Abstract
Biological imaging is an essential tool to visualise and obtain reference data. In this context, the programme ImageJ has been widely used in many disciplines to determine the surface areas of planar biological samples in marine and aquatic experimental biology. Despite its range of advantages, ImageJ is relatively time-consuming, because of the need to manually select the target areas for quantification. Hence, we here evaluated the freeware programme Photopea as a potential alternative by comparing the accuracy and time required for the surface area quantification of exemplary algae compared with established ImageJ analysis. Our results show that Photopea is equally accurate as ImageJ, but 45% more time efficient. This time efficiency originates from using colour contrast that reduces the time needed to analyse each picture. Photopea thus offers an accurate, rapid, and cost-free tool to easily obtain reference data from field and laboratory experiments. This tool is particularly useful for experiments with an extensive sample size of specimens and thus can increase the power of study results.
1. Introduction
Quantitative and computational imaging has evolved at an unprecedented pace, offering seemingly limitless possibilities for a wide range of scientific fields. Particularly within the field of biological imaging, novel computational technologies together with tools in microscopy [1], mass spectrometry [2] and others [3] are currently a powerful way to make scientific progress accessible and visible. A key player in the field of biological imaging is the pioneer scientific image processing programme ‘ImageJ’ (previously known as NIH Image) first introduced in 1987 [4,5]. The reasons for the programme’s success are as wide-ranging as the programme’s span of applications, proven by its almost 16,000 citations (to date), its improvements over the years through the introduction of new plug-ins, its straightforward handling and by being an open access software [3,4].
ImageJ is commonly used in the field of aquatic and marine experimental biology, for example, to quantify coral tissue loss [6] or to determine the surface area of biological specimens [7,8]. Concerning the latter, biological specimens are often irregularly shaped, and so are the parts of the images to be analysed. ImageJ offers a range of image processing tools; however, accuracy may vary, and it is particularly time-consuming for irregular, cryptically shaped and coloured images.
One of many underlying (and highly discussed) pillars of science is the concept of either direct (i.e., identical experimental conditions) or conceptual (i.e., adjusted experimental conditions) replication [9]. Despite still being discussed, a consensus on the need to have a considerable number of biological replicates in the field of aquatic and marine experimental biology is shared among scientists [9]. This subsequently increases the generated data that are commonly referred to as specimens surface area to allow further statistical analysis, and hence, comparison to other studies, organisms or experiments. Thus, the acquirement of this relevant data (i.e., surface area) is often as time-consuming as it is essential.
Conclusively, biological imaging techniques and technologies are key tools to determine the surface area of aquatic and marine biological substrates and organisms. Here, we introduce a method for processing images of planar aquatic/marine organisms/substrates using the programme Photopea as an alternative to the commonly used approach for surface area quantification with ImageJ. To illustrate the applicability of the new tool, Photopea, as a potential alternative to the established tool of ImageJ, we determined the surface area of 12 images of two exemplary and freshly collected Mediterranean algal species of different shapes using both the established ImageJ and the novel Photopea tools. Both algal species show a three-dimensional erect growth form that relies on the presence of water to grow vertically. Once removed from its natural environment, the algal material is flattened and can be analysed to obtain the planar substrate surface area.
2. Materials and Methods
2.1. Description of Exemplary Study Substrates
We used two exemplary algae species of different growth forms for this study: the mat-building red alga Phyllophora crispa that produces fleshy thalli of up to 15 cm length [10], and the canopy-forming brown alga Cystoseira sp. that builds fine filaments [11]. Benthic algal samples were collected via SCUBA diving from the Punta Fenaio dive site (42°23′19.98″ N 10°52′47.92″ E) on the north-western coast of Giglio Island, Tyrrhenian Sea, Italy, in September 2021. Both algal species can be considered exemplary representatives for other marine or aquatic specimens that do not contain hard skeletons (i.e., leaves, seaweeds, seagrasses).
2.2. Data Collection
To quantify the surface area of the two algal species used in the present study, algal material (i.e., P. crispa and Cystoseira sp., n = 6 each) of 5 to 9 g wet weight was laid out on a laminated grid DIN A4 paper with all branches carefully separated and thinned to one branch layer thick with the addition of ca. 10 mL water over the paper, and then pressed with a glass pane so that all parts were visible and compressed into a two-dimensional shape (i.e., planar surface). Pictures were taken with an Apple iPhone 11 Pro smartphone (12.00 megapixels resolution) from the top with a 90° angle within the A4 frame. These pictures were processed for surface area quantification with both ImageJ (according to Abràmoff et al., 2004 [4]) and Photopea (Figure 1). For the latter, we refer to Supplementary Material S1 for a step-by-step protocol and descriptive images. Briefly, images were uploaded to the Photopea webpage (https://www.photopea.com, accessed on 28 October 2021) before using the “Color Range” tool that automatically selects the algal material due to its different colour hues compared to the white background. By dividing the number of pixels of the algal specimen by the pixel number of a scale area, the final surface area value was obtained. For the present study, three persons with different levels of competency (beginner to advanced in image processing) in using the two programs determined the surface area of each alga. The time required for the surface area determination of each image was recorded accordingly. Images of the respective algal species were processed consecutively, starting with Cystoseira sp. I and ending with P. crispa VI (see Table 1).
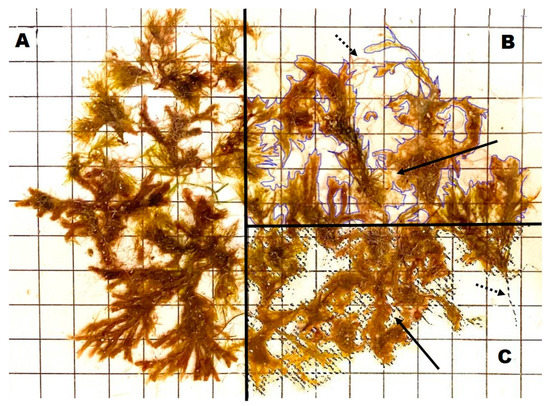
Figure 1.
Flattened Cystoseira sp. specimen (A) with manual selection using ImageJ (blue lines, (B)) and automated selection using Photopea (dashed lines, (C)). Bold arrows highlight included (B) and excluded (C) areas within the selected area. Dashed arrows highlight delicate algal filaments that are not included using ImageJ (B), but are automatically selected using Photopea (C).

Table 1.
Mean surface area and mean time saved to determine the surface area of Cystoseira sp. (Cys sp.) and Phyllophora crispa (P. crispa) samples (n = 6) using ImageJ and Photopea.
2.3. Statistical Analysis
The data were analysed using the software SigmaPlot (version 12.0) by testing the normality (Shapiro–Wilk test) and differences between the time required to obtain the surface area data using either ImageJ or Photopea. Two-tailed t-tests were used if the data were distributed normally, whereas Mann–Whitney rank sum tests were used if the data were not normally distributed.
3. Results
We were able to quantify the surface area of both Cystoseira sp. and P. crispa samples with surface areas being in a similar range, i.e., of equal accuracy (Mann–Whitney rank sum test with T = 1371, p = 0.525) using ImageJ compared to areas determined using Photopea (Table 1). The total as well as the average time required to determine the surface areas using ImageJ (total 170:27 min:ss, mean 14:12 ± 0:24 min:ss; Table 1) were significantly higher compared to the time required using Photopea (total 87:51 min:ss, mean 7:19 ± 00:18 min:ss, respectively; Table 1) (Mann–Whitney rank sum test with T = 705, p < 0.001). On average, about 45% less time was spent on the surface area quantification using Photopea compared to ImageJ. The standard error of the determined surface areas did not differ significantly between Photopea and ImageJ, i.e., both tools show a similar robustness in terms of observer bias.
4. Discussion
We were able to demonstrate that the surface areas obtained with ImageJ were similar, i.e., not statistically different, compared to those obtained with Photopea. The minor discrepancies occurring between the surface areas obtained from a single image can be clearly explained by the tools available within the respective programs that select the parts of the image that encompass exclusively and entirely the organism/substrate, and are considered in the surface area calculation (see Figure 1). Whereas this needs to be done manually with ImageJ, we make use of a colour range selection tool in Photopea that uses the colour contrasts of the image to automatically select the algal material and calculate the number of image pixels representative of the totality of the specimen to finally derive the surface area through a simple formula.
This method, using the Photopea programme offers three core comparative advantages to ImageJ.
Firstly, the automated selection using the colour range selection tool in Photopea allows a more efficient (i.e., time and labour saving) image processing and subsequent surface area determination than the manual selection in ImageJ. This is reflected in our study results (see Table 1), whereby we demonstrated that the time required for both previously mentioned tasks in Photopea is 45% shorter compared to ImageJ. This time difference is only larger when considering the Cystoseira sp. samples (up to 57%), likely due to their complex spatial arrangement. Furthermore, the user with a beginner competency who analysed the images with Photopea progressively reduced the time spent over the entire course of the image analysis (Person A, see Supplementary Table S1).
Secondly, the aforementioned tool in Photopea allows for an accurate selection compared to the manual selection in ImageJ for organisms/substrates that are cryptic or have a complex shape (Figure 1, step 4 in the Supplementary Materials S1). For Photopea, the programme automatically distinguishes the specimen from the background in the image and selects the specimen in its entirety, excluding the background, without the need for major fine-tuning of the total selection. Although the validation of the selection is conducted by visually checking the similitude between the original image and the selection and by making small adjustments of the selection if necessary to further improve its accuracy for surface area quantification, Photopea presents a superior tool for this type of image processing and quantification. When targeting organisms/substrates of increasing shape complexity (e.g., from simply flattened thalli to branched filamentous algae), manual selection in ImageJ becomes more difficult and prone to human error, which potentially leads to an overestimation of the total surface area. This effect of shape complexity was observed in our surface area quantification with more time spent using ImageJ than in Photopea, particularly for the Cystoseira sp. (i.e., branched filaments) samples, which have more complex shapes than the P. crispa (i.e., flattened thalli) samples (see Figure 1). However, the Cystoseira sp. samples presented a higher number of small interstitial spaces in the order of <1 mm in between branches, which is virtually impossible and laborious to remove from the manual selection in ImageJ for the surface area quantification. Nevertheless, these fine compartments of Cystoseira sp. seem not to contribute significantly to the overall surface area in the present study, as no significant differences were observed regarding the surface areas obtained with both tools. We hypothesise that this can be the case when comparing extremely complex organisms/substrates with more biomass (e.g., exceeding wet weights used in the present study) and postulate that the automated selection tool in Photopea is beneficial for these cases.
Thirdly, the task of image processing and surface area determination can be performed by individuals with different levels of competency in using the programme, while producing analogous results. The method using Photopea offers a comfortable measurement with similar variability compared to ImageJ and, thus, enables a reliable statistical analysis and the reproducibility of the results and experiment. Ultimately, the use of the newly proposed method promotes the integrity of research in aquatic/marine experimental biology.
Based on these three aspects, we conclude that Photopea offers not only a more time- and labour-efficient, but also an accurate alternative to ImageJ. It is noteworthy that our analyses would have produced even more indisputable values for the surface area and time required for processing and quantification if we had used a solely white background for the pictures. The grid background generated some confusion during the automated selection in Photopea as the programme identified at certain points the grid as algal material, and thus included some lines in the total selection. To create a better contrast between the background and the sample and facilitate an accurate automated selection during image processing, we suggest using a colour and pattern neutral (e.g., white with no grid) background with a distinct scale for reference when taking the pictures.
Nevertheless, both Photopea and ImageJ share some properties, such as being open access and intuitive to operate. Whereas ImageJ needs to be downloaded and installed according to the user operating system (e.g., Microsoft and macOS), Photopea is a web-based programme (i.e., no installation, but internet access required) that can be accessed from any device; however, it does not upload any file to external servers and stores all files locally. Both programs support a range of common image file types such as .jpeg, .png, .svg, .tiff, and others. Photopea displays an easy interface, particularly for those who are familiar with the professional image editing software Adobe Photoshop.
Although both programmes exhibit good performance in image processing, they are limited to the surface area quantification of planar (i.e., two-dimensional) organisms and substrates. However, compared to other approaches to determine the surface areas of planar organisms and substrates, for example, via simple geometry [12], we believe that both Photopea and ImageJ display more accurate and time-efficient solutions. This particularly holds true in the case of irregular, filamentous or rather cryptic substrates and organisms, as the simple geometry approach for which specimens are (partially) interpreted as geometric figures. For example, for the comparatively simple disc-shaped Fungiidae coral specimens, the oral and aboral disc surface areas were calculated as circles, and subsequently multiplied by the height of the specimens, which can be classified as a rather intermediate time investment per sample (see Table 2). However, allocating geometric figures to the algal specimens of the present study is doubtless a rather complex endeavour, as particularly fine filaments cannot be allowed for (Figure 1). Taken together, all aforementioned approaches are not limited to specimens of the marine environment, but organisms and substrates can be from terrestrial or aquatic ecosystems, which underlines the versatile applicability of the introduced tool.

Table 2.
Comparative overview regarding the costs, time investment, accuracy and efficiency of various approaches to determine surface areas of two- and three-dimensional biological specimens.
Both Photopea and ImageJ are tools that—without any add-ons—are still limited to two-dimensional specimens. Thus, the visualization and selection of three-dimensional structures, such as the surface area of corals or seagrass roots, is not yet possible. To quantify the surface areas of three-dimensional specimens, the advanced geometry [12] or cloud-based modelling [13,14] approaches offer versatile solutions. However, two-dimensional approaches could overcome the aforementioned limitation by using additional tools such as recently described approaches to predict three-dimensional surface areas from the two-dimensional planar surface areas of corals [15].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d14040272/s1. Supplementary Materials consist of step-by-step protocol for the surface area determination via Photopea (Supplementary Materials S1) and acquired raw data (Supplementary Table S1).
Author Contributions
Y.C.E.-K. and C.W.; methodology, Y.C.E.-K., A.K.L. and S.D.M.; software, Y.C.E.-K., S.D.M. and A.K.L.; validation, Y.C.E.-K. and C.W.; formal analysis, Y.C.E.-K.; investigation, Y.C.E.-K., A.K.L. and S.D.M.; resources, Y.C.E.-K. and C.W.; data curation, Y.C.E.-K., A.K.L. and S.D.M.; writing—original draft preparation, Y.C.E.-K.; writing—review and editing, Y.C.E.-K., A.K.L., S.D.M. and C.W.; visualization, Y.C.E.-K. and S.D.M.; supervision, C.W.; project administration, Y.C.E.-K. and C.W.; funding acquisition, C.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by baseline funding of University of Bremen and DFG project Wi 2677/16-1.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
All data used in the present study can be found in the Supplementary Material.
Acknowledgments
We are thankful to Jenny Tucek and Mischa Schwarzmeier from the Institute of Marine Biology, Giglio Island, Italy, for the maintenance of the algal specimens. Furthermore, we thank Luca Kler Lago for his support in developing the step-by-step protocol, and Hannah Sophie Börner for her support in quantifying the surface areas of algae using ImageJ and Photopea.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Müller, M.; Mönkemöller, V.; Hennig, S.; Hübner, W.; Huser, T. Open-source image reconstruction of super-resolution structured illumination microscopy data in ImageJ. Nat. Commun. 2016, 7, 10980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ward, A.M.; Bidet, K.; Yinglin, A.; Ler, S.G.; Hogue, K.; Blackstock, W.; Gunaratne, J.; Garcia-Blanco, M.A. Quantitative mass spectrometry of DENV-2 RNA-interacting proteins reveals that the DEAD-box RNA helicase DDX6 binds the DB1 and DB2 3′ UTR structures. RNA Biol. 2011, 8, 1173–1186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Abràmoff, M.D.; Magalhães, P.J.; Ram, S.J. Image processing with imageJ. Biophotonics Int. 2004, 11, 36–41. [Google Scholar] [CrossRef]
- Rasband, W. ImageJ. 1997. [Google Scholar]
- Wiedenmann, J.; D’Angelo, C.; Smith, E.G.; Hunt, A.N.; Legiret, F.E.; Postle, A.D.; Achterberg, E.P. Nutrient enrichment can increase the susceptibility of reef corals to bleaching. Nat. Clim. Chang. 2013, 3, 160–164. [Google Scholar] [CrossRef]
- Helber, S.B.; Winters, G.; Stuhr, M.; Belshe, E.F.; Bröhl, S.; Schmid, M.; Reuter, H.; Teichberg, M. Nutrient History Affects the Response and Resilience of the Tropical Seagrass Halophila stipulacea to Further Enrichment in Its Native Habitat. Front. Plant Sci. 2021, 12, 678341. [Google Scholar] [CrossRef] [PubMed]
- Bednarz, V.N.; Cardini, U.; Van Hoytema, N.; Al-Rshaidat, M.M.D.; Wild, C. Seasonal variation in dinitrogen fixation and oxygen fluxes associated with two dominant zooxanthellate soft corals from the northern Red Sea. Mar. Ecol. Prog. Ser. 2015, 519, 141–152. [Google Scholar] [CrossRef]
- Machery, E. What is a replication? Philos. Sci. 2020, 87, 545–567. [Google Scholar] [CrossRef]
- Zaitsev, Y. An Introduction to the Black Sea Ecology; Smil Edition and Publishing Agency Ltd.: Odessa, Ukraine, 2008; ISBN 9789668127830. [Google Scholar]
- Thibaut, T.; Pinedo, S.; Torras, X.; Ballesteros, E. Long-term decline of the populations of Fucales (Cystoseira spp. and Sargassum spp.) in the Albères coast (France, North-western Mediterranean). Mar. Pollut. Bull. 2005, 50, 1472–1489. [Google Scholar] [CrossRef] [PubMed]
- Naumann, M.S.; Niggl, W.; Laforsch, C.; Glaser, C.; Wild, C. Coral surface area quantification-evaluation of established techniques by comparison with computer tomography. Coral Reefs 2009, 28, 109–117. [Google Scholar] [CrossRef]
- Gutierrez-Heredia, L.; Benzoni, F.; Murphy, E.; Reynaud, E.G. End to End Digitisation and Analysis of Three-Dimensional Coral Models, from Communities to Corallites. PLoS ONE 2016, 11, e0149641. [Google Scholar] [CrossRef] [PubMed]
- Lavy, A.; Eyal, G.; Neal, B.; Keren, R.; Loya, Y.; Ilan, M. A quick, easy and non-intrusive method for underwater volume and surface area evaluation of benthic organisms by 3D computer modelling. Methods Ecol. Evol. 2015, 6, 521–531. [Google Scholar] [CrossRef]
- House, J.E.; Brambilla, V.; Bidaut, L.M.; Christie, A.P.; Pizarro, O.; Madin, J.S.; Dornelas, M. Moving to 3D: Relationships between coral planar area, surface area and volume. PeerJ 2018, 2018, e4280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).