Investigating Plant–Bird Co-Occurrence Patterns in Mediterranean Wetlands: Can They Reveal Signals of Ecosystem Connectivity?
Abstract
:1. Introduction
2. Materials and Methods
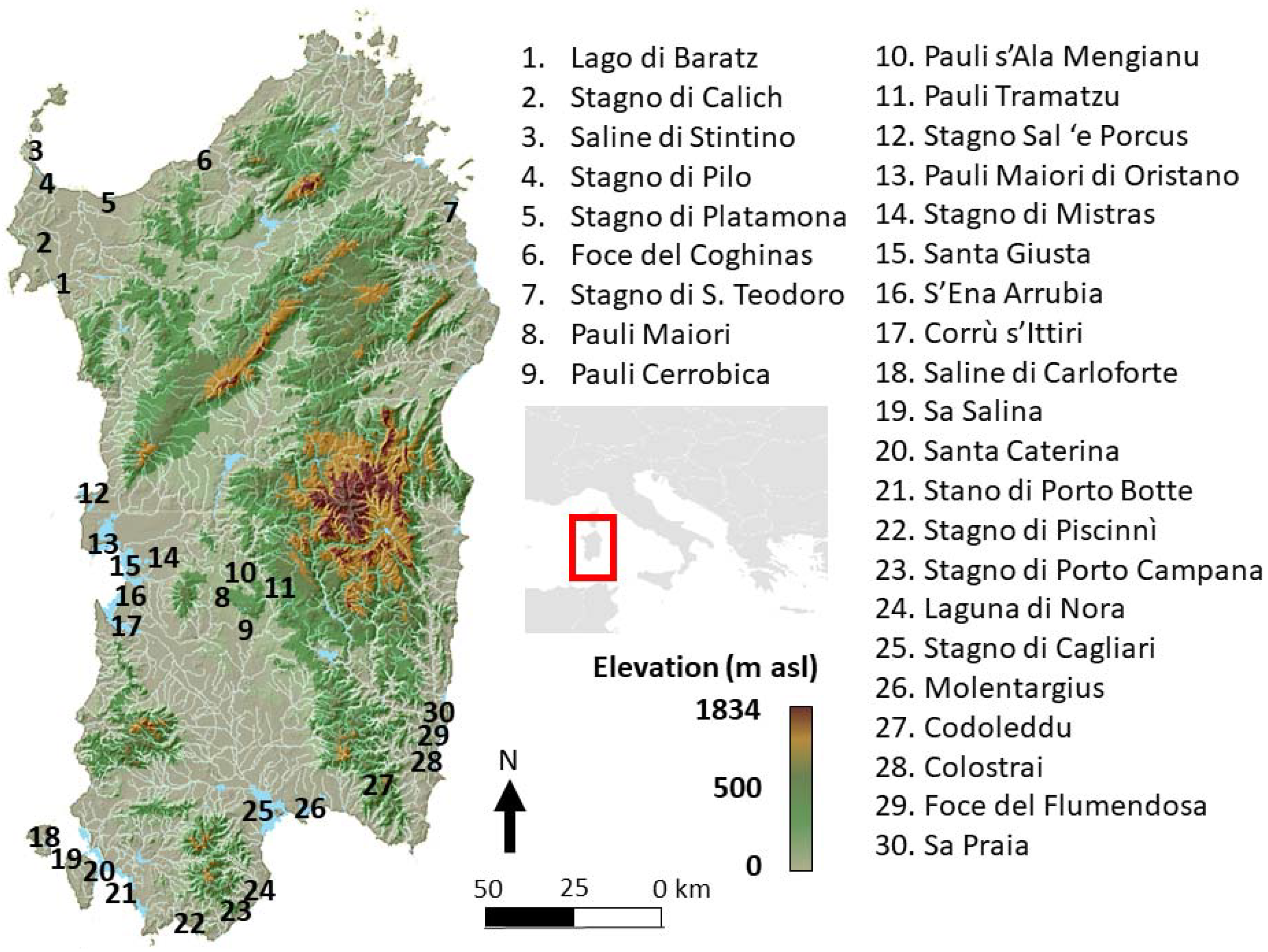
2.1. Study Area and Sampling
2.2. Analysis of Species Co-Occurrence
2.3. Species and Site Traits Data
2.4. Statistical Analysis
3. Results
3.1. Summary of Co-Occurrences among Site-Based and Species-Based Traits
3.2. Influence of Site- and Species-Based Traits on Plant–Bird Co-Occurrences
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blois, J.L.; Zarnetske, P.L.; Fitzpatrick, M.C.; Finnegan, S. Climate change and the past, present, and future of biotic interactions. Science 2013, 341, 499–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parejo, D. Informational mismatches: A neglected threat of climate change to interspecific interactions. Front. Ecol. Evol. 2016, 4, 31. [Google Scholar] [CrossRef] [Green Version]
- Stachowicz, J.J. Mutualism, facilitation, and the structure of ecological communities: Positive interactions play a critical, but underappreciated, role in ecological communities by reducing physical or biotic stresses in existing habitats and by creating new habitats on which many species depend. Bioscience 2001, 51, 235–246. [Google Scholar] [CrossRef]
- Griffith, S.C. Cooperation and coordination in socially monogamous birds: Moving away from a focus on sexual conflict. Front. Ecol. Evol. 2019, 7, 455. [Google Scholar] [CrossRef] [Green Version]
- Kissling, W.D.; Field, R.; Böhning-Gaese, K. Spatial patterns of woody plant and bird diversity: Functional relationships or environmental effects? Glob. Ecol. Biogeogr. 2008, 17, 327–339. [Google Scholar] [CrossRef]
- Farris, E.; Filigheddu, R. Patterns of plant population spatial variability in relation to vegetation dynamics: Vegetation series matter. Fitosociologia 2011, 48, 67–80. [Google Scholar]
- Viana, D.S.; Santamaría, L.; Figuerola, J. Optimal methods for fitting probability distributions to propagule retention time in studies of zoochorous dispersal. BMC Ecol. 2016, 16, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lovas-Kiss, Á.; Sánchez, M.I.; Wilkinson, D.M.; Coughlan, N.E.; Alves, J.A.; Green, A.J. Shorebirds as important vectors for plant dispersal in Europe. Ecography 2019, 42, 956–967. [Google Scholar] [CrossRef]
- Martín-Vélez, V.; Lovas-Kiss, Á.; Sánchez, M.I.; Green, A.J. Endozoochory of the same community of plants lacking fleshy fruits by storks and gulls. J. Veg. Sci. 2020, 32, e12967. [Google Scholar] [CrossRef]
- Böhm, S.M.; Wells, K.; Kalko, E.K. Top-down control of herbivory by birds and bats in the canopy of temperate broad-leaved oaks (Quercus robur). PLoS ONE 2011, 6, e17857. [Google Scholar] [CrossRef]
- D’Amen, M.; Mod, H.K.; Gotelli, N.J.; Guisan, A. Disentangling biotic interactions, environmental filters, and dispersal limitation as drivers of species co-occurrence. Ecography 2018, 41, 1233–1244. [Google Scholar] [CrossRef] [Green Version]
- Zurell, D.; Pollock, L.J.; Thuiller, W. Do joint species distribution models reliably detect interspecific interactions from co-occurrence data in homogenous environments? Ecography 2018, 41, 1812–1819. [Google Scholar] [CrossRef] [Green Version]
- Quitián, M.; Santillán, V.; Espinosa, C.I.; Homeier, J.; Böhning-Gaese, K.; Schleuning, M.; Neuschulz, E.L. Elevation-dependent effects of forest fragmentation on plant–bird interaction networks in the tropical Andes. Ecography 2018, 41, 1497–1506. [Google Scholar] [CrossRef] [Green Version]
- Testi, A.; Fanelli, G.; Crosti, R.; Castigliani, V.; D’Angeli, D. Characterizing river habitat quality using plant and animal bioindicators: A case study of Tirino River (Abruzzo Region, Central Italy). Ecol. Indic. 2012, 20, 24–33. [Google Scholar] [CrossRef]
- Battisti, C.; Fanelli, G. Applying indicators of disturbance from plant ecology to vertebrates: The hemeroby of bird species. Ecol. Indic. 2016, 61, 799–805. [Google Scholar] [CrossRef]
- Battisti, C. Synanthropic-dominated biomass in an insular landbird assemblage. Community Ecol. 2018, 19, 203–210. [Google Scholar] [CrossRef]
- Valiente-Banuet, A.; Aizen, M.A.; Alcántara, J.M.; Arroyo, J.; Cocucci, A.; Galetti, M.; García, M.B.; García, D.; Gómez, J.M.; Jordano, P.; et al. Beyond species loss: The extinction of ecological interactions in a changing world. Funct. Ecol. 2015, 29, 299–307. [Google Scholar] [CrossRef]
- Gotelli, N.J.; Ulrich, W. Statistical challenges in null model analysis. Oikos 2012, 121, 171–180. [Google Scholar] [CrossRef]
- Boulangeat, I.; Gravel, D.; Thuiller, W. Accounting for dispersal and biotic interactions to disentangle the drivers of species distributions and their abundances. Ecol. Lett. 2012, 15, 584–593. [Google Scholar] [CrossRef]
- Veech, J.A. A probabilistic model for analysing species co-occurrence. Glob. Ecol. Biogeogr. 2013, 22, 252–260. [Google Scholar] [CrossRef]
- Sebastián-González, E.; Green, A.J. Phylogenetic relatedness of co-occurring waterbird communities: A test of Darwin’s competition-relatedness hypothesis. J. Avian Biol. 2017, 48, 1372–1382. [Google Scholar] [CrossRef]
- Fanfarillo, E.; Latini, M.; Abbate, G. Patterns of co-occurrence of rare and threatened species in winter arable plant communities of Italy. Diversity 2020, 12, 195. [Google Scholar] [CrossRef]
- Cazelles, K.; Araújo, M.B.; Mouquet, N.; Gravel, D. A theory for species co-occurrence in interaction networks. Theor. Ecol. 2016, 9, 39–48. [Google Scholar] [CrossRef]
- Brazeau, H.A.; Schamp, B.S. Examining the link between competition and negative cooccurrence patterns. Oikos 2019, 128, 1358–1366. [Google Scholar] [CrossRef]
- Pollock, L.J.; Tingley, R.; Morris, W.K.; Golding, N.; O’Hara, R.B.; Parris, K.M.; Vesk, P.A.; McCarthy, M.A. Understanding co-occurrence by modelling species simultaneously with a Joint Species Distribution Model (JSDM). Methods Ecol. Evol. 2014, 5, 397–406. [Google Scholar] [CrossRef]
- Blanchet, F.G.; Cazelles, K.; Gravel, D. Co-occurrence is not evidence of ecological interactions. Ecol. Lett. 2020, 23, 1050–1063. [Google Scholar] [CrossRef]
- Cadotte, M.W.; Tucker, C.M. Should environmental filtering be abandoned? Trends Ecol. Evol. 2017, 32, 429–437. [Google Scholar] [CrossRef]
- Holt, R.D. Some thoughts about the challenge of inferring ecological interactions from spatial data. Biodivers. Inform. 2020, 15, 61–66. [Google Scholar] [CrossRef]
- Bolpagni, R.; Laini, A.; Stanzani, C.; Chiarucci, A. Aquatic plant diversity in Italy: Distribution, drivers and strategic conservation actions. Front. Plant Sci. 2018, 9, 116. [Google Scholar] [CrossRef] [Green Version]
- Cuena-Lombraña, A.; Fois, M.; Cogoni, A.; Bacchetta, G. Where we Come from and where to Go: Six Decades of Botanical Studies in the Mediterranean Wetlands, with Sardinia (Italy) as a Case Study. Wetlands 2021, 41, 69. [Google Scholar] [CrossRef]
- Bagella, S.; Caria, M.C.; Filigheddu, R. Gap analysis revealed a low efficiency of Natura 2000 network for the conservation of endemic species in Mediterranean temporary freshwater habitats. Plant Biosyst. 2013, 147, 1092–1094. [Google Scholar] [CrossRef]
- Spina, F.; Volponi, S. Atlante della Migrazione degli Uccelli in Italia; Ministerio dell’Ambiente e della Tutela del Territorio e del Mare; Tipografia SCR-Roma; Istituto Superiore per la Protezione e la Ricerca Ambientale (ISPRA): Roma, Italy, 2008.
- Ferrarini, A.; Celada, C.; Gustin, M. Preserving the Mediterranean bird flyways: Assessment and prioritization of 38 main wetlands under human and climate threats in Sardinia and Sicily (Italy). Sci. Total Environ. 2020, 751, 141556. [Google Scholar] [CrossRef] [PubMed]
- RAS. Regione Autonoma della Sardegna-Assessorato della Difesa dell’Ambiente. Servizio Tutela della Natura. Censimenti IWC. 2011. Available online: https://www.sardegnaambiente.it/documenti/18_269_20121204133947.pdf (accessed on 4 November 2021).
- Bagella, S.; Filigheddu, R.; Peruzzi, L.; Bedini, G. Wikiplantbase# Sardegna v. 2.1. 2016. Available online: http://bot.biologia.unipi.it/wpb/sardegna/index.html (accessed on 15 September 2020).
- Van Horn, G.; Mac Aodha, O.; Song, Y.; Cui, Y.; Sun, C.; Shepard, A.; Adam, H.; Perona, P.; Belongie, S. The INaturalist species classification and detection dataset. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Salt Lake City, UT, USA, 18–23 June 2018; pp. 8769–8778. [Google Scholar]
- Griffith, D.M.; Veech, J.A.; Marsh, C.J. Cooccur: Probabilistic species co-occurrence analysis in R. J. Stat. Softw. 2016, 69, 1108–1117. [Google Scholar] [CrossRef]
- Royan, A.; Reynolds, S.J.; Hannah, D.M.; Prudhomme, C.; Noble, D.G.; Sadler, J.P. Shared environmental responses drive co-occurrence patterns in river bird communities. Ecography 2016, 39, 733–742. [Google Scholar] [CrossRef] [Green Version]
- Tomàs-Vives, P. Inventory, Assessment and Monitoring of Mediterranean Wetlands: The pan-Mediterranean Wetland Inventorymodule; MedWet Publication, Tour du Valat: Arles, France, 2008; Available online: https://medwet.org/codde/wetlandinvetory.html (accessed on 25 April 2020).
- Kattge, J.; Bönisch, G.; Díaz, S.; Lavorel, S.; Prentice, I.C.; Leadley, P.; Tautenhahn, S.; Werner, G.D.A.; Aakala, T.; Abedi, M.; et al. TRY plant trait database-enhanced coverage and open access. Glob. Chang. Biol. 2020, 26, 119–188. [Google Scholar] [CrossRef] [Green Version]
- Kleyer, M.; Bekker, R.M.; Knevel, I.C.; Bakker, J.P.; Thompson, K.; Sonnenschein, M.; Poschlod, P.; van Groenendael, J.M.; Klimeš, L.; Klimešová, J.; et al. The LEDA Traitbase: A database of life-history traits of the Northwest European flora. J. Ecol. 2008, 96, 1266–1274. [Google Scholar] [CrossRef]
- Grussu, M. Checklist of the birds of Sardinia. Updated to December 2001. Aves Ichnusae 2001, 4, 2–55. [Google Scholar]
- Biondi, M.; Pietrelli, L.; Guerrieri, G.; Corso, A.; Grussu, M. Il Corriere piccolo, Charadrius dubius, nell’Italia centrale e meridionale. Riv. Ital. Ornitol. 2000, 70, 97–114. [Google Scholar]
- Grussu, M.; Floris, G.; Sanna, M. Terzo censimento delle Garzaie in Sardegna: 2002. Aves Ichnusae 2004, 7, 22–41. [Google Scholar]
- Galasso, G.; Conti, F.; Peruzzi, L.; Ardenghi, N.M.G.; Banfi, E.; Celesti-Grapow, L.; Albano, A.; Alessandrini, A.; Bacchetta, G.; Ballelli, S.; et al. An updated checklist of the vascular flora alien to Italy. Plant Biosyst. 2018, 152, 556–592. [Google Scholar] [CrossRef]
- Cook, R.D.; Weisberg, S. Residuals and Influence in Regression; Chapman and Hall: New York, NY, USA, 1982. [Google Scholar]
- Calcagno, V.; de Mazancourt, C. glmulti: An R package for easy automated model selection with (generalized) linear models. J. Stat. Softw. 2010, 34, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Burnham, K.; Anderson, D.; Huyvaert, K. AIC model selection and multimodel inference in behavioral ecology: Some background, observations, and comparisons. Behav. Ecol. Sociobiol. 2011, 65, 23–35. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Multimodel inference understanding AIC and BIC in model selection. Sociol. Methods Res. 2004, 33, 261–304. [Google Scholar] [CrossRef]
- Ma, X.; Geng, Q.; Zhang, H.; Bian, C.; Chen, H.Y.; Jiang, D.; Xu, X. Global negative effects of nutrient enrichment on arbuscular mycorrhizal fungi, plant diversity and ecosystem multi-functionality. New Phytol. 2020, 229, 2957–2969. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, A.M.; Brown, J.A.; Jimenez-Valverde, A.; Real, A. modEvA: Model Evaluation and Analysis. 2015. Available online: http://modeva.r-forge.r-project.org/ (accessed on 21 October 2018).
- Kallimanis, A.S.; Mazaris, A.D.; Tzanopoulos, J.; Halley, J.M.; Pantis, J.D.; Sgardelis, S.P. How does habitat diversity affect the species–area relationship? Glob. Ecol. Biogeogr. 2008, 17, 532–538. [Google Scholar] [CrossRef]
- Fois, M.; Fenu, G.; Bacchetta, G. Global analyses underrate part of the story: Finding applicable results for the conservation planning of small Sardinian islets’ flora. Biodivers. Conserv. 2016, 25, 1091–1106. [Google Scholar] [CrossRef]
- Rooney, R.C.; Azeria, E.T. The strength of cross-taxon congruence in species composition varies with the size of regional species pools and the intensity of human disturbance. J. Biogeogr. 2015, 42, 439–451. [Google Scholar] [CrossRef]
- Calvino-Cancela, M. Gulls (Laridae) as frugivores and seed dispersers. Plant Ecol. 2011, 212, 1149–1157. [Google Scholar] [CrossRef]
- Fois, M.; Cuena-Lombraña, A.; Bacchetta, G. Knowledge gaps and challenges for conservation of Mediterranean wetlands: Evidence from a comprehensive inventory and literature analysis for Sardinia. Aquat. Conserv. Mar. Freshw. Ecosyst. 2021, 31, 2621–2631. [Google Scholar] [CrossRef]
- Battisti, C.; Grosso, G.; Ioni, S.; Zullo, F.; Cerfolli, F. Response of specialized birds to reed-bed aging in a Mediterranean wetland: Significant changes in bird biomass after two decades. Isr. J. Ecol. Evol. 2020, 67, 17–22. [Google Scholar] [CrossRef]
- Gagnon, L.N.; Gauthier, G.; Lavoie, C. Effect of the invasive common reed on the abundance, richness and diversity of birds in freshwater marshes. Anim. Conserv. 2015, 18, 32–43. [Google Scholar] [CrossRef]
- Kawatsu, M.; Morimoto, G.; Kagami, M. Seasonal changes in the water bird community in Lake Inba: Influence of floating-leaved macrophytes on habitat selection. Aquat. Bot. 2015, 126, 32–37. [Google Scholar] [CrossRef]
- Muñoz-Gallego, R.; Fedriani, J.M.; Traveset, A. Non-native mammals are the main seed dispersers of the ancient Mediterranean palm Chamaerops humilis L. in the Balearic Islands: Rescuers of a lost seed dispersal service? Front. Ecol. Evol. 2019, 7, 161. [Google Scholar] [CrossRef] [Green Version]
- Mokotjomela, T.M.; Thabethe, V.; Downs, C. Comparing germination metrics of Opuntia ficus-indica and O. robusta between two sets of bird species (Pied Crows and two smaller species). Acta Oecol. 2021, 110, 103676. [Google Scholar] [CrossRef]
- Chupp, A.D.; Battaglia, L.L. Bird–plant interactions and vulnerability to biological invasions. J. Plant Ecol. 2016, 9, 692–702. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Brito, D.; Carrete, M.; Blanco, G.; Romero-Vidal, P.; Senar, J.C.; Mori, E.; White, T.H., Jr.; Luna, Á.; Tella, J.L. The Role of Monk Parakeets as Nest-Site Facilitators in Their Native and Invaded Areas. Biology 2021, 10, 683. [Google Scholar] [CrossRef] [PubMed]
- Mellado, A.; Zamora, R. Generalist birds govern the seed dispersal of a parasitic plant with strong recruitment constraints. Oecologia 2014, 176, 139–147. [Google Scholar] [CrossRef]
- Higgins, S.I.; Nathan, R.; Cain, M.L. Are long-distance dispersal events in plants usually caused by nonstandard means of dispersal? Ecology 2003, 84, 1945–1956. [Google Scholar] [CrossRef]
- MacArthur, R.H.; MacArthur, J.W. On Bird Species Diversity. Ecology 1961, 42, 594–598. [Google Scholar] [CrossRef]
- Warren, R.J.; Love, J.P.; Bradford, M.A. Nest-mediated seed dispersal. Plant Ecol. 2017, 218, 1213–1220. [Google Scholar] [CrossRef]
- Tews, J.; Brose, U.; Grimm, V.; Tielbörger, K.; Wichmann, M.C.; Schwager, M.; Jeltsch, F. Animal species diversity driven by habitat heterogeneity/diversity: The importance of keystone structures. J. Biogeogr. 2004, 31, 79–92. [Google Scholar] [CrossRef] [Green Version]
- Stephens, C.R.; González-Salazar, C.; Villabos-Segura, M.; Marquet, P.A. Can We Infer Species Interactions from co-occurrence Patterns? A Reply to Peterson et al. Biodivers. Inform. 2020, 15, 57–60. [Google Scholar] [CrossRef]
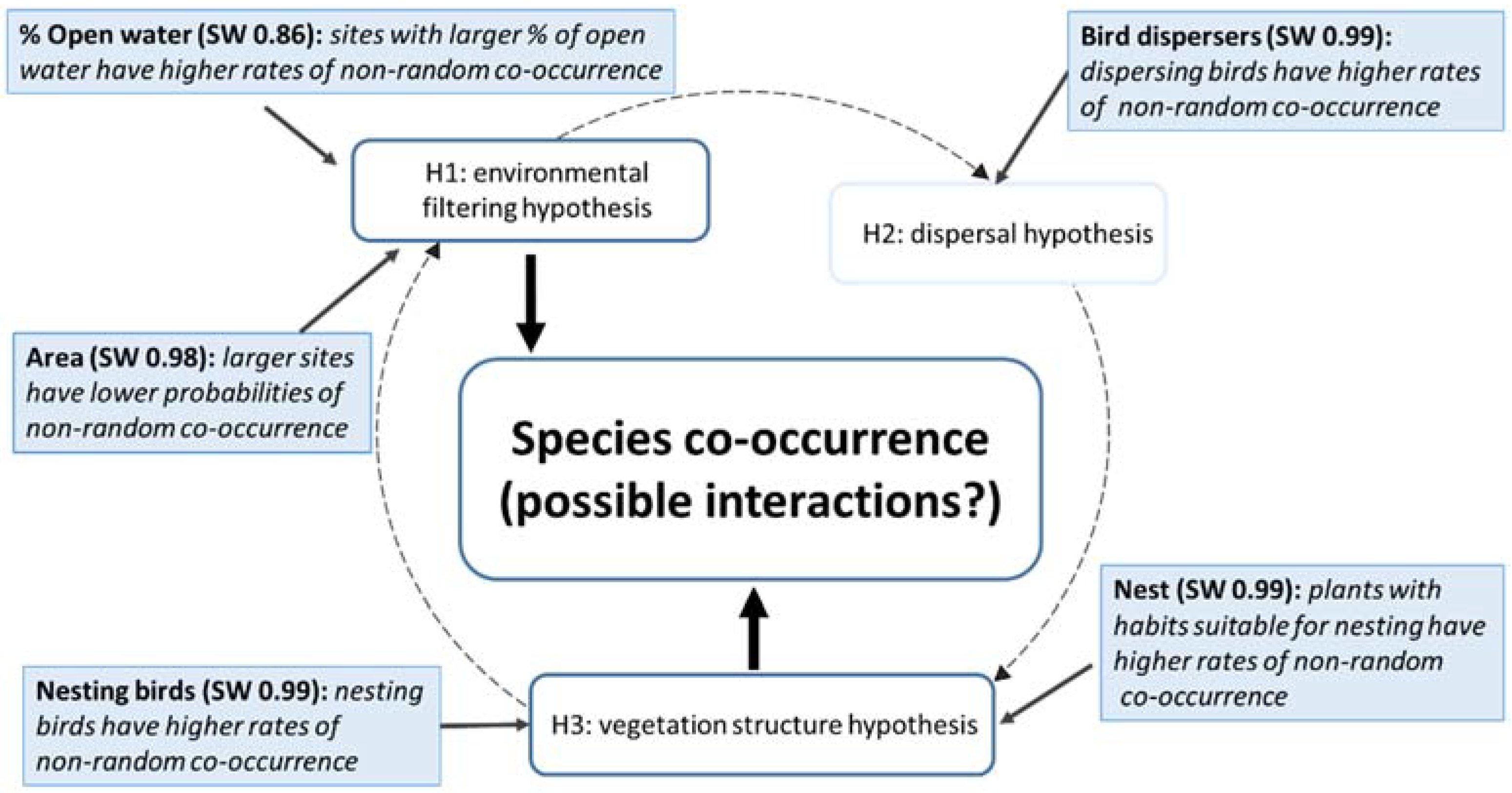
| Explanatory: site co-occurrence | ||||||||
| Model terms | Model performance | |||||||
| Area (ha) | N_Habitat | D_coast (km) | Open Water | Protection | df | Dsq | AICc | delta |
| −0.00 ** | 0.07 * | −0.03 * | 0.02 * | −0.99 | 23 | 0.66 | 66.86 | |
| −0.00 ** | −0.03 * | 0.02 * | 24 | 0.47 | 67.05 | 0.19 | ||
| −0.00 ** | 0.06 * | 0.03 *** | 24 | 0.45 | 68.12 | 1.26 | ||
| −0.00 ** | 0.00 *** | −0.01 | 24 | 0.45 | 68.56 | 1.51 | ||
| −0.00 ** | 0.05 | 0.03 *** | −0.91 | 23 | 0.48 | 68.70 | 1.65 | |
| −0.00 ** | −0.05 *** | 25 | 0.41 | 68.73 | 1.87 | |||
| −0.00 ** | −0.02 | 0.02 * | 23 | 0.48 | 68.80 | 1.94 | ||
| 0.98 | 0.35 | 0.66 | 0.86 | 0.46 | <-- Akaike weights | |||
| Explanatory: bird co-occurrence | ||||||||
| Model terms | Model performance | |||||||
| Disperser | Nesting | df | Dsq | AICc | delta | |||
| 1.70 *** | 2.71 *** | 142 | 0.19 | 739.63 | ||||
| 0.99 | 0.99 | <-- Akaike weights | ||||||
| Explanatory: plant co-occurrence | ||||||||
| Model terms | Model performance | |||||||
| Nest | Seeding | Exotic | df | Dsq | AICc | delta | ||
| 2.31 *** | 132 | 0.13 | 640.34 | |||||
| 2.20 *** | 0.47 | 132 | 0.13 | 641.73 | 1.39 | |||
| 2.26 *** | 0.41 | 131 | 0.13 | 642.13 | 1.79 | |||
| 0.99 | 0.32 | 0.28 | <-- Akaike weights | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fois, M.; Cuena-Lombraña, A.; Zucca, C.; Nissardi, S.; Bacchetta, G. Investigating Plant–Bird Co-Occurrence Patterns in Mediterranean Wetlands: Can They Reveal Signals of Ecosystem Connectivity? Diversity 2022, 14, 253. https://doi.org/10.3390/d14040253
Fois M, Cuena-Lombraña A, Zucca C, Nissardi S, Bacchetta G. Investigating Plant–Bird Co-Occurrence Patterns in Mediterranean Wetlands: Can They Reveal Signals of Ecosystem Connectivity? Diversity. 2022; 14(4):253. https://doi.org/10.3390/d14040253
Chicago/Turabian StyleFois, Mauro, Alba Cuena-Lombraña, Carla Zucca, Sergio Nissardi, and Gianluigi Bacchetta. 2022. "Investigating Plant–Bird Co-Occurrence Patterns in Mediterranean Wetlands: Can They Reveal Signals of Ecosystem Connectivity?" Diversity 14, no. 4: 253. https://doi.org/10.3390/d14040253
APA StyleFois, M., Cuena-Lombraña, A., Zucca, C., Nissardi, S., & Bacchetta, G. (2022). Investigating Plant–Bird Co-Occurrence Patterns in Mediterranean Wetlands: Can They Reveal Signals of Ecosystem Connectivity? Diversity, 14(4), 253. https://doi.org/10.3390/d14040253








