Abstract
We conducted an intercontinental biogeographic survey to analyze the effects of an invasive plant species in its native and invaded ranges. Our study system included tidal wetlands colonized by Iris pseudacorus L. (yellow flag iris, Iridaceae) along salinity gradients in two estuaries in its native European (Guadalquivir Estuary) and invaded North American (San Francisco Bay-Delta Estuary) ranges. We hypothesized I. pseudacorus would impart more negative community-level impacts on plant species diversity in the invaded range compared to the native range. Our results show that the colonization of Iris pseudacorus has very different effects on the diversity of tidal plant communities in its native and invaded ranges. In the native range, I. pseudacorus promoted plant diversity by increasing evenness and species richness. On the contrary, I. pseudacorus greatly reduced plant diversity in the invaded range, being this reduction higher in those communities with higher species richness and diversity levels. In view of these results, urgent management practices are needed to control and eradicate I. pseudacorus from the inland Sacramento-San Joaquin Delta Estuary since this invasive macrophyte is reducing plant diversity at local and landscape scales.
1. Introduction
The human-mediated movement of species has increased significantly with economic globalization and, as a result, biological invasions have become a main component of global change with increasing negative effects on ecosystems. In fact, the accumulation of multiple exotic invasive species within ecosystems around the globe is one of the most important causes of biodiversity loss [1]. However, such invader impacts on biological diversity can be highly variable at landscape scales and are influenced by processes in the native ecosystem [2]. At the same time, in a dialectic relationship, biological invasions are frequently associated with habitat disturbance and changing environmental conditions [3]. In this sense, exotic invasive species directly impact on native species through competitive displacement over common resources and through invasion-driven ecosystem modifications [4].
In this general scenario, conflicting evidence as to whether plant invasions pose a great threat to native plant diversity is still debated [5]. Some studies argue that invasive species can cause biotic differentiation, but in many cases biological invasions actually decrease biodiversity locally through biotic homogenization [6]. Moreover, competitive impacts of invasive species may vary across invaded ranges and local to regional scales [7]. Frequently, native species-rich communities are more successful at resisting invasion by exotic species than are species-poor communities at the local scale, according to the biotic resistance hypothesis [8], owing to competition for limiting resources [9]. Instead, following the biotic acceptance hypothesis, species-rich communities are often more vulnerable to invasions by exotic species than species-poor communities at larger spatial scales [10,11]. In fact, native species can facilitate invader abundance by changing environmental conditions, with positive interactions as important drivers of invasion success [12]. Thus, the impacts of plant invasions on plant species richness are not universal [13], and the relative role of species richness in predicting invasion success is not fully understood [14]. Furthermore, the characteristics of recipient communities seem to be one of the most important determinants of invasion impacts [5], but little is known about the full range of direct and indirect interactions among native and exotic species [15]. Ecological interactions rarely enable communities to resist invasion but instead constrain the abundance of invasive species once they have successfully established themselves [16]. Additionally, according to the invasional meltdown hypothesis (IMH), exotic species facilitate one another’s invasion and can benefit newly arriving invasive species by increasing their likelihood of survival. Moreover, they may synergistically increase habitat invasibility, promote further spread, and impart negative impacts in the native community [17,18]. Understanding the processes that influence invasion by exotic species remains a challenge in ecology.
Comparisons of the role of exotic species in relation to native plant communities in their native and invaded ranges represent a crucial step when studying invaders. In general, invasive plants are assumed to behave more aggressively in their invaded ranges than in their native one [19,20,21]. Some invasive plant species form dense mono-dominant stands in invaded areas while having sparse distribution in their native ranges, which has been linked to their impacts on biodiversity [22,23]. Higher abundance of invaders in the introduced range are often explained by a reduction in negative species interactions, although results are equivocal [12]. In addition, invasive species often evolve rapidly in response to the novel environmental conditions in their introduced range [24].
We conducted an intercontinental biogeographic survey to analyze the effects of an invasive plant species, Iris pseudacorus L. (yellow flag iris, Iridaceae), on plant community diversity in its native and invaded ranges. Climate drives plant diversity over large spatial scales more than any other factor [25]. The global distribution of I. pseudacorus encompasses a wide range of climate zones and bioregions, including populations in arctic, subarctic, temperate and subtropical climates [26,27,28]. Given the increasing high costs of controlling invasive plant species, it is important to understand the degree to which an invasive species drives significant negative impacts such as a decline in biological diversity and the magnitude of its influence at spatial scales where impacts potentially occur [29]. Therefore, to examine the role of invader impacts on diversity, we focused our investigation on tidal wetlands colonized by I. pseudacorus along broad tidewater salinity gradients in two estuaries with similar latitude (~37 °N) and warm Mediterranean climates in its native European and invaded North American ranges. This aquatic macrophyte has been introduced as an ornamental aquatic plant worldwide and has escaped cultivation and naturalized, becoming invasive in freshwater wetlands of South and North America, South Africa, New Zealand, Australia, India and Japan, where it is displacing native plant species [26,28,30,31]. Intertidal marsh habitat has not often been considered vulnerable to invasion by I. pseudacorus, yet unexpected colonization and spread of invasive plants into estuarine environments (e.g., [32]) underscores the need for improved understanding of invasion dynamics and impacts across expanding environmental gradients.
Exotic invasive species of aquatic macrophytes have been identified as a major cause of biodiversity loss [33]. Following the biotic acceptance hypothesis, we hypothesized I. pseudacorus would impart more negative community-level impacts on plant species diversity in the invaded range, where plant diversity was apparently higher compared to the native range. We also postulated impacts on plant community diversity would vary along a salinity gradient that corresponds to more diverse freshwater tidal areas or to less diverse brackish wetlands. Comparative studies of invasive plants in their native and naturalized ranges are crucial for understanding factors that limit species distributions and impacts, yet most studies focus on areas of introduction [34,35]. Results of this inquiry are useful for understanding the dynamics and impacts of I. pseudacorus on plant communities to inform management planning and contribute new knowledge to the on-going debate about the role of invasive species in native plant communities.
2. Materials and Methods
2.1. Study Sites
We studied the effects of Iris pseudacorus presence on plant diversity in its invaded and native ranges. We studied populations of I. pseudacorus naturalized in the San Francisco Bay-Delta Estuary (Pacific Coast of North America, CA, USA) (Figure 1 and Figure S1). This area has mixed semi-diurnal tides and a Mediterranean climate with cool and wet winters and hot and dry summers and some summer fog for locations closer to the Pacific Ocean. According to the climatic series (1981–2010), mean annual temperature is +15.2 °C; minimum mean daily temperature is +9.2 °C in January, and maximum mean daily temperature is +20.3 °C in July [36]. In the United States (USA), I. pseudacorus first established in the late 19th century in northeastern Atlantic coastal states, while first records in California were from rivers and inland lakes in the 1950s [37] and have long been limited to inland freshwater habitats. By 1969, an invasive population had established in freshwater tidal wetlands of the inland Sacramento-San Joaquin Delta [38]. Downstream spread into brackish tidal wetlands in the greater San Francisco Bay-Delta Estuary has recently occurred, raising concerns about persistent impacts and spread with sea level rise [39].
Figure 1.
Location map of study locations of Iris pseudacorus in the native range (Guadalquivir River Estuary, Andalusia, Spain, A1–A5) and the invaded range (Sacramento-San Joaquin Delta, California, USA, C1–C5). Source: Google Earth 2020 (accessed on 1 July 2021).
We established comparative study sites in the native European range of I. pseudacorus in the Guadalquivir River Estuary (Southwest Iberian Peninsula, Andalusia, Spain) (Figure 1 and Figure S1). This mesotidal estuary experiences semidiurnal tides (tidal range 3.5 m in spring tides) from the mouth of Guadalquivir River to the Alcalá del Río Dam located 110 km upstream [40]. Similar to the California sites, the climate is Mediterranean with Atlantic Ocean influence with a mean annual temperature of +18.7 °C, and mean daily minimum and maximum temperatures are +10.8 °C in January and +27.1 °C in July, respectively, for the climatic series 1981–2010 [41]. I. pseudacorus was found in fringing emergent intertidal wetlands adjacent to transitional riparian ecotones that included some tree species at both geographical ranges (Acer negundo L., Alnus rhombifolia Nutt., Juglans regia L. and Salix laevigata Bebb in the invaded range and Populus alba L., Salix alba L. and Ulmus minor Mill. in the native range). Observations of herbivory within populations were recorded. We observed signs of herbivory on I. pseudacorus by livestock (cows and sheep) at populations A2 and A5.
2.2. Data Collection
2.2.1. Establishment of Monitoring Plots
In July 2017, monitoring plots were established at five locations within population patches of I. pseudacorus representing invasions along an estuarine gradient from near the port of Stockton in fringing freshwater tidal wetlands to downstream brackish reach at Carquinez Strait in the San Francisco Bay-Delta Estuary (n = 7–8 patches per locations). In 2018, comparative monitoring plots with native I. pseudacorus were established in fringing shoreline wetlands along an estuarine gradient in the Guadalquivir River Estuary, from a freshwater tidal reach at Seville city to brackish tidal wetlands near the Gulf of Cadiz (Table S1). We established pairs of fixed square monitoring plots (1 m2), coincident with the central areas of discrete patches of I. pseudacorus and adjacent areas not colonized by I. pseudacorus. Methods for evaluation of vegetation and environmental variables were developed during the initial study in California, standardized for implementation across all study sites in both estuaries, and principal investigators directly collaborated on research in both estuaries.
2.2.2. Sediment Electrical Conductivity
Electrical conductivity (EC) of sediment interstitial water is a standard indirect measure of salinity in wetland sediment. Sediment EC was recorded in our study because the sampled tidal marshes were distributed along estuaries where salinity is the most important changing environmental stress factor [42], and because I. pseudacorus shows marked functional responses to salinity [43]. Sediment EC was recorded concurrently with plant community composition. Soil cores (4.5 cm diameter × 10 cm depth) were collected from each monitoring plot inside Iris clumps (1–3 cores per plot), stored in coolers with blue ice and transported to the laboratory. Sediment EC (mS cm−1) (invaded range: Oakton CON2700, Oakton Instruments, Vernon Hills, IL, USA; native range: Crison EC-meter Basic 30, Crison Instruments, Barcelona, Spain) was recorded using the standard method for saturation paste extraction with distilled water.
2.2.3. Plant Community Composition
The observed vegetation distribution pattern was complex in structure. Therefore, following [44], we established two linear transects up to 1.0 m long that originated at the centre of each fixed/paired monitoring plot (with I. pseudacorus; without I. pseudacorus) and were at 90° to one another. We recorded the presence of each plant taxon every 10 cm along both transects. These data were used to calculate the absolute cover of each plant taxa. A cover of 2.5% was adjudicated to those plant taxa observed to be present in a plot that were not recorded along the transects. In addition, to study the distribution of plant taxa in relation to the structure of Iris patches, both perpendicular transects were extended to the edge of Iris occupied patches for assessment of vegetation within the total area of each patch. Plant taxa were taxonomically evaluated and identified following [45,46] for California flora and [47,48] for the Guadalquivir Estuary. Vegetation assessment was limited to emergent species rooted in the plots and did not include minor areas with over-hanging canopies from nearby riparian tree species.
2.3. Data Analyses
Relative cover (%) for each species was calculated as the number of points in which it was present in relation to the total number of sampling points and multiplied by 100. To examine the impact of I. pseudacorus on the invaded plant communities, we calculated mean plant species richness (S; number of discrete plant taxa), evenness (J′), and Shannon–Wiener α-diversity (H′) for plant species in invaded and uninvaded plots [49] from plant abundance (relative cover from 1 m long transects) matrices using the ‘vegan: ecological diversity’ package of the R-software [50]. To evaluate the effect of I. pseudacorus on changes in plant community composition at landscape scale (comparing floristic differences among plots where I. pseudacorus was present or absent), we calculated the Morisita–Horn pairwise dissimilarity index from cover matrices using the ‘vegdist’ function of R-software to obtain a pairwise dissimilarity matrix, and the Sørensen dissimilarity index using the ‘betadist (sor method)’ function in the ‘vegan: ecological diversity’ package of R-software [51]. In addition, we used the function ‘indicators’ of the package ‘indicspecies’ of R-software that explores the indicator value of the simultaneous occurrence of sets of species. The method is described in [52] and is a generalization of the Indicator Value method of [53]. The statistical significance of the relationships was tested with a permutation test.
Deviations were calculated as standard errors of the mean (SE). A significance level (α) of 0.05 was applied for every analysis. Prior to conduct the analyses, data series were tested for normality using the Kolmogorov–Smirnov test and for homoscedasticity applying Levene’s test. When necessary, data series were transformed using 1/x, √x and ln(x) functions trying to address the assumptions needed for parametric tests. Since homogeneity of variance was not accomplished after data transformations, sediment EC was compared between distribution ranges (native and invaded) and between locations (ordered in each range sequentially according to their distance to the ocean) using the Gamma Generalized Linear Model (GLM) (Poisson distribution) with chi-square (χ2) de Wald [54]. I. pseudacorus cover from 1 m long transects was next compared between distribution ranges and between locations using a General Linear Model (LM) with sediment EC as covariant. Multivariate analysis of variance (MANOVA) and Pillai’s Trace were conducted for diversity indices (S was square root transformed) using distribution range, I. pseudacorus presence (with or without Iris) and location (ordered in each range sequentially according to their distance to the ocean) as grouping factors, with sediment EC as covariant. The analyses of multivariate variance protect subsequent analyses from type I error [55]. Once multivariate significance was confirmed via MANOVA, the main univariate differences were evaluated for each diversity and dissimilarity index with LMs. Particular differences between distribution ranges and between plots with and without I. pseudacorus were tested using Student t-test. When normality or homogeneity of variance was not achieved after data transformation, univariate differences were analyzed using GLM with chi-square (χ2) de Wald [54] and particular differences using Man–Whitney U-test. These analyses were repeated excluding exotic species, and the most interesting results are reported in comparison with analyses including native and exotic species. Relationships between sediment electrical conductivity, I. pseudacorus cover and diversity indices were analyzed using correlation analysis, Pearson (r) or Spearman (ρ) correlation coefficient, and lineal regression analysis. Data from the whole patch diameter were analyzed using regression analysis between mean distance from the center of the Iris patch and species richness. All these analyses were conducted using IBM SPSS V. 20 (IBM Corp., Armonk, NY, USA).
3. Results
3.1. Sediment Electrical Conductivity
Sediment EC changed significantly between the distribution ranges, as did the interaction between ranges (invaded, native) and population patch location (Table 1). Mean sediment EC was 18% higher in the native range (2400 ± 184 μS cm−1) than in the invaded range (1960 ± 344 μS cm−1). However, sediment EC increased 92% seaward among population sites in the invaded range varying from 412 ± 75 to 5327 ± 1114 μS cm−1. In contrast, sediment EC increased only 70% along the estuarine gradient in the native range, varying from 1127 ± 105 to 3768 ± 104 μS cm−1.

Table 1.
Generalized Linear Model for sediment electrical conductivity comparing between distribution ranges (native and invaded) and locations (ordered according to their distance to the ocean).
3.2. Iris pseudacorus Abundance
The relative cover of I. pseudacorus changed significantly between the distribution ranges, as did the interaction between ranges and population patch location (Table 2). I. pseudacorus cover was 17% lower in the native range (62 ± 4%) than cover of the species in the invaded range (75 ± 2%). The cover of I. pseudacorus varied just 15% between populations in its invaded range (changing between 69 ± 4% and 81 ± 4%), whereas it varied 48% in its native range (between 38 ± 9% and 74 ± 11%).

Table 2.
General Linear Model for the relative cover of Iris pseudacorus comparing between distribution ranges (native and invaded) and population locations (ordered in each range sequentially according to their distance to the ocean), with sediment electrical conductivity (EC) as covariate. Significant differences are marked in bold.
3.3. Iris pseudacorus Impacts on Plant Community Diversity
A total of 30 native plant species and only 2 exotic species were recorded in the native range of I. pseudacorus, whereas 55 native and 19 exotic species were recorded in the invaded range (Tables S2 and S3).
All calculated diversity indices of vegetation from plots with and without I. pseudacorus were independent of sediment EC in both distribution ranges when native and exotic species were included in the analyses (Pearson or Spearman correlation coefficient, p > 0.05). In contrast, when exotic species were excluded from the analysis, α-diversity and the number of plant species decreased at higher sediment EC in the invaded range (H′: r = −0.261, p = 0.022, n = 78; S: r = −0.260, p = −0.260, n = 78). Moreover, the differences in diversity indices between areas with and without I. pseudacorus were independent of EC along estuarine gradients studied in the native and invaded ranges, both including exotic and native plant species and only native species (Pearson or Spearman correlation coefficient, p < 0.05). In addition, all diversity indices decreased with increasing I. pseudacorus cover in the invaded range, showing the opposite response in the native range (Pearson or Spearman correlation coefficient, p < 0.05) (Figure 2).
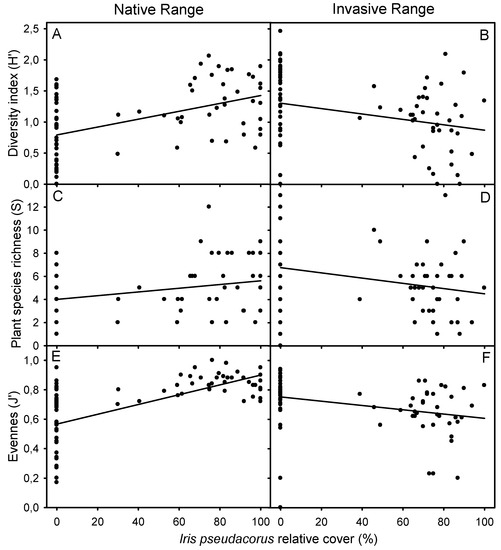
Figure 2.
Relationship between the α-diversity (H′), plant species richness (S) and evenness (J′) with Iris pseudacorus relative cover in native (Guadalquivir Estuary) and invaded (San Francisco Bay-Delta Estuary) distribution ranges. Regression equations: (A) y = 0.7921 + 6.335 × (R = 0.429, p < 0.0001, n = 76); (B) y = 1.304 − 4.333 × (R = 0.274, p < 0.05, n = 78); (C) y = 4.012 + 0.016 × (R = 0.302, p < 0.01, n = 76); (D) y = 6.764 − 0.023 × (R = 0.252, p < 0.05, n = 78); (E) y = 0.567 + 3.333 × (R = 0.624, p < 0.0001, n = 75); (F) y = 0.752 − 1.453 × (R = 0.302, p < 0.01, n = 73).
Shannon–Wiener α-diversity and number of plant species within I. pseudacorus patches increased together with their values recorded outside of occupied patches in both distribution ranges (Figure S2). In contrast, species evenness within I. pseudacorus patches only increased together with evenness outside of Iris patches in the native range (Figure S2). In addition, the differences in plant species diversity and richness between areas without and with I. pseudacorus increased together with values of diversity and richness without Iris, respectively, only in the invaded range; these relationships were non-significant for species evenness (Figure 3).
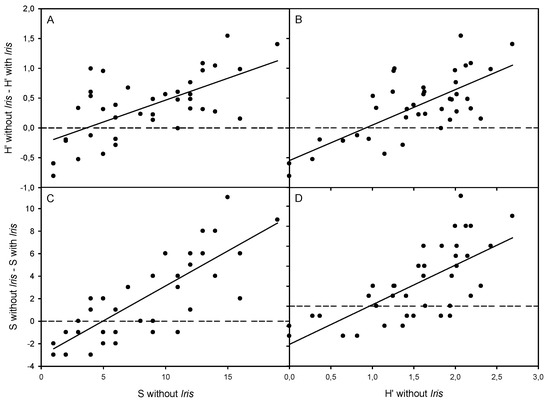
Figure 3.
Relationship between the differences in α-diversity (H′) and plant species richness (S) without and with Iris pseudacorus and H′ values at study populations in the invaded San Francisco Bay-Delta Estuary. Regression equations: (A) y = −0.266 + 0.073 × (R = 0.645, p < 0.0001, n = 39); (B) y = −0.548 + 0.595 × (R = 0.723, p < 0.0001, n = 39); (C) y = −3.074 + 0.621 × (R = 0.795, p < 0.0001, n = 39); (D) y = −3.822 + 3.963 × (R = 0.702, p < 0.0001, n = 39).
Distribution range, location along the estuarine gradient and the interactions between range and I. pseudacorus presence, range and location, and the three-way interaction among range, I. pseudacorus presence and location all had a significant effects on diversity indices (MANOVA, p < 0.05) (Table S4). According to the lineal models, α-diversity and species richness changed significantly between distribution ranges and locations. Species evenness varied significantly between I. pseudacorus presence and locations. In addition, there were also a number of interactive effects of distribution range, location and invader presence that influenced diversity indices (Table S5).
H′ α-diversity was 48% lower in the native than in the invaded range of I. pseudacorus in areas of the plant community where the invader was absent. These areas were characterized by lower species richness and evenness. In contrast, diversity and species richness within Iris-occupied plots were similar in both distribution ranges, whereas evenness was 22% higher within Iris-occupied plots in the native range than was documented in the invasive range (Figure 4; Table S6). When discarding exotic species from the analysis, α-diversity within Iris-occupied plots was 24% higher in the native than in the invaded range (Figure 4; Table S7). The reported differences in diversity indices between distribution ranges were reflected in lower values of the Morisita–Horn dissimilarity index in the native vs. the invaded range when comparing un-invaded plots with themselves and with invaded plots. In contrast, higher Morisita–Horn dissimilarity indices were found for plots with I. pseudacorus in the native compared to the invaded range (Figure 5A, Table S8). The Sørensen dissimilarity index was always higher in the native than in the invaded range for every plot combination with and without I. pseudacorus (Figure 5B, Table S9).
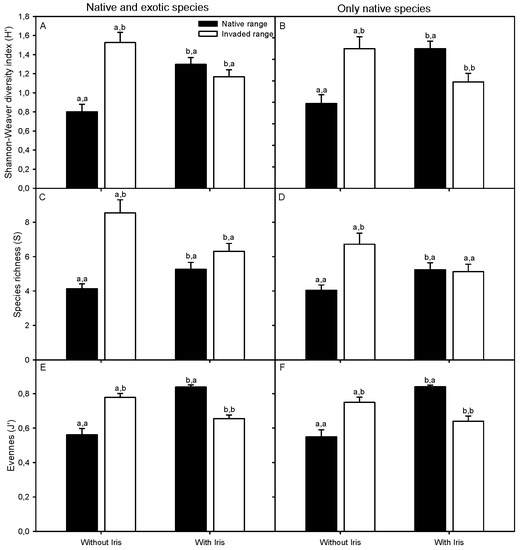
Figure 4.
Shannon–Wiener α-diversity (H′) (A,B), species richness (S) (C,D) and J′ evenness (E,F) with and without Iris pseudacorus in its native (Guadalquivir Estuary) and invaded (San Francisco Bay-Delta Estuary) distribution ranges, analyzing both native and exotic species (A,C,E) and only native species (B,D,F). Different letters indicate significant differences between paired plots with I. pseudacorus presence or absence within each range (first letter) and between ranges (second letter) (t-test or U-test, p < 0.05). Values are mean ± SE (n = 38–39).
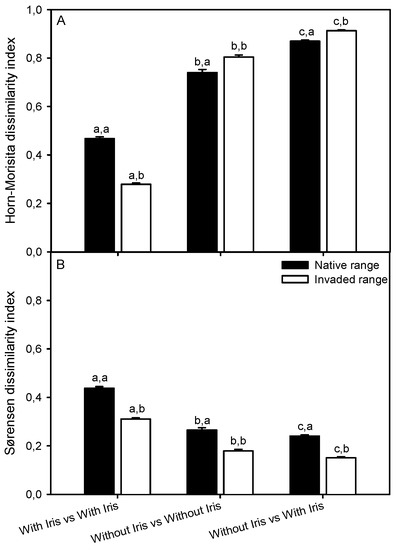
Figure 5.
Horn–Morisita (A) and Sørensen (B) dissimilarity indices between plant communities with and without Iris pseudacorus in its native (Guadalquivir Estuary) and invaded (San Francisco Bay-Delta Estuary) distribution ranges. Different letters indicate significant differences between plant communities within each range (first letter) (Kruskal–Wallis test and Man–Whitney U-test, p < 0.05) and between ranges for the same plant community (second letter) (Man–Whitney U-test, p < 0.05). Values are mean ± SE (n = 703–1521).
In the native range, α-diversity was lower by 38% in plots where I. pseudacorus was not present due to lower number of plant taxa and species evenness values than where the Iris was a community member (Figure 4; Table S6), but eight rare plant taxa (with a frequency of occurrence lower than 6–14%) were recorded growing out of I. pseudacorus patches and were absent from within those patches (Table S2). In contrast, results documented the opposite impact of I. pseudacorus on the plant community in the invaded California range where α-diversity, species richness, evenness were significantly reduced in plots with I. pseudacorus (Figure 4; Table S6). Species richness did not change significantly between areas with and without I. pseudacorus when exotic species were excluded from the analysis (Figure 4; Table S7). In this invaded range, α-diversity was 24% higher in plots where the I. pseudacorus was absent compared to species poor plant community composition associated with the focal species. At these study sites, 11 native and only 2 exotic plant taxa (12 taxa with frequencies <9%) were recorded growing beyond of Iris patches, but they were not present in adjacent Iris-invaded habitat with otherwise comparable environmental conditions. In contrast, only 5 native species (with frequency <9%) grew within Iris-occupied plots, and these same species were absent from areas free of Iris in the invaded range (Table S3). Iris pseudacorus was mainly associated with six native species (Polygonum salicifolium Brouss., Cynodon dactylon (L.) Pers., Oenanthe lachenalii C.C. Gmel., Phragmites australis (Cav.) Trin. ex Steud., Bolboschoenus maritimus (L.) Palla and Atriplex chenopodioides Batt.) in its native range and with four native species (Juncus balticus Willd. (syn. J. arcticus var. balticus (Willd) Trautvetter), Calystegia sepium (L.) R.Br., Schoenoplectus acutus (Muhl. ex J.M.Bigelow) Á. Löve & D. Löve and Schoenoplectus californicus (C.A. Mey.) Steud.) in its invaded range (Table S10). In general, the plant taxa with higher relative cover values beyond plots with I. pseudacorus also occurred at greater abundance (higher relative cover) within the Iris-occupied plots. Furthermore, most of the species that were in the greater community but did not occur within plots with I. pseudacorus had relative cover lower than 20% where without the Iris (Figure S3).
Species richness decreased towards the center of I. pseudacorus patches in three of the five study locations in the invaded range, while this pattern was only observed at one location in the native range. In contrast, the number of plant species increased towards the center of I. pseudacorus patches in only one study location in each distribution range. Moreover, the pattern of species richness was characterized by waves along the radius of I. pseudacorus patches, and at every location, the peaks of species richness were at nearly the same distance intervals from the patch centers (0.0–0.3 m, 0.5–0.8 m, 1.0–1.2 m and 1.4–1.7 m). The pattern of these peaks of species richness within I. pseudacorus patches was c. 20–30 cm long and c. 20–30 cm away from each other (Figure 6).
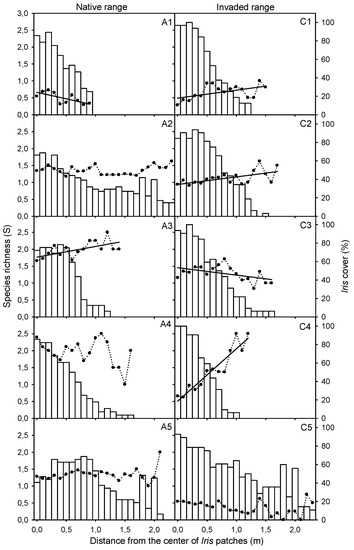
Figure 6.
Relationship between species richness (S) and distance (m) to the center of Iris pseudacorus population patches in Andalusia (A1–A5), Guadalquivir Estuary (native range), compared to this relationship in California (C1–C5), San Francisco Bay-Delta Estuary (invaded range). Columns indicate relative cover (%) of I. pseudacorus. Regression equations: (A1) y = 0.663 −0.385 × (R = −0.699, p = 0.05, n = 10); (C1) y = 0.490 + 0.235 × (R = 0.532, p = 0.034, n = 16); (C2) y = 0.939 + 0.224 × (R = 0.600, p = 0.008, n = 18); (A3) y = 1.768 + 0.315 × (R = 0.613, p = 0.015, n = 15); (C3) y = 1.455 − 0.221 × (R = −0.497, p = 0.042, n = 17); (C4) y = 0.506 + 1.552 × (R = 0.942, p < 0.0001, n = 13).
In both distribution ranges, the Horn–Morisita dissimilarity index was higher when comparing areas with and without I. pseudacorus than when comparing areas with I. pseudacorus among them (Figure 5A, Table S8). On the contrary, the Sørensen dissimilarity index showed the highest absolute values when comparing areas with I. pseudacorus among themselves (Figure 5B, Table S9).
4. Discussion
Our study reveals that the colonization of Iris pseudacorus has very different effects on the diversity of tidal plant communities in its native and invaded range. In the native range, I. pseudacorus was associated with plant diversity by increasing evenness and species richness. On the contrary, greatly reduced plant diversity was associated with I. pseudacorus in the invaded range. In addition, the effects of I. pseudacorus on every diversity index were independent of sediment EC along the estuarine gradients in both study ranges.
Plant α-diversity levels in plots without I. pseudacorus were 48% lower in the native than in the invaded range, due to lower species richness and evenness. This geographical difference could be the product of many factors. For example, our field observations suggest high herbivory levels by livestock as a main factor that could explain reduced plant diversity in most of the native study populations. In contrast, there was no grazing or other significant herbivory observed at our study sites in the invaded range. Heavy grazing is reported to eliminate sensitive species and increase the density of graminoids in high marsh habitats [56] as we recorded in the most grazed locations in the native range, where Cynodon dactylon dominated plant communities.
Plant α-diversity was similar within I. pseudacorus patches in both distribution ranges, even though higher α-diversity was recorded without I. pseudacorus in the invaded range. This can be explained by the contrasted effects of I. pseudacorus on plant diversity between geographical ranges: α-diversity increased 38% within I. pseudacorus patches in the native range and decreased 24% in the invaded range, in relation to areas not colonized by I. pseudacorus. These contrasted differences in α-diversity within I. pseudacorus invaded areas were related to differences in both evenness and species richness. Thus, we found native range microenvironments associated with I. pseudacorus harbored more species than were recorded outside of I. pseudacorus patches. In contrast, I. pseudacorus presence corresponded to decreasing local plant species richness in the invaded range. Iris pseudacorus cover was 17% lower in the native range than in the invaded range, which enabled some low-abundant plant taxa to colonize and coexist within I. pseudacorus patches in the native range where the presence of I. pseudacorus limits the abundance of dominant species. Nevertheless, I. pseudacorus presence limited the colonization of eight low-frequently occurring plant taxa that were recorded only outside of I. pseudacorus-occupied patches in the native range. This phenomenon of displacing low-abundant taxa was also recorded in the invaded range, where I. pseudacorus negatively impacted α-diversity levels. Nevertheless, diversity levels were independent of I. pseudacorus foliar cover in both distribution ranges, pointing to a complexity of factors at play that can control biodiversity. In this context, sediment EC changed between 70–92% along the estuarine gradients in the native and invaded study areas, but diversity indices for the plant communities and the effects of I. pseudacorus on them were surprisingly independent of this environmental factor. In the invaded range, we recorded more exotic species in intertidal marshes with lower sediment salinity than at locations with higher salinities, but this pattern did not relate to the effects of I. pseudacorus on diversity indices. These results indicate I. pseudacorus reaches a level of abundance as a successful invader that disrupts the composition, structure and biodiversity function of native plant communities along broad brackish estuarine gradients. Nevertheless, I. pseudacorus shows negative responses at 17 ppt salinity (EC c. 27.5 mS cm−1) [43], whereas the maximum sediment EC recorded in our study was 3.7 ± 0.1 mS cm−1. The competitive ability of I. pseudacorus could also differ between distribution ranges due to a range of other factors such as resource availability or genetic differences that are expressed as contrasting phenotypes. In this sense, invasive plant species are likely to be more competitive with native flora as a result of greater size (ex. height) and density [5,57,58]. For example, the invasive aquatic macrophyte Typha sp. is capable of rapidly colonizing habitats and forming monodominant vegetation stands due to traits such as robust size, rapid growth rate, and rhizomatic expansion [59], which are plant traits shared also by I. pseudacorus in the invaded range [43]. Moreover, invasion success of exotic species may be based on selective introduction of specific preadapted and plastic genotypes or on adaptive evolution to contrasted environmental conditions in the introduced range [19,24,60]. In addition, plant species interacting with I. pseudacorus may show different responses to I. pseudacorus colonization between distribution ranges. Thus, high competitive ability, biomass and diversity of recipient communities, long-term coexistence (coevolutionary history) and species filtering have been reported as responsible for lower impacts of invasive species in their native range [14,20,23].
In accordance with our hypothesis, I. pseudacorus had greater negative impacts on diversity where species richness and α-diversity were higher in plots without the Iris in the invaded range. In this sense, [5] suggested that invasion impacts may be a continuum across an entire invaded range. This changing impact of I. pseudacorus invasion on recipient plant communities depending on their diversity was due to dominant perennial rhizomatous native species, including Juncus balticus (Baltic rush), Schoenoplectus acutus (hardstem bulrush) and Schoenoplectus californicus (California bulrush). These four native emergent macrophytes persisted despite introduction and spread of I. pseudacorus in their community, suggesting they are more resistant to invasion by I. pseudacorus than rare and low-abundance species that succumb to competitive displacement. There was a spatial context to the invasion impact on diversity in our invaded study populations. Iris pseudacorus displaced the more rare, low-abundant species (cover < 20%) from more interior areas within its patches, where I. pseudacorus was present at higher cover levels and during longer periods than at the periphery of the invading patches. The interspecific displacement of native species by I. pseudacorus showed a spatial pattern organized in 20–30 cm wide concentric rings of high and low species richness alternating along the I. pseudacorus patch radius. This spatial pattern would respond to the clonal growth of I. pseudacorus in successive waves of aerial sprouts coming from 20–30 cm long rhizomes. This clonal growth pattern has been described before for other clonal plant species [61,62,63].
Our results agree with other inter-continental studies on exotic plant species comparing their effects on plant communities in their native versus invaded ranges. In this sense, Centaurea melitensis L. (Asteraceae) was more dominant in invaded sites than in native ones, which may be related to different climatic and soil nutrient conditions [19]. Reference [20] reported that the richness of native species responded more negatively to three dominant species cover in its invaded rather than native range, suggesting this was the result of long-term coexistence and species filtering. Reference [23] also suggested that coevolutionary history between native-range neighbors may lead to different outcomes of the interactions between Chromolaena odorata (L.) R.M. King & H. Rob. (Asteraceae) and neighbor plants in its native and invaded ranges. Moroever, [21] recorded negative impacts between macrophyte richness and cover and Eichhornia crassipes Kunth (Pontederiaceae) biomass only in its invaded range and mainly on rare species. This was likely due to the role of invasive E. crassipes as an ecosystem engineer that decreases habitat heterogeneity.
To support ecological restoration, it is important to understand how the invasiveness (abundance) and community-level impacts of an invasive plant species vary in response to changing environmental conditions [64]. The simplification of plant communities within invasive I. pseudacorus patches (relatively low α-diversity) was also accompanied by a homogenization at the landscape scale (β-diversity) along the estuarine gradient that was reflected on dissimilarity indexes. Thus, we recorded lower Morisita–Horn index values and higher Sørensen index values when comparing areas colonized by I. pseudacorus among themselves than with areas free of I. pseudacorus, showing that I. pseudacorus invasion promoted similarity in the relative abundance of species and dissimilarity in species presence [51]. Results of recent studies of I. pseudacorus support updated risk assessments highlighting the continued high invasion potential of I. pseudacorus under sea level rise and other changing environmental conditions. For example, high production of viable seeds that withstand fresh to high aqueous salinity ranges [39] and rapid hydrochorous dispersal of buoyant I. pseudacorus seeds can be expected to continue to contribute to invasion spread [65]. In view of our results, urgent management practices are needed to control and eradicate I. pseudacorus from the inland Sacramento-San Joaquin Delta Estuary since this invasive macrophyte is reducing plant diversity at local and landscape scales along an extensive estuarine gradient. In this context, eradication and control efforts should initially prioritize impacted freshwater tidal areas in the upper inland Delta, where more low-abundant native species are being displaced by invasive I. pseudacorus. Timing of management actions prior to seed production is also crucial, to prevent dispersal and further spread of the exotic species downstream in the estuary.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d14050326/s1. Figure S1: Field pictures of sampled locations colonized by Iris pseudacorus in its native (Guadalquivir River Estuary, Andalusia, Spain, A1–A5) and the invaded range (Sacramento-San Joaquin Delta Estuary, CA, USA, C1–C5).; Figure S2: Relationship between the α-diversity (H′), plant species richness (S) and evenness (J′) with and without Iris pseudacorus in native (Guadalquivir Estuary) and invaded (San Francisco Bay-Delta Estuary) distribution ranges. Dashed lines indicate means values. Regression equations: A, y = 0.871 + 0.533 × (R = 0.598, p < 0.0001, n = 38); B, y = 0.548 + 0.405 × (R = 0.581, p < 0.0001, n = 39); C, y = 1.581 + 0.891 × (R = 0.445, p < 0.005, n = 38); D, y = 3.074 + 0.379 × (R = 0.624, p < 0.0001, n = 39); E, y = 0.764 + 0.132 × (R = 0.397, p = 0.015, n = 37).; Figure S3: Relationship between cover (%) of plant taxa in paired plots with and without invasive Iris pseudacorus in Iris-invaded study locations in tidal wetlands along an estuarine gradient in the San Francisco Bay-Delta Estuary (CA, USA). Regression equation: y = 4.076 + 0.467 × (R = 0.408, p < 0.0001, n = 75). Table S1: Geographical coordinates and number of study patches for locations colonized by native and invasive Iris pseudacorus populations. Locations are numbered from the most inland location to the closest to the sea; Table S2: Native and exotic plant taxa growing without and with Iris pseudacorus along the Guadalquivir River Estuary; Table S3: Native and exotic plant taxa growing without and with Iris pseudacorus along the Sacramento-San Joaquin Delta Estuary; Table S4: F-statistics and Pillai’s trace from MANOVAs for diversity indices for the factors distribution range (native and invaded), Iris pseudacorus presence (with and without Iris), locations (grouped in each range sequentially according to their distance to the ocean) and their interactions, with sediment electrical conductivity as covariate, including main effect and model degrees of freedom (d.f.); Table S5: General Linear Model or Generalized Linear Model for the Shannon–Wiener index (H′), species richness (S) and evenness (J′) comparing between distribution ranges (native and invaded), presence of Iris pseudacorus (with and without Iris) and locations (ordered in each range sequentially according to their distance to the ocean) and their corresponding interactions, with sediment electrical conductivity (EC) as covariate. Significant differences are marked in bold; Table S6: Student t-test or Man-Whitney U-test comparing Shannon– Wiener index (H′) (A), number of plant species (S) (B), and evenness (J′) (C) without and with Iris pseudacorus in its native (Guadalquivir Estuary) and invaded (Sacramento-San Joaquin Delta Estuary) distribution range, including all recorded species in the analyses. Significant differences are marked in bold. d.f., degrees of freedom; Table S7: Student t-test or Man-Whitney U-test comparing Shannon–Wiener index (H′) (A), number of plant species (S) (B), and evenness (J′) (C) without and with Iris pseudacorus in intertidal marshes within its native (Guadalquivir Estuary) and invaded (Sacramento-San Joaquin Delta Estuary) distribution range, including only native species in the analyses. Significant differences are marked in bold. d.f., degrees of freedom; Table S8: Generalized Linear Model for Morisita–Horn dissimilarity index comparing plant communities with and without Iris pseudacorus and between its distribution ranges (native and invaded); Table S9: Generalized Linear Model for Sørensen dissimilarity index comparing plant communities with and without Iris pseudacorus and between its distribution ranges (native and invaded); Table S10: Indicator values for the simultaneous occurrence of plant species with Iris pseudacorus (Irps) in intertidal marshes within its native and invaded ranges. A, Positive predictive power of species combinations; B, Sensitivity of species combinations; sqrtIV, Square root of indicator value of species combinations; P, P-value of the permutation test of statistical significance. We show results with sqrtIV > 0.05.
Author Contributions
Conceptualization, B.G.-T., B.J.G., C.R.W., J.C.F. and J.M.C.; methodology, B.J.G., G.B.-M., J.C.F. and J.M.C.; formal analysis, B.G.-T., B.J.G., G.B.-M. and J.M.C.; writing—original draft preparation, B.G.-T., B.J.G. and J.M.C.; writing—review and editing, B.G.-T., B.J.G., C.R.W., J.C.F. and J.M.C.; funding acquisition, B.J.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by the U.S. Department of Agriculture, Agricultural Research Service. USDA is an equal opportunity provider and employer. Mention of trade names or commercial products is solely to provide specific information and does not imply recommendation or endorsement by USDA.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are openly available in FigShare. Available online: https://doi.org/10.6084/m9.figshare.19453667 (accessed on 21 April 2022).
Acknowledgments
The authors thank Jessica Drost, Christopher McCort and Christy Morgan (USDA); Anita Arenas (California State University Long Beach) and Francisca Real-Castro and Lourdes Bernal-Galiano (Universidad de Sevilla) for field assistance. We thank California State Parks, for a scientific research and collection permits and access to Brannon Island and Benicia State Recreation Areas.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jaggi, D.; Varun, M.; Pagare, S.; Tripathi, N.; Rathore, M.; Singh, R.; Kumar, B. Invasive Alien Weed Species: A Threat to Plant Biodiversity. Ansari, Plant Biodiversity. In Monitoring, Assessment and Conservation; CABI: Wallingford, UK, 2017; pp. 564–592. [Google Scholar]
- Pyšek, P.; Jarošík, V.; Hulme, P.E.; Pergl, J.; Hejda, M.; Schaffner, U.; Vilà, M. A global assessment of invasive plant impacts on resident species, communities and ecosystems: The interaction of impact measures, invading species’ traits and environment. Glob. Chang. Biol. 2017, 18, 1725–1737. [Google Scholar] [CrossRef]
- Melbourne, B.A.; Cornell, H.V.; Davies, K.F.; Dugaw, C.J.; Elmendorf, S.; Freestone, A.L.; Hall, R.J.; Harrison, S.; Hastings, A.; Holland, M.; et al. Invasion in a heterogeneous world: Resistance, coexistence or hostile takeover? Ecol. Lett. 2007, 10, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Carboni, M.; Livingstone, S.W.; Isaac, M.E.; Cadotte, M.W. Invasion drives plant diversity loss through competition and ecosystem modification. J. Ecol. 2021, 109, 3587–3601. [Google Scholar] [CrossRef]
- Dong, L.-J.; Yu, H.-W.; He, W.-M. What determines positive, neutral and negative impacts of Solidago canadensis invasion on native plant species richness? Sci. Rep. 2015, 5, 16804. [Google Scholar] [CrossRef]
- Chen, G.-Q.; Zhang, C.-B.; Ma, L.; Qiang, S.; Silander, J.A.; Qi, L.L. Biotic Homogenization Caused by the Invasion of Solidago canadensis in China. J. Integr. Agric. 2013, 12, 835–845. [Google Scholar] [CrossRef]
- Iacarella, J.C.; Mankiewicz, P.; Ricciardi, A. Negative competitive effects of invasive plants change with time since invasion. Ecosphere 2015, 6, 1–14. [Google Scholar] [CrossRef]
- Elton, C.S. The Ecology of Invasions by Animals and Plants; Methuen and Company: London, UK, 1958. [Google Scholar]
- Case, T.J. Invasion resistance arises in strongly interacting species-rich model competition communities. Proc. Natl. Acad. Sci. USA 1990, 87, 9610–9614. [Google Scholar] [CrossRef]
- Muthukrishnan, R.; Hansel-Welch, N.; Larkin, D.J. Environmental filtering and competitive exclusion drive biodiversity-invasibility relationships in shallow lake plant communities. J. Ecol. 2018, 106, 2058–2070. [Google Scholar] [CrossRef]
- Peng, S.; Kinlock, N.L.; Gurevitch, J.; Peng, S. Correlation of native and exotic species richness: A global meta-analysis finds no invasion paradox across scales. Ecology 2019, 100, e02552. [Google Scholar] [CrossRef]
- Gribben, P.E.; Poore, A.G.B.; Thomsen, M.S.; Quesey, P.; Weschke, E.; Wright, J.T. Habitat provided by native species facilitates higher abundances of an invader in its introduced compared to native range. Sci. Rep. 2020, 10, 6385. [Google Scholar] [CrossRef]
- Stohlgren, T.J.; Rejmánek, M. No universal scale-dependent impacts of invasive species on native plant species richness. Biol. Lett. 2014, 10, 20130939. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.L.; Burns, J.H.; Liao, Z.Y.; Li, Y.P.; Yang, J.; Chen, Y.J.; Zhang, J.L.; Zheng, Y.G. Species composition, functional and phylogenetic distances correlate with success of invasive Chromolaena odorata in an experimental test. Ecol. Lett. 2018, 21, 1211–1220. [Google Scholar] [CrossRef] [PubMed]
- Kuebbing, S.E.; Nuñez, M. Invasive non-native plants have a greater effect on neighbouring natives than other non-natives. Nat. Plants 2016, 2, 16134. [Google Scholar] [CrossRef] [PubMed]
- Levine, J.M.; Adler, P.B.; Yelenik, S.G. A meta-analysis of biotic resistance to exotic plant invasions. Ecol. Lett. 2004, 7, 975–989. [Google Scholar] [CrossRef]
- Braga, R.R.; Gómez-Aparicio, L.; Heger, T.; Vitule, J.R.S.; Jeske, J.M. Invasion Meltdown Hypothesis. In Invasion Biology: Hypotheses and Evidence; Jeske, J.M., Heger, T., Eds.; CABI Invasive Species Compendium: Oxfordshire, UK, 2018; pp. 79–91. [Google Scholar] [CrossRef]
- Braga, R.R.; Gómez-Aparicio, L.; Heger, T.; Vitule, J.R.S.; Jeschke, J.M. Structuring evidence for invasional meltdown: Broad support but with biases and gaps. Biol. Invasions 2018, 20, 923–936. [Google Scholar] [CrossRef]
- Moroney, J.R.; Rundel, P.W. Abundance and dispersion of the invasive Mediterranean annual, Centaurea melitensis in its native and non-native ranges. Biol. Invasions 2013, 15, 495–507. [Google Scholar] [CrossRef]
- Hejda, M.; Štajerová, K.; Pyšek, P. Dominance has a biogeographical component: Do plants tend to exert stronger impacts in their invaded rather than native range? J. Biogeogr. 2017, 44, 18–27. [Google Scholar] [CrossRef]
- Lolis, L.A.; Alves, D.C.; Fan, S.F.; Lv, T.; Yang, L.; Li, Y.; Liu, C.H.; Yu, D.; Thomaz, S.M. Negative correlations between native macrophyte diversity and water hyacinth abundance are stronger in its introduced than in its native range. Divers. Distrib. 2020, 26, 242–253. [Google Scholar] [CrossRef]
- Taylor, K.T.; Maxwell, B.D.; Pauchard, A.; Nuñez, M.A.; Rew, L.J. Native versus non-native invasions: Similarities and differences in the biodiversity impacts of Pinus contortain introduced and native ranges. Divers. Distrib. 2016, 22, 578–588. [Google Scholar] [CrossRef]
- Zheng, Y.; Liao, Z. High-density native-range species affects the invasive plant Chromolaena odorata more strongly than species from its invasive range. Sci. Rep. 2017, 7, 16075. [Google Scholar] [CrossRef]
- Bossdorf, O.; Lipowsky, A.; Prati, D. Selection of preadapted populations allowed Senecio inaequidens to invade Central Europe. Divers. Distrib. 2008, 14, 676–685. [Google Scholar] [CrossRef]
- Harrison, S.; Spasojevic, M.J.; Li, D. Climate and plant community diversity in space and time. Proc. Natl. Acad. Sci. USA 2020, 117, 4464–4470. [Google Scholar] [CrossRef] [PubMed]
- GBIF.org Global Biodiversity Information Facility. GBIF Occurrence Download. Available online: https://www.gbif.org/es/ (accessed on 15 March 2022).
- Minuti, G.; Stiers, I.; Coetzee, J.A. Climatic suitability and compatibility of the invasive Iris pseudacorus L. (Iridaceae) in the Southern Hemisphere: Considerations for biocontrol. Biol. Control. 2022, 169, 104886. [Google Scholar] [CrossRef]
- POWO. Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. Published on the Internet. 2022. Available online: http://www.plantsoftheworldonline.org (accessed on 15 March 2022).
- Panetta, F.D.; Gooden, B. Managing for biodiversity: Impact and action thresholds for invasive plants in natural ecosystems. NeoBiota 2017, 34, 53–66. [Google Scholar] [CrossRef]
- Mopper, S.; Wiens, K.C.; Goranova, G.A. Competition, salinity, and clonal growth in native and introduced irises. Am. J. Bot. 2016, 103, 1575–1581. [Google Scholar] [CrossRef]
- Hayasaka, D.; Fujiwara, S.; Uchida, T. Impacts of invasive Iris pseudacorus L. (yellow flag) establishing in an abandoned urban pond on native semi-wetland vegetation. J. Integr. Agric. 2018, 17, 1881–1887. [Google Scholar] [CrossRef]
- Whitcraft, C.R.; Talley, D.M.; Crooks, J.A.; Boland, J.; Gaskin, J. Invasion of tamarisk (Tamarix spp.) in a southern California salt marsh. Biol. Invasions 2007, 9, 875–879. [Google Scholar] [CrossRef]
- Fleming, J.P.; Dibble, E.D. Ecological mechanisms of invasion success in aquatic macrophytes. Hydrobiologia 2015, 746, 23–37. [Google Scholar] [CrossRef]
- Hierro, J.L.; Maron, J.L.; Callaway, R.M. A biogeographical approach to plant invasions: The importance of studying exotics in their introduced and native range. J. Ecol. 2004, 93, 5–15. [Google Scholar] [CrossRef]
- Guo, Q. Intercontinental biotic invasions: What can we learn from native populations and habitats? Biol. Invasions 2006, 8, 1451–1459. [Google Scholar] [CrossRef][Green Version]
- NCEI. NOAA’S National Centers for Environmental Information. 2020. Available online: https://www.ncdc.noaa.gov/ (accessed on 13 November 2020).
- Rubtzoff, P. Iris pseudacorus and Caltha palustris in California. Leaf West Bot. 1959, 9, 31–32. [Google Scholar]
- Light, T.; Grosholz, T.; Moyle, P. Delta Ecological Survey (Phase I): Nonindigenous Aquatic Species in the Sacramento-San Joaquin Delta, a Literature Review; US Fish and Wildlife Service: Stockton, CA, USA, 2005.
- Gillard, M.B.; Castillo, J.M.; Mesgaran, M.B.; Futrell, C.J.; Grewell, B.J. High aqueous salinity does not preclude germination of invasive Iris pseudacorus from estuarine populations. Ecosphere 2021, 12, e03486. [Google Scholar] [CrossRef]
- Díez-Minguito, M.; Baquerizo, A.; Sánchez, M.O.; Navarro, G.; Losada, M. Tide transformation in the Guadalquivir estuary (SW Spain) and process-based zonation. J. Geophys. Res. Earth Surf. 2012, 117. [Google Scholar] [CrossRef]
- AEMET. AEMET OpenData. 2020. Available online: https://opendata.aemet.es/centrodedescargas/inicio (accessed on 27 November 2020).
- La Peyre, M.K.G.; Grace, J.B.; Hahn, E.; Mendelssohn, I.A. The importance of competition in regulating plant species abundance along a salinity gradient. Ecology 2001, 82, 62–69. [Google Scholar] [CrossRef]
- Grewell, B.J.; Gallego-Tévar, B.; Gillard, M.B.; Futrell, C.J.; Reicholf, R.; Castillo, J.M. Salinity and inundation effects on Iris pseudacorus: Implications for tidal wetland invasion with sea level rise. Plant Soil 2021, 466, 275–291. [Google Scholar] [CrossRef]
- Castillo, J.M.; Gallego-Tévar, B.; Castellanos, E.M.; Figueroa, M.E.; Davy, A.J. Primary succession in an Atlantic salt marsh: From intertidal flats to mid-marsh platform in 35 years. J. Ecol. 2021, 109, 2909–2921. [Google Scholar] [CrossRef]
- Baldwin, B.G.; Goldman, D.H.; Keil, D.J.; Patterson, R.; Rosatti, T.J.; Wilken, D.H. The Jepson Manual: Vascular Plants of California, 2nd ed.; University of California Press: Berkeley, CA, USA, 2012; p. 1600. [Google Scholar]
- Flora of North America Editorial Committee. Flora of North America North of Mexico; Oxford University Press: New York, NY, USA; Oxford, UK, 1993. [Google Scholar]
- Castroviejo, S. Flora ibérica: Plantas Vasculares de la Península Ibérica e Islas Baleares; Real Jardín Botánico, CSIC: Madrid, Spain. 1986–2012; p. 784. Available online: http://www.floraiberica.es/ (accessed on 29 March 2022).
- Valdés, B.; Talavera, S.; Fernández-Galiano, E. Flora Vascular de Andalucía Occidental; Ketres Editora S.A.: Barcelona, Spain, 1987. [Google Scholar]
- Magurran, A.E. Ecological Diversity and Its Measurement; Princeton University Press: Princeton, NJ, USA, 1988. [Google Scholar]
- Oksanen, J.; Blanchet, M.F.; Friendly, R.; Kindt, P.; Legendre, D.; McGlinn, P.; Minchin, P.R.; O’hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. 2018. Available online: http://cran.r-project.org/package=vegan (accessed on 1 July 2021).
- Jost, L.; Chao, A.; Chazdon, R. Compositional Similarity and Beta Diversity. Biological Diversity: Frontiers in Measurement and Assessment; Magurran, A., McGill, B.J., Eds.; Oxford University Press: Oxford, UK, 2011; pp. 66–84. [Google Scholar]
- De Cáceres, M.; Legendre, P.; Wiser, S.; Brotons, L. Using species combinations in indicator value analyses. Methods Ecol. Evol. 2012, 3, 973–982. [Google Scholar] [CrossRef]
- Dufrêne, M.; Legendre, P. Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- Ng, V.K.; Cribbie, R.A. Using the Gamma Generalized Linear Model for Modeling Continuous, Skewed and Heteroscedastic Outcomes in Psychology. Curr. Psychol. 2016, 36, 225–235. [Google Scholar] [CrossRef]
- Scheiner, S. Multiple Response Variables and Multi-Species Interactions. In Design and Analysis of Ecological Experiments, 2nd ed.; Scheiner, S., Gurevitch, J., Eds.; Oxford University Press: Oxford, UK, 2001; pp. 99–115. [Google Scholar]
- Ungar, I.A. Are biotic factors significant in influencing the distribution of halophytes in saline habitats? Bot. Rev. 1998, 74, 176–199. [Google Scholar] [CrossRef]
- Hejda, M.; Pyšek, P.; Jarošík, V. Impact of invasive plants on the species richness, diversity and composition of invaded communities. J. Ecol. 2009, 97, 393–403. [Google Scholar] [CrossRef]
- Jelbert, K.; Stott, I.; McDonald, R.A.; Hodgson, D. Invasiveness of plants is predicted by size and fecundity in the native range. Ecol. Evol. 2015, 5, 1933–1943. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.; Lishawa, S.C.; Newman, S.; Tangen, B.A.; Wilcox, D.; Albert, D.; Anteau, M.J.; Chimney, M.J.; Cressey, R.L.; DeKeyser, E.; et al. Typha (Cattail) Invasion in North American Wetlands: Biology, Regional Problems, Impacts, Ecosystem Services, and Management. Wetlands 2019, 39, 645–684. [Google Scholar] [CrossRef]
- Castillo, J.M.; Gallego-Tévar, B.; Figueroa, E.; Grewell, B.J.; Vallet, D.; Rousseau, H.; Keller, J.; Lima, O.; Dréano, S.; Salmon, A.; et al. Low genetic diversity contrasts with high phenotypic variability in heptaploid Spartina densiflora populations invading the Pacific coast of North America. Ecol. Evol. 2018, 8, 4992–5007. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, P.-A. The Spatial Development of Spartina Colonies Growing without Competition. Ann. Bot. 1957, 21, 203–214. [Google Scholar] [CrossRef]
- Castellanos, E.M.; Figueroa, M.E.; Davy, A.J. Nucleation and Facilitation in Saltmarsh Succession: Interactions between Spartina Maritima and Arthrocnemum Perenne. J. Ecol. 1994, 82, 239–248. [Google Scholar] [CrossRef]
- Castillo, J.M.; Rubio-Casal, A.E.; Luque, T.; Figueroa, M.E.; Jiménez-Nieva, F.J. Intratussock tiller distribution and biomass of Spartina densiflora Brongn. in an invaded salt marsh. Lagascalia 2003, 23, 61–73. [Google Scholar]
- Drenovsky, R.E.; Grewell, B.J.; D’Antonio, C.M.; Funk, J.L.; James, J.J.; Molinari, N.; Parker, I.M.; Richards, C. A functional trait perspective on plant invasion. Ann. Bot. 2012, 110, 141–153. [Google Scholar] [CrossRef]
- Gaskin, J.F.; Pokorny, M.L.; Mangold, J.M. An unusual case of seed dispersal in an invasive aquatic; yellow flag iris (Iris pseudacorus). Biol. Invasions 2016, 7, 2067–2075. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).