Herbivory by Geese Inhibits Tidal Freshwater Wetland Restoration Success
Abstract
:1. Introduction
2. Materials and Methods
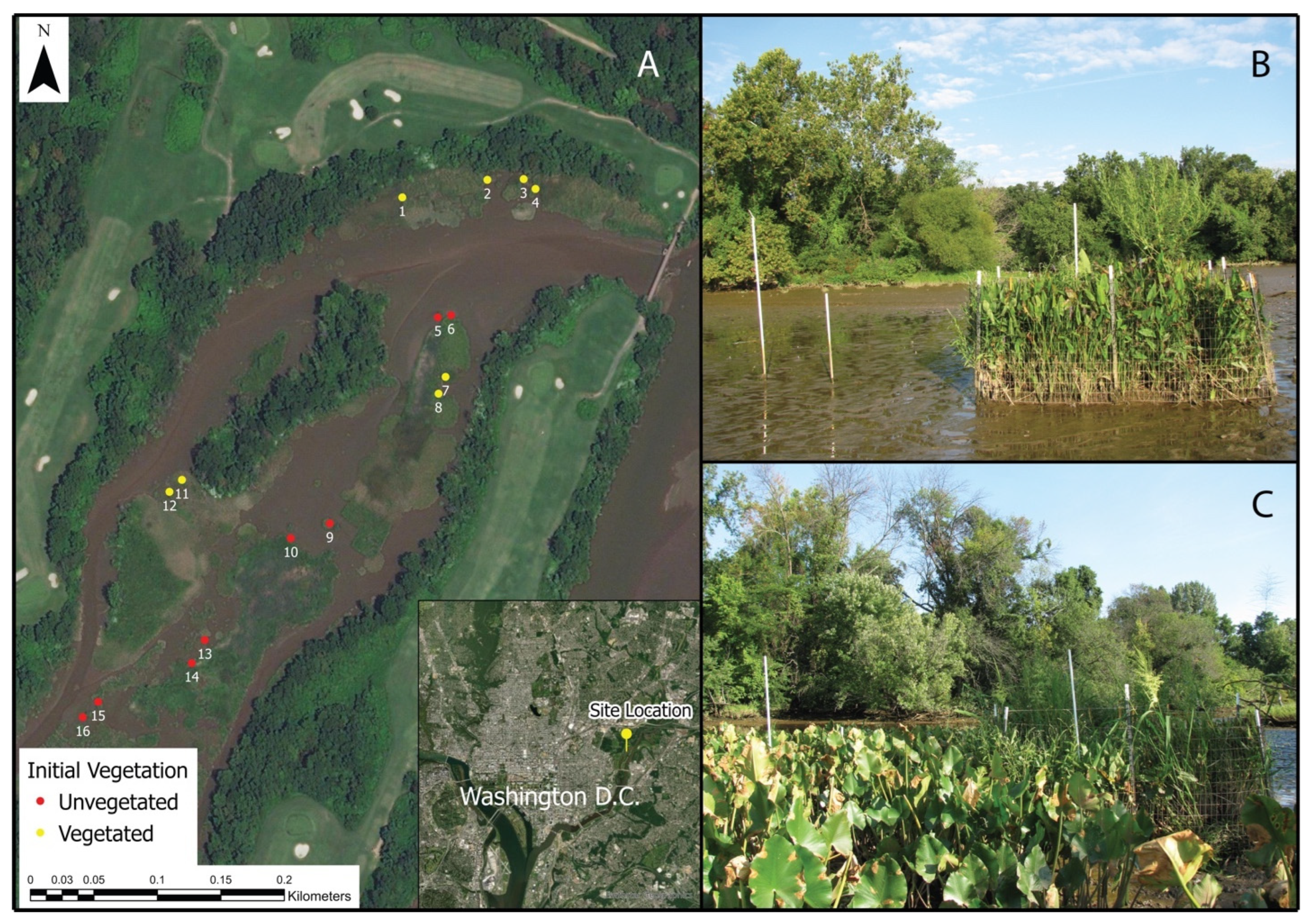
2.1. Study Site
2.2. Exclosure Experiment
2.3. Goose Population Census
2.4. Statistical Analyses
3. Results
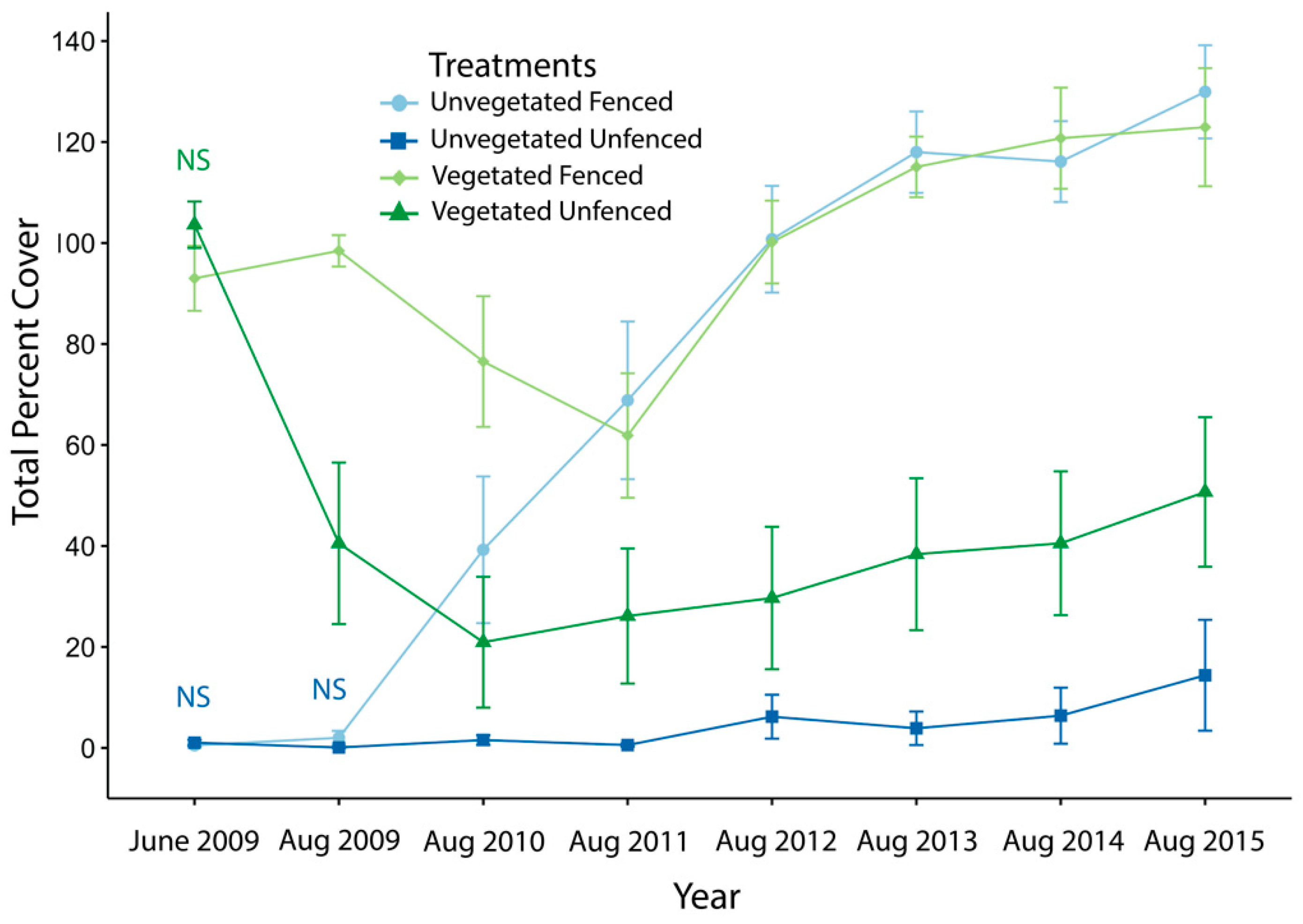
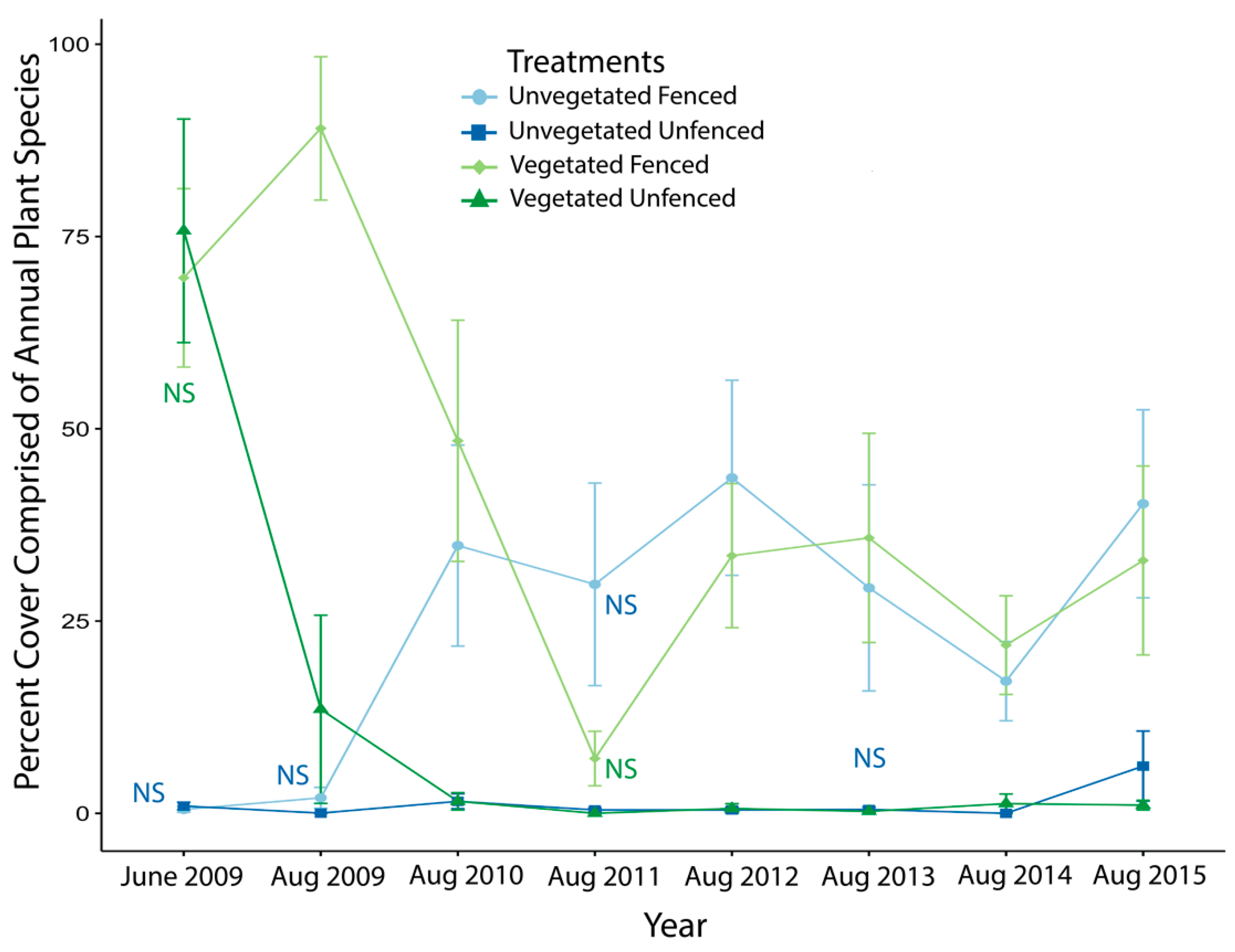
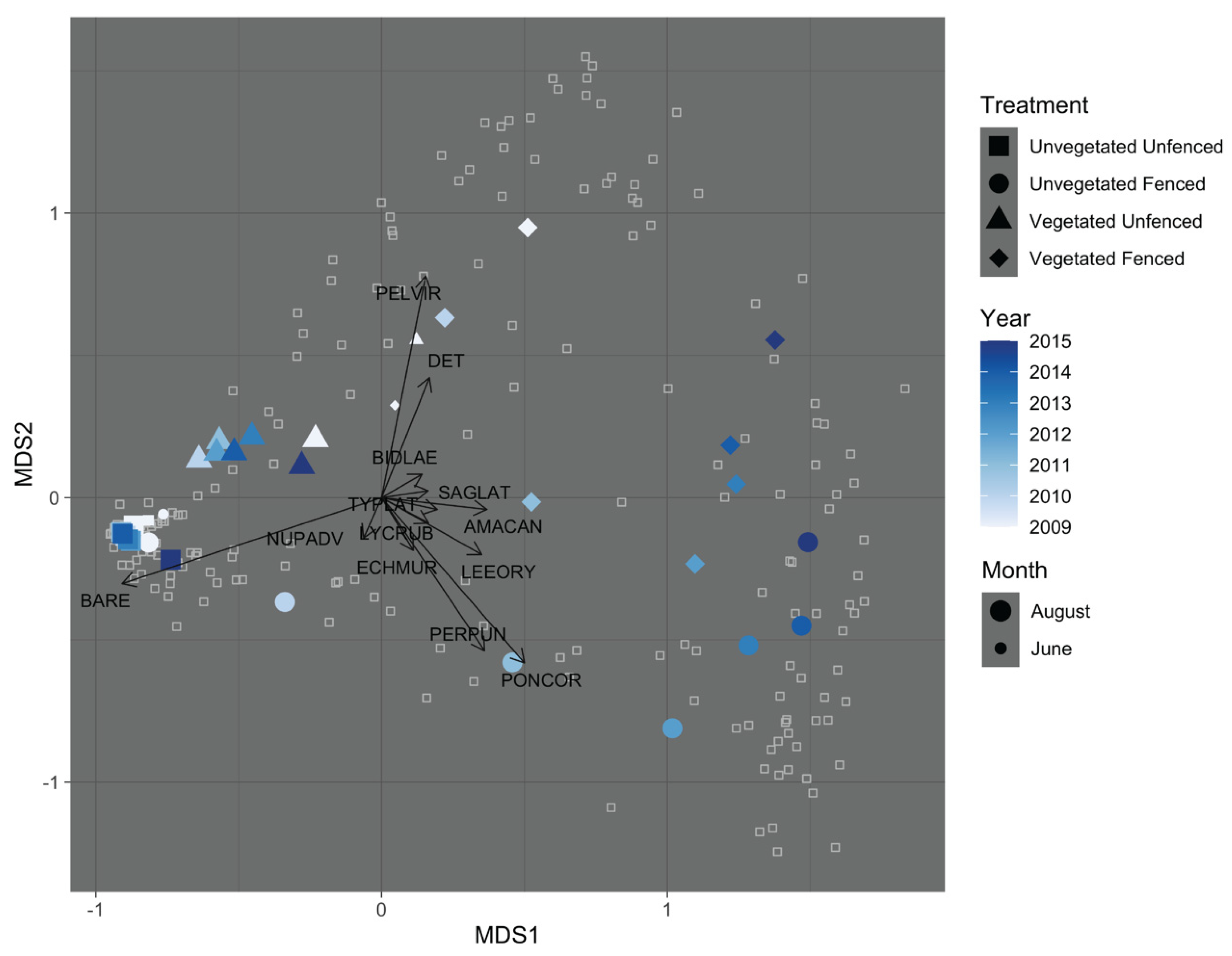
3.1. Exclosure Experiment
3.1.1. Vegetative and Annuals Cover
3.1.2. Diversity Measures
3.1.3. Community Composition
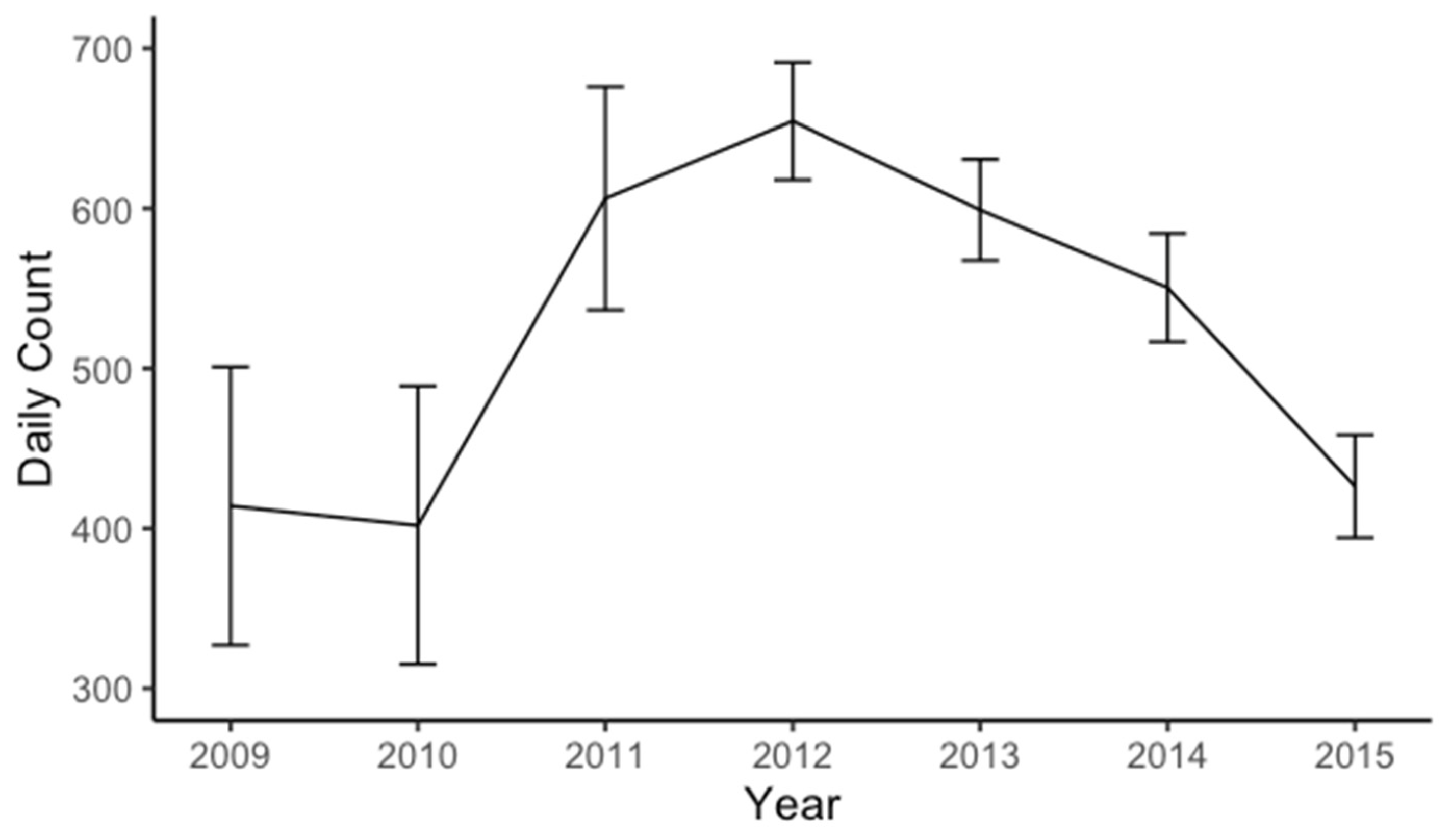
3.2. Goose Population Census
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krafft, C.C.; Hatfield, J.S.; Hammerschlag, R.S. Effects of Canada Goose Herbivory on the Tidal Freshwater Wetlands in Anacostia Park, 2009–2011; National Park Service: Washington, DC, USA, 2013.
- Krafft, C.C.; Hatfield, J.S.; Hammerschlag, R.S. Effects of Canada Goose Herbivory on the Tidal Freshwater Wetlands in Anacostia Park, 2009–2015; National Park Service: Washington, DC, USA, 2015.
- Sweeney, B.W.; Czapka, S.J.; Yerkes, T. Riparian Forest Restoration: Increasing Success by Reducing Plant Competition and Herbivory. Restor. Ecol. 2002, 10, 392–400. [Google Scholar] [CrossRef]
- Thyroff, E.C.; Burney, O.T.; Jacobs, D.F. Herbivory and Competing Vegetation Interact as Site Limiting Factors in Maritime Forest Restoration. Forests 2019, 10, 950. [Google Scholar] [CrossRef]
- Wasson, K.; Tanner, K.E.; Woofolk, A.; McCain, S.; Suraci, J.P. Top-down and Sideways: Herbivory and Cross-Ecosystem Connectivity Shape Restoration Success at the Salt Marsh-Upland Ecotone. PLoS ONE 2021, 16, e0247374. [Google Scholar] [CrossRef] [PubMed]
- Nichols, T.C. Ten Years of Resident Canada Goose Damage Management in a New Jersey Tidal Freshwater Wetland. Wildl. Soc. Bull. 2014, 38, 221–228. [Google Scholar] [CrossRef]
- Dolbeer, R.A.; Seubert, J.L.; Begier, M.J. Population Trends of Resident and Migratory Canada Geese in Relation to Strikes with Civil Aircraft. Hum.-Wildl. Interact. 2014, 8, 88–99. [Google Scholar]
- Conover, M.R. Population Growth and Movements of Canada Geese in New Haven County, Connecticut, during a 25-Year Period. Waterbirds Int. J. Waterbird Biol. 2011, 34, 412–421. [Google Scholar] [CrossRef]
- Baldwin, A.H.; Pendleton, F.N. Interactive Effects of Animal Disturbance and Elevation on Vegetation of a Tidal Freshwater Marsh. Estuaries 2003, 26, 905–915. [Google Scholar] [CrossRef]
- Haramis, G.M.; Kearns, G.D. Herbivory by Resident Geese: The Loss and Recovery of Wild Rice along the Tidal Patuxent River. J. Wildl. Manag. 2007, 71, 788–794. [Google Scholar] [CrossRef]
- Smith, A.E.; Craven, S.R.; Curtis, P.D. Managing Canada Geese in Urban Environments; Cornell Cooperative Extension: Ithaca, NY, USA, 2000. [Google Scholar]
- Simpson, R.L.; Good, R.E.; Leck, M.A.; Whigham, D.F. The Ecology of Freshwater Tidal Wetlands. BioScience 1983, 33, 255–259. [Google Scholar] [CrossRef]
- Ehrenfeld, J.G. Evaluating Wetlands within an Urban Context. Urban Ecosyst. 2000, 4, 69–85. [Google Scholar] [CrossRef]
- Whigham, D.F.; Baldwin, A.H.; Swarth, C. Conservation of Tidal Freshwater Wetlands in North America. In Tidal Freshwater Wetlands; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Baden, J., III; Batson, W.T.; Stalter, R. Factors Affecting the Distribution of Vegetation of Abandoned Rice Fields, Georgetown Co., South Carolina. Castanea 1975, 40, 171–184. [Google Scholar]
- Baldwin, A.H.; Barendregt, A.; Whigham, D.F. Tidal Freshwater Wetlands, an Introduction to the Ecosystem. In Tidal Freshwater Wetlands; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Swarth, C.W.; Delgado, P.; Whigham, D.F. Vegetation Dynamics in a Tidal Freshwater Wetland: A Long-Term Study at Differing Scales. Estuaries Coasts 2013, 36, 559–574. [Google Scholar] [CrossRef]
- Crain, C.M.; Gedan, K.B.; Dionne, M. Tidal Restrictions and Mosquito Ditching in New England Marshes. Human Impacts on Salt Marshes: A Global Perspective; University of California Press: Berkeley, CA, USA, 2009; pp. 149–169. [Google Scholar]
- Davidson, N.C. How Much Wetland Has the World Lost? Long-Term and Recent Trends in Global Wetland Area. Mar. Freshw. Res. 2014, 65, 934–941. [Google Scholar]
- Baldwin, A.H.; Hammerschlag, R.S.; Cahoon, D.R. Evaluating Restored Tidal Freshwater Wetlands. In Coastal Wetlands; Elsevier: Amsterdam, The Netherlands, 2019; pp. 889–912. [Google Scholar]
- Mazzotta, M.; Bousquin, J.; Berry, W.; Ojo, C.; McKinney, R.; Hyckha, K.; Druschke, C.G. Evaluating the Ecosystem Services and Benefits of Wetland Restoration by Use of the Rapid Benefit Indicators Approach. Integr. Environ. Assess. Manag. 2019, 15, 148–159. [Google Scholar]
- Collas, L.; Green, R.E.; Ross, A.; Wastell, J.H.; Balmford, A. Urban Development, Land Sharing and Land Sparing: The Importance of Considering Restoration. J. Appl. Ecol. 2017, 54, 1865–1873. [Google Scholar] [CrossRef]
- Baldwin, A.H. Restoring Complex Vegetation in Urban Settings: The Case of Tidal Freshwater Marshes. Urban Ecosyst. 2004, 7, 125–137. [Google Scholar]
- Bowers, J.K. Innovations in Tidal Marsh Restoration: The Kenilworth Marsh Account. Restor. Manag. Notes 1995, 13, 155–161. [Google Scholar] [CrossRef]
- Neff, K.P.; Rusello, K.; Baldwin, A.H. Rapid Seed Bank Development in Restored Tidal Freshwater Wetlands. Restor. Ecol. 2009, 17, 539–548. [Google Scholar]
- Scott, P. Capital Engineers: The US Army Corps of Engineers in the Development of Washington, DC 1790–2004; Army Corps of Engineers Alexandria VA Office of History: Alexandria, VA, USA, 2011. [Google Scholar]
- Hammerschlag, R.S.; Baldwin, A.H.; Krafft, C.C.; Neff, K.P.; Paul, M.M.; Brittingham, K.D.; Rusello, K.; Hatfield, J.S. Five Years of Monitoring Reconstructed Freshwater Tidal Wetlands in the Urban Anacostia River (2000–2004); USGS: Reston, VA, USA, 2006.
- Hammerschlag, R.S.; Krafft, C.C. Five-Year Post-Reconstruction Kingman Marsh Monitoring Project: Vegetation; USGS: Reston, VA, USA, 2006.
- ITIS Integrated Taxonomic Information System 2015. Available online: www.itis.gov (accessed on 12 January 2022).
- Weakley, A.S.; Ludwig, J.C.; Townsend, J.F.; Crowder, B. Flora of Virginia; Botanical Research Institute of Texas Press: Fort Worth, TX, USA, 2012; ISBN 978-1-889878-38-6. [Google Scholar]
- USDA. The PLANTS Database 2015. National Plant Data Team: Greensboro, NC, USA. Available online: http://plants.usda.gov (accessed on 12 January 2022).
- R Core Team R: The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 23 February 2022).
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Vegan: Community Ecology Package. R Package Version 2.0-10. 2013. 2015. Available online: https://CRAN.R-project.org/package=vegan (accessed on 23 February 2022).
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; Heisterkamp, S.; Van Willigen, B.; Maintainer, R. Package ‘Nlme.’ Linear Nonlinear Mixed Effects Models Version. 2017, Volume 3. Available online: https://cran.r-project.org/web/packages/nlme/index.html (accessed on 23 February 2022).
- Suding, K.N.; Gross, K.L.; Houseman, G.R. Alternative States and Positive Feedbacks in Restoration Ecology. Trends Ecol. Evol. 2004, 19, 46–53. [Google Scholar] [PubMed]
- Zedler, J.B. Progress in Wetland Restoration Ecology. Trends Ecol. Evol. 2000, 15, 402–407. [Google Scholar] [CrossRef]
- Whigham, D.F.; Simpson, R.L. Annual Variation in Biomass and Production of a Tidal Freshwater Wetland and Comparison with Other Wetland Systems. Va. J. Sci. 1992, 43, 5–14. [Google Scholar]
- Leck, M.A.; Crain, C.M. Northeastern North America Case Studies—New Jersey and New England. In Tidal Freshwater Wetlands; Elsevier: Amsterdam, The Netherlands, 2009; pp. 145–156. [Google Scholar]
- Zedler, J.B.; Leach, M.K. Managing Urban Wetlands for Multiple Use: Research, Restoration, and Recreation. Urban Ecosyst. 1998, 2, 189–204. [Google Scholar] [CrossRef]
- Callaway, J.C.; Zedler, J.B. Restoration of Urban Salt Marshes: Lessons from Southern California. Urban Ecosyst. 2004, 7, 107–124. [Google Scholar] [CrossRef]
- Balkcom, G.D. Demographic Parameters of Rural and Urban Adult Resident Canada Geese in Georgia. J. Wildl. Manag. 2010, 74, 120–123. [Google Scholar] [CrossRef]
- Holevinski, R.A.; Curtis, P.D.; Malecki, R.A. Hazing of Canada Geese Is Unlikely to Reduce Nuisance Populations in Urban and Suburban Communities. Hum. Wildl. Confl. 2007, 1, 257–264. [Google Scholar]

| Model Terms | Total Percent Cover | Cover by Annual Species | Species Richness | Shannon–Wiener Diversity Index | |||||
|---|---|---|---|---|---|---|---|---|---|
| d.f. | F | p | F | p | F | p | F | p | |
| Intercept | 1112 | 0.02 | 0.89 | 1.39 | 0.24 | 47.12 | <0.01 | 39.54 | <0.01 |
| Init. Vegetation | 1112 | 4.30 | 0.04 | 0.87 | 0.35 | 0.10 | 0.76 | 1.87 | 0.17 |
| Year | 7112 | 17.32 | <0.01 | 16.29 | <0.01 | 13.59 | <0.01 | 12.31 | <0.01 |
| Init. Vegetation × Year | 7112 | 2.80 | 0.01 | 2.85 | 0.01 | 1.23 | 0.29 | 0.79 | 0.59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jobe, J.; Krafft, C.; Milton, M.; Gedan, K. Herbivory by Geese Inhibits Tidal Freshwater Wetland Restoration Success. Diversity 2022, 14, 278. https://doi.org/10.3390/d14040278
Jobe J, Krafft C, Milton M, Gedan K. Herbivory by Geese Inhibits Tidal Freshwater Wetland Restoration Success. Diversity. 2022; 14(4):278. https://doi.org/10.3390/d14040278
Chicago/Turabian StyleJobe, Justus, Cairn Krafft, Mikaila Milton, and Keryn Gedan. 2022. "Herbivory by Geese Inhibits Tidal Freshwater Wetland Restoration Success" Diversity 14, no. 4: 278. https://doi.org/10.3390/d14040278
APA StyleJobe, J., Krafft, C., Milton, M., & Gedan, K. (2022). Herbivory by Geese Inhibits Tidal Freshwater Wetland Restoration Success. Diversity, 14(4), 278. https://doi.org/10.3390/d14040278








