How the Strength of Monsoon Winds Shape Forest Dynamics
Abstract
:1. Introduction
2. Materials and Methods
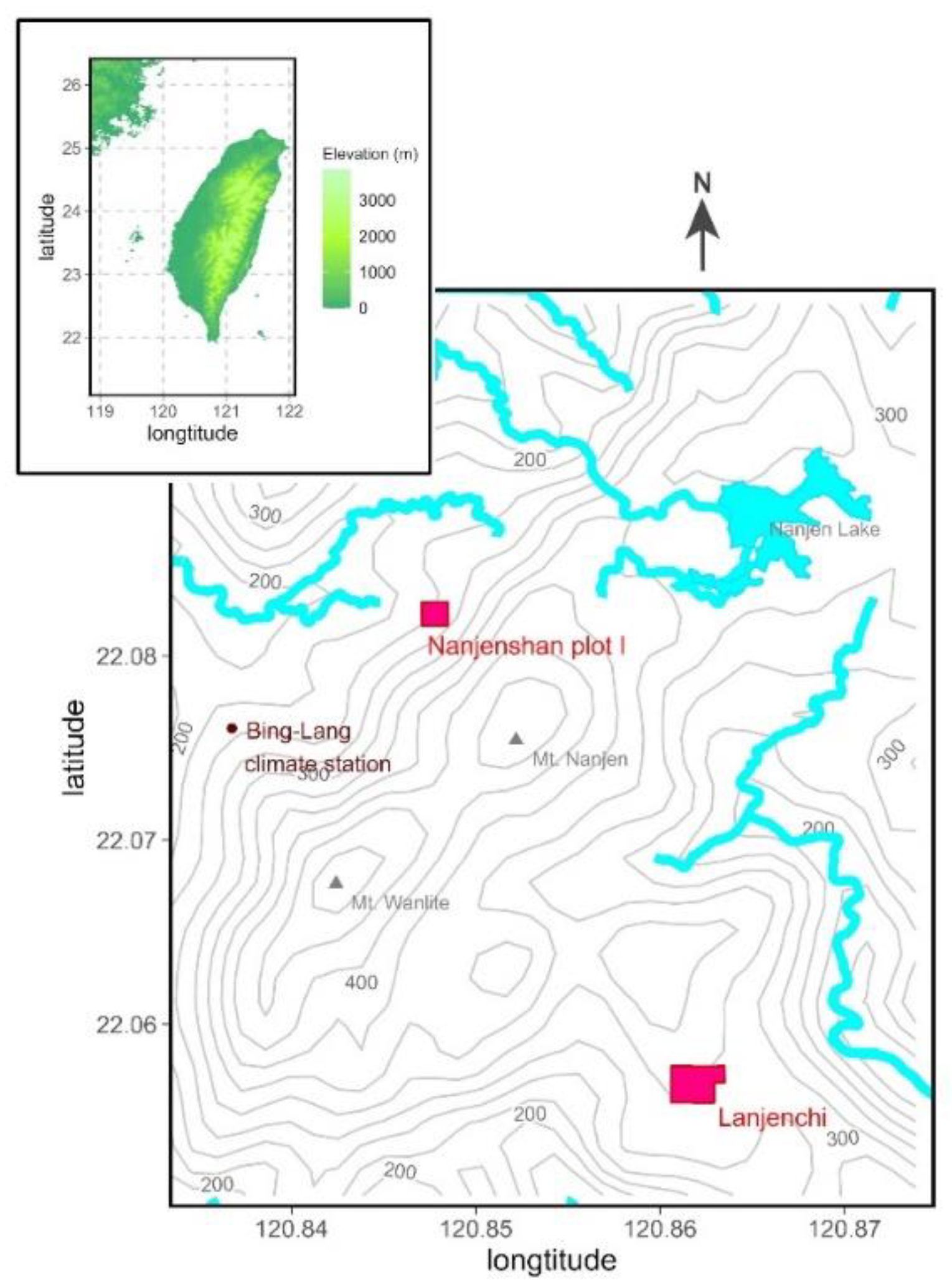
2.1. Study Site
2.2. Data from the Study Site
2.3. Data Analysis
2.3.1. Abundance and Dominance
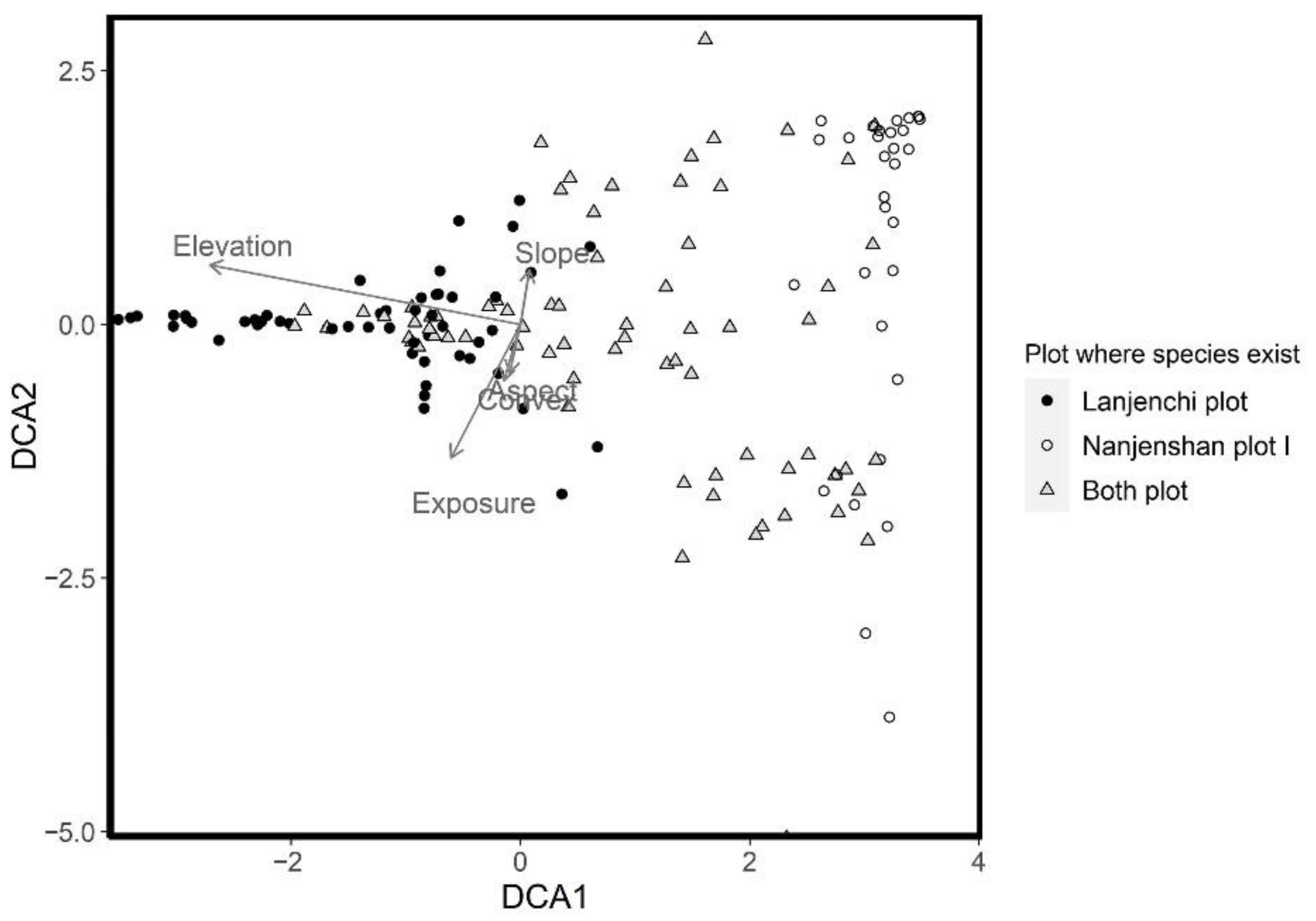
2.3.2. DCA Analysis and the Environmental Factors
2.3.3. Diversity Index
2.3.4. Dynamic Index
3. Results
3.1. Forest Structure and Forest Composition in the Lanjenchi and Nanjenshan Plots
3.2. Overall Dynamic Changes in the Plots
3.3. Changes in Abundant and Dominant Species in the Lanjenchi and Nanjenshan Plots I
4. Discussion
4.1. Different Wind Stressing Affects Forest Composition
4.2. From Species to Overall Change in Dynamics
4.3. How the Monsoon Wind Shaped the Forest of the Future
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Begon, M.; Townsend, C.R.; Harper, J.L. Ecology: From Individuals to Ecosystems; Blackwell: Oxford, UK, 2006. [Google Scholar]
- Condit, R.; Hubbell, S.P.; Foster, R.B. Short-term dynamics of a neotropical forest: Change within limits. Bioscience 1992, 42, 822–828. [Google Scholar] [CrossRef]
- Condit, R.; Hubbell, S.P.; Foster, R.B. Mortality rates of 205 neotropical tree and shrub species and the impact of a severe drought. Ecol. Monogr. 1995, 65, 419–439. [Google Scholar] [CrossRef]
- Condit, R. Research in large, long-term tropical forest plots. Trends Ecol. Evol. 1995, 10, 18–22. [Google Scholar] [CrossRef]
- Legendre, P.; Condit, R. Spatial and temporal analysis of beta diversity in the Barro Colorado Island forest dynamics plot, Panama. For. Ecosyst. 2019, 6, 7. [Google Scholar] [CrossRef] [Green Version]
- Chisholm, R.A.; Condit, R.; Rahman, K.A.; Baker, P.J.; Bunyavejchewin, S.; Chen, Y.Y.; Chuyong, G.; Dattaraja, H.; Davies, S.; Ewango, C.E. Temporal variability of forest communities: Empirical estimates of population change in 4000 tree species. Ecol. Lett. 2014, 17, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Condit, R.; Ashton, P.S.; Manokaran, N.; LaFrankie, J.V.; Hubbell, S.P.; Foster, R.B. Dynamics of the forest communities at Pasoh and Barro Colorado: Comparing two 50–ha plots. Philos. Trans. R. Soc. Lond. B 1999, 354, 1739–1748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, Y.; Santiago, L.S.; Ma, L.; Lin, G.-J.; Lian, J.-Y.; Cao, H.-L.; Ye, W.-H. Forest dynamics of a subtropical monsoon forest in Dinghushan, China: Recruitment, mortality and the pace of community change. J. Trop. Ecol. 2013, 29, 131–145. [Google Scholar] [CrossRef] [Green Version]
- Vincent, J.B.; Turner, B.L.; Alok, C.; Novotny, V.; Weiblen, G.D.; Whitfeld, T.J. Tropical forest dynamics in unstable terrain: A case study from New Guinea. J. Trop. Ecol. 2018, 34, 157–175. [Google Scholar] [CrossRef]
- Lai, J.; Mi, X.; Ren, H.; Ma, K. Species-habitat associations change in a subtropical forest of China. J. Veg. Sci. 2009, 20, 415–423. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, B.; Mallik, A.U.; Huang, F.; Xiang, W.; Ding, T.; Wen, S.; Lu, S.; Li, D.; He, Y. Topographic species–habitat associations of tree species in a heterogeneous tropical karst seasonal rain forest, China. J. Plant Ecol. 2017, 10, 450–460. [Google Scholar] [CrossRef]
- Hsieh, C.-F.; Chen, Z.-S.; Sun, I.; Hsieh, T.-H.; Zheng, Y.-B.; Wang, K.-H.; Su, M.-H.; Jiang, F.-Y. The Subtropical Rain Forest in Nanjenshan Area, Kenting National Park (Report No. RES085); Kenting National Park, Construction and Planning Agency, Ministry of the Interior: Pingtung County, Taiwan, 1992; p. 269. [Google Scholar]
- Su, M.H. The Leaf Structure of Canopy of Nanjenshan Subtropical Rain Forest. Master’s Thesis, National Taiwan University, Taipei, Taiwan, 1993. [Google Scholar]
- Chang, C.-Y. Effects of Wind Speed and Other Environmental Factors on Sap Flux of Three Species in a Subtropical Rainforest of Nanjenshan, South Taiwan. Master’s Thesis, National Taiwan University, Taipei, Taiwan, 2008. [Google Scholar]
- Hsieh, C.-F.; Sun, I.-F.; Yang, C.-C. Species composition and vegetation pattern of a lowland rain forest at the Nanjenshan LTER site, southern Taiwan. Taiwania 2000, 45, 107–119. [Google Scholar] [CrossRef]
- Chao, W.-C.; Song, G.-Z.M.; Chao, K.-J.; Liao, C.-C.; Fan, S.-W.; Wu, S.-H.; Hsieh, T.-H.; Sun, I.-F.; Kuo, Y.-L.; Hsieh, C.-F. Lowland rainforests in southern Taiwan and Lanyu, at the northern border of Paleotrophics and under the influence of monsoon wind. Plant Ecol. 2010, 210, 1–17. [Google Scholar] [CrossRef]
- Li, C.F.; Chytrý, M.; Zelený, D.; Chen, M.Y.; Chen, T.Y.; Chiou, C.R.; Hsia, Y.J.; Liu, H.Y.; Yang, S.Z.; Yeh, C.L. Classification of Taiwan forest vegetation. Appl. Veg. Sci. 2013, 16, 698–719. [Google Scholar] [CrossRef]
- Ku, C.-C.; Song, G.-Z.M.; Chao, K.-J.; Chao, W.-C. Species-habitat associations of tree species under the northeast monsoon wind-affected tropical forest at Lanjenchi Forest Dynamics Plot, Taiwan. Taiwania 2021, 66, 39–47. [Google Scholar] [CrossRef]
- Chao, K.-J.; Liao, P.-S.; Chen, Y.-S.; Song, G.-Z.M.; Phillips, O.L.; Lin, H.-J. Very Low Stocks and Inputs of Necromass in Wind-affected Tropical Forests. Ecosystems 2021. [Google Scholar] [CrossRef]
- Chao, W.-C.; Chao, K.-J.; Song, G.-Z.M.; Hsieh, C.-F. Species composition and structure of the lowland subtropical rainforest at Lanjenchi, Southern Taiwan. Taiwania 2007, 52, 253–269. [Google Scholar] [CrossRef]
- Ku, C.-C.; Tang, J.; Chao, W.-C.; Chao, K.-J.; Song, G.-Z.M. Changes in aboveground biomass during the past 22 years at Lanjenchi Forest Dynamics Plot tropical forest in southern Taiwan. Ecol. Evol. 2022. (under review). [Google Scholar]
- Chao, K.-J.; Chao, W.-C.; Chen, K.-M.; Hsieh, C.-F. Vegetation dynamics of a lowland rainforest at the Northern border of the Paleotropics at Nanjenshan, southern Taiwan. Taiwan J. For. Sci. 2010, 25, 29–40. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 24 February 2022).
- Højsgaard, S.; Halekoh, U.; Yan, J. The R package geepack for generalized estimating equations. J. Stat. Softw. 2005, 15, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Huang, T.-C.; Hsuan, K.; Tsai, J.-L. Editorial Committee of the Flora of Taiwan, 2nd ed.; Department of Botany, National Taiwan University: Taipei, Taiwan, 2003. [Google Scholar]
- Hill, M.O.; Gauch, H.G. Detrended correspondence analysis: An improved ordination technique. Vegetatio 1980, 42, 47–58. [Google Scholar] [CrossRef]
- Oksanen, J.; Kindt, R.; Legendre, P.; O’Hara, B.; Stevens, M.H.H.; Oksanen, M.J.; Suggests, M. The vegan package. Community Ecol. Package 2007, 10, 719. [Google Scholar]
- Telewski, F.W. Is windswept tree growth negative thigmotropism? Plant Sci. 2012, 184, 20–28. [Google Scholar] [CrossRef]
- Schimper, A.F.W. Pflanzen-Geographie auf Physiologischer Grundlage; G. Fischer: Jena, Germany, 1898; Volume 2. [Google Scholar]
- MacFarlane, D.W.; Kane, B. Neighbour effects on tree architecture: Functional trade-offs balancing crown competitiveness with wind resistance. Funct. Ecol. 2017, 31, 1624–1636. [Google Scholar] [CrossRef] [Green Version]
- Archibald, S.; Bond, W.J. Growing tall vs growing wide: Tree architecture and allometry of Acacia karroo in forest, savanna, and arid environments. Oikos 2003, 102, 3–14. [Google Scholar] [CrossRef]
- Hubbell, S.P. The Unified Neutral Theory of Biodiversity and Biogeography (MPB-32); Princeton University Press: Princeton, NJ, USA, 2001; Volume 32. [Google Scholar]
- Editorial Committee of the Red List of Taiwan Plants. The Red List of Vascular Plants of Taiwan; Endemic Species Research Institute, Forestry Bureau, Council of Agriculture, Executive Yuan and Taiwan Society of Plant Systematics: Nantou, Taiwan, 2017; p. 186. [Google Scholar]
- Kuo, Y.-L.; Lin, T.-Y.; Yang, Y.-Y.; Chen, H.-L.; Yang, C.-K.; Yu, S.-Y. Photosynthetic characteristics and shade tolerance of 440 native woody species in Taiwan. Taiwan J. For. Sci. 2021, 36, 189–220. [Google Scholar]
- Velázquez, E.; Wiegand, T. Competition for light and persistence of rare light-demanding species within tree-fall gaps in a moist tropical forest. Ecology 2020, 101, e03034. [Google Scholar] [CrossRef] [Green Version]
- Hubbell, S.P.; Foster, R.B. Plant Ecology; Blackwell Scientific: Oxford, UK, 1986. [Google Scholar]
- Lutz, J.A.; Furniss, T.J.; Johnson, D.J.; Davies, S.J.; Allen, D.; Alonso, A.; Anderson-Teixeira, K.J.; Andrade, A.; Baltzer, J.; Becker, K.M. Global importance of large-diameter trees. Glob. Ecol. Biogeogr. 2018, 27, 849–864. [Google Scholar] [CrossRef] [Green Version]
- Lutz, J.A.; Larson, A.J.; Swanson, M.E.; Freund, J.A. Ecological importance of large-diameter trees in a temperate mixed-conifer forest. PLoS ONE 2012, 7, e36131. [Google Scholar] [CrossRef]
- Bertness, M.D.; Callaway, R. Positive interactions in communities. Trends Ecol. Evol. 1994, 9, 191–193. [Google Scholar] [CrossRef]
- Olsen, S.L.; Töpper, J.P.; Skarpaas, O.; Vandvik, V.; Klanderud, K. From facilitation to competition: Temperature-driven shift in dominant plant interactions affects population dynamics in seminatural grasslands. Glob. Change Biol. 2016, 22, 1915–1926. [Google Scholar] [CrossRef]
- Chu, C.J.; Maestre, F.T.; Xiao, S.; Weiner, J.; Wang, Y.S.; Duan, Z.H.; Wang, G. Balance between facilitation and resource competition determines biomass-density relationships in plant populations. Ecol. Lett. 2008, 11, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Anthelme, F.; Cavieres, L.A.; Dangles, O. Facilitation among plants in alpine environments in the face of climate change. Front. Plant Sci. 2014, 5, 387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crabtree, D.; Ellis, C.J. Species interaction and response to wind speed alter the impact of projected temperature change in a montane ecosystem. J. Veg. Sci. 2010, 21, 744–760. [Google Scholar] [CrossRef]
- He, Q.; Bertness, M.D.; Altieri, A.H. Global shifts towards positive species interactions with increasing environmental stress. Ecol. Lett. 2013, 16, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.-w.; Kao, P.-k. Weakening of the winter monsoon and abrupt increase of winter rainfalls over northern Taiwan and southern China in the early 1980s. J. Clim. 2010, 23, 2357–2367. [Google Scholar] [CrossRef]
- Wang, L.; Lu, M.-M. The East Asian winter monsoon. In The Global Monsoon System: Research and Forecast, 2nd ed.; Chang, C.-P., Ding, Y., Lau, N.-C., Johnson, R.H., Wang, B., Yasunari, T., Eds.; World Scientific: Singapore, 2017; pp. 51–61. [Google Scholar]

| Census | Lanjenchi Plot | Nanjenshan Plot I | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Stem Density (Individual ha−1) | Basal Area (m2 ha−1) | Species | H’ Ϯ2 | DϮ3 | Stem Density (Individual ha−1) | Basal Area (m2 ha−1) | Species | H’ Ϯ2 | DϮ3 | |
| 1 Ϯ1 | 12,179 ± 6689 d | 46.3 ± 36.4 a | 119 | 3.86 | 0.96 | 3253 ± 1208 a | 42.7 ± 32.9 a | 105 | 3.31 | 0.93 |
| 2 | 10,406 ± 5603 d | 46.6 ± 32.1 a | 132 | 3.90 | 0.97 | 3610 ± 1582 a | 45.1 ± 41.3 a | 106 | 3.26 | 0.93 |
| 3 | 9681 ± 4760 b | 45.3 ± 29.0 a | 131 | 3.89 | 0.96 | 4058 ± 1792 b | 44.3 ± 40.6 a | 106 | 3.30 | 0.93 |
| 4 | 9532 ± 4306 a | 46.1 ± 26.1 a | 130 | 3.86 | 0.96 | 3991 ± 1772 b | 44.6 ± 40.5 a | 107 | 3.28 | 0.93 |
| 5 | 9694 ± 3924 b,c | 46.0 ± 26.3 a | 126 | 3.80 | 0.96 | 4019 ± 1791 b | 46.6 ± 43.0 a | 105 | 3.26 | 0.93 |
| Census | Lanjenchi Plot | Nanjenshan Plot I | ||||
|---|---|---|---|---|---|---|
| r (% yr−1) | m (% yr−1) | λ (% yr−1) | r (% yr−1) | m (% yr−1) | λ (% yr−1) | |
| 1 Ϯ1 | - | - | - | - | - | - |
| 2 | 2.56 | 2.50 | 0.06 | 3.01 | 1.66 | 1.35 |
| 3 | 1.66 | 2.56 | −0.9 | 3.55 | 2.09 | 1.46 |
| 4 | 1.92 | 2.12 | −0.2 | 2.49 | 2.83 | −0.34 |
| 5 | 3.14 | 2.86 | 0.28 | 2.31 | 2.43 | −0.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ku, C.-C.; Chao, K.-J.; Song, G.-Z.M.; Lin, H.-Y.; Fan, S.-W.; Chao, W.-C. How the Strength of Monsoon Winds Shape Forest Dynamics. Diversity 2022, 14, 169. https://doi.org/10.3390/d14030169
Ku C-C, Chao K-J, Song G-ZM, Lin H-Y, Fan S-W, Chao W-C. How the Strength of Monsoon Winds Shape Forest Dynamics. Diversity. 2022; 14(3):169. https://doi.org/10.3390/d14030169
Chicago/Turabian StyleKu, Chen-Chia, Kuo-Jung Chao, Guo-Zhang M. Song, Huan-Yu Lin, Su-Wei Fan, and Wei-Chun Chao. 2022. "How the Strength of Monsoon Winds Shape Forest Dynamics" Diversity 14, no. 3: 169. https://doi.org/10.3390/d14030169
APA StyleKu, C.-C., Chao, K.-J., Song, G.-Z. M., Lin, H.-Y., Fan, S.-W., & Chao, W.-C. (2022). How the Strength of Monsoon Winds Shape Forest Dynamics. Diversity, 14(3), 169. https://doi.org/10.3390/d14030169






