1. Introduction
The date palm,
Phoenix dactylifera L. (Arecales Arecaceae), is a common crop that has been widely cultivated in arid and semiarid regions of the Middle East and North Africa for about 5000 years. In many countries, the fruit of date palm has become a staple food, rich in carbohydrates and other nutrients [
1]. During the last decade of the 20th century, the RPW,
Rhynchophorus ferrugineus Olivier (order: Coleoptera, family: Curculionidae), was reported as an important pest in date palm plantations in Egypt [
2,
3]. This curculionid is considered the most serious pest, and it severely ravages palms of date and coconut in several regions of the world [
4,
5].
R. ferrugineus originated in South-East Asia [
6] and spread to the Near East and North Africa. For the last two decades, RPW has invaded several Middle Eastern countries such as Iran, Iraq, Saudi Arabia, the Emirates, and recently, Egypt. It has been reported to attack 19 palm species belonging to 15 different genera [
7]. Because of its wide distribution,
R. ferrugineus is the most important studied pest of palms in the world [
8].
RPWs are concealed tissue borers which spend all their life stages inside palm trees. The symptoms of infestation include the presence of tunnels made by larvae in the leaf petioles and trunks, the oozing out of viscous liquid (yellow to brown) from the palm tree, the appearance of chewed plant material (frass) in and around openings in the trunk, a fermented odour from liquid in infected tunnels, adults and cocoons present in the leaf axils, empty cocoons seen on the ground, the presence the pellets of frass on the ground around the palm, and toppling of the palm crown or trunk breaking [
9].
However, symptoms of attack during the early stage of an infestation cannot be detected until the crown of the date palm tree has been badly damaged and collapses. Therefore, this weevil poses a threat to the date palm tree and other palms. The integration of morphometric and molecular techniques represents a very important tool in resolving taxonomic uncertainties and the identification and characterization of genetic diversity [
10]. Continuous introduction of new date palm varieties into the region leads to changes in the structure of the insect population. The high distribution capacity of the red palm weevil may be attributed to its strong flight capabilities, and its population is suspected to show diversity [
11]. The study of genetic variability between invasive species is fundamental for their management strategies, including biosecurity. It offers quick and accurate identification of these invasive species and their populations. [
12,
13]. DNA technologies based on DNA molecular markers have significantly contributed to the rapid growth of molecular studies of genetic relatedness, population dynamics of gene and genome mapping, genetic diversity, and phylogeny. [
14]. Previously, the genetic variation of RPW was detected using RAPD markers. These research studies were limited to comparing seven individual RPW from the UAE, individuals from Egypt, Saudi Arabia, and Indonesia, as well as diverse morphological types of RPW from Egypt and Saudi Arabia. [
11,
15,
16,
17]. A long life cycle is characteristic of all three developmental stages of RPW inhabiting palm tree trunks [
18].
The present study dealt, for the first time in Egypt, with characterization of RPW at the ultrastructural level using a scanning microscope (SEM). To support this study, characterization at morphological and molecular levels was achieved by using vernier callipers and RAPD and ISSR markers. It is hoped that the information from this study may aid in a better understanding of the taxonomy of R. ferrugineus, which will be the first step in the development of effective integrated pest management of this weevil.
2. Materials and Methods
2.1. Sampling of RPW Adults
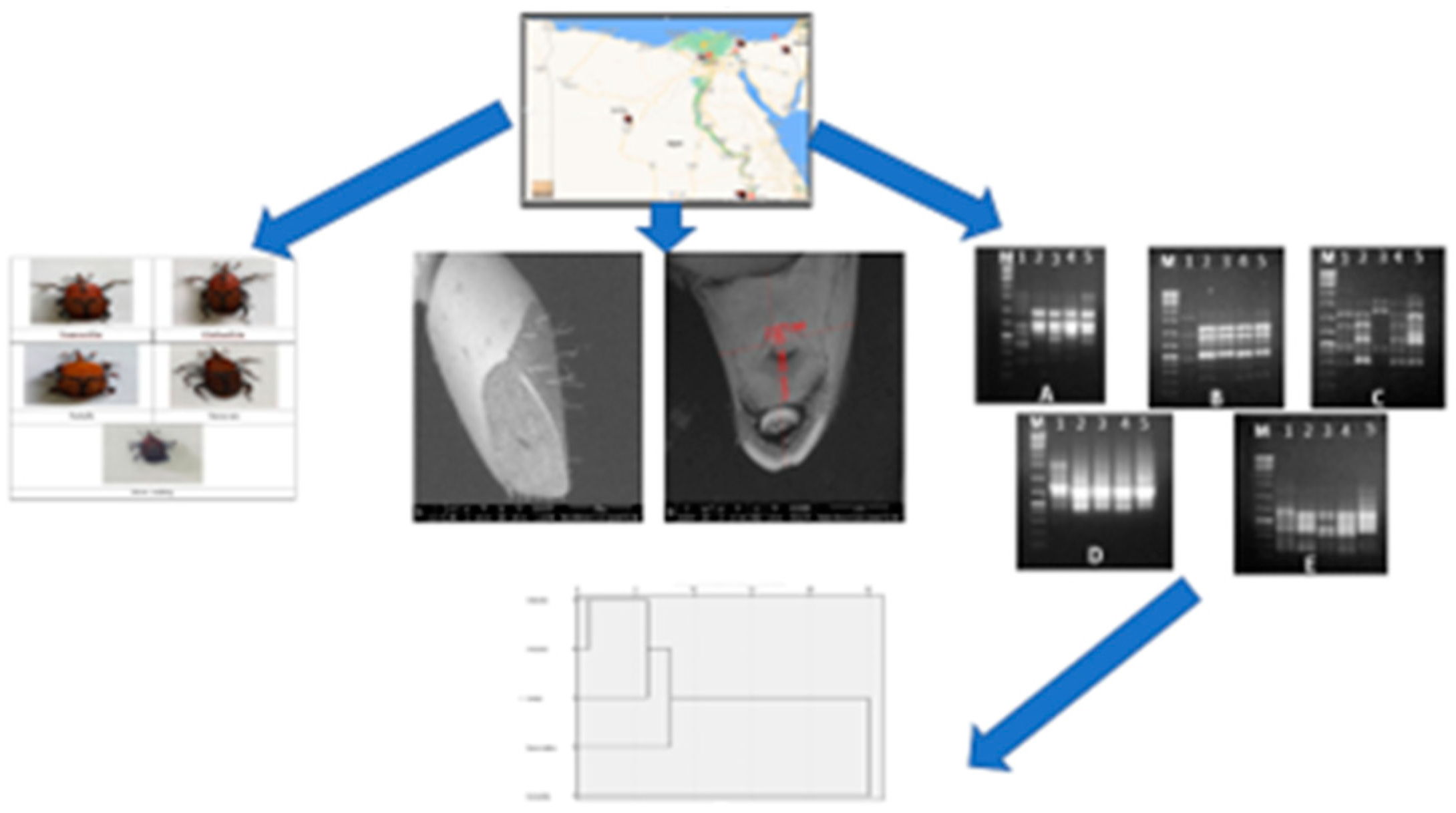
RPW adult samples were taken from heavily infested date palm plantations. Random samples of
R. ferrugineus males and females utilized were originally collected from 5 geographic Egyptian locations, as illustrated in
Figure 1, during July 2019, namely Ismailia, Qalyubia, El Arish, New valley and Aswan.
2.2. Study Area and Site Selection
Ismaeilia samples were collected from Kassassin village of Lower Egypt, 22 miles (35 km) by rail west of Ismailia (31°8′55.716″ N and 30°38′53.376″ E).
The Qalubia sample was collected from Kafr Taha, Shibin Al Qanater, Qalubia, Egypt. Kafr Taha is a small district in Egypt, located in the prefecture of Shibin Al Qanater, Qalyubia region, to the north of Cairo (30°16′8.382″ N and 31°18′23868″ E).
Arish samples were collected from Arish, which is the capital and largest city of the North Sinai Governorate of Egypt (16°58′35.764″ N and 42°50′52.371″ E).
The Aswan sample was collected from Kom Umbo, Aswan Governorate, Egypt (24°5′20.176″ N and 32°53′59.388″ E).
The New Valley sample was collected from Farafra oasis, New Valley Governorate, central Egypt (25°26′51.413″ N and 30°33′18.138″ E).
2.3. Sampling Design
The conditions for selecting palms for sampling, including the meeting of inclusion and exclusion criteria, were as follows:
Three to five feddans of farmland was chosen. The palms were in one place as much as possible, were as similar as possible, and were not more than 20 years old. They were medium-infested palms devoid of ground and high offshoots. The injuries were at a suitable height for sampling. Every member of the population had an equal opportunity to be chosen, and the sampling frame included the whole population. The number of replications for each region was not less than 30 palms. We numbered the palm trees from which the replicates were taken. A portable wood saw was utilized to facilitate collecting weevils from heavily infested palm trees. Adults were placed into individual plastic boxes with press-on tight-fitting lids (30 × 20 × 15 cm).
2.4. Dealing with Collected Adults
The insects were cultured in the Insect Research Laboratory at the Plant Protection Department, Faculty of Agricultural at Moshtohor, Benha University. The insects were maintained at 25 ± 2 °C, 60–70% RH, and 12:12 LD. Adults were supplied with fresh sugarcane stems for feeding. Adults were sampled from palm trees growing in Qalyubia governorate. These adults were sexed and examined by the naked eye and then using a magnification lens were certified that all the weevils/sample are of the same species, Rhynchophorus ferrugineus. Every couple of adults was kept, separately, in small jars (5 × 10 cm) to begin observational measurements of morphological characters and electron microscopy inspections. Sexing of adult weevils was determined based on the absence of a series of black hairs on the dorsal–frontal part of the snouts of females, and their presence in the males.
2.5. Identification of Male and Female of Red Palm Weevil
The differentiation between males and females was carried out using the taxonomic work of [
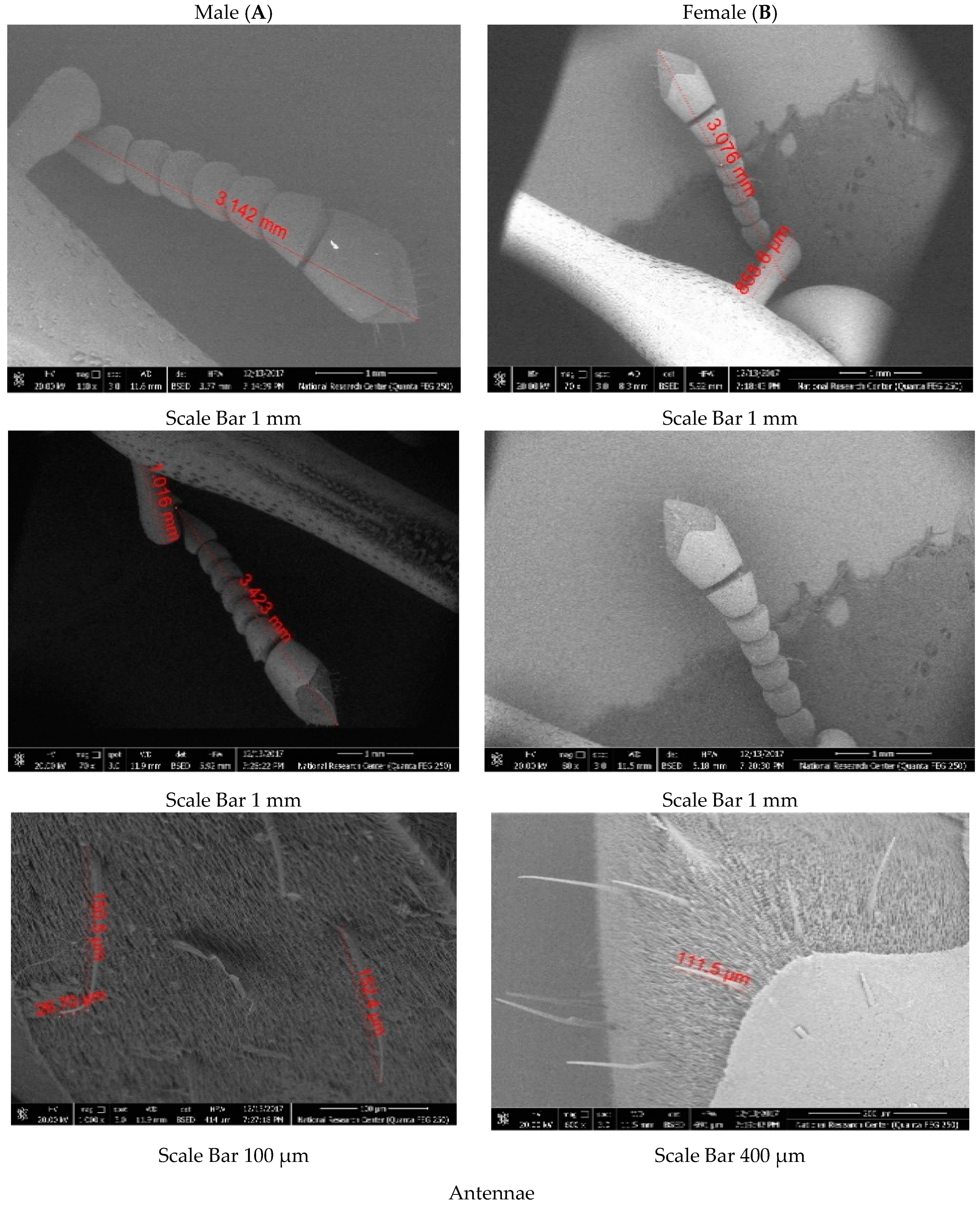
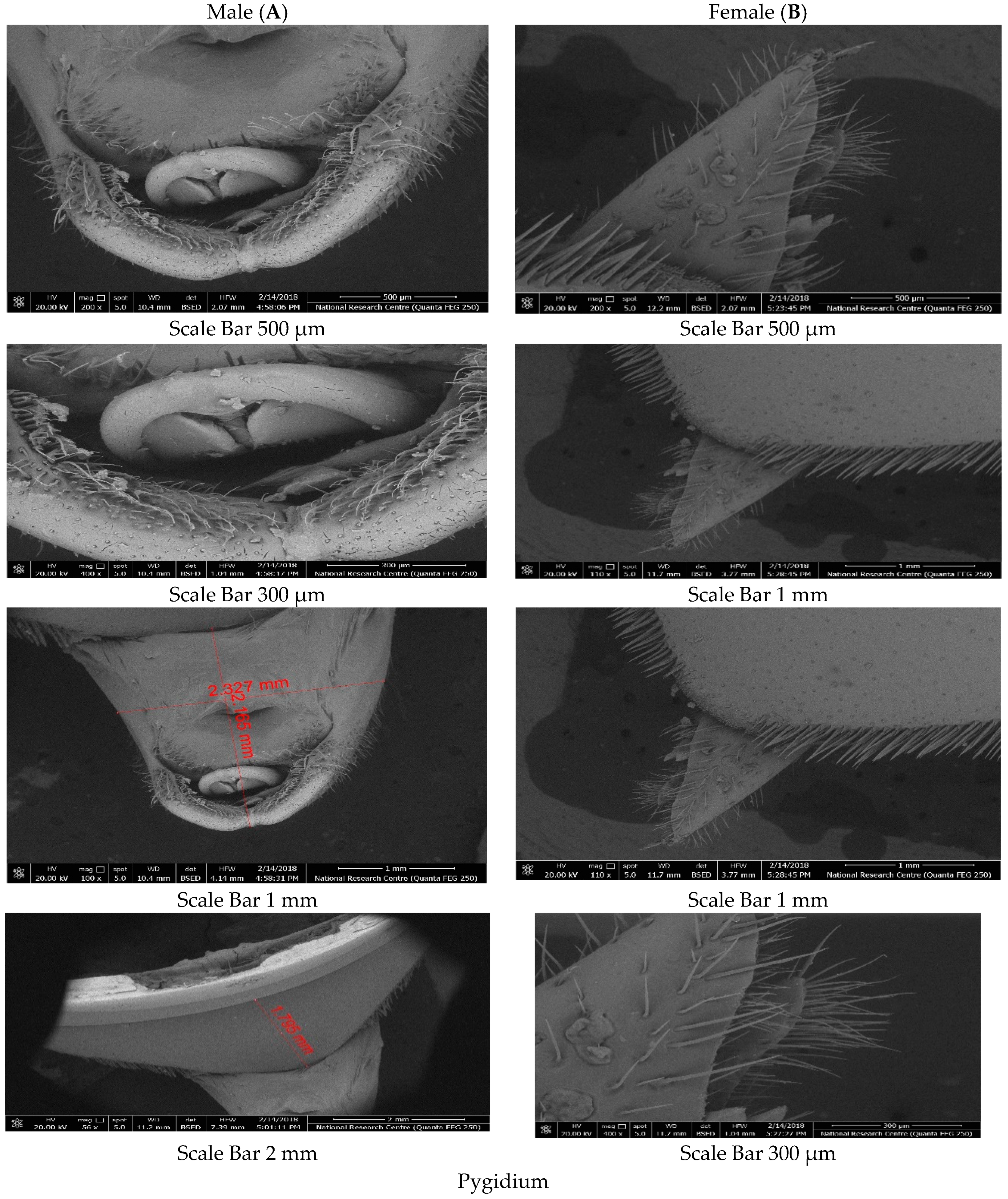
18]. This involved observing the tuft of brown hairs on the half rostrum that are present in males and absent in females. The adult weevils collected were utilized for measuring various parts of the body using a binocular stereomicroscope. Characterization was carried out in both male and female representatives of the population to identify differences at morphometric and ultrastructural levels. These differences will help differentiate between males and females when tracked.
2.6. Morphological Variation in Adult Red Palm Weevil
The morphological variables measured were the length of body (L) and abdomen (AL), width of abdomen (AW), pronotum length (PL), pronotum width (PW), Rostrum length (RL), size of head (HS), length of the leg (foreleg), and length from tip of rostrum to antennal insertion (TA), according to [
19].
Male and female RPW were collected and prepared to ensure that they were free from dust or palm fibres to avoid debris accumulation on the specimens. Head, body and legs of males and females were decapitated with fine scissors, and immediately placed in tubes filled with glutaraldehyde (4%) for fixation. After fixation, the samples were dehydrated in increasing alcohol baths of 50%, 70%, 80%, 95% and 100% (twice for 5 min) for further clearing and fixation. Finally, before observation, the samples were rinsed once, dried, fixed in the cylindrical stub and then spurred-coated with a thin golden layer using an apparatus (S150A SPUTTER COATER, Crawley, UK) for 1 min. An electron microscope (QUANTA FEG 250, Zaragoza, Spain) located at the Scientific Imaging Unit, National Research Centre, Cairo, Egypt, was used to observe the specimens. SEM photomicrographs were taken from different locations of each morphological part for each RPW male and female.
2.7. DNA Extraction
Total genomic DNA was extracted from red palm weevil using genomic prep cells and a tissue DNA isolation kit (QIAgene DNeasy Blood and Tissue Kit, QIAGEN Inc. — Canada, Montréal) according to manufacturer’s instructions. The integrity of extracted DNA was detected via 1% agarose gel electrophoresis. The concentration of the isolated DNA was measured with a spectrophotometer at 260 nm. The purity of DNA was checked using a spectrophotometer with a ratio of 260/280 nm absorbance.
2.8. RAPD and ISSR Analysis
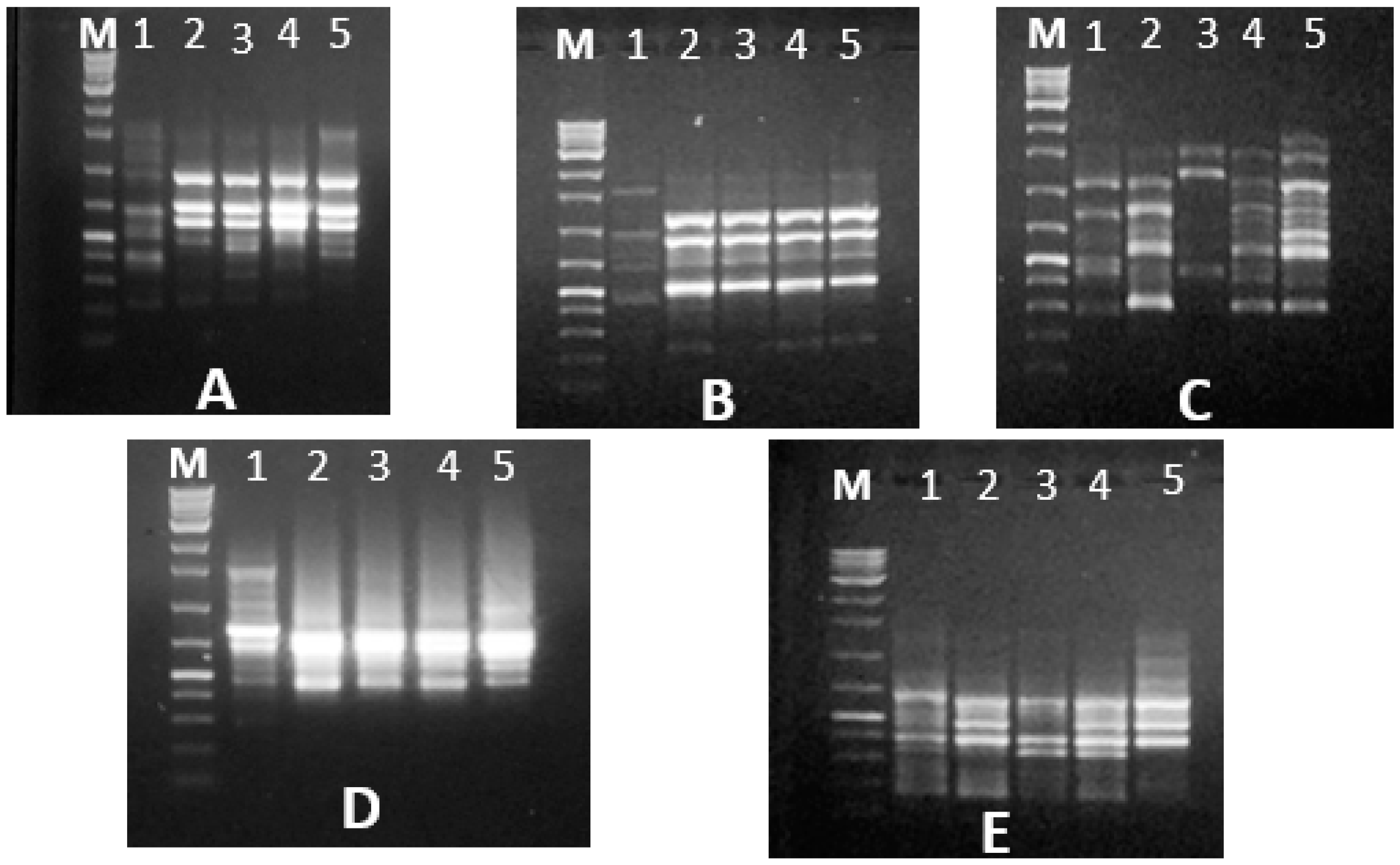
PCRs were performed using 2× superhot PCR Master Mix (Bioron; Germany) with 10 Pmol of each 5 RAPD and five ISSR primers. The names and sequences of the used primers are illustrated in results section. RAPD reactions were carried out in a Biometra’s T-personal Thermal Cycler using the following PCR program: 1 cycle at 94 °C, 4 min; 35 cycles of 94 °C for 5 s, 37 °C for 20 s, 72 °C for 20 s and finally 72 °C for 10 min. The PCR program for ISSR analysis was as follows: Initial denaturation at 94 °C for 2 min, followed by 35 cycles consisting of denaturation at 94 °C for 30 s, annealing at 55 °C for 30 s, extension at 72 °C for 2 min and a final extension at 72 °C for 2 min. After amplification, the PCR-amplified products were migrated on agarose gel electrophoresis against GeneRuler 100 bp and 100 bp plus DNA ladder marker (Thermo Scientific, Horsham, UK) for the ISSR and RAPD PCR-amplified products, respectively, to determine the bands’ molecular sizes. The electrophoresis process was carried out though 10 × 14 cm 1.5%-agarose gel (Burlington, Ontario, Canada) for 25 min using TAE (Tris-acetate-EDTA, 50×) buffer. The gels were stained with 0.5 ug/mL of ethidium bromide (Bioshop, Burlington, Canada), visualized under UV light and documented using a GeneSnap 4.00—Gene Genius Bio Imaging System (Syngene; Frederick, MD, USA).
2.9. Data Analysis
The statistical analysis was carried out using one-way ANOVA using SPSS, ver. 25 (IBM Corp. Released 2013). Data were treated as a complete randomization design according to [
20]. Multiple comparisons were carried out applying Tukey’s test. The significance level was set at <0.05 [
20].
RAPD and ISSR amplified fragments were scored as (1) for band presence and (0) for band absence, and a binary qualitative data matrix was constructed. Data analyses were performed using the NTSYS PC version 2.02 computer package program [
21]. The similarity values were used to generate a dendrogram via UPGMA (Unweighted Pair Group Method) with arithmetic average (UPGMA).
4. Discussion
The most devastating pest of farmed palms, particularly date palm plants, is the red palm weevil (R. ferrugineus). It was first discovered in the early twentieth century in Asia’s southern and south-eastern regions. It then expanded throughout the Middle East, Europe, and North Africa. At the end of the 20th century, it was reported in Australia. It was discovered in 2010 in western North America.
There was no sexual dimorphism found in RPW in this study. In both sexes, all pronotal markings were observed. An earlier study of RPW markings has shown that there are 12 colour morphs recognized in KSA [
22]. A non-significant difference was found between mean length and width of the body of adults between different geographic locations. In similar studies [
23], it has been reported that adult RPW are large—30–40 mm long. They are characterized by a high degree of colour polymorphism. Currently, there are two colour morphs of RPW that are recognized as a single species of
R. ferrugineus: (1) a black with a red stripe ≈ “vulneratus” colour morph and (2) orange with black markings ≈ “
ferrugineus” colour morph.
Limited information regarding a morphometric description of this weevil is available. In this respect, it has been reported [
24] that adults of RPW from Meghalaya, India measured 33.2–34.05 mm (mean 33.62 mm) in length and 11.9–13.1 mm (mean 12.50 mm) in width [
24].
Based on a previous study [
25], the body measurements of
R.ferrugineus adult males ranged from 19.0 to 42.0 mm in length and from 8 to 16 mm in width, whereas those of female adults were 29.00–40.0 and 10–16 mm, respectively. other studies [
16,
25] showed that the mean length of adult males ranged 29.0–44.0 mm, with 11.50–18.00 mm in width, whereas adult female length measured 26.00–42.00 mm, with width of 11.0–17.0 mm.
Distinguishing
R. ferrugineus from other species can be easy when using dorsal characteristics of the pronotum [
26]. The pronotum is characterized by its black colour, and it is opaque, velvety to shining, longer than it is wide, flat, narrowed towards the apex, and constricted anterolaterally. Its base is generated posteriorly, covered with brown setae under the posterior border, bisinuate on both sides, with a fine elevated margin, and it is finely and diffusely pierced, with indications of a median longitudinal carina [
27]. Other morphological characters are required to separate between males and females.
Data obtained from [
27] suggest that the rostral setae consist of a group of bristles, which function as chemoreceptors and contain neuron sensors which are important for mating, and possibly for response to odour stimuli. However, the authors speculated that wax or fluid, which may be hydrocarbon based, is emitted from the wall markings, acting as a lubricant for the rostrum to grind the soft tissue of palms.
The most trustworthy characteristics were discussed in [
19]. These include a combination of traits, including the pronotum, dorsal, lateral, and ventral aspects of the head.
Different measurements of RPW body parts taken in this study, including total body length, rostrum length, and rostrum length from apex to antennal insertion, could be utilized to effectively discriminate between male and female RPW.
The results from [
27] reported that the flagellum comprises six segments (
Figure 6), being reddish-brown in colour and broadly triangular with several dorsal and ventral setae (antennal sensilla), especially at the apex.
The RAPD and ISSR markers were successfully used in genetic fingerprinting and characterization of red palm weevil populations and for detecting population diversity. Among DNA markers, ISSR and RAPD require small quantities of DNA and do not require radioactive labels; they are simple and fast. These markers are reproducible, powerful, simple, quick, and relatively cheap for use in estimating genetic variation and identifying differences between populations [
11,
13,
28]. In the present study, both RAPD and ISSR markers generated reproducible and distinct banding patterns. The polymorphic banding patterns resulting from using RAPD and ISSR primers confirmed that these markers, based on DNA from five
R. ferrugineus individuals from each population, revealed genetic variability between these Egyptian populations, thus confirming the extreme heterogeneity within them [
29].
These findings revealed a random pattern of amplification as well as the heterogeneity and polymorphism of the RPW population, implying that Egypt has many unique genotypes. In the present study, RAPD and ISSR techniques generated variable numbers and sizes of amplified bands. These results could be due to size of amplified fragments related to primer sequences annealed with the DNA template [
30]. Insertions and/or deletions could alter the size of the amplified product [
31].
The number of amplified bands is varied due to primer structure, where there is variation in the primer annealing sites. Some primers identify a large number of annealing sites, which are more beneficial than primers that recognize a smaller number. As a consequence, the number of amplified bands will be higher, which provides us with a better opportunity to detect DNA polymorphisms between individuals [
32,
33,
34].
In total, 47 various and specific positive RAPD and ISSR markers were observed amongst the studied R. ferrugineus populations. The markers used in this study were highly effective in distinguishing between the various Egyptian populations.
The highest number of ISSR markers was generated using the HB15 and OPA 13 primers, which could be used for early detection and molecular diagnosis of
R. ferrugineus infestation [
35].
The specific markers obtained in the present study could be used for rapid R. ferrugineus characterization, identification, and accurate tracking.
The highest genetic distance was observed between Ismaeilia and New Valley populations (82%), while the lowest genetic distance and thus closest relationship was between the Qalubia and Aswan populations (39%). This low genetic distance suggests they are closely related and have a recent common ancestor. Conversely, the large genetic distance between New valley and Isamaeila suggests that the two regions have a fairly distant relation. Two factors may explain the genetic structure of populations identified in the represented study. First, although adult RPW are capable of long flights, they usually stay under the base of the frond petioles during the day [
36]. Although they usually prefer dying or damaged palms, undamaged ones also can be attacked. They move short distances throughout their adult flight [
37]. Second, the palms trads from native to new areas caused irregular dispersal of RPW, probably as immature stages bored into palm trees.