Three New Species of Cystolepiota from Laos and Thailand
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collecting and Material Examination
2.2. Phylogenetic Study
3. Results and Discussion
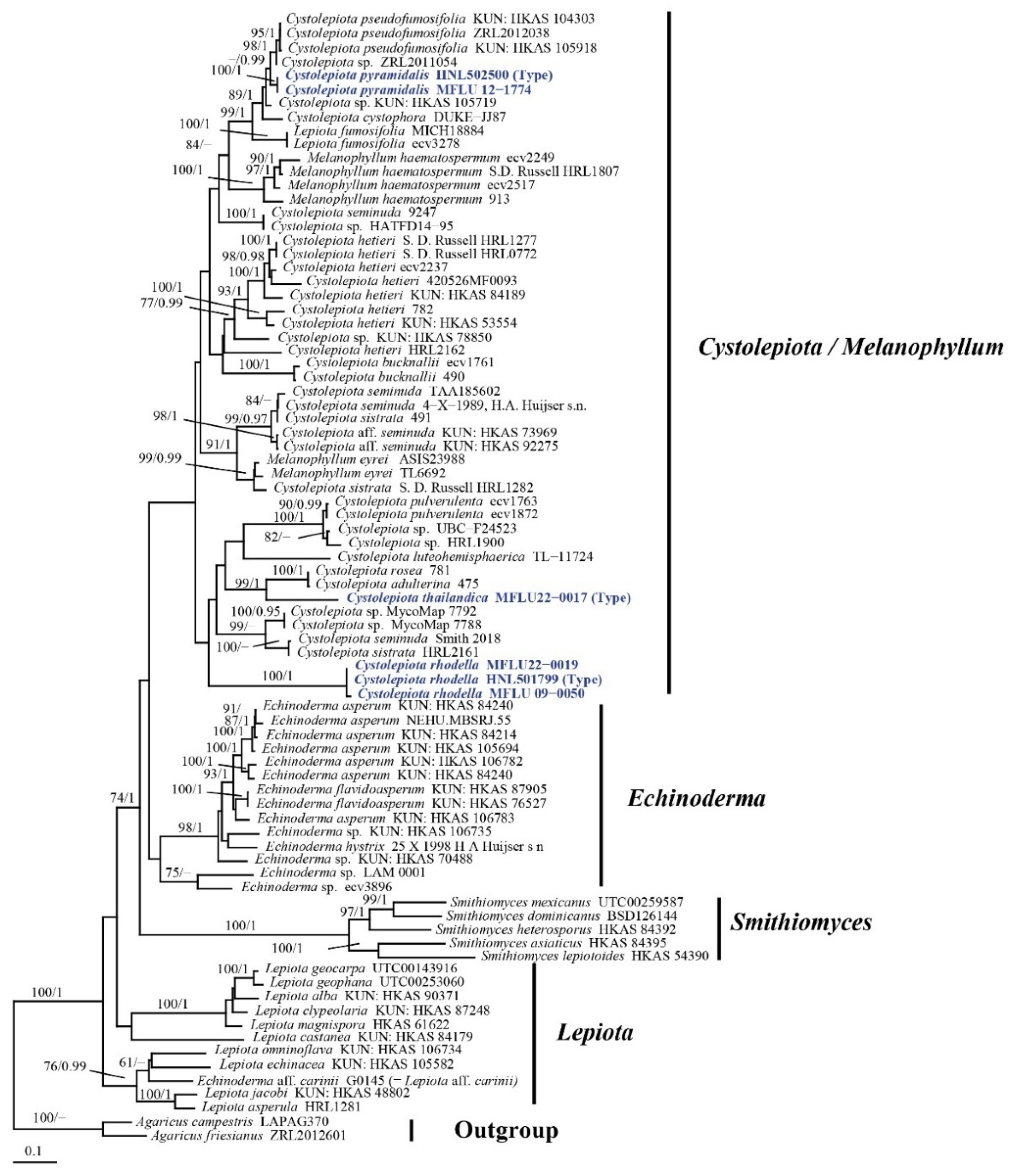
3.1. Phylogeny
3.2. Taxonomy
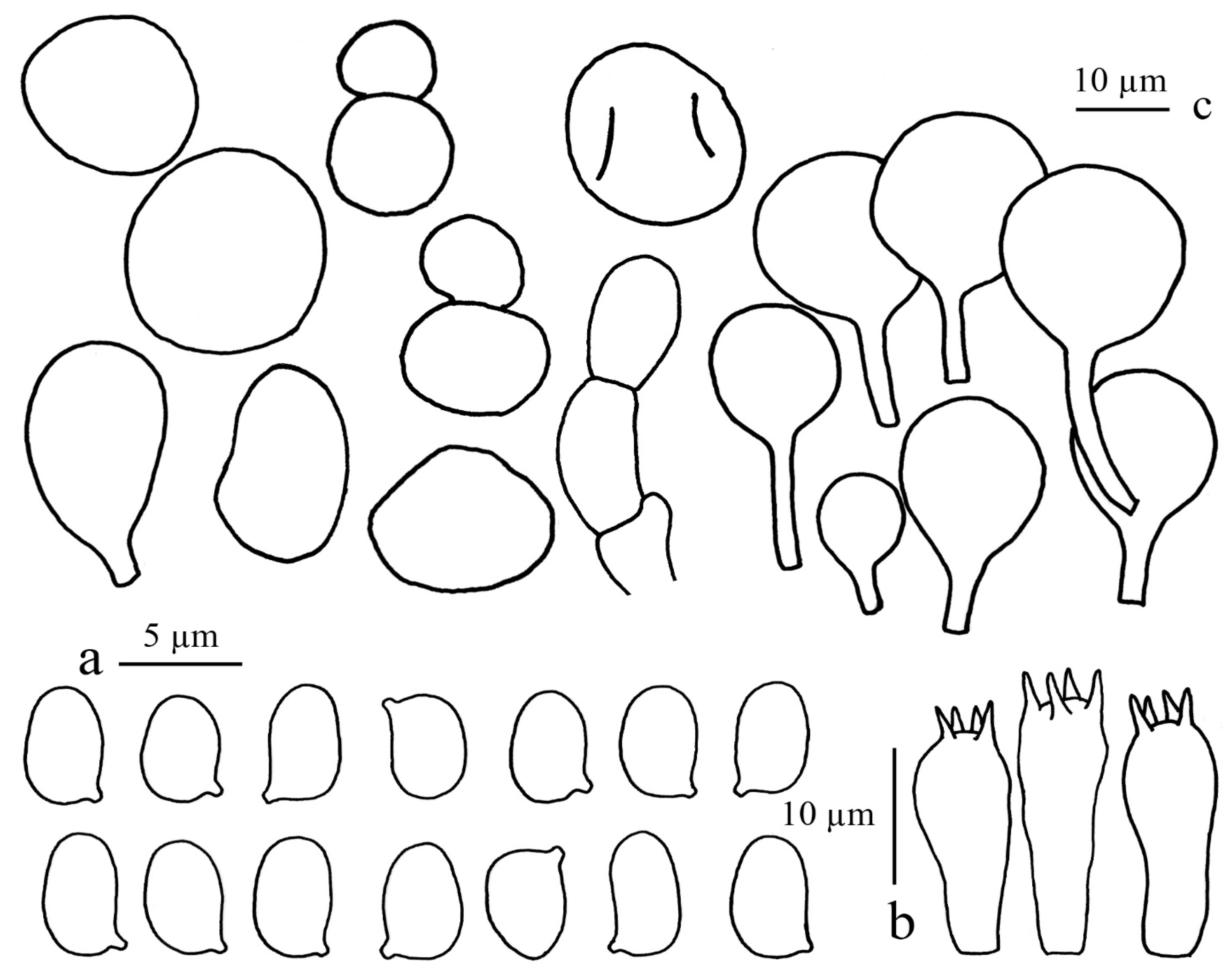
3.2.1. Cystolepiota pyramidalis Sysoup. and Thongkl. sp. nov.
3.2.2. Cystolepiota thailandica Yuan S. Liu, Sysouph. and Thongkl. sp. nov.
3.2.3. Cystolepiota rhodella Sysoup. and Thongkl., sp. nov.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, M.Q.; Zhao, R.L.; Hyde, K.D.; Begerow, D.; Kemler, M.; Yurkov, A.; McKenzie, E.H.C.; Raspé, O.; Kakishima, M.; SánchezRamírez, S.; et al. Notes, outline and divergence times of Basidiomycota. Fungal Divers. 2019, 99, 105–367. [Google Scholar]
- Kalichman, J.; Kirk, P.M.; Matheny, P.B. A compendium of generic names of agarics and Agaricales. Taxon 2020, 69, 425–447. [Google Scholar] [CrossRef]
- Bon, M. Novitates 4. Famille Lepiotaceae Roze ex Overeen. Doc. Mycol. 1993, 22, 27–32. [Google Scholar]
- Vellinga, E.C.; Noordeloos, M.E. Glossary. In Flora Agaricina Neerlandica 5; Noordeloos, M.E., Kuyper, T.W., Vellinga, E.C., Eds.; A.A. Balkema Publishers: Tokyo, Japan, 2001; pp. 6–11. [Google Scholar]
- Vellinga, E.C. Genera in the family Agaricaceae—Evidence from nrITS and nrLSU sequences. Mycol. Res. 2004, 108, 354–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, Y.J.; Ge, Z.W. New species of Echinoderma and Lepiota (Agaricaceae) from China. Phytotaxa 2020, 447, 221–236. [Google Scholar] [CrossRef]
- Donk, M.A. The generic names proposed for Agaricaceae. Bei. Nova Hedwigia 1962, 5, 1–320. [Google Scholar]
- Fayod, V. Prodrome d’une histoire naturelle des agaricinés. Ann. Soc. Nat. Bot. VII 1889, 9, 181–411. [Google Scholar]
- Knudsen, H. Notes on Cystolepiota Sing. and Lepiota S.F. Gray. Bot. Tidsskr. 1978, 73, 124–136. [Google Scholar]
- Bon, M. Les lépiotes de l’herbier Boudier au Muséum National d’Histoire Naturelle de Paris. Doc. Mycol. 1977, 7, 11–22. [Google Scholar]
- Bon, M. Les genres Echinoderma (Locq. ex Bon) st. nov. et Rugosomyces Raithelhuber ss. lato. Doc. Mycol. 1991, 21, 61–66. [Google Scholar]
- Vellinga, E.C. Phylogeny of Lepiota (Agaricaceae)—evidence from nrITS and nrLSU sequences. Mycol. Prog. 2003, 2, 305–322. [Google Scholar] [CrossRef]
- Chandrasrikul, A.; Suwanarit, P.; Sangwanit, U.; Lumyong, S.; Payapanon, A.; Sanoamuang, N.; Pukahuta, C.; Petcharat, V.; Sardsud, U.; Duengkae, K.; et al. Checklist of Mushrooms (Basidiomycetes) in Thailand; Office of Natural Resources and Environmental Policy and Planning: Bangkok, Thailand, 2011. [Google Scholar]
- Sysouphanthong, P.; Bouamanivong, S.; Salichan, T. Some mushrooms in Houayyang Preserves; Biotechnology and Ecology Institute: Vientiane, Laos. (In Lao)
- Kornerup, A.; Wanscher, J.H. Methuen Handbook of Colour, 3rd ed.; Eyre Methuen: London, UK, 1978. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes—Application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Rehner, S.A.; Samuels, G.J. Taxonomy and phylogeny of Gliocladium analysed from nuclear large subunit ribosomal DNA sequences. Mycol. Res. 1994, 98, 625–634. [Google Scholar] [CrossRef]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.J.; Whelen, S.; Hall, B.D. Phylogenetic relationships among Ascomycetes: Evidence from an RNA polymerase II subunit. Mol. Biol. Evol. 1999, 16, 1799–1808. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katoh, K.; Toh, H. Recent developments in the MAFFT multiple sequence alignment program. Brief. Bioinform. 2008, 9, 286–298. [Google Scholar] [CrossRef] [Green Version]
- Stamatakis, A.; Hoover, P.; Rougemont, J. A rapid bootstrap algorithm for the RAxML webservers. Syst. Biol. 2008, 75, 758–771. [Google Scholar] [CrossRef]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef] [Green Version]
- Nylander, J.A.A. Modeltest v2: Program Distributed by the Author; Evolutionary Biology Centre, Uppsala University: Uppsala, Sweden, 2004. [Google Scholar]
- Page, R.D. TreeView: An application to display phylogenetic trees on personal computers. Comput. Appl. Biosci. 1996, 12, 357–358. [Google Scholar]
- Vellinga, E.C. Lepiotaceous fungi in California, U.S.A. 4. Type studies of Lepiota fumosifolia and L. petasiformis. Mycotaxon 2007, 98, 225–232. [Google Scholar]
- Vellinga, E.C.; Huijser, H.A. Notes on Cystolepiota: Sections Cystolepiota and Pulverolepiota. Persoonia 1998, 16, 513–526. [Google Scholar]
- Vellinga, E.C. Notes on Cystolepiota seminuda. Persoonia 1987, 13, 321–325. [Google Scholar]
- Paraíso, M.; Maurice, J.P.; Normand, A.C.; Fouchier, F.; Roux, P. Cystolepiota oliveirae sp. nov., récoltée au Portugal sur tronc de fougère arborescente morte. Mycol. Montenegrina 2016, 19, 21–31. [Google Scholar]
- Pegler, D.N. Agaric Flora of Sri Lanka; Kew Bulletin Additional Series XII; HMSO: London, UK, 1986. [Google Scholar]
- Seok, S.J.; Jin, Y.J.; Kwon, S.W.; Kim, Y.S.; Kim, W.G. A taxonomic study of genus Melanophyllum in Korea. Korean J. Mycol. 2013, 41, 205–211. [Google Scholar] [CrossRef]
- Vellinga, E.C. Lepiota (Pers.: Fr.) S.F. Gray, Cystolepiota Sing. and Melanophyllum Velen. In Flora Agaricina Neerlandica; Noordeloos, M.E., Kuyper, T.W., Vellinga, E.C., Eds.; A.A. Balkema Publishers: Rotterdam, The Netherlands, 2001; Volume 5, pp. 109–151. [Google Scholar]





| Taxon | Country | Voucher Number | GenBank Accession Number | ||
|---|---|---|---|---|---|
| ITS | LSU | RPB2 | |||
| Agaricus campestris | China | LAPAG370 | KM657927 | KR006607 | KT951556 |
| Agaricus friesianus | China | ZRL2012601 | KX657026 | KX656970 | KX685048 |
| Cystolepiota adulterina | Italy | 475 | JF907978 | - | - |
| Cystolepiota bucknallii | The Netherlands | ecv1761 | AY176458 | - | - |
| Cystolepiota bucknallii | Italy | 490 | JF907979 | - | - |
| Cystolepiota cystidiosa | USA | MICH18884 | U85333 | U85298 | - |
| Cystolepiota cystophora | Costa Rica | DUKE-JJ87 | U85332 | U85297 | - |
| Cystolepiota fumosifolia | USA | ecv3278 | EF121817 | - | - |
| Cystolepiota hetieri | The Netherlands | ecv2237 | AY176459 | - | - |
| Cystolepiota hetieri | Italy | 782 | JF907982 | - | - |
| Cystolepiota hetieri | China | 420526MF0093 | MG694259 | - | - |
| Cystolepiota hetieri | Canada | HRL0772 | MH979434 | - | - |
| Cystolepiota hetieri | Canada | HRL1277 | MH979438 | - | - |
| Cystolepiota hetieri | USA | HRL2162 | MH979463 | - | - |
| Cystolepiota hetieri | China | HKAS 84189 | MN810139 | MN810094 | MN820976 |
| Cystolepiota hetieri | China | HKAS 53554 | MN810143 | MN810102 | MN820977 |
| Cystolepiota luteohemisphaerica | Ecuador | TL_11724 | AM946477 | AM946476 | - |
| Cystolepiota pseudofumosifolia | China | HKAS 104303 | MN810150 | MN810095 | MN820973 |
| Cystolepiota pseudofumosifolia | China | HKAS 105918 | MN810152 | MN810108 | MN820974 |
| Cystolepiota pulverulenta | USA | ecv1872 | AF391036 | AY176349 | - |
| Cystolepiota pulverulenta | USA | ecv1763 | AF391037 | - | - |
| Cystolepiota pyramidalis | Laos | HNL502500 | MZ574554 | MZ569511 | - |
| Cystolepiota pyramidalis | Thailand | MFLU 12-1774 | MZ574555 | MZ569512 | - |
| Cystolepiota rosea | Italy | 781 | JF907981 | - | - |
| Cystolepiota seminuda | The Netherlands | H.A. Huijser s.n. | AY176350 | AY176351 | - |
| Cystolepiota seminuda | Italy | 9247 | JF907983 | - | - |
| Cystolepiota seminuda | USA | Smith 2018 | MK573889 | - | - |
| Cystolepiota aff. seminuda | China | HKAS 73969 | MN810144 | MN810100 | MN820979 |
| Cystolepiota aff. seminuda | China | HKAS 92275 | MN810149 | MN810101 | MN820980 |
| Cystolepiota sistrata | Canada | HRL1282 | MH979429 | - | - |
| Cystolepiota sistrata | Italy | 491 | JF907980 | - | - |
| Cystolepiota sistrata | USA | HRL2161 | MH979462 | - | - |
| Cystolepiota sp. | China | ZRL2011054 | KF804000 | - | - |
| Cystolepiota sp. | China | ZRL2012038 | KF804001 | - | - |
| Cystolepiota sp. | Canada | UBC_F24523 | MF955171 | - | - |
| Cystolepiota sp. | USA | HRL1900 | MH979456 | - | - |
| Cystolepiota sp. | USA | MycoMap_7788 | MK560110 | - | - |
| Cystolepiota sp. | USA | MycoMap_7792 | MK560111 | - | - |
| Cystolepiota sp. | China | HKAS 78850 | MN810142 | MN810103 | MN820978 |
| Cystolepiota sp. | China | HKAS 105719 | MN810151 | MN810109 | MN820975 |
| Cystolepiota sp. (Echinoderma) | Thailand | ecv3896 | - | HM488789 | - |
| Cystolepiota sp. (Echinoderma) | Malaysia | LAM 0001 | - | KY090841 | - |
| Cystolepiota sp. | India | HATFD14_95 | KU847887 | - | - |
| Cystolepiota thailandica | Thailand | MFLU 22-0017 | MZ574556 | MZ569513 | - |
| Cystolepiota rhodella | Laos | HNL501799 | MZ574551 | MZ569508 | MZ508496 |
| Cystolepiota rhodella | Thailand | MFLU 22-0019 | MZ574552 | MZ569509 | MZ574090 |
| Cystolepiota rhodella | Thailand | MFLU 09-0050 | MZ574553 | MZ569510 | - |
| Echinoderma asperum | North Macedonia | HKAS 106783 | MN810133 | MN810088 | MN820967 |
| Echinoderma asperum | USA | HKAS 84214 | MN810135 | MN810089 | MN820964 |
| Echinoderma asperum | USA | HKAS 84240 | MN810136 | MN810090 | MN820965 |
| Echinoderma asperum | China | HKAS 106782 | MN810134 | MN810087 | MN820962 |
| Echinoderma asperum | China | HKAS 105694 | MN810153 | MN810106 | MN820966 |
| Echinoderma asperum | China | HKAS 77440 | MN810145 | MN810096 | MN820963 |
| Echinoderma asperum | India | NEHU.MBSRJ.55 | KP843884 | MG253012 | - |
| Echinoderma flavidoasperum | China | HKAS 87905 | MN810147 | MN810098 | MN820969 |
| Echinoderma flavidoasperum | China | HKAS 76527 | MN810146 | MN810097 | MN820968 |
| Echinoderma hystrix | France | H.A. Huijser | AY176377 | AY176378 | - |
| Echinoderma sp. | China | HKAS 70488 | MN810148 | MN810099 | MN820970 |
| Echinoderma sp. | China | HKAS 106735 | MN810154 | MN810107 | MN820971 |
| Lepiota aff. carinii | Hungary | NL-2202 | - | MK277953 | - |
| Lepiota alba | China | HKAS 90371 | MN810115 | MN810075 | MN820946 |
| Lepiota asperula | Canada | S.D.Russell HRL1281 | MH979440 | - | - |
| Lepiota castanea | China | HKAS.84179 | MN810119 | MN810077 | MN820960 |
| Lepiota clypeolaria | China | HKAS 87248 | MN810123 | MN810080 | MN820941 |
| Lepiota echinacea | China | HKAS 105582 | MN810155 | MN810104 | MN820954 |
| Lepiota geocarpa | USA | UTC00143916 | HQ020412 | EU130550 | MN820945 |
| Lepiota geophana | USA | UTC00253060 | HQ020411 | HQ020421 | MN820944 |
| Lepiota jacobi | China | HKAS 48802 | MN810138 | GU199356 | MN820953 |
| Lepiota magnispora | China | HKAS 61622 | JN944089 | JN940285 | JN993693 |
| Lepiota omninoflava | China | HKAS 106734 | MN810157 | MN810092 | MN820951 |
| Melanophyllum eyrei | South Korea | ASIS23988 | KF953546 | - | - |
| Melanophyllum eyrei | Sweden | TL6692 | AY176493 | - | - |
| Melanophyllum haematospermum | USA | ecv2517 | AF391039 | AY176456 | - |
| Melanophyllum haematospermum | The Netherlands | ecv2249 | AF391038 | AY176455 | - |
| Melanophyllum haematospermum | Canada | S.D.Russell HRL1807 | MH979452 | - | - |
| Melanophyllum haematospermum | Italy | 913 | JF908498 | - | - |
| Smithiomyces asiaticus | China | HKAS 84395 | MW522986 | MW716269 | MW736566 |
| Smithiomyces dominicanus | Dominican Republic | BSD126144 | KR604686 | MW716266 | MW736563 |
| Smithiomyces heterosporus | China | HKAS 84392 | MW522985 | MW716268 | MW736565 |
| Smithiomyces lepiotoides | China | HKAS 54390 | MW522984 | MW716270 | - |
| Smithiomyces mexicanus | Switzerland | UTC259587 | MW723225 | MW716267 | MW736564 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sysouphanthong, P.; Thongklang, N.; Liu, Y.S.; Vellinga, E.C. Three New Species of Cystolepiota from Laos and Thailand. Diversity 2022, 14, 449. https://doi.org/10.3390/d14060449
Sysouphanthong P, Thongklang N, Liu YS, Vellinga EC. Three New Species of Cystolepiota from Laos and Thailand. Diversity. 2022; 14(6):449. https://doi.org/10.3390/d14060449
Chicago/Turabian StyleSysouphanthong, Phongeun, Naritsada Thongklang, Yuan S. Liu, and Else C. Vellinga. 2022. "Three New Species of Cystolepiota from Laos and Thailand" Diversity 14, no. 6: 449. https://doi.org/10.3390/d14060449
APA StyleSysouphanthong, P., Thongklang, N., Liu, Y. S., & Vellinga, E. C. (2022). Three New Species of Cystolepiota from Laos and Thailand. Diversity, 14(6), 449. https://doi.org/10.3390/d14060449








