtschaft Berl.-Dahl. 1976, 169, 1–117. [Google Scholar]
- Nelson, P.E.; Toussoun, T.A.; Marasas, W.F.O. Fusarium Species: An. Illustrated Manual for Identification; Pennsylvania State University Press: State College, PA, USA, 1983. [Google Scholar]
- Jacobs, A.; Van Wyk, P.S.; Marasas, W.F.; Wingfield, B.D.; Wingfield, M.J.; Coutinho, T.A. Fusarium ananatum sp. nov. in the Gibberella fujikuroi species complex from pineapples with fruit rot in South Africa. Fungal Biol. 2010, 114, 515–527. [Google Scholar] [CrossRef]
- De Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research. R Package Version 1.3-2. Available online: https://CRAN.R-project.org/package=agricolae2020 (accessed on 23 March 2021).
- Rayner, R.W. A Mycological Colour Chart; CMI and British Mycological Society: London, UK, 1970. [Google Scholar]
- Modi, A.T. Growth temperature and plant age influence on nutritional quality of Amaranthus leaves and seed germination capacity. Water SA 2007, 33, 369–376. [Google Scholar] [CrossRef][Green Version]
- Sakalidis, M.L.; Ray, J.D.; Lanoiselet, V.; Hardy, G.E.S.; Burgess, T.I. Pathogenic Botryosphaeriaceae associated with Mangifera indica in the Kimberley Region of Western Australia. Eur. J. Plant Pathol. 2011, 130, 379–391. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 3 March 2021).
- RStudio Team. RStudio: Integrated Development Environment for R (Version 1.2.5042) [Computer Software]; RS-Team-Inc.: Boston, MA, USA, 2020. [Google Scholar]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; D’Agostino McGowan, L.; François, R.; Grolemund, G.A.; Henry, L.; Hester, J.; Kuhn, M.; et al. Welcome to the tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Marasas, W.F.O.; Rabie, C.J.; Lübben, A.; Nelson, P.E.; Toussoun, T.A.; van Wijk, P.S. Fusarium napiforme, a new species from millet and sorghum in southern Africa. Mycologia 1978, 79, 910–914. [Google Scholar] [CrossRef]
- Nirenberg, H.I.; O’Donnell, K. New Fusarium species and combinations within the Gibberella fujikuroi species complex. Mycologia 1998, 90, 434–458. [Google Scholar] [CrossRef]
- Van Hove, F.; Waalwijk, C.; Logrieco, A.; Munaut, F.; Moretti, A. Gibberella musae (Fusarium musae) sp. nov., a recently discovered species from banana is sister to F. verticillioides. Mycologia 2011, 103, 570–585. [Google Scholar] [CrossRef] [PubMed]
- Gams, W. Cephalosporium-Artige Schimmelpilze (Hyphomycetes); Fischer: Stuttgart, Germany, 1971. [Google Scholar]
- Aoki, T.; O’Donnell, K.; Ichikawa, K. Fusarium fractiflexum sp. nov. and two other species within the Gibberella fujikuroi species complex recently discovered in Japan that form aerial conidia in false heads. Mycoscience 2001, 42, 461–478. [Google Scholar] [CrossRef]
- Yilmaz, N.; Sandoval-Denis, M.; Lombard, L.; Visagie, C.M.; Wingfield, B.D.; Crous, P.W. Redefining species limits in the Fusarium fujikuroi species complex. Pers. Mol. Phylogeny Evol. Fungi 2021, 46, 129–162. [Google Scholar]
- Britz, H.; Steenkamp, E.T.; Coutinho, T.A.; Wingfield, B.D.; Marasas, W.F.O.; Wingfield, M.J. Two new species of Fusarium section Liseola associated with mango malformation. Mycologia 2002, 94, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Otero-Colina, G.; Rodríguez-Alvarado, G.; Fernández-Pavía, S.; Maymon, M.; Ploetz, R.C.; Aoki, T.; O’Donnell, K.; Freeman, S. Identification and characterization of a novel etiological agent of mango malformation disease in Mexico. Phytopathology 2010, 100, 1176–1184. [Google Scholar] [CrossRef] [PubMed]
- Lima, C.S.; Pfenning, L.H.; Costa, S.S.; Abreu, L.M.; Leslie, J.F. Fusarium tupiense sp. nov., a member of the Gibberella fujikuroi complex that causes mango malformation in Brazil. Mycologia 2012, 104, 1408–1419. [Google Scholar] [CrossRef]
- Gerlach, W.; Nirenberg, H. Mitteilungen aus der Bioloischen Bundesansalt fur Land- und Forstwirschaft. In The Genus Fusarium: A Pictorial Atlas; Parey: Berlin, Germany, 1982; Volume 209, pp. 1–406. [Google Scholar]
- Kerry O’Donnell, K.; Humber, R.A.; Geiser, D.M.; Kang, S.; Park, B.; Robert, V.A.R.G.; Crous, P.W.; Johnston, P.; Aoki, T.; Rooney, A.P.; et al. Phylogenetic diversity of insecticolous fusaria inferred from multilocus DNA sequence data and their molecular identification via FUSARIUM-ID and Fusarium MLST. Mycologia 2012, 104, 427–445. [Google Scholar] [CrossRef]

| Species | Host/Substrate | Origin | Culture Collection(A)/Strain Reference | ßT GenBank Accession | TEF1α GenBank Accession | RPB2 GenBank Accession |
|---|---|---|---|---|---|---|
| Fusarium acutatum(T) | Unknown | India | NRRL 13308 | U34431 | AF160276 | KR674009 |
| F. agapanthi(T) | Agapanthus praecox | Australia | NRRL 54464 | KU900637 | KU900632 | KU900627 |
| F. ananatum(T) | Ananas comosus | South Africa | CBS 118516 | LT996112 | LT996091 | LT996137 |
| F. andiyazi(T) | Sorghum bicolor | Ethiopia | CBS 119857 | KP662894 | KR071718 | KT154004 |
| F. anthophilum | Hippeastrum sp. | Germany | NRRL 25214 | KU171776 | KF466414 | KU171696 |
| F. begoniae(T) | Begonia elatior | Germany | NRRL 25300 | U61543 | AF160293 | LT996140 |
| F. bulbicola(T) | Nerine bowdenii | Germany | NRRL 13618 | U61546 | AF160294 | KF466404 |
| F. casha1(T) | Lesions in Amaranthus cruentus associated with Athesapeuta dodonis and Baris amaranti weevils | South Africa | PPRI 21883/PREM 61342 | MF787255 | MF787261 | MN605059/MN605065 |
| F. casha1 | Athesapeuta dodonis | South Africa | PPRI 20462/PREM 61343 | MF787256 | MF787262 | MN605060/MN605066 |
| F. casha1 | Athesapeuta dodonis | South Africa | PPRI 20468/PREM 61344 | MF787257 | MF787263 | MN605061/MN605067 |
| F. casha | Isolated from galleries in Amaranthus hybridus associated with Hypolixus haerens larva | South Africa | Blodget et al., 2004 [JB143FS] | MT304823 | MF787264 | MN605068 |
| F. circinatum(T) | Pinus radiata | USA | NRRL 25331 | U61547 | AF160295 | JX171623 |
| F. coicis (T) | Coix gasteenii | Australia | NRRL 66233 | LT996115 | KP083251 | KP083274 |
| F. curculicola1 (T) | Athesapeuta dodonis | South Africa | PPRI 20458/PREM 61345 | MF787258 | MF787266 | MN605062/MN605069 |
| F. curculicola1 | Athesapeuta dodonis | South Africa | PPRI 20464/PREM 61346 | MF787259 | MF787267 | MN605063/MN605070 |
| F. curculicola1 | Isolated from lesion in Amaranthus cruentus associated with Athesapeuta dodonis weevils | South Africa | PPRI 20386/PREM 61347 | MF787260 | MF787268 | MN605064/MN605071 |
| F. denticulatum | Ipomoea batatas | USA | NRRL 25302 | U61550 | AF160269 | LT996143 |
| F. dlaminii(T) | Soil | South Africa | NRRL 13164 | U34430 | AF160277 | KU171701 |
| F. ficicrescens | Ficus carica | Iran | CBS 125181 | KP662897 | KP662900 | KT154003 |
| F. fracticaudum(T) | Pinus maximinoi | Colombia | CBS 137234 | KJ541048 | KJ541058 | LT996144 |
| F. fractiflexum(T) | Cymbidium sp. | Japan | NRRL 28852 | AF160315 | AF160288 | LT575064 |
| F. fredkrugeri(T) | Melhania acuminata rhizophere | South Africa | CBS 144209 | LT996117 | LT996097 | LT996147 |
| F. fujikuroi | Oryza sativa | Taiwan | NRRL 13566 | U34415 | AF160279 | JX171570 |
| F. globosum(T) | Zea mays | South Africa | NRRL 26131 | U61557 | AF160285 | KF466406 |
| F. inflexum(T) | Vicia faba | Germany | NRRL 20433 | U34435 | AF008479 | JX171583 |
| F. konzum(T) | Sorghastrum nuttans | USA | CBS 119849 | LT996118 | LT996098 | LT996148 |
| F. lactis(T) | Ficus carica | USA | NRRL 25200 | U61551 | AF160272 | LT996149 |
| F. mangiferae | Mangifera indica | India | NRRL 25226 | U61561 | AF160281 | HM068353 |
| F. musaeT) | Musa sp. | Honduras | NRRL 25059 | FN545368 | FN552086 | FN552108 |
| F. napiforme(T) | Pennisetum typhoides | South Africa | NRRL 13604 | U34428 | AF160266 | EF470117 |
| F. nygamai(T) | Sorghum bicolor | Australia | NRRL 13448 | U34426 | AF160273 | EF470114 |
| F. oxysporum | Pseudotsuga menziesii | USA | NRRL 22902 | U34424 | AF160312 | LT575065 |
| F. parvisorum (T) | Pinus patula | Colombia | CBS 137236 | KJ541055 | KJ541060 | LT996150 |
| F. phyllophilum(T) | Dracaena deremensis | Italy | NRRL 13617 | U34432 | AF160274 | KF466410 |
| F. proliferatum | Cattleya sp. | Germany | NRRL 22944 | U34416 | AF160280 | HM068352 |
| F. pseudocircinatum(T) | Solanum sp. | Ghana | NRRL 22946 | U34427 | AF160271 | LT996151 |
| F. pseudonygamai(T) | Pennisetum typhoides | Nigeria | NRRL 13592 | U34421 | AF160263 | LT996152 |
| F. ramigenum(T) | Ficus carica | USA | NRRL 25208 | U61554 | AF160267 | KF466412 |
| F. sacchari | Saccharum officinarum | India | NRRL 13999 | U34414 | AF160278 | JX171580 |
| F. siculi(T) | Citrus sinensis | Italy | CBS 142422 | LT746346 | LT746214 | LT746327 |
| F. sororula(T) | Pinus patula | Colombia | CBS 137242 | KJ541057 | KJ541067 | LT996153 |
| F. subglutinans | Zea mays | USA | NRRL 22016 | U34417 | AF160289 | JX171599 |
| F. succisae | Succisa pratensis | Germany | NRRL 13613 | U34419 | AF160291 | LT996154 |
| F. sudanense(T) | Striga hermonthica | Sudan | CBS 454.97 | KU603909 | KU711697 | LT996155 |
| F. temperatum | Unknown | Unknown | UNRC RCFT0983 | KP270978 | KP270949 | KP270986 |
| F. terricola(T) | Soil | Australia | CBS 483.94 | KU603908 | KU711698 | LT996156 |
| F. thapsinum | Sorghum bicolor | South Africa | NRRL 22045 | U34418 | AF160270 | JX171600 |
| F. tjaetaba(T) | Sorghum interjectum | Australia | NRRL 66243 | LT996119 | KP083263 | KP083275 |
| F. tupiense | Unknown | Spain | UMAF F1194 | KP753392 | KP753406 | KP753446 |
| F. udum | Lactarius pubescens | Germany | NRRL 22949 | U34433 | AF160275 | LT996172 |
| F. verticillioides | Zea mays | Germany | NRRL 22172 | U34413 | AF160262 | EF470122 |
| F. volatile(T) | Human bronchoalveolar lavage liquid | French Guiana | CBS 143874 | LR596008 | LR596007 | LR596006 |
| F. xylarioides | Coffea sp. | Ivory Coast | NRRL 25486 | AY707118 | AY707136 | JX171630 |
| Statistics | ßT | TEF1α | RPB2 | Combined ßT, TEF1α and RPB2 |
|---|---|---|---|---|
| Number of sequences | 53 | 53 | 53 | 53 |
| Aligned characters | 505 | 668 | 1637 | 2810 |
| Parsimony-informative characters | 82 | 161 | 236 | 479 |
| Tree length | 164 | 496 | 734 | 1507 |
| Consistency index (CI) | 0.750 | 0.550 | 0.377 | 0.447 |
| Retention index (RI) | 0.939 | 0.810 | 0.682 | 0.746 |
| Model | TrN+G | TIM2+G | TIM2+G | TIM2ef+G |
| Gamma shape | 0.4850 | 0.3570 | 0.1430 | 0.2160 |
| P-inv | No | No | No | No |
| Species | Isolate Number | Average Growth (mm) at Different Incubation Temperatures after Day 8 | Average Growth/d 25 °C | |||||
|---|---|---|---|---|---|---|---|---|
| 10 °C | 15 °C | 20 °C | 25 °C | 30 °C | 35 °C | |||
| Fusarium casha | PPRI 21883 | 9.1 n | 26.6 k | 40.5 i | 50 fgh | 41.9 i | 11.1 mn | 6.25 |
| F. casha | PPRI 20462 | 11.4 lmn | 27.6 jk | 51.1 fg | 69.1 b | 50.7 fgh | 8.5 n | 8.63 |
| F. casha | PPRI 20468 | 12.2 lmn | 31.4 j | 65.9 bc | 82.5 a | 65.8 bc | 13.9 lm | 10.31 |
| F. curculicola | PPRI 20386 | 9.1 n | 27.2 jk | 50.4 fgh | 60.9 de | 59.9 e | 14 lm | 7.62 |
| F. curculicola | PPRI 20458 | 8.0 n | 28.5 jk | 53.9 f | 82.2 a | 64.6 cd | 15.5 l | 10.28 |
| F. curculicola | PPRI 20464 | 9.8 mn | 26.1 k | 47.8 gh | 61.8 cde | 46.7 gh | 11.2 mn | 7.73 |
| Mean Lesion Length (mm) | |||
|---|---|---|---|
| Species | Isolate Number | Trial 1 | Trial 2 |
| Fusarium casha | PPRI 21883 | 21.73 a | 19.68 A |
| F. casha | PPRI 20462 | 11.28 c | 10.88 C |
| F. casha | PPRI 20468 | 17.35 ab | 9.36 CD |
| F. curculicola | PPRI 20458 | 15.00 bc | 9.68 C |
| F. curculicola | PPRI 20386 | 17.50 ab | 8.93 CD |
| F. curculicola | PPRI 20464 | 14.08 bc | 14.61 B |
| Control | 10.73 c | 5.82 D | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vermeulen, M.; Rothmann, L.A.; Swart, W.J.; Gryzenhout, M. Fusarium casha sp. nov. and F. curculicola sp. nov. in the Fusarium fujikuroi Species Complex Isolated from Amaranthuscruentus and Three Weevil Species in South Africa. Diversity 2021, 13, 472. https://doi.org/10.3390/d13100472
Vermeulen M, Rothmann LA, Swart WJ, Gryzenhout M. Fusarium casha sp. nov. and F. curculicola sp. nov. in the Fusarium fujikuroi Species Complex Isolated from Amaranthuscruentus and Three Weevil Species in South Africa. Diversity. 2021; 13(10):472. https://doi.org/10.3390/d13100472
Chicago/Turabian StyleVermeulen, Marcele, Lisa A. Rothmann, Wijnand J. Swart, and Marieka Gryzenhout. 2021. "Fusarium casha sp. nov. and F. curculicola sp. nov. in the Fusarium fujikuroi Species Complex Isolated from Amaranthuscruentus and Three Weevil Species in South Africa" Diversity 13, no. 10: 472. https://doi.org/10.3390/d13100472
APA StyleVermeulen, M., Rothmann, L. A., Swart, W. J., & Gryzenhout, M. (2021). Fusarium casha sp. nov. and F. curculicola sp. nov. in the Fusarium fujikuroi Species Complex Isolated from Amaranthuscruentus and Three Weevil Species in South Africa. Diversity, 13(10), 472. https://doi.org/10.3390/d13100472






