Black Mantle Tissue of Endolithic Mussels (Leiosolenus spp.) Is Cloaking Borehole Orifices in Caribbean Reef Corals
Abstract
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jones, B.; Pemberton, S.G. Lithophaga borings and their influence on the diagenesis of corals in the Pleistocene Ironshore Formation of Grand Cayman Island, British West Indies. Palaios 1988, 3, 3–21. [Google Scholar] [CrossRef]
- Owada, M. Functional morphology and phylogeny of the rock-boring bivalves Leiosolenus and Lithophaga (Bivalvia: Mytilidae): A third functional clade. Mar. Biol. 2007, 150, 853–860. [Google Scholar] [CrossRef]
- Scaps, P.; Denis, V. Can organisms associated with live scleractinian corals be used as indicators of coral reef status? Atoll Res. Bull. 2008, 566, 1–18. [Google Scholar] [CrossRef][Green Version]
- Sorauf, J.E.; Harries, P.J. Rotatory colonies of the corals Siderastrea radians and Solenastraea ssp. (Cnidaria, Scleractinia), from the Pleistocene Bermont formation, south Florida, USA. Palaeontology 2009, 52, 111–126. [Google Scholar] [CrossRef]
- Kázmér, M.; Taborosi, D. Bioerosion on the small scale–examples from the tropical and subtropical littoral. Hantkeniana 2012, 7, 37–94. [Google Scholar]
- Bagur, M.; Richardson, C.A.; Gutiérrez, J.L.; Arribas, L.P.; Doldan, M.S.; Palomo, M.G. Age, growth and mortality in four populations of the boring bivalve Lithophaga patagonica from Argentina. J. Sea Res. 2013, 81, 49–56. [Google Scholar] [CrossRef]
- Glynn, P.W.; Manzello, D.P. Bioerosion and coral reef growth: A dynamic balance. In Coral Reefs in the Anthropocene; Birkeland, C., Ed.; Springer: Dordrecht, The Netherlands, 2015; pp. 67–97. [Google Scholar] [CrossRef]
- Ricci, S.; Perasso, C.S.; Antonelli, F.; Petriaggi, B.D. Marine bivalves colonizing Roman artefacts recovered in the Gulf of Pozzuoli and in the Blue Grotto in Capri (Naples, Italy): Boring and nestling species. Int. Biodeter. Biodegrad. 2015, 98, 89–100. [Google Scholar] [CrossRef]
- Wizemann, A.; Nandini, S.D.; Stuhldreier, I.; Sánchez-Noguera, C.; Wisshak, M.; Westphal, H.; Rixen, T.; Christian, W.; Reymond, C.E. Rapid bioerosion in a tropical upwelling coral reef. PLoS ONE 2018, 13, e0202887. [Google Scholar] [CrossRef]
- MolluscaBase eds. MolluscaBase. Lithophaginae H. Adams & A. Adams, 1857. World Register of Marine Species. 2022. Available online: https://www.marinespecies.org/aphia.php?p=taxdetails&id=510723 (accessed on 20 April 2022).
- Zann, L.P. Living Together in the Sea; T.F.H. Publications: Neptune, NY, USA, 1980; p. 416. [Google Scholar]
- Highsmith, R.C. Coral bioerosion: Damage relative to skeletal density. Am. Nat. 1981, 117, 193–198. [Google Scholar] [CrossRef]
- Hutchings, P.A. Biological destruction of coral reefs. Coral Reefs 1986, 4, 239–252. [Google Scholar] [CrossRef]
- Scott, P.J.B.; Risk, M.J. The effect of Lithophaga (Bivalvia: Mytilidae) boreholes on the strength of the coral Porites lobata. Coral Reefs 1988, 7, 145–151. [Google Scholar] [CrossRef]
- Rice, M.M.; Maher, R.L.; Correa, A.M.S.; Moeller, H.V.; Lemoine, N.P.; Shantz, A.A.; Burkepile, D.E.; Silbiger, N.J. Macroborer presence on corals increases with nutrient input and promotes parrotfish bioerosion. Coral Reefs 2020, 39, 409–418. [Google Scholar] [CrossRef]
- Wong, K.T.; Tsang, R.H.L.; Ang, P. Did borers make corals more susceptible to a catastrophic disease outbreak in Hong Kong? Mar. Biodivers. 2016, 46, 325–326. [Google Scholar] [CrossRef]
- Yonge, C.M. Adaptation to rock boring in Botula and Lithophaga (Lamellibranchia, Mytilidae) with a discussion on the evolution of this habit. J. Cell Sci. 1955, 3, 383–410. [Google Scholar] [CrossRef]
- Audino, J.A.; Serb, J.M.; Marian, J.E.A.R. Phylogeny and anatomy of marine mussels (Bivalvia: Mytilidae) reveal convergent evolution of siphon traits. Zool. J. Linn. Soc. 2020, 190, 592–612. [Google Scholar] [CrossRef]
- Soliman, G.N. Ecological aspects of some coral-boring gastropods and bivalves of the northwestern Red Sea. Am. Zool. 1969, 9, 887–894. [Google Scholar] [CrossRef]
- Simone, L.R.L.; Gonçalves, E.P. Anatomical study on Myoforceps aristatus, an invasive boring bivalve in S.E. Brazilian coast (Mytilidae). Pap. Avulsos Zool. 2006, 46, 57–65. [Google Scholar] [CrossRef]
- Kleemann, K.H. Lithophaga (Bivalvia) from dead coral from the Great Barrier Reef, Australia. J. Molluscan Stud. 1984, 50, 192–230. [Google Scholar] [CrossRef]
- Kleemann, K. Boring and growth in chemically boring bivalves from the Caribbean, Eastern Pacific and Australia’s Great Barrier Reef. Senckenberg. Marit. 1990, 22, 101–154. [Google Scholar]
- Gohar, H.A.F.; Soliman, G.N. On three mytilid species boring in living corals. Publ. Mar. Biol. Sta. Al-Ghardaqa 1963, 12, 65–98. [Google Scholar]
- Zottoli, R.A.; Carriker, M.R. Burrow morphology, tube formation, and microarchitecture of shell dissolution by the spionid polychaete Polydora websteri. Mar. Biol. 1974, 27, 307–316. [Google Scholar] [CrossRef]
- Hoeksema, B.W. Excavation patterns and spiculae dimensions of the boring sponge Cliona celata from the SW Netherlands. Senckenb. Marit. 1983, 15, 55–85. [Google Scholar]
- Liu, P.J.; Hsieh, H.L. Burrow architecture of the spionid polychaete Polydora villosa in the corals Montipora and Porites. Zool. Stud. 2000, 39, 47–54. [Google Scholar]
- Buschbaum, C.; Buschbaum, G.; Schrey, I.; Thieltges, D.W. Shell-boring polychaetes affect gastropod shell strength and crab predation. Mar. Ecol. Prog. Ser. 2007, 329, 123–130. [Google Scholar] [CrossRef]
- Pulido Mantas, T.; Pola, L.; Cerrano, C.; Gambi, M.C.; Calcinai, B. Bioerosion features of boring polydorid polychaetes in the North Adriatic Sea. Hydrobiologia 2022, 849, 1969–1980. [Google Scholar] [CrossRef]
- de Bakker, D.M.; Webb, A.E.; van den Bogaart, L.A.; van Heuven, S.M.A.C.; Meesters, E.H.; van Duyl, F.C. Quantification of chemical and mechanical bioerosion rates of six Caribbean excavating sponge species found on the coral reefs of Curaçao. PLoS ONE 2018, 13, e0197824. [Google Scholar] [CrossRef]
- Stearley, R.F.; Ekdale, A.A. Modern marine bioerosion by macroinvertebrates, northern Gulf of California. Palaios 1989, 4, 453–467. [Google Scholar] [CrossRef]
- Highsmith, R.C. Burrowing by the bivalve mollusc Lithophaga curta in the living reef coral Montipora berryi and a hypothesis of reciprocal larval recruitment. Mar. Biol. 1980, 56, 155–162. [Google Scholar] [CrossRef]
- Kleemann, K.H. Boring bivalves and their host corals from the Great Barrier Reef. J. Molluscan Stud. 1980, 46, 13–54. [Google Scholar] [CrossRef]
- Nielsen, C. Notes on boring bivalves from Phuket, Thailand. Ophelia 1976, 15, 141–148. [Google Scholar] [CrossRef]
- Cantera, J.R.; Contreras, R. Bivalvos perforadores de esqueletos de corales escleractiniarios en la Isla de Gorgona, Pacífico Colombiano. Rev. Biol. Trop. 1988, 36, 151–158. [Google Scholar]
- Nogueira, M.M.; Menezes, N.M.; Johnsson, R.; Neves, E. The adverse effects of cryptochirid crabs (Decapoda: Brachyura) on Siderastrea stellata Verril, 1868 (Anthozoa: Scleractinia): Causes and consequences of cavity establishment. Cah. Biol. Mar. 2014, 55, 155–162. [Google Scholar] [CrossRef]
- Hoeksema, B.W.; van der Meij, S.E.T. Gall crab city: An aggregation of endosymbiotic crabs inhabiting a colossal colony of Pavona clavus. Coral Reefs 2013, 32, 59. [Google Scholar] [CrossRef]
- García-Hernández, J.E.; de Gier, W.; van Moorsel, G.W.N.M.; Hoeksema, B.W. The scleractinian Agaricia undata as a new host for the coral-gall crab Opecarcinus hypostegus at Bonaire, southern Caribbean. Symbiosis 2020, 81, 303–311. [Google Scholar] [CrossRef]
- Lymperaki, M.M.; Hill, C.E.; Hoeksema, B.W. The effects of wave exposure and host cover on coral-associated fauna of a centuries-old artificial reef in the Caribbean. Ecol. Eng. 2022, 176, 106536. [Google Scholar] [CrossRef]
- Hoeksema, B.W.; Harper, C.E.; Langdon-Down, S.J.; van der Schoot, R.J.; Smith-Moorhouse, A.; Spaargaren, R.; Timmerman, R.F. Host range of the coral-associated worm snail Petaloconchus sp. (Gastropoda: Vermetidae), a newly discovered cryptogenic pest species in the southern Caribbean. Diversity 2022, 14, 196. [Google Scholar] [CrossRef]
- Hoeksema, B.W.; Timmerman, R.F.; Spaargaren, R.; Smith-Moorhouse, A.; van der Schoot, R.J.; Langdon-Down, S.J.; Harper, C. Morphological modifications and injuries of corals caused by feather duster worms (Sabellidae: Anamobaea sp.) in the Caribbean. Diversity 2022, 14, 332. [Google Scholar] [CrossRef]
- Fang, L.-S.; Shen, P. A living mechanical file: The burrowing mechanism of the coral-boring bivalve Lithophaga nigra. Mar. Biol. 1988, 97, 349–354. [Google Scholar] [CrossRef]
- Yahel, G.; Marie, D.; Beninger, P.G.; Eckstein, S.; Genin, A. In situ evidence for pre-capture qualitative selection in the tropical bivalve Lithophaga simplex. Aquat. Biol. 2009, 6, 235–246. [Google Scholar] [CrossRef]
- Chan, B.K.K.; Tan, J.C.H.; Ganmanee, M. Living in a growing host: Growth pattern and dwelling formation of the scallop Pedum spondyloideum in massive Porites spp. corals. Mar. Biol. 2020, 167, 95. [Google Scholar] [CrossRef]
- Scaps, P. Association between the scallop Pedum spondyloideum (Bivalvia: Pteriomorphia: Pectinidae) and scleractinian coralsfrom Nosy Be, Madagascar. Cah. Biol. Mar. 2020, 61, 73–80. [Google Scholar] [CrossRef]
- Scott, P.J.B. Aspects of living coral associates in Jamaica. In Proceedings of the 5th International Coral Reef Congress, Tahiti, France, 27 May–1 June 1985; Volume 5, pp. 345–350. [Google Scholar]
- Scott, P.J.B. A new species of Lithophaga (Bivalvia: Lithophaginae) boring corals in the Caribbean. J. Molluscan Stud. 1986, 52, 55–61. [Google Scholar] [CrossRef]
- Scott, P.J.B. Associations between corals and macro-infaunal invertebrates in Jamaica, with a list of Caribbean and Atlantic coral associates. Bull. Mar. Sci. 1987, 40, 271–286. [Google Scholar]
- Scott, P.J.B. Distribution, habitat and morphology of the Caribbean coral and rock-boring bivalve, Lithophaga bisulcata (d’Orbigny) (Mytilidae: Lithophaginae). J. Molluscan Stud. 1988, 5, 83–95. [Google Scholar] [CrossRef]
- Scott, P.J.B. Initial settlement behaviour and survivorship of Lithophaga bisulcata (d’Orbigny) (Mytilidae: Lithophaginae). J. Molluscan Stud. 1988, 54, 97–108. [Google Scholar] [CrossRef]
- Vinagre, C.; Silva, R.; Mendonça, V.; Flores, A.A.V.; Baeta, A.; Marques, J.C. Food web organization following the invasion of habitat-modifying Tubastraea spp. corals appears to favour the invasive borer bivalve Leiosolenus aristatus. Ecol. Ind. 2018, 85, 1204–1209. [Google Scholar] [CrossRef]
- Valentich-Scott, P.; Dinesen, G.E. Rock and coral boring Bivalvia (Mollusca) of the middle Florida Keys, USA. Malacologia 2004, 46, 339–354. [Google Scholar]
- Bromley, R.G. Biocrosion of Bermuda reefs. Paleogeogr. Paleoclimatol. Paleoecol. 1978, 23, 169–197. [Google Scholar] [CrossRef]
- McCloskey, L.R. The dynamics of the community associated with a marine scleractinian coral. Int. Rev. Gesamt. Hydrobiol. 1970, 55, 13–81. [Google Scholar] [CrossRef]
- Oigman-Pszczol, S.S.; Creed, J.C. Distribution and abundance of fauna on living tissues of two Brazilian hermatypic corals (Mussismilia hispida (Verril, 1902) and Siderastrea stellata Verril, 1868). Hydrobiologia 2006, 563, 143–154. [Google Scholar] [CrossRef]
- Blanchon, P.; Perry, C.T. Taphonomic differentiation of Acropora palmata facies in cores from Campeche Bank reefs, Gulf of México. Sedimentology 2004, 51, 53–76. [Google Scholar] [CrossRef]
- Donovan, S.K.; Hensley, C. Gastrochaenolites Leymerie in the Cenozoic of the Antillean region (review). Ichnos 2006, 13, 11–19. [Google Scholar] [CrossRef]
- Kleemann, K.H. Gastrochaenolites hospitium sp. nov., trace fossil by a coral-associated boring bivalve from the Eocene and Miocene of Austria. Geol. Carpath. 2009, 60, 339–342. [Google Scholar] [CrossRef]
- Wisshak, M.; Knaust, D.; Bertling, M. Bioerosion ichnotaxa: Review and annotated list. Facies 2019, 65, 24. [Google Scholar] [CrossRef]
- Bassi, D.; Braga, J.C.; Owada, M.; Aguirre, J.; Lipps, J.H.; Takayanagi, H.; Iryu, Y. Boring bivalve traces in modern reef and deeper-water macroid and rhodolith beds. Prog. Earth Planet. Sci. 2020, 7, 41. [Google Scholar] [CrossRef]
- Mokady, O.; Bonar, D.B.; Arazi, G.; Loya, Y. Coral host specificity in settlement and metamorphosis of the date mussel Lithophaga lessepsiana (Vaillant, 1865). J. Exp. Mar. Biol. Ecol. 1991, 146, 205–216. [Google Scholar] [CrossRef]
- Mokady, O.; Arazi, G.; Bonar, D.B.; Loya, Y. Settlement and metamorphosis specificity of Lithophaga simplex Iredale (Bivalvia: Mytilidae) on Red Sea corals. J. Exp. Mar. Biol. Ecol. 1992, 162, 243–251. [Google Scholar] [CrossRef]
- Liu, J.C.W.; Hoeg, J.T.; Chan, B.K.K. How do coral barnacles start their life in their hosts? Biol. Lett. 2016, 12, 20160124. [Google Scholar] [CrossRef]
- Dreyer, N.; Tsai, P.-C.; Olesen, J.; Kolbasov, G.A.; Høeg, J.T.; Chan, B.K.K. Independent and adaptive evolution of phenotypic novelties driven by coral symbiosis in barnacle larvae. Evolution 2022, 76, 139–157. [Google Scholar] [CrossRef]
- Marsden, J.R. Coral preference behaviour by planktotrophic larvae of Spirobranchus giganteus corniculatus (Serpulidae: Polychaeta). Coral Reefs 1987, 6, 71–74. [Google Scholar] [CrossRef]
- Marsden, J.R.; Conlin, B.E.; Hunte, W. Habitat selection in the tropical polychaete Spirobranchus giganteus. Mar. Biol. 1990, 104, 93–99. [Google Scholar] [CrossRef]
- Moretzsohn, F.; Tsuchyia, M. Preliminary survey of the coral boring Bivalvia fauna of Okinawa, southern Japan. In Proceedings of the 7th International Coral Reef Symposium, Guam, 22–26 June 1992; Volume 1, pp. 404–412. [Google Scholar]
- Mokady, O.; Rozenblatt, S.; Graur, D.; Loya, Y. Coral-host specificity of Red Sea Lithophaga bivalves: Interspecific and intraspecific variation in 12S mitochondrial. Mol. Mar. Biol. Biotechnol. 1994, 3, 158–164. [Google Scholar] [PubMed]
- Kleemann, K.H. Association of coral and boring bivalves: Lizard Island (Great Barrier Reef, Australia) versus Safaga (N Red Sea). Beitr. Paläontol. 1995, 20, 31–39. [Google Scholar]
- Kleemann, K.; Hoeksema, B.W. Lithophaga (Bivalvia: Mytilidae), including a new species, boring in mushroom corals (Scleractinia: Fungiidae) at South Sulawesi, Indonesia. Basteria 2002, 66, 11–24. [Google Scholar]
- Hoeksema, B.W.; Kleemann, K. New records of Fungiacava eilatensis Goreau et al., 1968 (Bivalvia: Mytilidae) boring in Indonesian mushroom corals (Scleractinia: Fungiidae). Basteria 2002, 66, 25–30. [Google Scholar]
- Mohammed, T.A.; Yassien, M.H. Bivalve assemblages on living coral species in the Northern Red Sea, Egypt. J. Shellfish Res. 2008, 27, 1217–1223. [Google Scholar] [CrossRef]
- Owada, M. The first record of Leiosolenus simplex (Iredale, 1939) (Bivalvia: Mytilidae) Boring into Plesiastrea versipora from Minamata Bay in Japan. Venus 2008, 67, 81–84. [Google Scholar]
- Owada, M.; Hoeksema, B.W. Molecular phylogeny and shell microstructure of Fungiacava eilatensis Goreau et al. 1968, boring into mushroom corals (Scleractinia: Fungiidae), in relation to other mussels (Bivalvia: Mytilidae). Contrib. Zool. 2011, 80, 169–178. [Google Scholar] [CrossRef][Green Version]
- Kleemann, K.; Maestrati, P. Pacific Lithophaga (Bivalvia, Mytilidae) from recent French expeditions with the description of two new species. Boll. Malacol. 2012, 48, 73–102. [Google Scholar]
- Printrakoon, C.; Yeemin, T.; Valentich-Scott, P. Ecology of endolithic bivalve mollusks from Ko Chang, Thailand. Zool. Stud. 2016, 55, 50. [Google Scholar] [CrossRef]
- Liu, J.; Liu, H.; Zhang, H. Phylogeny and evolutionary radiation of the marine mussels (Bivalvia: Mytilidae) based on mitochondrial and nuclear genes. Mol. Phylogenet. Evol. 2018, 126, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Stella, J.S.; Pratchett, M.S.; Hutchings, P.A.; Jones, G.P. Coral-associated invertebrates: Diversity, ecology importance and vulnerability to disturbance. Oceanogr. Mar. Biol. Ann. Rev. 2011, 49, 43–104. [Google Scholar]
- Hoeksema, B.W.; van der Meij, S.E.T.; Fransen, C.H.J.M. The mushroom coral as a habitat. J. Mar. Biol. Assoc. 2012, 92, 647–663. [Google Scholar] [CrossRef]
- Hoeksema, B.W. The hidden biodiversity of tropical coral reefs. Biodiversity 2017, 18, 8–12. [Google Scholar] [CrossRef]
- Hoeksema, B.W.; van Beusekom, M.; ten Hove, H.A.; Ivanenko, V.N.; van der Meij, S.E.T.; van Moorsel, G.W.N.M. Helioseris cucullata as a host coral at St. Eustatius. Dutch Caribbean. Mar. Biodivers. 2017, 47, 71–78. [Google Scholar] [CrossRef]
- Montano, S. The extraordinary importance of coral-associated fauna. Diversity 2020, 12, 357. [Google Scholar] [CrossRef]
- Ng, P.K.L.; Meyer, C. A new species of pea crab of the genus Serenotheres Ahyong & Ng, 2005 (Crustacea, Brachyura, Pinnotheridae) from the date mussel Leiosolenus Carpenter, 1857 (Mollusca, Bivalvia, Mytilidae, Lithophaginae) from the Solomon Islands. ZooKeys 2016, 623, 31–41. [Google Scholar] [CrossRef][Green Version]
- de Gier, W.; Becker, C. A Review of the ecomorphology of pinnotherine pea crabs (Brachyura: Pinnotheridae), with an updated list of symbiont-host associations. Diversity 2020, 12, 431. [Google Scholar] [CrossRef]
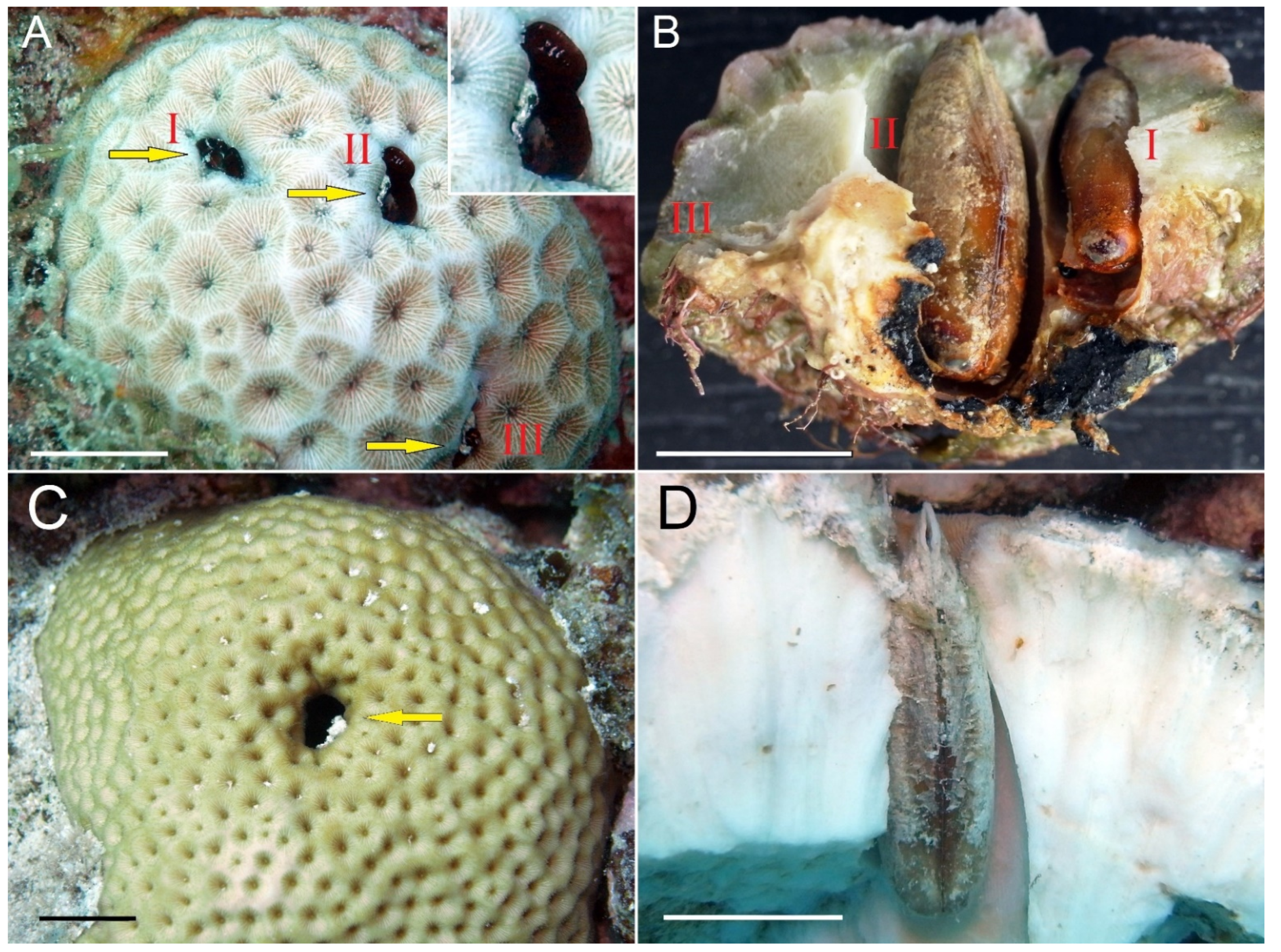

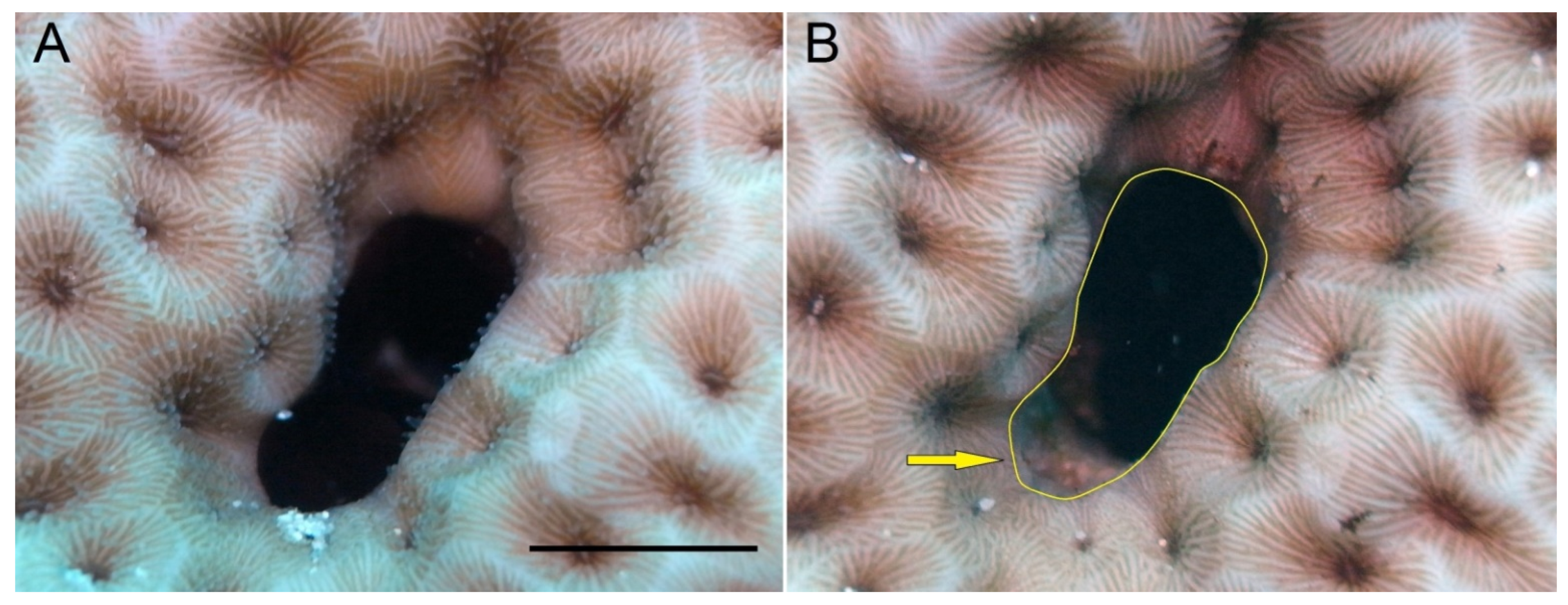
| Host Taxon | Orifice Shape |
|---|---|
| Cnidaria: Anthozoa: Scleractinia | |
| Agariciidae | |
| Agaricia agaricites (Linnaeus, 1758) | O |
| Agaricia humilis (Verrill, 1901) * | T O |
| Agaricia lamarcki Milne Edwards & Haime, 1851 * | O |
| Astrocoeniidae | |
| Stephanocoenia intersepta (Esper, 1795) | O |
| Faviidae: Faviinae | |
| Colpophyllia natans (Houttuyn, 1772) * | T O |
| Diploria labyrinthiformis (Linnaeus, 1758) * | |
| Favia fragum (Esper, 1793) | O |
| Pseudodiploria strigosa (Dana, 1846) | T O |
| Meandrinidae | |
| Eusmilia fastigiata (Pallas, 1766) * | O |
| Meandrina meandrites (Linnaeus, 1758) * | O |
| Merulinidae | |
| Orbicella annularis (Ellis & Solander, 1786) | O |
| Orbicella faveolata (Ellis & Solander, 1786) * | T O |
| Orbicella franksi (Gregory, 1895) * | T O |
| Montastraeidae | |
| Montastraea cavernosa (Linnaeus, 1767) | O |
| Pocilloporidae | |
| Madracis auretenra Locke, Weil & Coates, 2007 | O |
| Madracis decactis (Lyman, 1859) | T O |
| Madracis pharensis (Heller, 1868) * | T |
| Madracis senaria Wells, 1973 * | T O |
| Poritidae | |
| Porites astreoides Lamarck, 1816 | O |
| Siderastreidae | |
| Siderastrea siderea (Ellis & Solander, 1768) | O |
| Cnidaria: Hydrozoa: Anthoathecata | |
| Milleporidae | |
| Millepora alcicornis Linnaeus, 1758 * | O |
| Millepora complanata Lamarck, 1816 * | T O |
| Dead coral | T |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoeksema, B.W.; Smith-Moorhouse, A.; Harper, C.E.; van der Schoot, R.J.; Timmerman, R.F.; Spaargaren, R.; Langdon-Down, S.J. Black Mantle Tissue of Endolithic Mussels (Leiosolenus spp.) Is Cloaking Borehole Orifices in Caribbean Reef Corals. Diversity 2022, 14, 401. https://doi.org/10.3390/d14050401
Hoeksema BW, Smith-Moorhouse A, Harper CE, van der Schoot RJ, Timmerman RF, Spaargaren R, Langdon-Down SJ. Black Mantle Tissue of Endolithic Mussels (Leiosolenus spp.) Is Cloaking Borehole Orifices in Caribbean Reef Corals. Diversity. 2022; 14(5):401. https://doi.org/10.3390/d14050401
Chicago/Turabian StyleHoeksema, Bert W., Annabel Smith-Moorhouse, Charlotte E. Harper, Roel. J. van der Schoot, Rosalie F. Timmerman, Roselle Spaargaren, and Sean J. Langdon-Down. 2022. "Black Mantle Tissue of Endolithic Mussels (Leiosolenus spp.) Is Cloaking Borehole Orifices in Caribbean Reef Corals" Diversity 14, no. 5: 401. https://doi.org/10.3390/d14050401
APA StyleHoeksema, B. W., Smith-Moorhouse, A., Harper, C. E., van der Schoot, R. J., Timmerman, R. F., Spaargaren, R., & Langdon-Down, S. J. (2022). Black Mantle Tissue of Endolithic Mussels (Leiosolenus spp.) Is Cloaking Borehole Orifices in Caribbean Reef Corals. Diversity, 14(5), 401. https://doi.org/10.3390/d14050401










