Evolutionary and Biogeographical History of Penguins (Sphenisciformes): Review of the Dispersal Patterns and Adaptations in a Geologic and Paleoecological Context
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fossil Record and Penguin Phylogenies
2.2. Species Considered in the Present Analysis
2.3. Paleobiogeographical Analyses
3. Results and Discussion
3.1. Paleogene History of Penguins
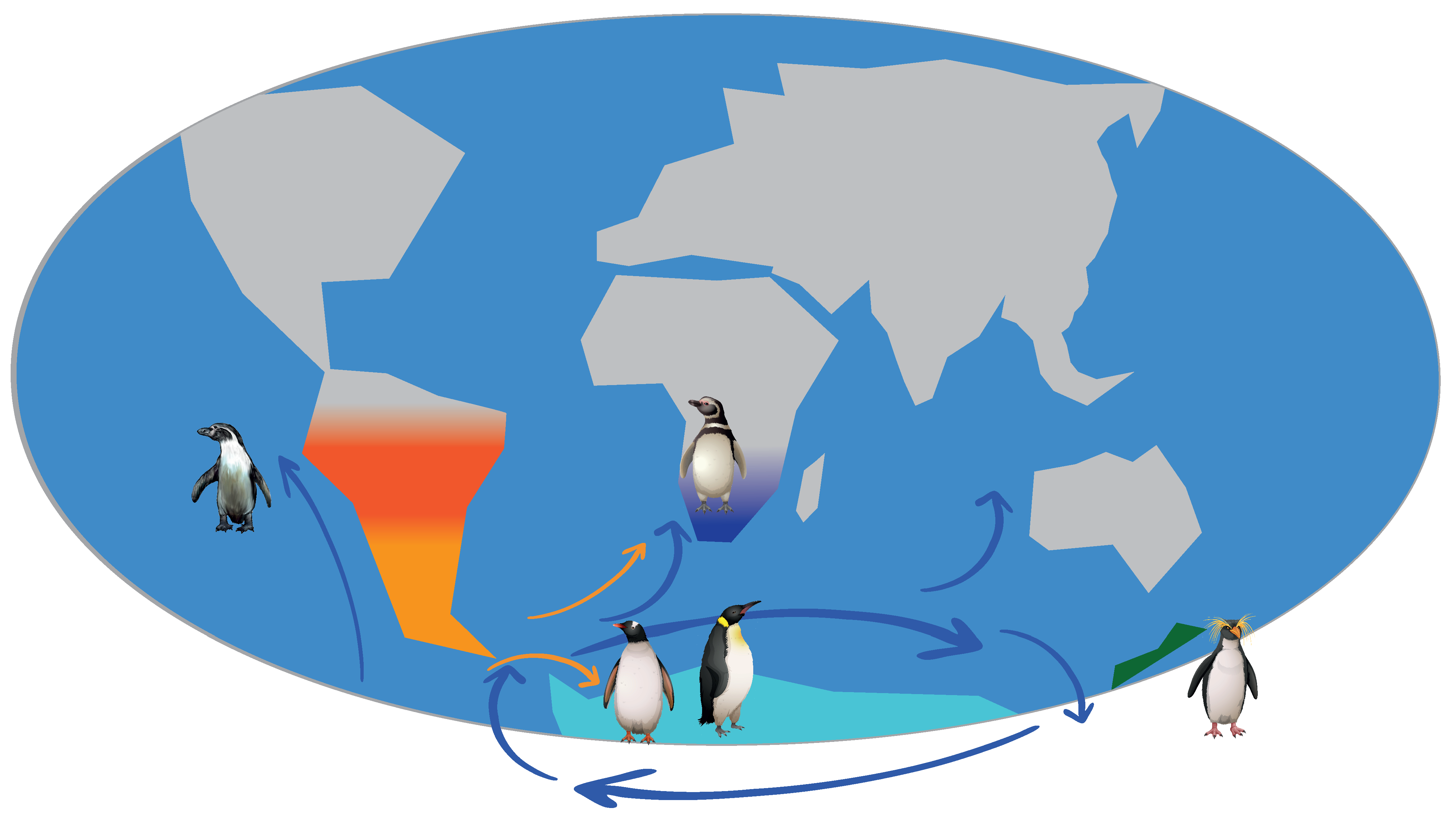
3.2. Neogene History of Penguins
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Williams, T.D.; Busby, J. Bird Families of the World. 2. The Penguins: Spheniscidae; Oxford University Press: Oxford, UK, 1995. [Google Scholar]
- Winkler, D.W.; Billerman, S.M.; Lovette, I.J. Bird Families of the World: An Invitation to the Spectacular Diversity of Birds; Lynx Editions: Cerdanyola del Vallès, Spain, 2015. [Google Scholar]
- Mayr, G. Metaves, Mirandornithes, Strisores and other novelties–a critical review of the higher-level phylogeny of neornithine birds. J. Zoolog. Syst. Evol. 2011, 49, 58–76. [Google Scholar] [CrossRef]
- Jarvis, E.D.; Mirarab, S.; Aberer, A.J.; Li, B.; Houde, P.; Li, C.; Zhang, G. Whole-genome analyses resolve early branches in the tree of life of modern birds. Science 2014, 346, 1320–1331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prum, R.O.; Berv, J.S.; Dornburg, A.; Field, D.J.; Townsend, J.P.; Lemmon, E.M.; Lemmon, A.R. A comprehensive phylogeny of birds (Aves) using targeted next-generation DNA sequencing. Nature 2015, 526, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Mayr, G. Avian Evolution: The Fossil Record of Birds and Its Paleobiological Significance; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Simpson, G.G. Penguins: Past and Present, Here and There; Yale University Press: New Haven, CT, USA, 1976. [Google Scholar]
- Kooyman, G.L.; Cherel, Y.; Le Maho, J.; Croxall, P.; Thorson, P.H.; Ridoux, V.; Kooyman, C.A. Diving behavior and energetics during foraging cycles in King Penguins. Ecol. Monogr. 1992, 62, 143–163. [Google Scholar] [CrossRef]
- Cooper, A.; Penny, D. Mass survival of birds across the Cretaceous-Tertiary boundary: Molecular evidence. Science 1997, 275, 1109–1113. [Google Scholar] [CrossRef]
- Hedges, S.B.; Parker, P.H.; Sibley, C.G.; Kumar, S. Continental breakup and the ordinal diversification of birds and mammals. Nature 1996, 381, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Slack, K.E.; Jones, C.M.; Ando, T.; Harrison, G.L.; Fordyce, R.E.; Arnason, U.; Penny, D. Early penguin fossils, plus mitochondrial genomes, calibrate avian evolution. Mol. Biol. Evol. 2006, 23, 1144–1155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feduccia, A. Explosive evolution in Tertiary birds and mammals. Science 1995, 267, 637–638. [Google Scholar] [CrossRef] [PubMed]
- Feduccia, A. ‘Big bang’ for Tertiary birds? Trends Ecol. Evol. 2003, 18, 172–176. [Google Scholar] [CrossRef]
- Gaina, C.; Müller, D.R.; Royer, J.Y.; Stock, J.; Hardebeck, J.; Symonds, P. The tectonic history of the Tasman Sea: A puzzle with 13 pieces. J. Geophys. Res. 1998, 103, 12413–12433. [Google Scholar] [CrossRef] [Green Version]
- Ksepka, D.T.; Ando, T. Penguins past, present, and future: Trends in the evolution of the Sphenisciformes. In Living Dinosaurs; John Wiley & Sons: Hoboken, NJ, USA, 2011; pp. 155–186. [Google Scholar]
- Fordyce, R.E.; Jones, C.M. Penguin history and new fossil material from New Zealand. In Penguin Biology; Davis, L.S., Darby, J.T., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 419–446. [Google Scholar]
- Mayr, G.; De Pietri, V.L.; Scofield, R.P. A new fossil from the mid-Paleocene of New Zealand reveals an unexpected diversity of world’s oldest penguins. Sci. Nat. 2017, 104, 9. [Google Scholar] [CrossRef]
- Mayr, G.; Scofield, R.P.; De Pietri, V.L.; Tennyson, A.J. A Paleocene penguin from New Zealand substantiates multiple origins of gigantism in fossil Sphenisciformes. Nat. Commun. 2017, 8, 1927. [Google Scholar] [CrossRef]
- Mayr, G.; De Pietri, V.L.; Love, L.; Mannering, A.A.; Scofield, R.P. A well-preserved new mid-Paleocene penguin (Aves, Sphenisciformes) from the Waipara Greensand in New Zealand. J. Vertebr. Paleontol. 2018, 37, e1398169. [Google Scholar] [CrossRef]
- Mayr, G.; De Pietri, V.L.; Love, L.; Mannering, A.; Scofield, R.P. Leg bones of a new penguin species from the Waipara Greensand add to the diversity of very large-sized Sphenisciformes in the Paleocene of New Zealand. Alcheringa 2020, 44, 194–201. [Google Scholar] [CrossRef]
- Thomas, D.B.; Fordyce, R.E. Biological plasticity in penguin heat-retention structures. Anat. Rec. 2012, 295, 249–256. [Google Scholar] [CrossRef]
- Thomas, D.B.; Ksepka, D.; Fordyce, E. Penguin heat-retention structures evolved in a greenhouse Earth. Biol. Lett. 2011, 7, 461–464. [Google Scholar] [CrossRef] [Green Version]
- Acosta Hospitaleche, C.; De Los Reyes, M.; Santillana, S.; Reguero, M. First fossilized skin of a giant penguin from the Eocene of Antarctica. Lethaia 2010, 53, 409–420. [Google Scholar] [CrossRef]
- Clarke, J.A.; Ksepka, D.T.; Salas-Gismondi, R.; Altamirano, A.J.; Shawkey, M.D.; D’Alba, L.; Vinther, J.; DeVries, T.J.; Baby, P. Fossil evidence for evolution of the shape and color of penguin feathers. Science 2010, 330, 954–957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanuki, Y.; Burger, A.E. Body mass and dive duration in Alcids and Penguins. Can. J. Zool. 1999, 77, 1838–1999. [Google Scholar] [CrossRef]
- Acosta Hospitaleche, C. New giant penguin bones from Antarctica: Systematic and paleobiological significance. C. R. Palevol. 2014, 13, 555–560. [Google Scholar] [CrossRef]
- Acosta Hospitaleche, C.; Reguero, M.A.; Santillana, S. Aprosdokitos mikrotero gen. et sp. nov., the tiniest Sphenisciformes that lived in Antarctica during the Paleogene. Neues Jahrb. Geol. Palaontol. Abh. 2017, 283, 25–34. [Google Scholar] [CrossRef]
- Clarke, J.A.; Ksepka, D.T.; Stucchi, M.; Urbina, M.; Giannini, N.; Bertelli, S.; Narváez, Y.; Boyd, C.A. Paleogene equatorial penguins challenge the proposed relationship between biogeography, diversity, and Cenozoic climate change. Proc. Natl. Acad. Sci. USA 2007, 104, 11545–11550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sallaberry, M.A.; Yury-Yáñez, R.E.; Otero, R.A.; Soto-Acuña, S.; Torres, T. Eocene birds from the western margin of southernmost South America. J. Paleontol. 2010, 84, 1061–1070. [Google Scholar] [CrossRef]
- Acosta Hospitaleche, C.; Olivero, E. Re-evaluation of the fossil penguin Palaeeudyptes gunnari from the Eocene Leticia Formation, Argentina: Additional material, systematics and palaeobiology. Alcheringa 2016, 40, 373–382. [Google Scholar] [CrossRef]
- Ando, T.; Fordyce, R.E. Evolutionary drivers for flightless, wing-propelled divers in the Northern and Southern Hemispheres. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2014, 400, 50–61. [Google Scholar] [CrossRef]
- Gavryushkina, A.; Heath, T.A.; Ksepka, D.T.; Stadler, T.; Welch, D.; Drummond, A.J. Bayesian total-evidence dating reveals the recent crown radiation of penguins. Syst. Biol. 2017, 66, 57–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blokland, J.C.; Reid, C.M.; Worthy, T.H.; Tennyson, A.J.; Clarke, J.A.; Scofield, R.P. Chatham Island Paleocene fossils provide insight into the palaeobiology, evolution, and diversity of early penguins (Aves, Sphenisciformes). Palaeontol. Electron. 2019, 22, 78. [Google Scholar] [CrossRef] [Green Version]
- Subramanian, S.; Beans-Picón, G.; Swaminathan, S.K.; Millar, C.D.; Lambert, D.M. Evidence for a recent origin of penguins. Biol. Lett. 2013, 9, 20130748. [Google Scholar] [CrossRef]
- Vianna, J.A.; Fernandes, F.A.; Frugone, M.J.; Figueiró, H.V.; Pertierra, L.R.; Noll, D.; Bowie, R.C. Genome-wide analyses reveal drivers of penguin diversification. Proc. Natl. Acad. Sci. USA 2020, 117, 22303–22310. [Google Scholar] [CrossRef]
- Chávez-Hoffmeister, M.; Briceño, J.D.; Nielsen, S.N. The evolution of seabirds in the Humboldt Current: New clues from the Pliocene of central Chile. PLoS ONE 2014, 9, e90043. [Google Scholar] [CrossRef]
- Boessenkool, S.; Austin, J.J.; Worthy, T.H.; Scofield, P.; Cooper, A.; Seddon, P.J.; Waters, J.M. Relict or colonizer? Extinction and range expansion of penguins in southern New Zealand. Proc. R. Soc. B 2009, 276, 815–821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, D.B.; Tennyson, A.J.D.; Scofield, R.P.; Heath, T.A.; Pett, W.; Ksepka, D.T. Ancient crested penguin constrains timing of recruitment into seabird hotspot. Proc. R. Soc. B 2020, 287, 20201497. [Google Scholar] [CrossRef] [PubMed]
- Paleobiology Database. The Paleobiology Database. Checklist Dataset. 2021. Available online: GBIF.org (accessed on 23 December 2021). [CrossRef]
- Acosta Hospitaleche, C.; Canto, J.; Tambussi, C.P. Pingüinos (Aves, Spheniscidae) en Coquimbo (Mioceno medio-Plioceno tardío), Chile y su vinculación con las corrientes oceánicas. Span. J. Palaeont. 2006, 21, 115–121. [Google Scholar] [CrossRef]
- Ksepka, D.T.; Clarke, J.A. The basal penguin (Aves: Sphenisciformes) Perudyptes devriesi and a phylogenetic evaluation of the penguin fossil recordBull. Am. Mus. Nat. Hist. 2010, 337, 1–77. [Google Scholar] [CrossRef]
- Ksepka, D.T.; Fordyce, R.E.; Ando, T.; Jones, C.M. New fossil penguins (Aves, Sphenisciformes) from the Oligocene of New Zealand reveal the skeletal plan of stem penguins. J. Vertebr. Paleontol. 2012, 32, 235–254. [Google Scholar] [CrossRef]
- Degrange, F.J.; Ksepka, D.T.; Tambussi, C.P. Redescription of the oldest crown clade penguin: Cranial osteology, jaw myology, neuroanatomy, and phylogenetic affinities of Madrynornis mirandus. J. Vertebr. Paleontol. 2018, 38, e1445636. [Google Scholar] [CrossRef]
- Matzke, N.J. BioGeoBEARS: Biogeography with Bayesian (and Likelihood) Evolutionary Analyses in R Scripts; BioGeoBEARS; The Comprehensive R Archive Network: Berkeley, CA, USA, 2013; Available online: http://CRAN.R-project.org/package (accessed on 23 November 2021).
- Matzke, N.J. Probabilistic historical biogeography: New models for founder event speciation, imperfect detection, and fossils allow improved accuracy and model-testing. Front. Biogeogr. 2013, 5, 242–248. [Google Scholar] [CrossRef] [Green Version]
- Tambussi, C.P.; Reguero, M.A.; Marenssi, S.A.; Santillana, S.N. Crossvallia unienwillia, a new Spheniscidae (Sphenisciformes, Aves) from the late Paleocene of Antarctica. Geobios 2005, 38, 667–675. [Google Scholar] [CrossRef]
- Jadwiszczak, P.; Acosta Hospitaleche, C.; Reguero, M. Redescription of Crossvallia unienwillia: The only Paleocene Antarctic penguin. Ameghiniana 2013, 50, 545–553. [Google Scholar] [CrossRef]
- Fordyce, R.E.; Thomas, D.B. Kaiika maxwelli, a new Early Eocene archaic penguin (Sphenisciformes, Aves) from Waihao Valley, South Canterbury, New Zealand. N. Z. J. Geol. Geophys. 2011, 54, 43–51. [Google Scholar] [CrossRef]
- Myrcha, A.; Jadwiszczak, P.; Tambussi, C.P.; Noriega, J.I.; Gaździcki, A.; Tatur, A.; del Valle, R.A. Taxonomic revision of Eocene Antarctic penguins based on tarsometatarsal morphology. Pol. Polar Res. 2002, 23, 5–46. [Google Scholar]
- Jadwiszczak, P.; Mörs, T. First partial skeleton of Delphinornis larseni Wiman, 1905, a slender-footed penguin from the Eocene of Antarctic Peninsula. Palaeontol. Electron. 2019, 22, 1–31. [Google Scholar] [CrossRef] [Green Version]
- Jadwiszczak, P.; Reguero, M.; Mörs, T. A new small-sized penguin from the late Eocene of Seymour Island with additional material of Mesetaornis polaris. GFF 2021, 143, 283–291. [Google Scholar] [CrossRef]
- Acosta Hospitaleche, C.; Jadwiszczak, P.; Clarke, J.A.; Cenizo, M. The fossil record of birds from the James Ross Basin, West Antarctica. Adv. Polar Sci. 2019, 30, 251–273. [Google Scholar] [CrossRef]
- Jadwiszczak, P. Partial limb skeleton of a “giant penguin” Anthropornis from the Eocene of Antarctic Peninsula. Pol. Polar Res. 2012, 3, 259–274. [Google Scholar] [CrossRef]
- Acosta Hospitaleche, C.; Reguero, M.; Scarano, A. Main pathways in the evolution of Antarctic fossil penguins. J. S. Am. Earth Sci. 2013, 43, 101–111. [Google Scholar] [CrossRef]
- Ando, T. New Zealand Fossil Penguins: Origin, Pattern, and Process. Ph.D. Thesis, University of Otago, Dunedin, New Zealand, 2007. [Google Scholar]
- Park, T.; Fitzgerald, E.M. A review of Australian fossil penguins (Aves: Sphenisciformes). Mem. Mus. Vic. 2012, 69, 309–325. [Google Scholar] [CrossRef] [Green Version]
- Tambussi, C.P.; Acosta Hospitaleche, C.; Reguero, M.A.; Marenssi, S.A. Late Eocene penguins from West Antarctica: Systematics and biostratigraphy. Geol. Soc. Spec. Publ. 2006, 258, 145–161. [Google Scholar] [CrossRef]
- Jadwiszczak, P.; Acosta Hospitaleche, C. Distinguishing between two Antarctic species of Eocene Palaeeudyptes penguins: A statistical approach using tarsometatarsi. Pol. Polar Res. 2013, 34, 237–252. [Google Scholar] [CrossRef] [Green Version]
- Acosta Hospitaleche, C.; Reguero, M. Palaeeudyptes klekowskii, the best-preserved penguin skeleton from the Eocene–Oligocene of Antarctica: Taxonomic and evolutionary remarks. Geobios 2014, 47, 77–85. [Google Scholar] [CrossRef]
- Giovanardi, S.; Ksepka, D.T.; Thomas, D.B. A giant Oligocene fossil penguin from the North Island of New Zealand. J. Vertebr. Paleontol. 2021, 41, e1953047. [Google Scholar] [CrossRef]
- Acosta Hospitaleche, C. Systematic revision of Arthrodytes Ameghino, 1905 (Aves, Spheniscidae) and its assignment to the Paraptenodytinae. Neues Jahrb. Geol. Paläontol. 2005, 7, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Parras, A.; Dix, G.R.; Griffin, M. Sr-isotope chronostratigraphy of Paleogene–Neogene marine deposits: Austral Basin, southern Patagonia (Argentina). J. S. Am. Earth Sci. 2012, 37, 122–135. [Google Scholar] [CrossRef]
- Acosta Hospitaleche, C.; Tambussi, C.; Cozzuol, M. Eretiscus tonnii (Simpson) (Aves, Sphenisciformes): Materiales adicionales, status taxonómico y distribución geográfica. Rev. Mus. Argent. Cienc. Nat. 2004, 6, 233–237. [Google Scholar]
- Acosta Hospitaleche, C.; Griffin, M.; Asensio, M.; Cione, A.L.; Tambussi, C. Middle Cenozoic penguin remains from the Patagonian Cordillera. Andean Geol. 2013, 40, 490–503. [Google Scholar]
- Acosta Hospitaleche, C. Revisión sistemática de Palaeospheniscus biloculata (Simpson) nov. comb.(Aves, Spheniscidae) de la Formación Gaiman (Mioceno Temprano), Chubut, Argentina. Ameghiniana 2007, 44, 417–426. [Google Scholar]
- Acosta Hospitaleche, C.; Castro, L.; Tambussi, C.; Scasso, R.A. Palaeospheniscus patagonicus (Aves, Sphenisciformes): New discoveries from the early Miocene of Argentina. J. Paleontol. 2008, 82, 565–575. [Google Scholar] [CrossRef]
- Walsh, S.A.; Suárez, M.E. New penguin remains from the Pliocene of northern Chile. Hist. Biol. 2006, 18, 119–130. [Google Scholar] [CrossRef]
- Gohlich, U.B. The oldest fossil record of the extant penguin genus Spheniscus-a new species from the Miocene of Peru. Acta Palaeontol. Pol. 2007, 52, 285–298. [Google Scholar]
- Acosta Hospitaleche, C.; Paulina-Carabajal, A.; Yury-Yáñez, R. The skull of the Miocene Spheniscus urbinai (Aves, Sphenisciformes): Osteology, brain morphology, and the cranial pneumatic systems. J. Anat. 2021, 239, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Acosta Hospitaleche, C.; Tambussi, C.; Donato, M.; Cozzuol, M. A new Miocene penguin from Patagonia and its phylogenetic relationships. Acta Palaeontol. Pol. 2007, 52, 299–314. [Google Scholar]
- Viglino, M.; Buono, M.; Acosta Hospitaleche, C.; Cione, A.; Cuitiño, J.; Gaetán, M.; Sterli, J.; Paolucci, F. Vertebrados marinos del Cenozoico. In Relatorio XXI Congreso Geológico Argentino; Geología y Recursos Naturales de la Provincia del Chubut: Puerto Madryn, Argentine, 2021; pp. 335–358. [Google Scholar]
- Acosta Hospitaleche, C.; Chávez-Hoffmeister, M.; Fritis, O. Pingüinos fósiles (Pygoscelis calderensis sp. nov.) en la Formación Bahía Inglesa (Mioceno Medio-Plioceno), Chile. Rev. Geol. Chile 2006, 33, 327–338. [Google Scholar] [CrossRef]
- Chávez-Hoffmeister, M. Fossil birds of Chile and Antarctic Peninsula. Arq. Mus. Nac. Rio Janeiro 2007, 65, 551–572. [Google Scholar]
- Stucchi, M. Los Pingüinos Fósiles de la Formación Pisco (Neógeno). In Proceedings of the 4° European Meeting on the Palaeontology and Stratigraphy of Latin America, Cuadernos del Museo Geominero 8, Perú, Tres Cantos, Madrid, 12–14 September 2007; Díaz–Martínez, E., Rábano, I., Eds.; Instituto Geológico y Minero de España: Madrid, Spain, 2007. [Google Scholar]
- Park, T. Redescription of the Miocene penguin Pseudaptenodytes macraei Simpson (Aves: Sphenisciformes) and redefinition of the taxonomic status of? Pseudaptenodytes minor Simpson. Alcheringa. 2014, 38, 450–454. [Google Scholar] [CrossRef]
- Simpson, G.G. A new genus of late Tertiary penguin from Langebaanweg, South Africa. Ann. S. Afr. Mus. 1979, 78, 1–9. [Google Scholar]
- Ksepka, D.T.; Thomas, D.B. Multiple cenozoic invasions of Africa by penguins (Aves, Sphenisciformes). Proc. R. Soc. B 2012, 279, 1027–1032. [Google Scholar] [CrossRef]
- Emslie, S.D.; Guerra Correa, C. A new species of penguin (Spheniscidae: Spheniscus) and other birds from the late Pliocene of Chile. Proc. Biol. Soc. Wash. 2003, 116, 308–316. [Google Scholar]
- Marples, B.J. Fossil penguins from the mid-Tertiary of Seymour Island. FIDS Sci. Rep. 1953, 5, 1–15. [Google Scholar]
- Heled, J.; Bouckaert, R.R. Looking for trees in the forest: Summary tree from posterior samples. BMC Evol. Biol. 2013, 13, 221. [Google Scholar] [CrossRef] [Green Version]
- Chávez-Hoffmeister, M. The humerus and stratigraphic range of Palaeospheniscus (Aves, Sphenisciformes). Ameghiniana 2014, 51, 159–172. [Google Scholar] [CrossRef]
- Kinsky, F.C.; Falla, R.A. A subspecific revision of the Australasian Blue Penguin (Eudyptula minor) in the New Zealand area. Rec. Nat. Mus. N 1976, 2, 105–126. [Google Scholar]
- Grosser, S.; Burridge, C.P.; Peucker, A.J.; Waters, J.M. Coallescent modelling reveals recent secondary contact of cryptic penguin species. PLoS ONE 2015, 10, e0144966. [Google Scholar] [CrossRef]
- Grosser, S.; Scofield, R.P.; Waters, J.M. Multivariate skeletal analyses support a taxonomic distinction between New Zealand and Australian Eudyptula penguins Sphenisciformes: Spheniscidae). Emu 2017, 117, 276–283. [Google Scholar] [CrossRef]
- Worthy, T.H.; Grant-Mackie, J.A. Late-Pleistocene avifaunas from Cape Wanbrow, Otago, South Island, New Zealand. J. R. Soc. N. Z. 2003, 33, 427–485. [Google Scholar] [CrossRef]
- Benson, R.D. A new species of penguin from the late Miocene of Chile with comments on the stratigraphic range of Palaeospheniscus. Sci. Pub. Sci. Mus. MN 2015, 8, 22. [Google Scholar]
- Strogen, D.P.; Seebeck, H.; Nicol, A.; King, P.R. Two-phase Cretaceous–Paleocene rifting in the Taranaki Basin region, New Zealand; implications for Gondwana break-up. J. Geol. Soc. 2017, 174, 929–946. [Google Scholar] [CrossRef]
- Storey, B.C.; Granot, R. Tectonic history of Antarctica over the past 200 million years. Geol. Soc. Lond. Mem. 2021, 55, 9–17. [Google Scholar] [CrossRef]
- Holbourn, A.; Kuhnt, W.; Frank, M.; Haley, B.A. Changes in Pacific Ocean circulation following the Miocene onset of permanent Antarctic ice cover. Earth Planet Sci. Lett. 2013, 365, 38–50. [Google Scholar] [CrossRef]
- Funk, E.R.; Burns, K.J. Biogeographic origins of Darwin’s finches (Thraupidae: Coerebinae). Auk 2018, 135, 561–571. [Google Scholar] [CrossRef] [Green Version]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference; Springer: New York, NY, USA, 2002. [Google Scholar]
- Ree, R.H.; Sanmartín, I. Conceptual and statistical problems with the DEC+ J model of founder-event speciation and its comparison with DEC via model selection. J. Biogeogr. 2018, 45, 741–749. [Google Scholar] [CrossRef]
- Klaus, K.V.; Matzke, N.J. Statistical comparison of trait-dependent biogeographical models indicates that Podocarpaceae dispersal is influenced by both seed cone traits and geographical distance. Syst. Biol. 2020, 69, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.B.; Birks, S.M.; Leaché, A.D. Incubator birds: Biogeographical origins and evolution of underground nesting in megapodes (Galliformes: Megapodiidae). J. Biogeogr. 2014, 41, 2045–2056. [Google Scholar] [CrossRef]
- Van Els, P.; Norambuena, H.V.; Etienne, R.S. From pampa to puna: Biogeography and diversification of a group of Neotropical obligate grassland birds (Anthus: Motacillidae). J. Zool. Syst. Evol. Res. 2019, 57, 485–496. [Google Scholar] [CrossRef]
- McCullough, J.M.; Moyle, R.G.; Smith, B.T.; Andersen, M.J. A Laurasian origin for a pantropical bird radiation is supported by genomic and fossil data (Aves: Coraciiformes). Proc. R. Soc. B 2019, 286, 20190122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveros, C.H.; Andersen, M.J.; Hosner, P.A.; Mauck, W.M., III; Sheldon, F.H.; Cracraft, J.; Moyle, R.G. Rapid Laurasian diversification of a pantropical bird family during the Oligocene-Miocene transition. IBIS 2020, 162, 137–152. [Google Scholar] [CrossRef]
- Garcia-R, J.C.; Matzke, N.J. Trait-dependent dispersal in rails (Aves: Rallidae): Historical biogeography of a cosmopolitan bird clade. Mol. Phylogenet. Evol. 2021, 159, 107106. [Google Scholar] [CrossRef] [PubMed]
- Ding, A.; Pittman, M.; Upchurch, P.; O’Connor, J.M.; Field, D.J.; Xu, X. The biogeography of coelurosaurian theropods and its impact on their evolutionary history. Bull. Am. Mus. Nat. Hist. 2020, 40, 117–157. [Google Scholar]
- Cantalapiedra, J.L.; Prado, J.L.; Hernández Fernández, M.; Alberdi, M.T. Decoupled ecomorphological evolution and diversification in Neogene-Quaternary horses. Science 2017, 355, 627–630. [Google Scholar] [CrossRef] [Green Version]
- Peucker, A.J.; Dann, P.; Burridge, C.P. Range-wide phylogeography of the little penguin (Eudyptula minor): Evidence of long-distance dispersal. Auk 2009, 126, 397–408. [Google Scholar] [CrossRef]
- Baker, A.J.; Pereira, S.L.; Haddrath, O.P.; Edge, K.A. Multiple gene evidence for expansion of extant penguins out of Antarctica due to global cooling. Proc. R. Soc. B 2006, 273, 11–17. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Harris, A.J.; Blair, C.; He, X. RASP (Reconstruct Ancestral State in Phylogenies): A tool for historical biogeography. Mol. Phylogenet. Evol. 2015, 87, 46–49. [Google Scholar] [CrossRef]
- Mayr, G.; De Pietri, V.L.; Love, L.; Mannering, A.A.; Bevitt, J.J.; Scofield, R.P. First complete wing of a stem group sphenisciform from the Paleocene of New Zealand sheds light on the evolution of the penguin flipper. Diversity 2020, 12, 46. [Google Scholar] [CrossRef] [Green Version]
- Stilwell, J.D.; Consoli, C.P. Tectono-stratigraphic history of the Chatham Islands, SW Pacific—The emergence, flooding and reappearance of eastern ‘Zealandia’. Proc. Geol. Assoc. 2012, 123, 170–181. [Google Scholar] [CrossRef]
- Tulloch, A.J.; Mortimer, N.; Ireland, T.R.; Waight, T.E.; Maas, R.; Palin, J.; Sahoo, T.; Seebeck, H.; Sagar, M.W.; Barrier, A. Reconnaissance basement geology and tectonics of South Zealandia. Tectonics 2019, 38, 516–551. [Google Scholar] [CrossRef] [Green Version]
- Bache, F.; Mortimer, N.; Sutherland, R.; Collot, J.; Rouillard, P.; Stagpoole, V.; Nicol, A. Seismic stratigraphic record of transition from Mesozoic subduction to continental breakup in the Zealandia Sector of eastern Gondwana. Gondwana Res. 2014, 26, 1060–1078. [Google Scholar] [CrossRef]
- Rouillard, P.; Collot, J.; Sutherland, R.; Bache, F.; Patriat, M.; Etienne, S.; Maurizot, P. Seismic stratigraphy and paleogeographic evolution of Fairway Basin, Northern Zealandia, Southwest Pacific: From Cretaceous Gondwana breakup to Cenozoic Tonga–Kermadec subduction. Basin Res. 2015, 29, 189–212. [Google Scholar] [CrossRef] [Green Version]
- Poole, I.; Cantrill, D.; Utescher, T. A mulitproxy approach to determine Antarctic terrestrial palaeoclimate during the Late Cretaceous and Early Tertiary. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2005, 222, 95–121. [Google Scholar] [CrossRef]
- Pross, J.; Contreras, L.; Bijl, P.K.; Greenwood, D.R.; Bohaty, S.M.; Schouten, S.; Brinkhuis, H. Persistent near-tropical warmth on the Antarctic continent during the early Eocene epoch. Nature 2012, 488, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Kemp, D.B.; Robinson, S.A.; Crame, J.A.; Francis, J.E.; Ineson, J.; Whittle, R.J.; O’Brien, C. A cool temperate climate on the Antarctic Peninsula through the latest Cretaceous to early Paleogene. Geology 2014, 42, 583–586. [Google Scholar] [CrossRef] [Green Version]
- Bijl, P.; Schouten, S.; Slujis, A.; Reichart, G.; Zachos, J.C.; Brinkhuis, H. Early Palaeogene temperature evolution of the southwest Pacific Ocean. Nature 2009, 461, 776–779. [Google Scholar] [CrossRef] [PubMed]
- Hollis, C.J.; Taylor, K.W.; Handley, L.; Pancost, R.D.; Huber, M.; Creech, J.B.; Zachos, J.C. Early Paleogene temperature history of the Southwest Pacific Ocean: Reconciling proxies and models. Earth Planet Sci. Lett. 2012, 349, 53–66. [Google Scholar] [CrossRef]
- Marenssi, S.A. Eustatically controlled sedimentation recorded by Eocene strata of the James Ross Basin, Antarctica. Geol. Soc. Spec. Publ. 2006, 258, 125–133. [Google Scholar] [CrossRef]
- Haidr, N.; Acosta Hospitaleche, C. Feeding habits of Antarctic Eocene penguins from a morphofunctional perspective. Neues Jahrb. Geol. Paläontol. Abh. 2012, 263, 125–131. [Google Scholar] [CrossRef]
- Chávez-Hoffmeister, M. Bill disparity and feeding strategies among fossil and modern penguins. Paleobiology 2020, 46, 176–192. [Google Scholar] [CrossRef]
- Zachos, J.; Pagani, M.; Sloan, L.; Thomas, E.; Billups, K. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science 2001, 292, 686–693. [Google Scholar] [CrossRef]
- Lawver, L.A.; Gahagan, L.M. Evolution of Cenozoic seaways in the circum-Antarctic region. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2003, 198, 11–37. [Google Scholar] [CrossRef]
- Houben, A.J.; Bijl, P.K.; Sluijs, A.; Schouten, S.; Brinkhuis, H. Late Eocene Southern Ocean cooling and invigoration of circulation preconditioned Antarctica for full-scale glaciation. Geochem. Geophys. 2019, 20, 2214–2234. [Google Scholar] [CrossRef] [Green Version]
- Pandey, M.; Pant, N.C.; Arora, D.; Gupta, R. A review of Antarctic ice sheet fluctuations records during Cenozoic and its cause and effect relation with the climatic conditions. Polar Sci. 2021, 30, 100720. [Google Scholar] [CrossRef]
- Acosta Hospitaleche, C.; Stucchi, M. Nuevos restos terciarios de Spheniscidae (Aves, Sphenisciformes) procedentes de la costa del Perú. Span. J. Paleontol. 2005, 20, 1–5. [Google Scholar] [CrossRef]
- Ramos, B.; González-Acuña, D.; Loyola, D.E.; Johnson, W.E.; Parker, P.G.; Massaro, M.; Dantas, G.; Marcelo, M.; Vianna, J.A. Landscape genomics: Natural selection drives the evolution of mitogenome in penguins. BMC Genom. 2018, 19, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kooyman, G.L. Evolutionary and ecological aspects of some Antarctic and sub-Antarctic penguin distributions. Oecologia 2001, 130, 485–495. [Google Scholar] [CrossRef]
- Dantas, G.P.; Oliveira, L.R.; Santos, A.M.; Flores, M.D.; Melo, D.R.; Simeone, A.; González-Acuña, D.; Luna-Jorquera, G.; Le Bohec, C.; Valdés-Velásquez, A.; et al. Uncovering population structure in the Humboldt penguin (Spheniscus humboldti) along the Pacific coast at South America. PLoS ONE 2019, 14, e0215293. [Google Scholar] [CrossRef] [PubMed]
- Glasser, N.F.; Jansson, K.N.; Harrison, S.; Kleman, J. The glacial geomorphology and Pleistocene history of South America between 38 S and 56 S. Quat. Sci. Rev. 2018, 27, 365–390. [Google Scholar] [CrossRef]
- Frugone, M.J.; Lowther, A.; Noll, D.; Ramos, B.; Pistorius, P.; Dantas, G.P.M.; Vianna, J.A. Contrasting phylogeographic pattern among Eudyptes penguins around the Southern Ocean. Sci. Rep. 2018, 8, 17481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
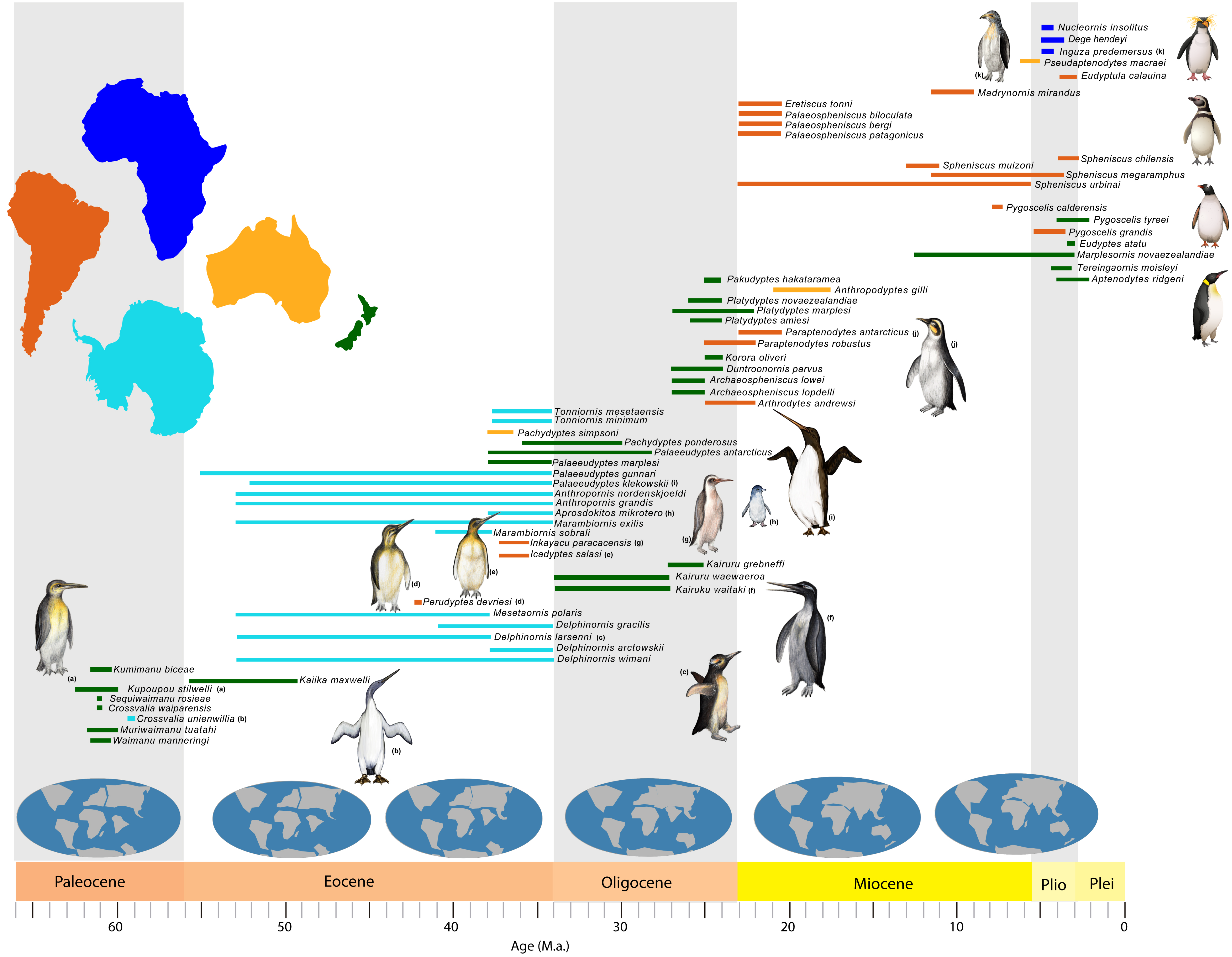
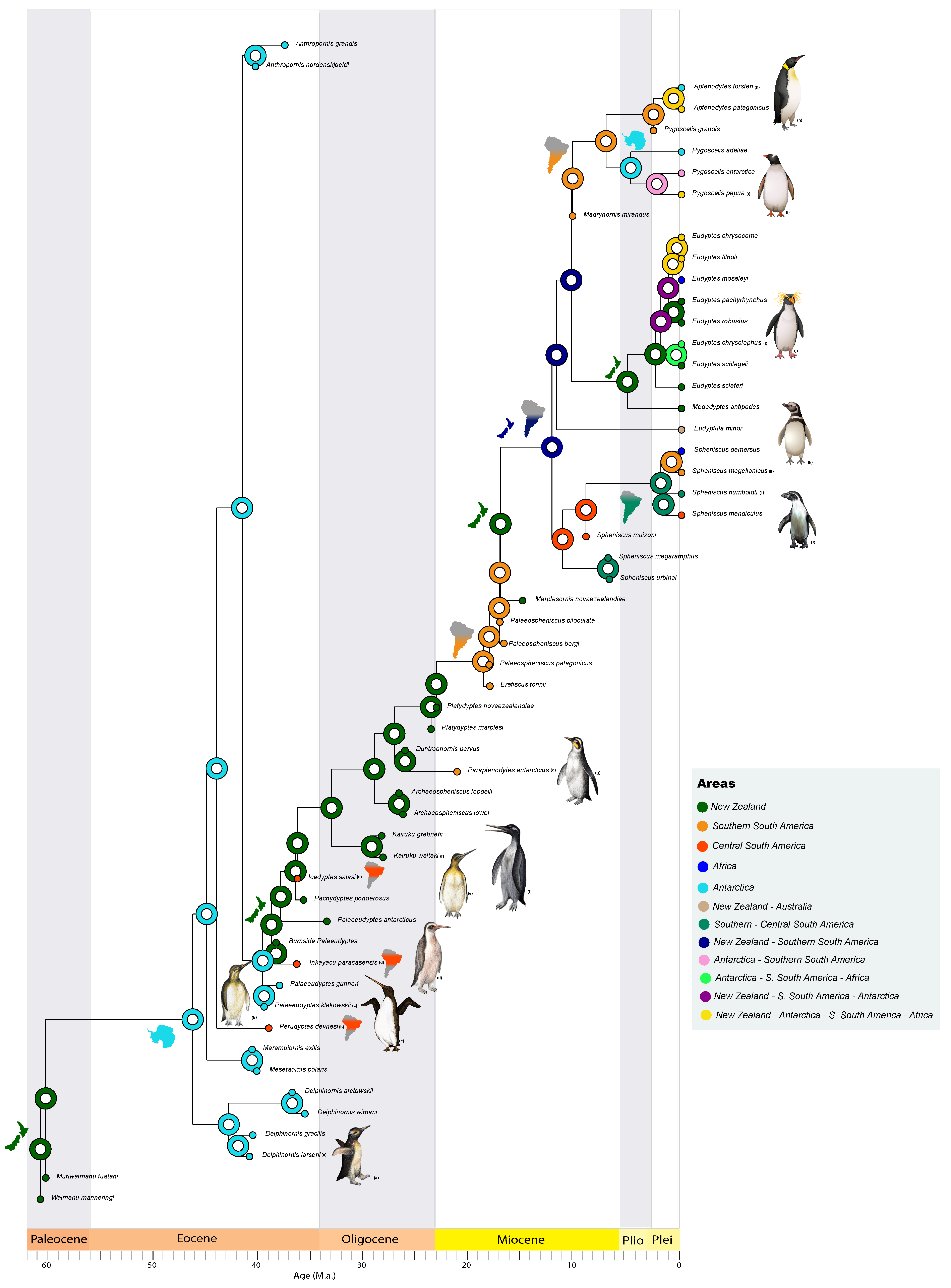
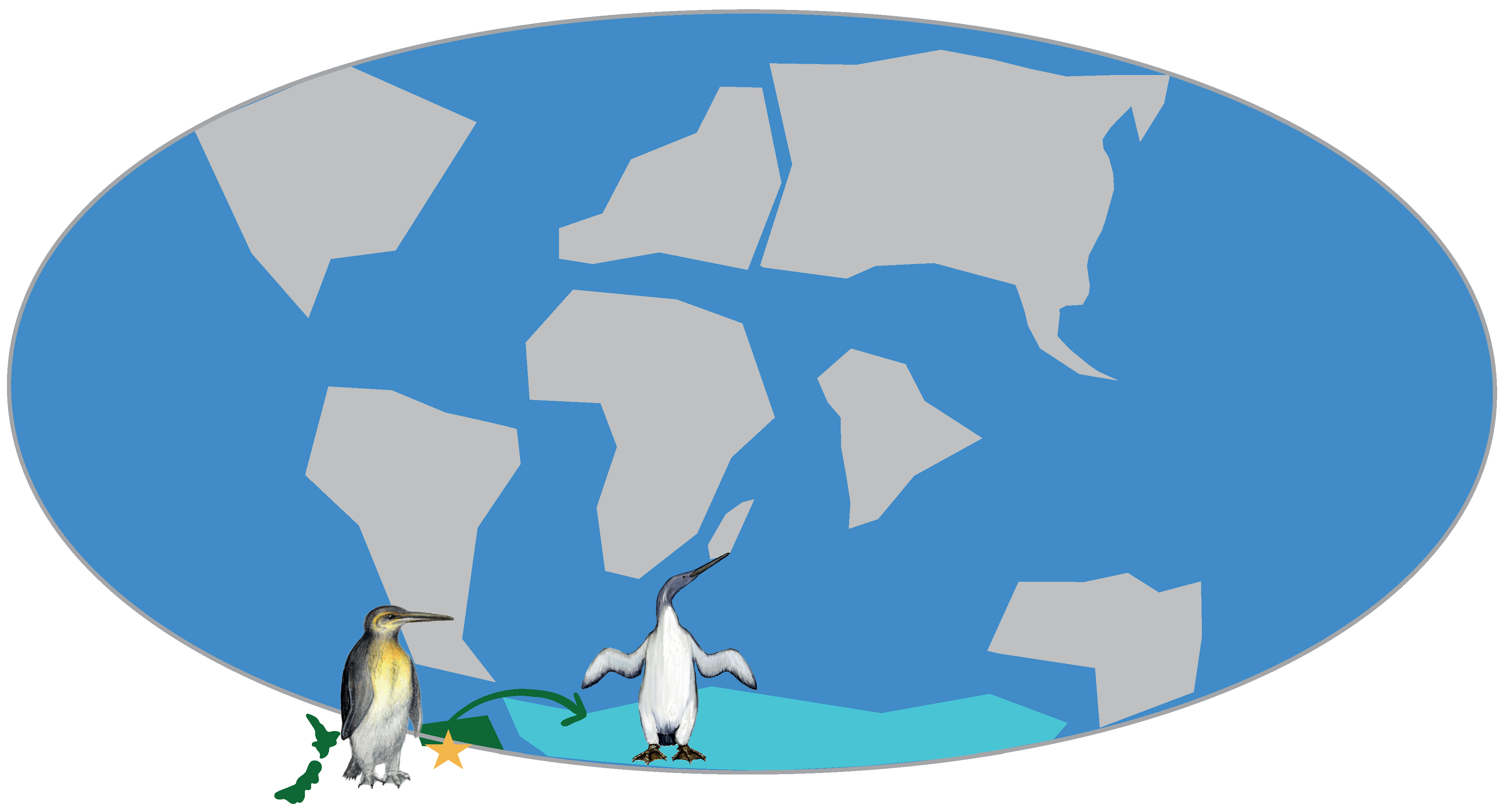
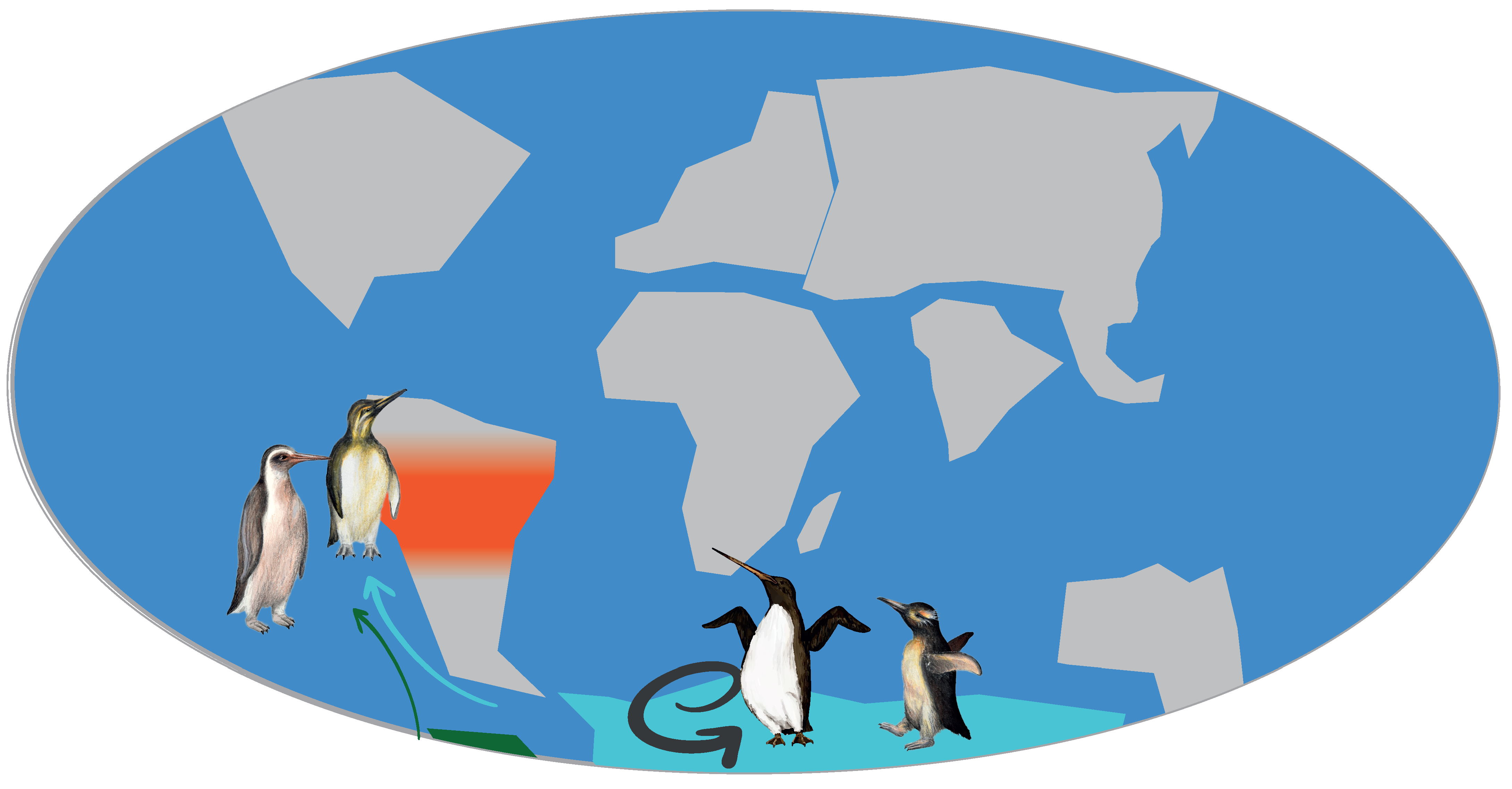


| Species | Location | Epoch | SR (Ma) | Reference |
|---|---|---|---|---|
| Kupoupou stilwelli | New Zealand | Paleocene | 62.5–60 | [33] |
| Crossvallia waiparensis | New Zealand | Paleocene | ~61 | [20] |
| Muriwaimanu tuatahi * | New Zealand | Paleocene | 58–60 | [20] |
| Sequiwaimanu rosieae | New Zealand | Paleocene | ~61 | [19] |
| Waimanu manneringi * | New Zealand | Paleocene | 60.5–61.6 | [11] |
| Crossvalia unienwillia | Antarctica | Paleocene | 59.2 | [46,47] |
| Kumimanu biceae | New Zealand | Paleocene | 60.5–61.6 | [17] |
| Kaiika maxwelli | New Zealand | Eocene | 55.8–49.3 | [48] |
| Perudyptes devriesi * | Peru | Eocene | ~42 | [28,41] |
| Delphinornis gracilis * | Antarctica | Eocene | 41–34 | [49] |
| Delphinornis larsenni * | Antarctica | Eocene | 53–34 | [50] |
| Mesetaornis polaris * | Antarctica | Eocene | 53–34 | [49,51] |
| Anthropornis grandis * | Antarctica | Eocene | 53–34 | [52,53,54] |
| Anthropornis nordenskjöldi * | Antarctica | Eocene | 53–34 | [52,54] |
| Aprosdokitos mikrotero | Antarctica | Eocene | 38–34 | [27] |
| Marambiornis exilis * | Antarctica | Eocene | 53–34 | [49,54] |
| Delphinornis arctowskii * | Antarctica | Eocene | 38–34 | [49,54] |
| Delphinornis wimani *,a | Antarctica | Eocene | 53–34 | [49,54] |
| Icadyptes salasi * | Peru | Eocene | 37.2–35.7 | [28] |
| Inkayaku paracacensis * | Peru | Eocene | 37.2–35.7 | [24] |
| Marambiornopsis sobrali | Antarctica | Eocene | 37.8–41.1 | [51] |
| Pachydyptes ponderosus * | New Zealand | Eocene | 36–30 | [55] |
| Pachydyptes simpsoni | Australia | Eocene | 38–36.5 | [56] |
| Palaeeudyptes antarcticus * | New Zealand | Eocene–Oligocene | 38–28 | [42] |
| Palaeeudyptes marplesi | New Zealand | Eocene | 38–34 | [42] |
| Tonniornis mesetaensis | Antarctica | Eocene | 37.8–34 | [54,57] |
| Tonniornis minimum | Antarctica | Eocene | 37.8–34 | [54,57] |
| Palaeeudyptes gunnari * | Antarctica | Eocene | 55–34 | [30,54,58] |
| Palaeeudyptes klekowskii * | Antarctica | Eocene | 52–34 | [54,58,59] |
| Kairuku waewaeroa | New Zealand | Oligocene | 34–27.3 | [60] |
| Archaeospheniscus lopdelli * | New Zealand | Oligocene | 27–25 | [55] |
| Archaeospheniscus lowei * | New Zealand | Oligocene | 27–25 | [55] |
| Kairuku grebneffi * | New Zealand | Oligocene | 27.3–25.2 | [42] |
| Kairuku waitaki * | New Zealand | Oligocene | 27.3–34.5 | [42] |
| Korora oliveri | New Zealand | Oligocene | 25–24 | [55] |
| Pakudyptes hakataramea | New Zealand | Oligocene | 25–24 | [55] |
| Platydyptes amiesi | New Zealand | Oligocene | 26–24 | [55] |
| Platydyptes marplesi * | New Zealand | Oligocene–Miocene | 27–22 | [55] |
| Platydyptes novaezealandiae * | New Zealand | Oligocene | 26–24 | [55] |
| Duntroonornis parvus * | New Zealand | Oligocene–Miocene | 27–24 | [55] |
| Paraptenodytes robustus | Argentina | Oligocene–Miocene | 25–22 | [61,62] |
| Arthrodytes andrewsi | Argentina | Oligocene–Miocene | 25–22 | [61,62] |
| Eretiscus tonni * | Argentina | Miocene | 23–20.44 | [63] |
| Palaeospheniscus bergi * | Argentina | Miocene | 23–20.44 | [64] |
| Palaeospheniscus biloculata * | Argentina | Miocene | 23–20.44 | [65] |
| Palaeospheniscus patagonicus * | Argentina | Miocene | 23–20.44 | [66] |
| Paraptenodytes antarcticus * | Argentina | Miocene | 23–20.44 | [61] |
| Anthropodyptes gilli | Australia | Miocene | 21–17.6 | [67] |
| Spheniscus muizoni * | Peru | Miocene | 13–11 | [68] |
| Spheniscus urbinai * | Argentina, Chile, Perú | Miocene | 23–5 | [69] |
| Madrynornis mirandus * | Argentina | Miocene | 11.4–9 | [70,71] |
| Pygoscelis calderensis | Chile | Miocene | ~7.6 | [69,72] |
| Marplesornis novaezealandiae * | New Zealand | Miocene–Pliocene | 12.7–2.4 | [55] |
| Spheniscus megaramphus * | Chile, Peru | Miocene–Pliocene | 11.6–3.6 | [73,74] |
| Pseudaptenodytes macraei | Australia | Miocene–Pliocene | 6.2–5 | [75] |
| Dege hendeyi | South Africa | Pliocene | 5.3–3.6 | [76] |
| Inguza predemersus | South Africa | Pliocene | 5 | [77] |
| Nucleornis insolitus | South Africa | Pliocene | 5 | [77] |
| Eudyptula calauina | Chile | Pliocene | 3.6–2.6 | [36] |
| Spheniscus chilensis | Chile | Pliocene | 3.6–2.6 | [78] |
| Eudyptes atatu | New Zealand | Pliocene | 3.3–3 | [38] |
| Tereingaornis moisleyi | New Zealand | Pliocene | 3–4 | [55] |
| Pygoscelis grandis * | Chile | Pliocene | 5.3–3.6 | [67] |
| Pygoscelis tyreei | New Zealand | Pliocene–Pleistocene | 4–2 | [55] |
| Aptenodytes ridgeni | New Zealand | Pliocene–Pleistocene | 4–2 | [55] |
| Model | LnL | p | AICc | AICc wt. |
|---|---|---|---|---|
| DEC | −139.9 | 2 | 284 | 2.6 × 10−6 |
| DEC + J | −133 | 3 | 272.5 | 8 × 10−4 |
| DIVALIKE | −151.4 | 2 | 307.1 | 2.5 × 10−11 |
| DIVALIKE + J | −143.7 | 3 | 294 | 1.8 × 10−8 |
| BAYAREALIKE | −150.2 | 2 | 304.6 | 8.9 × 10−11 |
| BAYAREALIKE + J | −125.9 | 3 | 258.3 | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pelegrín, J.S.; Acosta Hospitaleche, C. Evolutionary and Biogeographical History of Penguins (Sphenisciformes): Review of the Dispersal Patterns and Adaptations in a Geologic and Paleoecological Context. Diversity 2022, 14, 255. https://doi.org/10.3390/d14040255
Pelegrín JS, Acosta Hospitaleche C. Evolutionary and Biogeographical History of Penguins (Sphenisciformes): Review of the Dispersal Patterns and Adaptations in a Geologic and Paleoecological Context. Diversity. 2022; 14(4):255. https://doi.org/10.3390/d14040255
Chicago/Turabian StylePelegrín, Jonathan S., and Carolina Acosta Hospitaleche. 2022. "Evolutionary and Biogeographical History of Penguins (Sphenisciformes): Review of the Dispersal Patterns and Adaptations in a Geologic and Paleoecological Context" Diversity 14, no. 4: 255. https://doi.org/10.3390/d14040255
APA StylePelegrín, J. S., & Acosta Hospitaleche, C. (2022). Evolutionary and Biogeographical History of Penguins (Sphenisciformes): Review of the Dispersal Patterns and Adaptations in a Geologic and Paleoecological Context. Diversity, 14(4), 255. https://doi.org/10.3390/d14040255







