Incorporating Effect Factors into the Relationship between Biodiversity and Ecosystem Functioning (BEF)
Abstract
:1. Introduction
2. Community-Related Factors Affecting BEF
2.1. Community Structure
2.2. Trophic Level
2.3. Parasite-Host Relations
3. External Factors Affecting BEF
3.1. Effects of Environmental Factors on BEF
3.2. Effects of Climate Change on BEF
4. Spatial and Temporal Scale Effects on BEF
4.1. Spatial Scale Effects on BEF
4.2. Effects of Temporal Scale on BEF
5. Generating-Presentation Model
5.1. Model Significance and Structure
5.2. Generating Layer
5.3. Presentation Layer
5.4. Effect Layer
6. Discussion
6.1. Measuring Factors Affecting EFs
6.2. Integrating Factors Affecting BEF with the Generating-Presentation Model
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paul, C.; Hanley, N.; Meyer, S.T.; Fürst, C.; Weisser, W.W.; Knoke, T. On the functional relationship between biodiversity and economic value. Sci. Adv. 2020, 6, eaax7712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loreau, M.; Oteng-Yeboah, A.; Arroyo, M.T.K.; Babin, D.; Barbault, R.; Donoghue, M.; Gadgil, M.; Hauser, C.; Heip, C.; Larigauderie, A.; et al. Diversity without representation. Nature 2006, 442, 245–246. [Google Scholar] [CrossRef] [PubMed]
- van der Plas, F. Biodiversity and ecosystem functioning in naturally assembled communities. Biol. Rev. Camb. Philos. Soc. 2019, 94, 1220–1245. [Google Scholar] [CrossRef] [PubMed]
- Isbell, F.; Calcagno, V.; Hector, A.; Connolly, J.; Harpole, W.S.; Reich, P.B.; Scherer-Lorenzen, M.; Schmid, B.; Tilman, D.; van Ruijven, J.; et al. High plant diversity is needed to maintain ecosystem services. Nature 2011, 477, 199–202. [Google Scholar] [CrossRef]
- Leibold, M.A.; Chase, J.M.; Ernest, S.K.M. Community assembly and the functioning of ecosystems: How metacommunity processes alter ecosystems attributes. Ecology 2017, 98, 909–919. [Google Scholar] [CrossRef]
- Jaillard, B.; Rapaport, A.; Harmand, J.; Brauman, A.; Nunan, N. Community assembly effects shape the biodiversity-ecosystem functioning relationships. Funct. Ecol. 2014, 28, 1523–1533. [Google Scholar] [CrossRef]
- Cao, X.; Chai, L.; Jiang, D.; Wang, J.; Liu, Y.; Huang, Y. Loss of biodiversityalters ecosystem function in freshwater streams: Potential evidence from benthic macroinvertebrates. Ecosphere 2018, 9, e02445. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Chen, Y.; Castro-Izaguirre, N.; Baruffol, M.; Brezzi, M.; Lang, A.; Li, Y.; Hardtle, W.; von Oheimb, G.; Yang, X.; et al. Impacts of species richness on productivity in a large-scale subtropical forest experiment. Science 2018, 362, 80–83. [Google Scholar] [CrossRef] [Green Version]
- Bannar-Martin, K.H.; Kremer, C.T.; Ernest, S.K.M.; Leibold, M.A.; Auge, H.; Chase, J.; Declerck, S.A.J.; Eisenhauer, N.; Harpole, S.; Hillebrand, H.; et al. Integrating community assembly and biodiversity to better understand ecosystem function: The Community Assembly and the Functioning of Ecosystems (CAFE) approach. Ecol. Lett. 2018, 21, 167–180. [Google Scholar] [CrossRef] [Green Version]
- Thompson, P.L.; Gonzalez, A. Ecosystem multifunctionality in metacommunities. Ecology 2016, 97, 2867–2879. [Google Scholar] [CrossRef]
- Wang, M.-Q.; Li, Y.; Chesters, D.; Anttonen, P.; Bruelheide, H.; Chen, J.-T.; Durka, W.; Guo, P.-F.; Haerdtle, W.; Ma, K.; et al. Multiple components of plant diversity loss determine herbivore phylogenetic diversity in a subtropical forest experiment. J. Ecol. 2019, 107, 2697–2712. [Google Scholar] [CrossRef] [Green Version]
- Wright, A.J.; Wardle, D.A.; Callaway, R.; Gaxiola, A. The Overlooked Role of Facilitation in Biodiversity Experiments. Trends Ecol. Evol. 2017, 32, 383–390. [Google Scholar] [CrossRef]
- Barry, K.E.; Mommer, L.; van Ruijven, J.; Wirth, C.; Wright, A.J.; Bai, Y.; Connolly, J.; De Deyn, G.B.; de Kroon, H.; Isbell, F.; et al. The Future of Complementarity: Disentangling Causes from Consequences. Trends Ecol. Evol. 2019, 34, 167–180. [Google Scholar] [CrossRef] [Green Version]
- Heilpern, S.A.; Weeks, B.C.; Naeem, S. Predicting ecosystem vulnerability to biodiversity loss from community composition. Ecology 2018, 99, 1099–1107. [Google Scholar] [CrossRef]
- Soliveres, S.; van der Plas, F.; Manning, P.; Prati, D.; Gossner, M.M.; Renner, S.C.; Alt, F.; Arndt, H.; Baumgartner, V.; Binkenstein, J.; et al. Biodiversity at multiple trophic levels is needed for ecosystem multifunctionality. Nature 2016, 536, 456–459. [Google Scholar] [CrossRef]
- Schuldt, A.; Assmann, T.; Brezzi, M.; Buscot, F.; Eichenberg, D.; Gutknecht, J.; Hardtle, W.; He, J.S.; Klein, A.M.; Kuhn, P.; et al. Biodiversity across trophic levels drives multifunctionality in highly diverse forests. Nat. Commun. 2018, 9, 2989. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Brose, U.; Gravel, D. Intraguild predation enhances biodiversity and functioning in complex food webs. Ecology 2019, 100, e02616. [Google Scholar] [CrossRef]
- Enquist, B.J.; Abraham, A.J.; Harfoot, M.B.J.; Malhi, Y.; Doughty, C.E. The megabiota are disproportionately important for biosphere functioning. Nat. Commun. 2020, 11, 699. [Google Scholar] [CrossRef]
- Zavaleta, E.S.; Pasari, J.R.; Hulvey, K.B.; Tilman, G.D. Sustaining multiple ecosystem functions in grassland communities requires higher biodiversity. Proc. Natl. Acad. Sci. USA 2010, 107, 1443–1446. [Google Scholar] [CrossRef] [Green Version]
- Lefcheck, J.S.; Byrnes, J.E.K.; Isbell, F.; Gamfeldt, L.; Griffin, J.N.; Eisenhauer, N.; Hensel, M.J.S.; Hector, A.; Cardinale, B.J.; Duffy, J.E. Biodiversity enhances ecosystem multifunctionality across trophic levels and habitats. Nat. Commun. 2015, 6, 6936. [Google Scholar] [CrossRef]
- Barnes, A.D.; Jochum, M.; Lefcheck, J.S.; Eisenhauer, N.; Scherber, C.; O’Connor, M.I.; de Ruiter, P.; Brose, U. Energy Flux: The Link between Multitrophic Biodiversity and Ecosystem Functioning. Trends Ecol. Evol. 2018, 33, 186–197. [Google Scholar] [CrossRef]
- Maynard, D.S.; Crowther, T.W.; Bradford, M.A. Competitive network determines the direction of the diversity–function relationship. Proc. Natl. Acad. Sci. USA 2017, 114, 11464–11469. [Google Scholar] [CrossRef] [Green Version]
- Frainer, A.; McKie, B.G.; Amundsen, P.A.; Knudsen, R.; Lafferty, K.D. Parasitism and the Biodiversity-Functioning Relationship. Trends Ecol. Evol. 2018, 33, 260–268. [Google Scholar] [CrossRef] [Green Version]
- Ferlian, O.; Cesarz, S.; Craven, D.; Hines, J.; Barry, K.E.; Bruelheide, H.; Buscot, F.; Haider, S.; Heklau, H.; Herrmann, S.; et al. Mycorrhiza in tree diversity–ecosystem function relationships: Conceptual framework andexperimental implementation. Ecosphere 2018, 9, e02226. [Google Scholar] [CrossRef]
- Merrild, M.P.; Ambus, P.; Rosendahl, S.; Jakobsen, I. Common arbuscular mycorrhizal networks amplify competition for phosphorus between seedlings and established plants. New Phytol. 2013, 200, 229–240. [Google Scholar] [CrossRef]
- Scheublin, T.R.; Van Logtestijn, R.S.P.; Van Der Heijden, M.G.A. Presence and identity of arbuscular mycorrhizal fungi influence competitive interactions between plant species. J. Ecol. 2007, 95, 631–638. [Google Scholar] [CrossRef]
- Laforest-Lapointe, I.; Paquette, A.; Messier, C.; Kembel, S.W. Leaf bacterial diversity mediates plant diversity and ecosystem function relationships. Nature 2017, 546, 145–147. [Google Scholar] [CrossRef]
- Hu, A.; Wang, J.; Sun, H.; Niu, B.; Si, G.; Yeh, C.F.; Zhu, X.; Lu, X.; Zhou, J.; Yang, Y.; et al. Mountain biodiversity and ecosystem functions: Interplay between geology and contemporary environments. ISME J. 2020, 14, 931–944. [Google Scholar] [CrossRef]
- Lewington-Pearce, L.; Parker, B.; Narwani, A.; Nielsen, J.M.; Kratina, P. Diversity and temperature indirectly reduce CO2 concentrations in experimental freshwater communities. Oecologia 2020, 192, 515–527. [Google Scholar] [CrossRef] [Green Version]
- Theis, S.; Ruppert, J.L.W.; Roberts, K.N.; Minns, C.K.; Koops, M.; Poesch, M.S. Compliance with and ecosystem function of biodiversity offsets in North American and European freshwaters. Conserv. Biol. J. Soc. Conserv. Biol. 2020, 34, 41–53. [Google Scholar] [CrossRef]
- Nunes, C.A.; Braga, R.F.; de Moura Resende, F.; de Siqueira Neves, F.; Figueira, J.E.C.; Fernandes, G.W. Linking Biodiversity, the Environment and Ecosystem Functioning: Ecological Functions of Dung Beetles Along a Tropical Elevational Gradient. Ecosystems 2018, 21, 1244–1254. [Google Scholar] [CrossRef]
- Zirbel, C.R.; Grman, E.; Bassett, T.; Brudvig, L.A. Landscape context explains ecosystem multifunctionality in restored grasslands better than plant diversity. Ecology 2019, 100, e02634. [Google Scholar] [CrossRef] [PubMed]
- Smeti, E.; von Schiller, D.; Karaouzas, I.; Laschou, S.; Vardakas, L.; Sabater, S.; Tornes, E.; Monllor-Alcaraz, L.S.; Guillem-Argiles, N.; Martinez, E.; et al. Multiple stressor effects on biodiversity and ecosystem functioning in a Mediterranean temporary river. Sci. Total Environ. 2019, 647, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Dib, V.; Pires, A.P.F.; Casa Nova, C.; Bozelli, R.L.; Farjalla, V.F. Biodiversity-mediated effects on ecosystem functioning depend on the type and intensity of environmental disturbances. Oikos 2020, 129, 433–443. [Google Scholar] [CrossRef]
- Ratcliffe, S.; Wirth, C.; Jucker, T.; van der Plas, F.; Scherer-Lorenzen, M.; Verheyen, K.; Allan, E.; Benavides, R.; Bruelheide, H.; Ohse, B.; et al. Biodiversity and ecosystem functioning relations in European forests depend on environmental context. Ecol. Lett. 2017, 20, 1414–1426. [Google Scholar] [CrossRef]
- Gammal, J.; Järnström, M.; Bernard, G.; Norkko, J.; Norkko, A. Environmental Context Mediates Biodiversity–Ecosystem Functioning Relationships in Coastal Soft-sediment Habitats. Ecosystems 2018, 22, 137–151. [Google Scholar] [CrossRef] [Green Version]
- Spaak, J.W.; Baert, J.M.; Baird, D.J.; Eisenhauer, N.; Maltby, L.; Pomati, F.; Radchuk, V.; Rohr, J.R.; Van den Brink, P.J.; De Laender, F. Shifts of community composition and population density substantially affect ecosystem function despite invariant richness. Ecol. Lett. 2017, 20, 1315–1324. [Google Scholar] [CrossRef]
- Liu, J.; Wilson, M.; Hu, G.; Liu, J.; Wu, J.; Yu, M. How does habitat fragmentation affect the biodiversity and ecosystem functioning relationship? Landsc. Ecol. 2018, 33, 341–352. [Google Scholar] [CrossRef]
- Smith, M.D.; Knapp, A.K. Dominant species maintain ecosystem function with non-random species loss. Ecol. Lett. 2003, 6, 509–517. [Google Scholar] [CrossRef] [Green Version]
- Poisot, T.; Mouquet, N.; Gravel, D. Trophic complementarity drives the biodiversity-ecosystem functioning relationship in food webs. Ecol. Lett. 2013, 16, 853–861. [Google Scholar] [CrossRef]
- Frank, D.M. Science and values in the biodiversity-ecosystem function debate. Biol. Philos. 2022, 37, 7. [Google Scholar] [CrossRef]
- Rogers, A.D.; Frinault, B.A.V.; Barnes, D.K.A.; Bindoff, N.L.; Downie, R.; Ducklow, H.W.; Friedlaender, A.S.; Hart, T.; Hill, S.L.; Hofmann, E.E.; et al. Antarctic Futures: An Assessment of Climate-Driven Changes in Ecosystem Structure, Function and Service Provisioning in the Southern Ocean. Annu. Rev. Mar. Sci. 2020, 12, 7.1–7.34. [Google Scholar] [CrossRef] [Green Version]
- Pires, A.P.F.; Srivastava, D.S.; Marino, N.A.C.; MacDonald, A.A.M.; Figueiredo-Barros, M.P.; Farjalla, V.F. Interactive effects of climate change and biodiversity loss on ecosystem functioning. Ecology 2018, 99, 1203–1213. [Google Scholar] [CrossRef]
- Gazol, A.; Julio Camarero, J. Functional diversity enhances silver fir growth resilience to an extreme drought. J. Ecol. 2016, 104, 1063–1075. [Google Scholar] [CrossRef]
- Wieczynski, D.J.; Boyle, B.; Buzzard, V.; Duran, S.M.; Henderson, A.N.; Hulshof, C.M.; Kerkhoff, A.J.; McCarthy, M.C.; Michaletz, S.T.; Swenson, N.G.; et al. Climate shapes and shifts functional biodiversity in forests worldwide. Proc. Natl. Acad. Sci. USA 2019, 116, 587–592. [Google Scholar] [CrossRef] [Green Version]
- Garcia, F.C.; Bestion, E.; Warfield, R.; Yvon-Durocher, G. Changes in temperature alter the relationship between biodiversity and ecosystem functioning. Proc. Natl. Acad. Sci. USA 2018, 115, 10989–10994. [Google Scholar] [CrossRef] [Green Version]
- Eisenhauer, N.; Hines, J.; Isbell, F.; van der Plas, F.; Kazanski, C.E.; Lehmann, A.; Liu, M.; Hobbie, S.E.; Lochner, A.; Rillig, M.C.; et al. Plant diversity maintains multiple soil functions in future environments. eLife 2018, 7, e41228. [Google Scholar] [CrossRef]
- Wright, A.J.; Ebeling, A.; de Kroon, H.; Roscher, C.; Weigelt, A.; Buchmann, N.; Buchmann, T.; Fischer, C.; Hacker, N.; Hildebrandt, A.; et al. Flooding disturbances increase resource availability and productivity but reduce stability in diverse plant communities. Nat. Commun. 2015, 6, 6092. [Google Scholar] [CrossRef]
- Hisano, M.; Searle, E.B.; Chen, H.Y.H. Biodiversity as a solution to mitigate climate change impacts on the functioning of forest ecosystems. Biol. Rev. Camb. Philos. Soc. 2018, 93, 439–456. [Google Scholar] [CrossRef]
- Isbell, F.; Craven, D.; Connolly, J.; Loreau, M.; Schmid, B.; Beierkuhnlein, C.; Bezemer, T.M.; Bonin, C.; Bruelheide, H.; de Luca, E.; et al. Biodiversity increases the resistance of ecosystem productivity to climate extremes. Nature 2015, 526, 574–577. [Google Scholar] [CrossRef]
- Poorter, L.; Bongers, F.; Aide, T.M.; Almeyda Zambrano, A.M.; Balvanera, P.; Becknell, J.M.; Boukili, V.; Brancalion, P.H.S.; Broadbent, E.N.; Chazdon, R.L.; et al. Biomass resilience of Neotropical secondary forests. Nature 2016, 530, 211–214. [Google Scholar] [CrossRef]
- Oehri, J.; Schmid, B.; Schaepman-Strub, G.; Niklaus, P.A. Terrestrial land-cover type richness is positively linked to landscape-level functioning. Nat. Commun. 2020, 11, 154. [Google Scholar] [CrossRef]
- Gonzalez, A.; Germain, R.M.; Srivastava, D.S.; Filotas, E.; Dee, L.E.; Gravel, D.; Thompson, P.L.; Isbell, F.; Wang, S.; Kefi, S.; et al. Scaling-up biodiversity-ecosystem functioning research. Ecol. Lett. 2020, 23, 757–776. [Google Scholar] [CrossRef] [Green Version]
- Lefcheck, J.S.; Innes-Gold, A.A.; Brandl, S.J.; Steneck, R.S.; Torres, R.E.; Rasher, D.B. Tropical fish diversity enhances coral reef functioning across multiple scales. Sci. Adv. 2019, 5, eaav6420. [Google Scholar] [CrossRef] [Green Version]
- Bracken, M.E.S.; Douglass, J.G.; Perini, V.; Trussell, G.C. Spatial scale mediates the effects of biodiversity on marine primary producers. Ecology 2017, 98, 1434–1443. [Google Scholar] [CrossRef] [Green Version]
- Hautier, Y.; Isbell, F.; Borer, E.T.; Seabloom, E.W.; Harpole, W.S.; Lind, E.M.; MacDougall, A.S.; Stevens, C.J.; Adler, P.B.; Alberti, J.; et al. Local loss and spatial homogenization of plant diversity reduce ecosystem multifunctionality. Nat. Ecol. Evol. 2018, 2, 50–56. [Google Scholar] [CrossRef]
- van Moorsel, S.J.; Schmid, M.W.; Hahl, T.; Zuppinger-Dingley, D.; Schmid, B. Selection in response to community diversity alters plant performance and functional traits. Perspect. Plant Ecol. Evol. Syst. 2018, 33, 51–61. [Google Scholar] [CrossRef]
- Fargione, J.; Tilman, D.; Dybzinski, R.; Hille Ris Lambers, J.; Clark, C.; Harpole, W.S.; Knops, J.M.H.; Reich, P.B.; Loreau, M. From selection to complementarity: Shifts in the causes of biodiversity-productivity relationships in a long-term biodiversity experiment. Proc. R. Soc. B Biol. Sci. 2007, 274, 871–876. [Google Scholar] [CrossRef]
- Guerrero-Ramírez, N.R.; Craven, D.; Reich, P.B.; Ewel, J.J.; Isbell, F.; Koricheva, J.; Parrotta, J.A.; Auge, H.; Erickson, H.E.; Forrester, D.I.; et al. Diversity-dependent temporal divergence of ecosystem functioning in experimental ecosystems. Nat. Ecol. Evol. 2017, 1, 1639–1642. [Google Scholar] [CrossRef]
- Hautier, Y.; Seabloom, E.W.; Borer, E.T.; Adler, P.B.; Harpole, W.S.; Hillebrand, H.; Lind, E.M.; MacDougall, A.S.; Stevens, C.J.; Bakker, J.D.; et al. Eutrophication weakens stabilizing effects of diversity in natural grasslands. Nature 2014, 508, 521–525. [Google Scholar] [CrossRef]
- Roscher, C.; Temperton, V.M.; Buchmann, N.; Schulze, E.-D. Community assembly and biomass production in regularly and never weeded experimental grasslands. Acta Oecol. Int. J. Ecol. 2009, 35, 206–217. [Google Scholar] [CrossRef]
- Doherty, J.M.; Callaway, J.C.; Zedler, J.B. Diversity-function relationships changed in a long-term restoration experiment. Ecol. Appl. 2011, 21, 2143–2155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bezemer, T.M.; van der Putten, W.H. Ecology—Diversity and stability in plant communities. Nature 2007, 446, E6–E7. [Google Scholar] [CrossRef] [PubMed]
- Grace, J.B.; Anderson, T.M.; Seabloom, E.W.; Borer, E.T.; Adler, P.B.; Harpole, W.S.; Hautier, Y.; Hillebrand, H.; Lind, E.M.; Paertel, M.; et al. Integrative modelling reveals mechanisms linking productivity and plant species richness. Nature 2016, 529, 390–393. [Google Scholar] [CrossRef]
- Veen, G.F.; Putten, W.H.; Bezemer, T.M. Biodiversity-ecosystem functioning relationships in a long-term non-weeded field experiment. Ecology 2018, 99, 1836–1846. [Google Scholar] [CrossRef] [Green Version]
- Delgado-Baquerizo, M.; Reich, P.B.; Trivedi, C.; Eldridge, D.J.; Abades, S.; Alfaro, F.D.; Bastida, F.; Berhe, A.A.; Cutler, N.A.; Gallardo, A.; et al. Multiple elements of soil biodiversity drive ecosystem functions across biomes. Nat. Ecol. Evol. 2020, 4, 210–220. [Google Scholar] [CrossRef]
- Bestion, E.; Barton, S.; Garcia, F.C.; Warfield, R.; Yvon-Durocher, G. Abrupt declines in marine phytoplankton production driven by warming and biodiversity loss in a microcosm experiment. Ecol. Lett. 2020, 23, 457–466. [Google Scholar] [CrossRef] [Green Version]
- Smith, M.D.; Koerner, S.E.; Knapp, A.K.; Avolio, M.L.; Chaves, F.A.; Denton, E.M.; Dietrich, J.; Gibson, D.J.; Gray, J.; Hoffman, A.M.; et al. Mass ratio effects underlie ecosystem responses to environmental change. J. Ecol. 2020, 108, 855–864. [Google Scholar] [CrossRef]
- Perkins, D.M.; Bailey, R.A.; Dossena, M.; Gamfeldt, L.; Reiss, J.; Trimmer, M.; Woodward, G. Higher biodiversity is required to sustain multiple ecosystem processes across temperature regimes. Glob. Chang. Biol. 2015, 21, 396–406. [Google Scholar] [CrossRef] [Green Version]
- Mason, N.W.H.; Mouillot, D.; Lee, W.G.; Wilson, J.B. Functional richness, functional evenness and functional divergence: The primary components of functional diversity. Oikos 2005, 111, 112–118. [Google Scholar] [CrossRef]
- Hou, J.; Wang, H.; Fu, B.; Zhu, L.; Wang, Y.; Li, Z. Effects of plant diversity on soil erosion for different vegetation patterns. Catena 2016, 147, 632–637. [Google Scholar] [CrossRef]
- Isbell, F.; Gonzalez, A.; Loreau, M.; Cowles, J.; Diaz, S.; Hector, A.; Mace, G.M.; Wardle, D.A.; O’Connor, M.I.; Duffy, J.E.; et al. Linking the influence and dependence of people on biodiversity across scales. Nature 2017, 546, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Cardinale, B.J.; Duffy, J.E.; Gonzalez, A.; Hooper, D.U.; Perrings, C.; Venail, P.; Narwani, A.; Mace, G.M.; Tilman, D.; Wardle, D.A.; et al. Biodiversity loss and its impact on humanity. Nature 2012, 486, 59–67. [Google Scholar] [CrossRef]
- Titeux, N.; Henle, K.; Mihoub, J.-B.; Regos, A.; Geijzendorffer, I.R.; Cramer, W.; Verburg, P.H.; Brotons, L. Biodiversity scenarios neglect future land-use changes. Glob. Chang. Biol. 2016, 22, 2505–2515. [Google Scholar] [CrossRef] [Green Version]
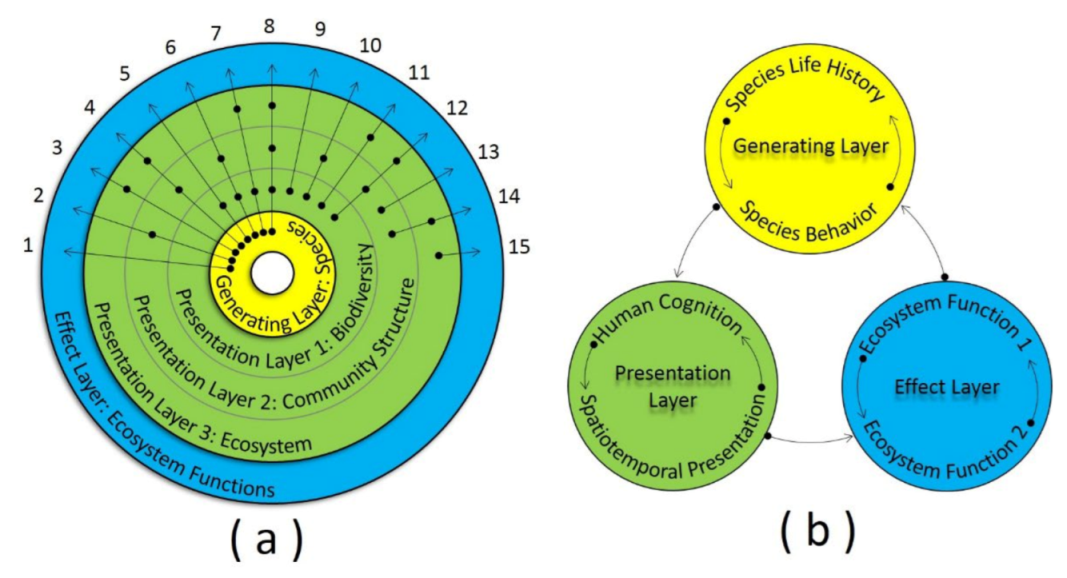
| Approach | Meaning |
|---|---|
| 1 | EF is affected by species life history or species behavior directly. |
| 2 | EF is affected by the community structure, and species life history or species behavior. |
| 3 | EF is affected by the ecosystem, and species life history or species behavior. |
| 4 | EF is affected by the ecosystem, community structure, and species life history or species behavior. |
| 5 | EF is affected by biodiversity, and species life history or species behavior. Examples can be found in studies [23,24,57,65]. |
| 6 | EF is affected by the community structure, biodiversity, and species life history or species behavior. Examples can be found in studies [14]. |
| 7 | EF is affected by the ecosystem, biodiversity, and species life history or species behavior. Examples can be found in studies [43]. |
| 8 | EF is affected by the ecosystem, community structure, biodiversity, and species life history or behavior. Examples can be found in studies [29,34,37,45]. |
| 9 | EF is affected by biodiversity directly. Examples can be found in studies [53,55,66]. |
| 10 | EF is affected by the community structure and biodiversity. Examples can be found in studies [11,16,21]. |
| 11 | EF is affected by the ecosystem and biodiversity. Examples can be found in studies [32,35,49,54,59]. |
| 12 | EF is affected by the ecosystem, community structure, and biodiversity. Examples can be found in studies [7,28,31,33,38,47]. |
| 13 | EF is affected by the community structure directly. |
| 14 | EF is affected by the ecosystem and community structure. |
| 15 | EF is affected by the ecosystem directly. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, J.; Feng, H.; Wu, M. Incorporating Effect Factors into the Relationship between Biodiversity and Ecosystem Functioning (BEF). Diversity 2022, 14, 274. https://doi.org/10.3390/d14040274
Hou J, Feng H, Wu M. Incorporating Effect Factors into the Relationship between Biodiversity and Ecosystem Functioning (BEF). Diversity. 2022; 14(4):274. https://doi.org/10.3390/d14040274
Chicago/Turabian StyleHou, Jian, Haobo Feng, and Menghan Wu. 2022. "Incorporating Effect Factors into the Relationship between Biodiversity and Ecosystem Functioning (BEF)" Diversity 14, no. 4: 274. https://doi.org/10.3390/d14040274
APA StyleHou, J., Feng, H., & Wu, M. (2022). Incorporating Effect Factors into the Relationship between Biodiversity and Ecosystem Functioning (BEF). Diversity, 14(4), 274. https://doi.org/10.3390/d14040274





