Filling a Gap: A Population of Eunicella verrucosa (Pallas, 1766) (Anthozoa, Alcyonacea) in the Tavolara-Punta Coda Cavallo Marine Protected Area (NE Sardinia, Italy)
Abstract
1. Introduction
2. Materials and Methods
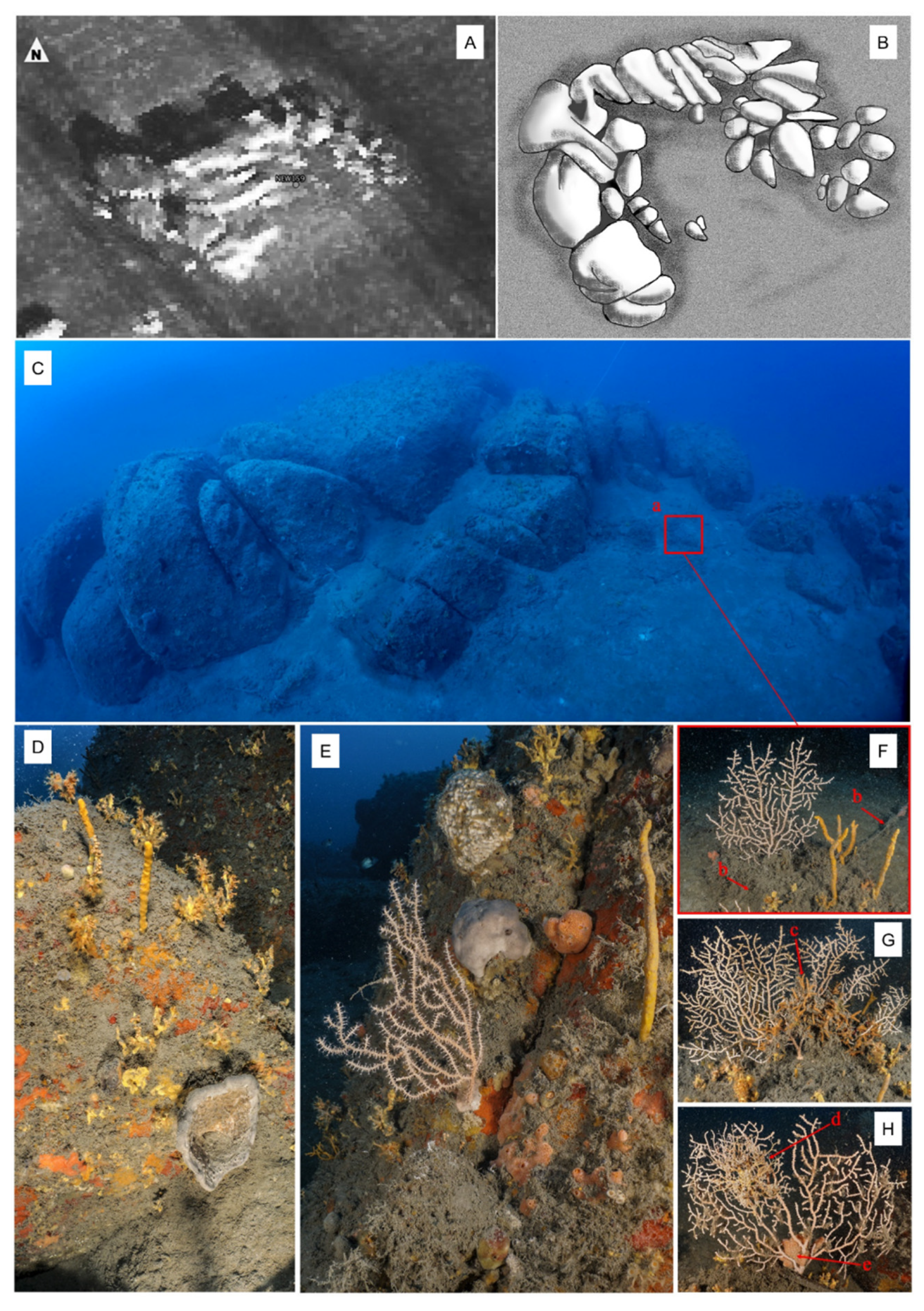
3. Results
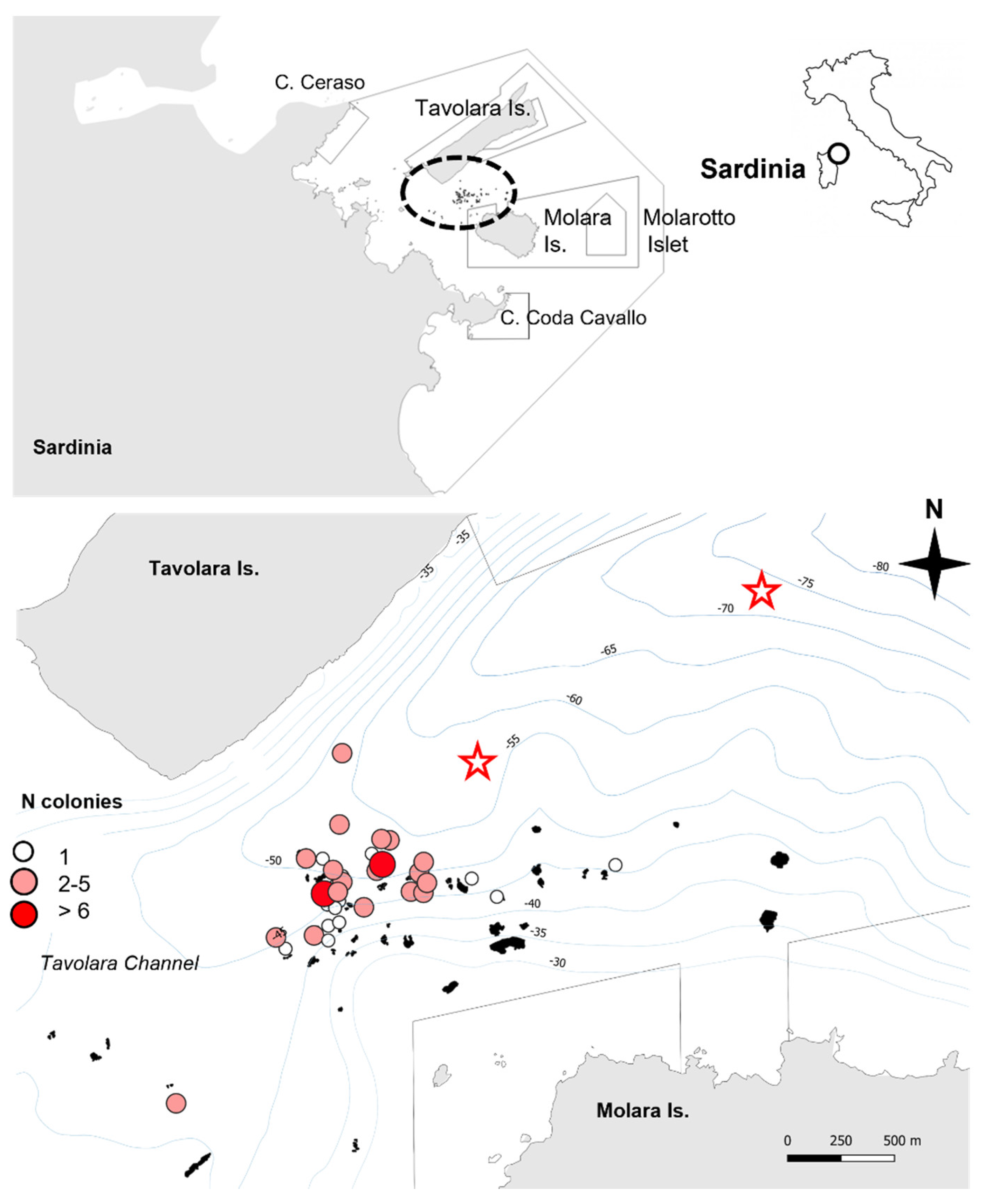
3.1. Distribution and Occurrence
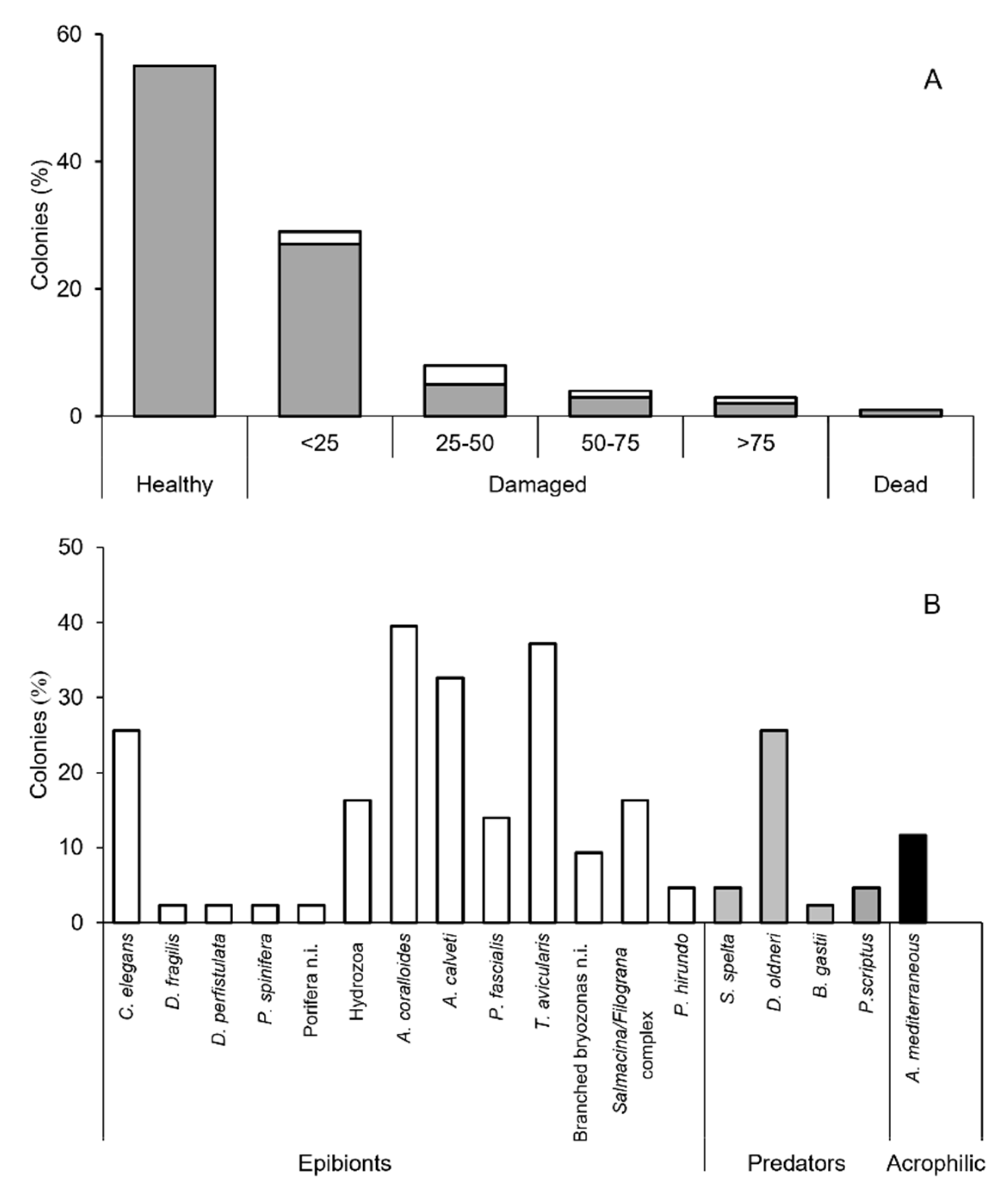
3.2. Epibiosis and Damages
4. Discussion
4.1. Distribution of Eunicella verrucosa and Population Singularity
4.2. Predators and Acrophilic Epibionts
4.3. Epibiosis and Health Status of the Population
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carpine, C. Les Gorgonaries de la Mediterranée. Bull. Musee Oceanogr. Monaco 1975, 71, 1–140. [Google Scholar]
- Manuel, R.L. British Anthozoa (Coelenterata: Octocorallia and Hexacorallia); Estuarine and Brackish-Water Sciences Association: Leiden, The Netherlands, 1988. [Google Scholar]
- Readman, J.A.J.; Hiscock, K. Eunicella verrucosa Pink sea fan. In Marine Life Information Network: Biology and Sensitivity Key Information Reviews; Tyler-Walters, H., Hiscock, K., Eds.; Marine Biological Association of the United Kingdom: Plymouth, UK, 2017; Available online: https://www.marlin.ac.uk/species/detail/1121 (accessed on 9 February 2022).
- Lafargue, F. Peuplements sessiles de l’Archipel de Glénan L’Inventaire: Anthozoaires. Vie Milieu 1969, 20, 415–436. [Google Scholar]
- Hiscock, K. Changes in the marine life of Lundy. Rep. Lundy Field Soc. 2003, 53, 86–95. [Google Scholar]
- Sartoretto, S.; Francour, P. Bathymetric distribution and growth rates of Eunicella verrucosa (Cnidaria: Gorgoniidae) populationsalong the Marseilles coast (France). Sci. Mar. 2012, 76, 349–355. [Google Scholar] [CrossRef]
- Di Camillo, C.G.; Ponti, M.; Bavestrello, G.; Krzelj, M.; Cerrano, C. Building a baseline for habitat-forming corals by a multi-source approach, including Web Ecological Knowledge. Biodivers. Conserv. 2018, 27, 1257–1276. [Google Scholar] [CrossRef]
- Enrichetti, F.; Dominguez-Carrió, C.; Toma, M.; Bavestrello, G.; Betti, F.; Canese, S.; Bo, M. Megabenthic communities of the Ligurian deep continental shelf and shelf break (NW Mediterranean Sea). PLoS ONE 2019, 14, e0223949. [Google Scholar] [CrossRef]
- IUCN (International Union for Conservation of Nature and Natural Resources). IUCN Red List of Threatened Species. 2022. Available online: www.iucnredlist (accessed on 9 February 2022).
- Grinyó, J.; Gori, A.; Ambroso, S.; Purroy, A.; Calatayud, C.; Dominguez-Carrió, C.; Coppari, M.; Lo Iacono, C.; López-González, P.J.; Gili, J.M. Diversity, distribution and population size structure of deep Mediterranean gorgonian assemblages (Menorca Channel, Western Mediterranean Sea). Progr. Oceanogr. 2016, 145, 42–56. [Google Scholar] [CrossRef]
- Chimienti, G.; Stithou, M.; Dalle Mura, I.; Mastrototaro, F.; D’Onghia, G.; Tursi, A.; Izzi, C.; Fraschetti, S. An explorative assessment of the importance of Mediterranean Coralligenous habitat to local economy: The case of recreational diving. J. Environ. Account. Manag. 2017, 5, 315–325. [Google Scholar] [CrossRef]
- Velimirov, B. Orientation in the sea fan Eunicella cavolinii related to water movement. Helgol. Wiss. Meeresunters. 1973, 24, 163–173. [Google Scholar] [CrossRef]
- Velimirov, B. Variations in growth forms of Eunicella cavolini Koch (Octocorallia) related to intensity of water movement. J. Exp. Mar. Biol. Ecol. 1976, 21, 109–117. [Google Scholar] [CrossRef]
- West, J.M. Plasticity in the sclerites of a gorgonian coral: Tests of water motion, light level, and damage cues. Biol. Bull. 1997, 192, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Weinbauer, M.G.; Velimirov, B. Morphological variations in the Mediterranean sea fan Eunicella cavolini (Coelenterata: Gorgonacea) in relation to exposure, colony size and colony region. Bull. Mar. Sci. 1995, 56, 283–295. [Google Scholar]
- Kim, K.; Lasker, H.R. Allometry of resource capture in colonial cnidarians and constraints on modular growth. Funct. Ecol. 1998, 12, 646–654. [Google Scholar] [CrossRef]
- Skoufas, G. Comparative biometry of Eunicella singularis (Gorgonian) sclerites at East Mediterranean Sea (North Aegean Sea, Greece). Mar. Biol. 2006, 149, 1365–1370. [Google Scholar] [CrossRef]
- Chang, W.L.; Chi, K.J.; Fan, T.Y.; Dai, C.F. Skeletal modification in response to flow during growth in colonies of the sea whip, Junceella fragilis. J. Exp. Mar. Biol. Ecol. 2007, 347, 97–108. [Google Scholar] [CrossRef]
- Munro, L.; Munro, C. Reef Research. Determining the Reproductive Cycle of Eunicella verrucosa; Interim report March 2003. RR Report 10 Nov. 2003. Available online: http://www.marine-bio-images.com/RR_Eunicella_PDFS/Report_RR12Jul2004reproductive%20cycle%20pdf.pdf (accessed on 9 February 2022).
- Goffredo, S.; Lasker, H.R. Modular growth of a gorgonian coral can generate predictable patterns of colony growth. J. Exp. Mar. Biol. Ecol. 2006, 336, 221–229. [Google Scholar] [CrossRef]
- Hiscock, K. Eunicella verrucosa. Pink Sea Fan. Marine Life Information Network: Biology and Sensitivity Key Information Sub-programme. 2007. Available online: https://www.marlin.ac.uk/assets/pdf/species/marlin_species_1121_2019-03-21.pdf (accessed on 9 February 2022).
- Coz, R.; Ouisse, V.; Artero, C.; Carpentier, A.; Crave, A.; Feunteun, E.; Olivier, J.M.; Perrin, B.; Ysnel, F. Development of a new standardised method for sustainable monitoring of the vulnerable pink sea fan Eunicella verrucosa. Mar. Biol. 2012, 159, 1375–1388. [Google Scholar] [CrossRef]
- Coma, R.; Pola, E.; Ribes, M.; Zabala, M. Long-term assessment of temperate octocoral mortality patterns, protected vs. unprotected areas. Ecol. Appl. 2004, 14, 1466–1478. [Google Scholar] [CrossRef]
- Linares, C.; Coma, R.; Zabala, M. Restoration of threatened red gorgonian populations: An experimental and modelling approach. Biol. Conserv. 2008, 141, 427–437. [Google Scholar] [CrossRef]
- Lloret, J.; Zaragoza, N.; Caballero, D.; Riera, V. Impacts of recreational boating on the marine environment of Cap de Creus (Mediterranean Sea). Ocean Coast. Manag. 2008, 51, 749–754. [Google Scholar] [CrossRef]
- Enrichetti, F.; Bava, S.; Bavestrello, G.; Betti, F.; Lanteri, L.; Bo, M. Artisanal fishing impact on deep coralligenous animal forests: A Mediterranean case study of marine vulnerability. Ocean Coast. Manag. 2019, 177, 112–126. [Google Scholar] [CrossRef]
- Otero, M.M.; Numa, C.; Bo, M.; Orejas, C.; Garrabou, J.; Cerrano, C.; Kružić, P.; Antoniadou, C.; Aguilar, R.; Kipson, S.; et al. Overview of the Conservation Status of Mediterranean Anthozoans; IUCN: Málaga, Spain, 2017; pp. 1–73. [Google Scholar]
- Chimienti, G. Vulnerable Forests of the Pink Sea Fan Eunicella verrucosa in the Mediterranean Sea. Diversity 2020, 12, 176. [Google Scholar] [CrossRef]
- Bianchi, C.N.; Morri, C.; Navone, A. I popolamenti delle scogliere rocciose sommerse dell’Area Marina Protetta di Tavolara Punta Coda Cavallo (Sardegna nord-orientale). Sci. Rep. Port Cros Natl. Park 2014, 24, 39–85. [Google Scholar]
- ANDROMEDE. Etude et Cartographie des Biocénoses Marines de l’Aire Marine Protégée de Tavolara—Punta Coda Cavallo, Sardaigne (Italie); Initiative pour les Petites Iles de Méditerranée; Contrat Œil d’ Andromède/Agence de L’Eau: Lion, France, 2012; p. 149. [Google Scholar]
- ANDROMEDE. Inventaire et Cartographie des Assemblages Coralligènes de l’Aire Marine Protégée de Tavolara—Punta Coda Cavallo, Sardaigne (Italie); Initiative pour les Petites Iles de Méditerranée; Andromède Océanologie/Agence de l’Eau: Lion, France, 2016; p. 100. [Google Scholar]
- Canessa, M.; Bavestrello, G.; Bo, M.; Trainito, E.; Panzalis, P.; Navone Caragnano, A.; Betti, F.; Cattaneo-Vietti, R. Coralligenous assemblages differ between limestone and granite: A case study at the Tavolara-Punta Coda Cavallo Marine Protected Area (NE Sardinia, Mediterranean Sea). Reg. Stud. Mar. Sci. 2020, 35, 101159. [Google Scholar] [CrossRef]
- Canessa, M.; Bavestrello, G.; Trainito, E.; Bianchi, C.N.; Morri, C.; Navone, A.; Cattaneo-Vietti, R. A large and erected sponge assemblage on granite outcrops in a Mediterranean Marine Protected Area (NE Sardinia). Reg. Stud. Mar. Sci. 2021, 44, 101734. [Google Scholar] [CrossRef]
- Bavestrello, G.; Cerrano, C.; Zanzi, D.; Cattaneo-Vietti, R. Damage by fishing activities to the Gorgonian coral Paramuricea clavata in the Ligurian Sea. Aquat. Conserv. 1997, 7, 253–262. [Google Scholar] [CrossRef]
- Bo, M.; Bava, S.; Canese, S.; Angiolillo, M.; Cattaneo-Vietti, R.; Bavestrello, G. Fishing impact on deep Mediterranean rocky habitats as revealed by ROV investigation. Biol. Conserv. 2014, 171, 167–176. [Google Scholar] [CrossRef]
- Pititto, F.; Trainito, E.; Macic, V.; Rais, C.; Torchia, G. The resolution in benthic carthography: A detailed mapping technique and a multiscale GIS approach with applications to coralligenous assemblages. In Proceedings of the Conference: 2° Mediterranean Symposium on the Conservation of Coralligenous & Other Calcareous Bio-Concretions, Portorož, Slovenia, 29–30 October 2014. [Google Scholar] [CrossRef]
- Rasband, W.S. ImageJ: Image Processing and Analysis in Java; ascl-1206; National Institutes of Health: Bethesda, MD, USA, 2012. [Google Scholar]
- Coma, R.; Ribes, M.; Zabala, M.; Gili, J.M. Growth in a modular colonial marine invertebrate. Estuar. Coast. Shelf Sci. 1998, 47, 459–470. [Google Scholar] [CrossRef]
- Chimienti, G.; Di Nisio, A.; Lanzolla, A.M.L. Size/Age Models for Monitoring of the Pink Sea Fan Eunicella verrucosa (Cnidaria: Alcyonacea) and a Case Study Application. J. Mar. Sci. Eng. 2020, 8, 951. [Google Scholar] [CrossRef]
- Bo, M.; Bavestrello, G.; Angiolillo, M.; Calcagnile, L.; Canese, S.; Cannas, R.; Cau, A.; D’Elia, M.; D’Oriano, F.; Follesa, M.C.; et al. Persistence of pristine deep-sea coral gardens in the Mediterranean Sea (SW Sardinia). PLoS ONE 2015, 10, e0119393. [Google Scholar] [CrossRef]
- Cau, A.; Follesa, M.C.; Moccia, D.; Alvito, A.; Bo, M.; Angiolillo, M.; Canese, S.; Paliaga, E.M.; Orrù, P.E.; Sacco, F.; et al. Deepwater corals biodiversity along roche du large ecosystems with different habitat complexity along the south Sardinia continental margin (CW Mediterranean Sea). Mar. Biol. 2015, 162, 1865–1878. [Google Scholar] [CrossRef]
- Gori, A.; Grinyó, J.; Dominguez-Carrió, C.; Ambroso, S.; López-González, P.J.; Gili, J.M.; Bavestrello, G.; Bo, M. 20 Gorgonian and black coral assemblages in deep coastal bottoms and continental shelves of the Mediterranean Sea. In Mediterranean Cold-Water Corals: Past, Present and Future; Springer: Cham, Switzerland, 2019; pp. 245–248. [Google Scholar]
- Moccia, D.; Alvito, A.; Follesa, M.C. Preliminary ROV surveys data on deep-coral assemblages from South-East Sardinian Sea (central western Mediterranean). In Proceedings of the Marine Imaging Workshop, Southampton, UK, 7–10 April 2014. [Google Scholar]
- Moccia, D.; Cau, A.; Bramanti, L.; Carugati, L.; Canese, S.; Follesa, M.C.; Cannas, R. Spatial distribution and habitat characterization of marine animal forest assemblages along nine submarine canyons of Eastern Sardinia (central Mediterranean Sea). Deep. Sea Res. Part I Oceanogr. Res. Pap. 2021, 167, 103422. [Google Scholar] [CrossRef]
- Weinberg, S.; Weinberg, F. The life cycle of a gorgonian: Eunicella singularis (Esper, 1794). Bijdr. Dierkd. 1979, 48, 127–140. [Google Scholar] [CrossRef]
- López Jurado, L.F.; González Barbuzano, J.; Hildebrandt, S. La foca Monje y las Islas Canarias: Biología, Ecología y Conservación de una Especie; Consejería de Política Territorial, Gobierno de Canarias: Las Palmas de Gran Canaria, Spain, 1995; ISDN 8460623610. [Google Scholar]
- Pinot, J.M.; Tintoré, J.; Gomis, D. Multivariate analysis of the surface circulation in the Balearic Sea. Progr. Oceanogr. 1995, 36, 343–376. [Google Scholar] [CrossRef]
- Vélez-Belchí, P.; Tintoré, J. Vertical velocities at an ocean front. Sci. Mar. 2001, 65 (Suppl. S1), 291–300. [Google Scholar] [CrossRef]
- García, A.; Alemany, F.; Vélez-Belchí, P.; López Jurado, J.L.; Cortés, D.; de la Serna, J.M.; González Pola, C.; Rodríguez, J.M.; Jansá, J.; Ramírez, T. Characterization of the bluefin tuna spawning habitat off the Balearic Archipelago in relation to key hydrographic features and associated environmental conditions. Collect. Vol. Sci. Pap. ICCAT 2003, 58, 535–549. [Google Scholar]
- Oray, I.; Karakulak, S.; Garcia, A.; Piccinetti, C.; Rollandi, L.; de la Serna, J.M. Report on the Mediterranean BYP tuna larval meeting. Coll. Vol. Sci. Pap. ICCAT 2005, 58, 1429–1435. [Google Scholar]
- Alemany, F.; Deudero, S.; Morales-Nin, B.; Lopez-Jurado, J.L.; Jansa, J.; Palmer, M.; Palomera, I. Influence of physical environmental factors on the composition and horizontal distribution of summer larval fish assemblages off Mallorca Island (Balearic archipelago, Western Mediterranean). J. Plankton Res. 2006, 28, 473–487. [Google Scholar] [CrossRef]
- Vergnaud-Grazzini, C.; Borsetti, A.M.; Cati, F.; Colantoni, P.; D’Onofrio, S.; Saliege, J.F.; Sartori, R.; Tampieri, R. Palaeoceanographic record of the last deglaciation in the Strait of Sicily. Mar. Micropaleontol. 1988, 13, 1–21. [Google Scholar] [CrossRef]
- Furfaro, G.; Trainito, E.; De Lorenzi, F.; Fantin, M.; Doneddu, M. Tritonia nilsodhneri Marcus Ev., 1983 (Gastropoda, Heterobranchia, Tritoniidae): First records for the Adriatic Sea and new data on ecology and distribution of Mediterranean populations. Acta Adriat. 2017, 58, 261–270. [Google Scholar] [CrossRef]
- Theodor, J. Contribution a l’étude des gorgones (VI): La dénudation des branches de gorgones par des mollusques prédateurs. Vie Milieu 1967, 18, 73–78. [Google Scholar]
- Manconi, R.; Mori, M. New records of Balssia gasti (BALSS, 1921) (Decapoda, Palaemonidae) in the western Mediterranean Sea. Crustaceana 1990, 59, 96–100. [Google Scholar] [CrossRef]
- Manconi, R.; Mori, M. Caridean shrimps (Decapoda) found among Corallium rubrum (L., 1758). Crustaceana 1992, 62, 105–110. [Google Scholar] [CrossRef]
- Mori, M.; Morri, C.; Bianchi, C.N. Notes on the life history of the pontonine shrimp Balssia gasti (Balss, 1921). Oebalia 1994, 20, 129–137. [Google Scholar]
- Trainito, E.; Baldacconi, R. Atlante di Flora e Fauna del Mediterraneo; Il Castello Editore: Cornaredo, Italy, 2021; pp. 262–448. [Google Scholar]
- Ponti, M.; Grech, D.; Mori, M.; Perlini, R.A.; Ventra, V.; Panzalis, P.A.; Cerrano, C. The role of gorgonians on the diversity of vagile benthic fauna in Mediterranean rocky habitats. Mar. Biol. 2016, 163, 120. [Google Scholar] [CrossRef]
- Canessa, M.; Bavestrello, G.; Guidetti, P.; Navone, A.; Trainito, E. Marine rocky reef assemblages and lithological properties of substrates are connected at different ecological levels. Eur. Zool. J. 2022, in press.
- Zibrowius, H. Observations biologiques au large du Lavandou (côte méditerranéenne de France) à l’aide du sous-marin Griffon de la Marine Nationale. Trav. Sci. Parc. Natl. Port Cros 1978, 4, 171–176. [Google Scholar]
- Montasell Bartrés, M. Astrospartus mediterraneus (Echinodermata: Ophiuroidea) in the Cap de Creus Marine Area: Ecological Characterization of an Emblematic Species. Bachelor’s Thesis, Universitat Central de Catalunya, Vic, Spain, 2020. [Google Scholar]
- Puce, S.; Bavestrello, G.; Di Camillo, C.G.; Boero, F. Long-term changes in hydroid (Cnidaria, Hydrozoa) assemblages: Effect of Mediterranean warming? Mar. Ecol. 2009, 30, 313–326. [Google Scholar] [CrossRef]
- Bavestrello, G.; Bertone, S.; Cattaneo-Vietti, R.; Cerrano, C.; Gaino, E.; Zanzi, D. Mass mortality of Paramuricea clavata (Anthozoa, Cnidaria) on Portofino Promontory cliffs, Ligurian Sea, Mediterranean Sea. Mar. Life 1994, 4, 15–19. [Google Scholar]
- Cerrano, C.; Bavestrello, G. Medium-term effects of die-off of rocky benthos in the Ligurian Sea. What can we learn from gorgonians? Chem. Ecol. 2008, 24 (Suppl. S1), 73–82. [Google Scholar] [CrossRef]
- Torrents, O.; Tambutté, E.; Caminiti, N.; Garrabou, J. Upper thermal thresholds of shallow vs. deep populations of the precious Mediterranean red coral Corallium rubrum (L.): Assessing the potential effects of warming in the NW Mediterranean. J. Exp. Mar. Biol. Ecol. 2008, 357, 7–19. [Google Scholar] [CrossRef]
- Huete-Stauffer, C.; Vielmini, I.; Palma, M.; Navone, A.; Panzalis, P.; Vezzulli, L.; Misic, C.; Cerrano, C. Paramuricea clavata (Anthozoa, Octocorallia) loss in the Marine Protected Area of Tavolara (Sardinia, Italy) due to a mass mortality event. Mar. Ecol. 2011, 32, 107–116. [Google Scholar] [CrossRef]
- Wahl, M. Ecological lever and interface ecology: Epibiosis modulates the interactions between host and environment. Biofouling 2008, 24, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Harmelin, J.G.; Marinopoulos, J. Population structure and partial mortality of the gorgonian Paramuricea clavata (Risso) in the north-western Mediterranean (France, Port-Cros Island). Mar. Life 1994, 4, 5–13. [Google Scholar]
- Riegl, B.; Riegl, A. Studies on coral community structure and damage as a basis for zoning marine reserves. Biol. Conserv. 1996, 77, 269–277. [Google Scholar] [CrossRef]
- Cerrano, C.; Arillo, A.; Azzini, F.; Calcinai, B.; Castellano, L.; Muti, C.; Valisano, L.; Zega, G.; Bavestrello, G. Gorgonian population recovery after a mass mortality event. Aquat. Conserv. 2005, 15, 147–157. [Google Scholar] [CrossRef]
- Groot, S.; Weinberg, S. Biogeography, taxonomical status and ecology of Alcyonium (Parerythropodium) coralloides (Pallas, 1766). Mar. Ecol. 1982, 3, 293–312. [Google Scholar] [CrossRef]
- Cerrano, C.; Bavestrello, G.; Bianchi, C.N.; Cattaneo-Vietti, R.; Bava, S.; Morganti, C.; Morri, C.; Picco, P.; Sara, G.; Schiaparelli, S.; et al. A catastrophic mass- mortality episode of gorgonians and other organisms in the Ligurian Sea (northwestern Mediterranean), summer 1999. Ecol. Lett. 2000, 3, 284–293. [Google Scholar] [CrossRef]
- Quintanilla, E.; Gili, J.-M.; Lòpez-Gonzàlez, P.J.; Tsounis, G.; Madurell, T.; Fiorillo, I.; Rossi, S. Sexual reproductive cycle of the epibiotic soft coral Alcyonium coralloides (Octocorallia, Alcyonacea). Aquat. Biol. 2013, 18, 113–124. [Google Scholar] [CrossRef]
- Hall-Spencer, J.M.; Pike, J.; Munn, C.B. Diseases affect cold-water corals too: Eunicella verrucosa (Cnidaria: Gorgonacea) necrosis in SW England. Dis. Aquat. Org. 2007, 76, 87–97. [Google Scholar] [CrossRef]
- Tsounis, G.; Martinez, L.; Bramanti, L.; Viladrich, N.; Gili, J.M.; Martinez, Á.; Rossi, S. Anthropogenic effects on reproductive effort and allocation of energy reserves in the Mediterranean octocoral Paramuricea clavata. Mar. Ecol. Prog. Ser. 2012, 449, 161–172. [Google Scholar] [CrossRef]
- Angiolillo, M.; di Lorenzo, B.; Farcomeni, A.; Bo, M.; Bavestrello, G.; Santangelo, G.; Cau, A.; Mastascusa, V.; Cau, A.; Sacco, F.; et al. Distribution and assessment of marine debris in the deep Tyrrhenian Sea (NW Mediterranean Sea, Italy). Mar. Pollut. Bull. 2015, 92, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Sini, M.; Kipson, S.; Linares, C.; Koutsoubas, D.; Garrabou, J. The yellow gorgonian Eunicella cavolini: Demography and disturbance levels across the Mediterranean Sea. PLoS ONE 2015, 10, e0126253. [Google Scholar] [CrossRef] [PubMed]
- Gori, A.; Bavestrello, G.; Grinyó, J.; Dominguez-Carrió, C.; Ambroso, S.; Bo, M. Animal forests in deep coastal bottoms and continental shelf of the Mediterranean Sea. In Marine Animal Forests: The Ecology of Benthic Biodiversity Hotspots; Springer: Berlin/Heidelberg, Germany, 2017; pp. 207–233. [Google Scholar]
- D’Onghia, G.; Calculli, C.; Capezzuto, F.; Carlucci, R.; Carluccio, A.; Grehan, A.; Indennidate, P.; Maiorano, F.; Mastrototaro, A.; Pollice, T.; et al. Anthropogenic impact in the Santa Maria di Leuca cold-water coral province (Mediterranean Sea): Observations and conservation straits. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2017, 145, 87–101. [Google Scholar] [CrossRef]
- Enrichetti, F.; Dominguez-Carrió, C.; Toma, M.; Bavestrello, G.; Canese, S.; Bo, M. Assessment and distribution of seafloor litter on the deep Ligurian continental shelf and shelf break (NW Mediterranean Sea). Mar. Pollut. Bull. 2020, 151, 110872. [Google Scholar] [CrossRef]
- Rovere, A.; Ferraris, F.; Parravicini, V.; Navone, A.; Morri, C.; Bianchi, C.N. Characterization and evaluation of a marine protected area: ‘Tavolara–Punta Coda Cavallo’ (Sardinia, NW Mediterranean). J. Maps 2013, 9, 279–288. [Google Scholar] [CrossRef]



| Site | Depth Range (m) | Outcrop Area (m2) | N Colony | N Dives |
|---|---|---|---|---|
| N1 | 37–44 | 428 | 6 | 3 |
| N2 | 39–45 | 1336 | 1 | 1 |
| N24 | 38–44 | 1408 | 1 | 1 |
| N25 | 43–54 | 677 | 6 | 2 |
| N27 | 38–47 | 1024 | 14 | 6 |
| N28 | 38–47 | 3343 | 4 | 3 |
| N29 | 42–49 | 410 | 2 | 1 |
| N30 | 46–50 | 435 | 4 | 1 |
| N32 | 47–52 | 371 | 3 | 1 |
| N33 | 50–56 | 574 | 2 | 1 |
| N34 | 38–45 | 421 | 3 | 1 |
| N37 | 35–41 | 736 | 3 | 2 |
| N73 | 36–40 | 100 | 1 | 1 |
| N74 | 40–45 | 345 | 1 | 1 |
| N75 | 40–45 | 595 | 1 | 1 |
| N83 | 48–52 | 1022 | 2 | 1 |
| N95 | 41 | 221 | 2 | 1 |
| N99 | 45–50 | 1253 | 1 | 2 |
| N100 | 38–45 | 498 | 1 | 1 |
| N101 | 46–52 | 820 | 1 | 1 |
| N102 | 47–53 | 264 | 1 | 1 |
| N118 | 47–52 | 580 | 3 | 2 |
| N119 | 39–44 | 286 | 1 | 1 |
| N120 | 40–45 | 331 | 1 | 1 |
| N140 | 42–48 | 900 | 4 | 1 |
| N148 | 48–54 | 471 | 3 | 1 |
| N150 | 43–49 | 165 | 4 | 1 |
| N151 | 38–46 | 198 | 3 | 3 |
| N159 | 48–59 | 272 | 12 | 1 |
| N160 | 44–49 | 760 | 3 | 1 |
| N165 | 44–48 | 197 | 3 | 1 |
| N171 | 40–44 | 141 | 1 | 1 |
| N180 | 40–45 | 92 | 1 | 1 |
| N182 | 40–45 | 280 | 1 | 1 |
| Site | Height (cm) | Surface (cm2) | Age (years) | Epiosis % | Naked Skeleton% | Damage % | Site | Height (cm) | Surface (cm2) | Age (years) | Epiosis % | Naked Skeleton % | Damage % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N1 | 41.03 | 688.07 | 30.5 | 5 | 0 | 5 | N74 | 22.56 | 10.5 | 0 | 0 | 0 | |
| N1 | 39.21 | 823.51 | 27.4 | 15 | 0 | 15 | N75 | 59.06 | 85.9 | 0 | 0 | 0 | |
| N1 | 43.27 | 1028.3 | 34.7 | 0 | 0 | 0 | N83 | 40.04 | 610.01 | 28.8 | 0 | 0 | 0 |
| N1 | 35.02 | 21.57 | 0 | 0 | 0 | N83 | 18.17 | 291.49 | 8.2 | 0 | 0 | 0 | |
| N1 | 19.49 | 183.14 | 8.8 | 0 | 0 | 0 | N95 | 48.59 | 1873.8 | 47.1 | 0 | 0 | 0 |
| N1 | 36.14 | 23 | 0 | 0 | 0 | N95 | 34.2 | 588.98 | 20.6 | 0 | 0 | 0 | |
| N2 | 27.31 | 490.01 | 13.8 | 30 | 0 | 30 | N99 | 25.37 | 397.06 | 12.4 | 40 | 0 | 40 |
| N24 | 10.73 | 26.92 | 5.3 | 0 | 0 | 0 | N100 | 35.7 | 713.1 | 22.5 | 0 | 0 | 0 |
| N25 | 26.68 | 13.4 | 0 | 0 | 0 | N101 | 35.72 | 22.5 | 0 | 0 | 0 | ||
| N25 | 29.12 | 15.4 | 5 | 0 | 5 | N102 | 27.73 | 230.85 | 14.2 | 0 | 0 | 0 | |
| N25 | 27.4 | 13.9 | 0 | 0 | 0 | N118 | 51.03 | 2344.6 | 54.2 | 25 | 0 | 25 | |
| N25 | 46.34 | 41.4 | 20 | 0 | 20 | N118 | 45.04 | 38.4 | 0 | 0 | 0 | ||
| N25 | 48.64 | 47.2 | 0 | 100 | 100 | N118 | 12.355 | 5.9 | 80 | 0 | 80 | ||
| N25 | 52.3 | 58.3 | 40 | 0 | 40 | N119 | 18.9 | 159.4 | 8.5 | 0 | 0 | 0 | |
| N27 | 20.5 | 176.32 | 9.4 | 0 | 0 | 0 | N120 | 10 | 0 | 0 | 0 | ||
| N27 | 31.43 | 423.55 | 17.5 | 0 | 0 | 0 | N140 | 63.55 | 111.3 | 0 | 0 | 0 | |
| N27 | 24.04 | 11.5 | 65 | 0 | 65 | N140 | 55.9 | 1754.9 | 71.7 | 5 | 0 | 5 | |
| N27 | 48.3 | 46.3 | 10 | 0 | 10 | N140 | 36.22 | 23.1 | 0 | 0 | 0 | ||
| N27 | 37.27 | 24.5 | 0 | 0 | 0 | N140 | 48.7 | 1222.9 | 47.4 | 5 | 0 | 5 | |
| N27 | 34.45 | 1985.9 | 20.9 | 15 | 0 | 15 | N148 | 33.31 | 722.6 | 19.5 | 0 | 0 | 0 |
| N27 | 24.93 | 490.38 | 12.1 | 10 | 0 | 10 | N148 | 46.43 | 1302.1 | 41.6 | 5 | 0 | 5 |
| N27 | 39.55 | 823.07 | 28.0 | 5 | 0 | 5 | N148 | 36.8 | 821.99 | 23.9 | 5 | 0 | 5 |
| N27 | 34.5 | 595.65 | 20.9 | 0 | 0 | 0 | N150 | 39.02 | 492.64 | 27.1 | 5 | 0 | 5 |
| N27 | 20 | 9.1 | 30 | 5 | 35 | N150 | 26.4 | 13.1 | 0 | 0 | 0 | ||
| N27 | 55.64 | 70.6 | 15 | 0 | 15 | N150 | 28.2 | 14.6 | 20 | 0 | 20 | ||
| N27 | 16.86 | 7.6 | 0 | 0 | 0 | N150 | 19.95 | 9.1 | 0 | 0 | 0 | ||
| N27 | 40.7 | 29.9 | 20 | 0 | 20 | N151 | 41.16 | 1318.0 | 30.7 | 0 | 0 | 0 | |
| N27 | 40.7 | 267.05 | 29.9 | 15 | 0 | 15 | N151 | 49.86 | 982.98 | 50.6 | 10 | 10 | 20 |
| N28 | 41.53 | 220.39 | 31.4 | 0 | 0 | 0 | N151 | 36.59 | 691.24 | 23.6 | 0 | 0 | 0 |
| N28 | 48.32 | 382.91 | 46.3 | 0 | 0 | 0 | N159 | 29.67 | 539.54 | 15.9 | 5 | 0 | 5 |
| N28 | 40.65 | 1219.6 | 29.8 | 0 | 0 | 0 | N159 | 43.78 | 668.55 | 35.7 | 0 | 0 | 0 |
| N28 | 34.67 | 811.27 | 21.1 | 0 | 0 | 0 | N159 | 38.33 | 492.06 | 26.1 | 0 | 0 | 0 |
| N29 | 36.14 | 23.0 | 0 | 0 | 0 | N159 | 37.72 | 733.66 | 25.2 | 0 | 0 | 0 | |
| N29 | 34.46 | 20.9 | 5 | 0 | 5 | N159 | 18.24 | 169.71 | 8.2 | 0 | 0 | 0 | |
| N30 | 66.32 | 1375.7 | 130.5 | 0 | 0 | 0 | N159 | 32.6 | 765.03 | 18.8 | 0 | 0 | 0 |
| N30 | 53.49 | 1688.0 | 62.4 | 20 | 5 | 25 | N159 | 34.21 | 693.07 | 20.6 | 0 | 0 | 0 |
| N30 | 19.32 | 8.7 | 85 | 0 | 85 | N159 | 46.09 | 1832.4 | 40.8 | 30 | 0 | 30 | |
| N30 | 36.13 | 932.27 | 23.0 | 0 | 0 | 0 | N159 | 37.02 | 49.97 | 24.2 | 0 | 0 | 0 |
| N32 | 15.11 | 6.9 | 50 | 0 | 50 | N159 | 23.59 | 11.2 | 0 | 0 | 0 | ||
| N32 | 55.13 | 1261.8 | 68.6 | 20 | 5 | 25 | N159 | 37.58 | 1325.2 | 25.0 | 10 | 0 | 10 |
| N32 | 50.98 | 1085.5 | 54.0 | 0 | 0 | 0 | N159 | 48.11 | 1326.8 | 45.8 | 5 | 0 | 5 |
| N33 | 25.76 | 12.7 | 0 | 0 | 0 | N160 | 35.54 | 720.5 | 22.2 | 0 | 0 | 0 | |
| N33 | 26.31 | 233.29 | 13.1 | 0 | 0 | 0 | N160 | 43.31 | 495.95 | 34.7 | 0 | 0 | 0 |
| N34 | 32.34 | 528.52 | 18.5 | 50 | 40 | 90 | N160 | 43.42 | 863.23 | 35.0 | 0 | 0 | 0 |
| N34 | 32.04 | 338.25 | 18.2 | 5 | 0 | 5 | N165 | 56.75 | 1796.2 | 75.3 | 5 | 0 | 5 |
| N34 | 55.65 | 1892.9 | 70.6 | 15 | 0 | 15 | N165 | 40.96 | 703.3 | 30.4 | 0 | 0 | 0 |
| N37 | 16.85 | 7.6 | 5 | 0 | 5 | N165 | 35.5 | 22 | 0 | 0 | 0 | ||
| N37 | 18.22 | 8.2 | 0 | 0 | 0 | N171 | 43.93 | 36.0 | 5 | 5 | 10 | ||
| N37 | 14.61 | 6.7 | 0 | 50 | 50 | N180 | 26.42 | 385.42 | 13.2 | 5 | 0 | 5 | |
| N73 | 19.8 | 299.26 | 9.0 | 50 | 0 | 50 | N182 | 31.48 | 599.32 | 17.6 | 5 | 0 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canessa, M.; Bavestrello, G.; Bo, M.; Enrichetti, F.; Trainito, E. Filling a Gap: A Population of Eunicella verrucosa (Pallas, 1766) (Anthozoa, Alcyonacea) in the Tavolara-Punta Coda Cavallo Marine Protected Area (NE Sardinia, Italy). Diversity 2022, 14, 405. https://doi.org/10.3390/d14050405
Canessa M, Bavestrello G, Bo M, Enrichetti F, Trainito E. Filling a Gap: A Population of Eunicella verrucosa (Pallas, 1766) (Anthozoa, Alcyonacea) in the Tavolara-Punta Coda Cavallo Marine Protected Area (NE Sardinia, Italy). Diversity. 2022; 14(5):405. https://doi.org/10.3390/d14050405
Chicago/Turabian StyleCanessa, Martina, Giorgio Bavestrello, Marzia Bo, Francesco Enrichetti, and Egidio Trainito. 2022. "Filling a Gap: A Population of Eunicella verrucosa (Pallas, 1766) (Anthozoa, Alcyonacea) in the Tavolara-Punta Coda Cavallo Marine Protected Area (NE Sardinia, Italy)" Diversity 14, no. 5: 405. https://doi.org/10.3390/d14050405
APA StyleCanessa, M., Bavestrello, G., Bo, M., Enrichetti, F., & Trainito, E. (2022). Filling a Gap: A Population of Eunicella verrucosa (Pallas, 1766) (Anthozoa, Alcyonacea) in the Tavolara-Punta Coda Cavallo Marine Protected Area (NE Sardinia, Italy). Diversity, 14(5), 405. https://doi.org/10.3390/d14050405









