Ethnobotanical Diversity of Cassava (Manihot esculenta Crantz) in the Peruvian Amazon
Abstract
:1. Introduction
2. Materials and Methods
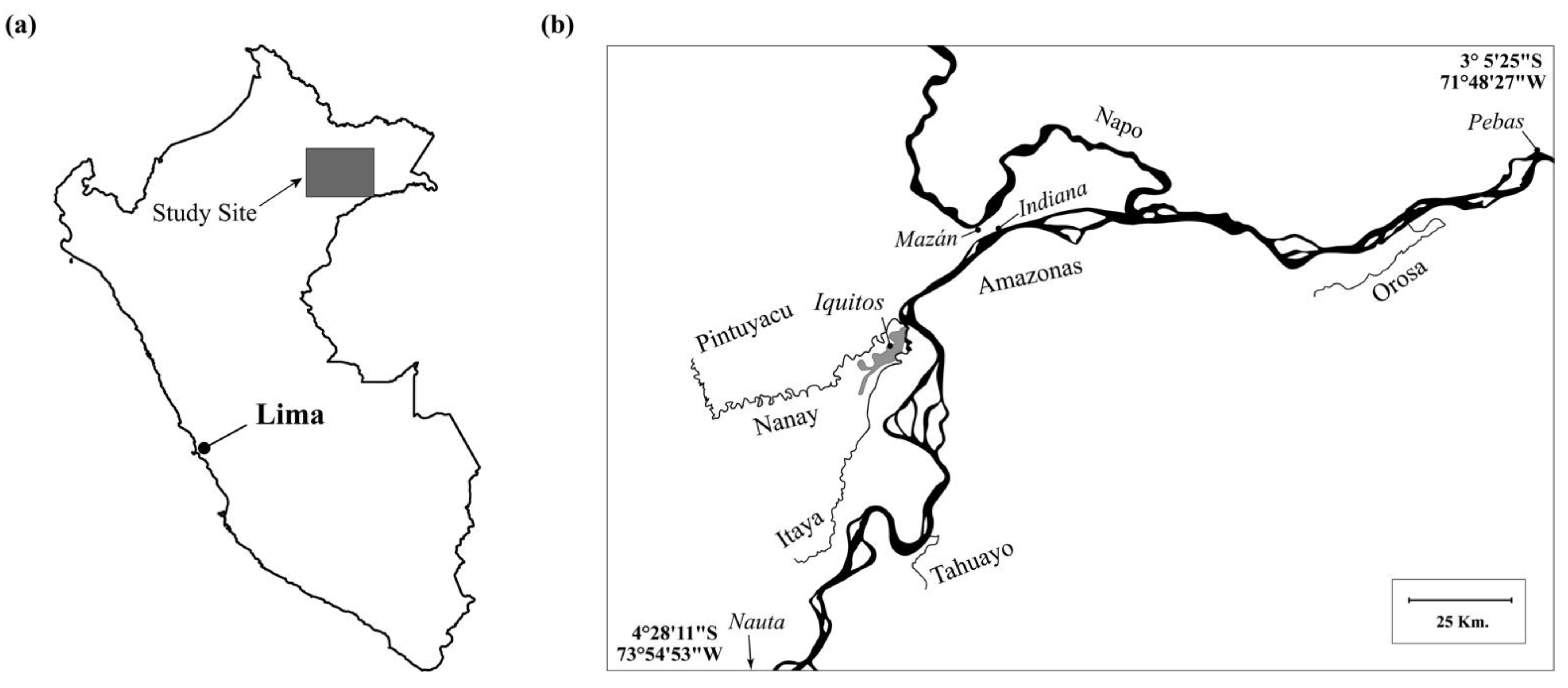
2.1. Study Site
2.2. Collection Sites
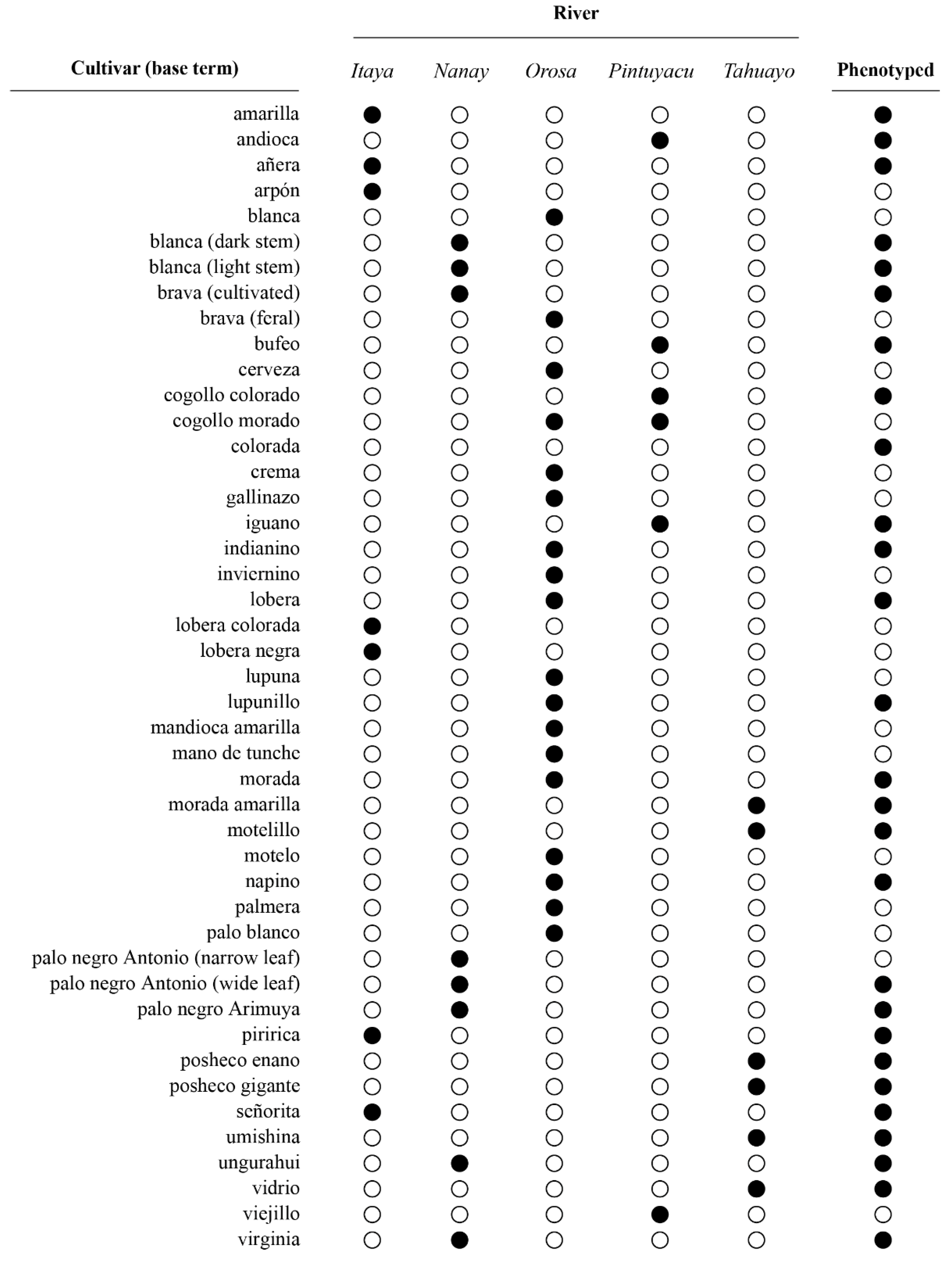
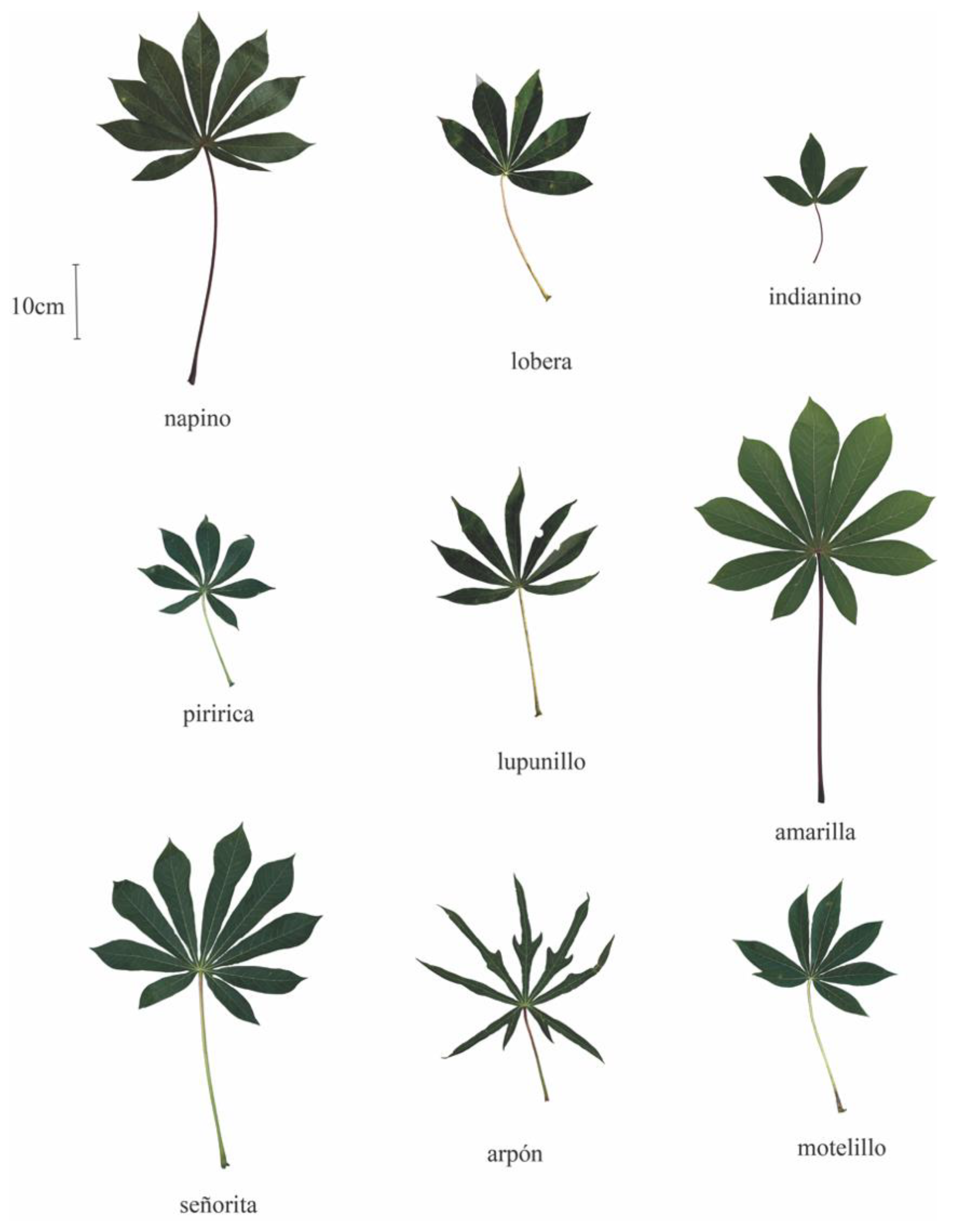
2.3. Plant Phenotypes
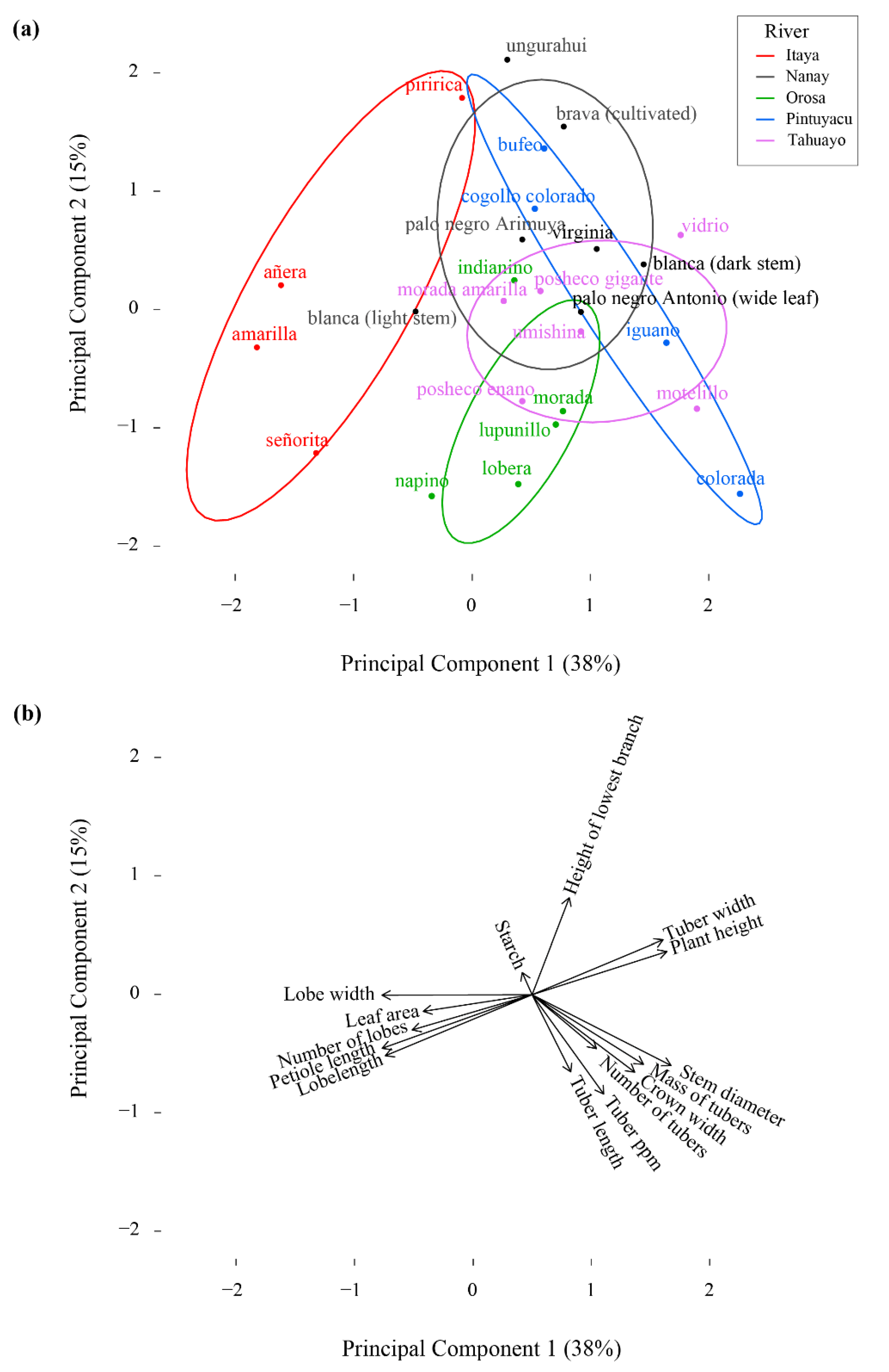
2.4. Statistical Analysis
3. Results
4. Discussion
4.1. Nomenclature
4.2. Chacra Organization
4.3. Phenotypic Variation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allem, A.C. The Origins and Taxonomy of Cassava. In Cassava: Biology, Production, and Utilization; Hillocks, R.J., Thresh, J.M., Bellotti, A.C., Eds.; CAB International: Wallingford, UK, 2002; pp. 1–16. [Google Scholar]
- Balagopalan, C. Cassava Utilization in Food, Feed, and Industry. In Cassava: Biology, Production, and Utilization; Hillocks, R.J., Thresh, J.M., Bellotti, A.C., Eds.; CAB International: Wallingford, UK, 2002; pp. 301–318. [Google Scholar]
- FAOSTAT. Agricultural Production Data. 2009. Available online: http://faostat.fao.org/site/339/default.aspx (accessed on 2 December 2021).
- Alves, A.A.C. Cassava Botany and Physiology. In Cassava: Biology, Production, and Utilization; Hillocks, R.J., Thresh, J.M., Bellotti, A.C., Eds.; CAB International: Wallingford, UK, 2002; pp. 67–90. [Google Scholar]
- McMahon, J.; White, W.; Sayre, R. Cyanogenesis in cassava (Manihot esculenta Crantz). J. Exp. Bot. 1999, 46, 731–741. [Google Scholar] [CrossRef]
- Arias, J.; Ramos, L.; Acosta, L.; Camacho, H.; Marín, G. Diversidad de yucas entre los Ticuna: Riqueza Cultural y Genética de un Producto Tradicional; Instituto Amazónico de Investigaciones Científicas: Sinchi, Bogotá, 2004; 42p. [Google Scholar]
- Boster, J. Exchange of varieties and information between Aguaruna manioc cultivators. Am. Anthropol. 1986, 88, 428–436. [Google Scholar] [CrossRef]
- Fraser, J.A. The diversity of bitter manioc (Manihot esculenta Crantz) cultivation in a whitewater Amazonian landscape. Diversity 2010, 2, 586–609. [Google Scholar] [CrossRef] [Green Version]
- Salick, J.; Cellinese, N.; Knapp, S. Indigenous diversity of cassava: Generation maintenance, use and loss among the Amuesha, Peruvian upper Amazon. Econ. Bot. 1997, 51, 6–19. [Google Scholar] [CrossRef]
- Wilson, W.M.; DuFour, D.L. Why “bitter” cassava? Productivity of “bitter” and “sweet” cassava in a Tukanoan Indian settlement in the northwest Amazon. Econ. Bot. 2002, 56, 49–57. [Google Scholar]
- Sánchez, H.; López, P. Diversidad de yuca (Manihot esculenta Crantz) en Jenaro Herrera, Loreto, Perú; Documento Técnico No. 28; IIAP: Iquitos, Peru, 2001. [Google Scholar]
- Chiwona-Karltun, L.; Brimer, L.; Saka, J.; Mhone, A.; Mkumbira, J.; Johansson, L.; Bokanga, M.; Mahungu, N.M.; Rosling, H. Bitter taste in cassava roots correlates with cyanogenic glucoside levels. J. Sci. Food Agric. 2004, 84, 581–590. [Google Scholar] [CrossRef]
- Rogers, D.; Fleming, H. A monograph of Manihot esculenta with an explanation of the taximetrics methods used. Econ. Bot. 1973, 27, 1–113. [Google Scholar] [CrossRef]
- Duputié, A.; Massol, F.; David, P.; Haxaire, C.; McKey, D. Traditional Amerindian cultivators combine directional and ideotypic selection for sustainable management of cassava genetic diversity. J. Evol. Biol. 2009, 22, 1317–1325. [Google Scholar] [CrossRef]
- Wang, W.; Feng, B.; Xiao, J.; Xia, Z.; Zhou, X.; Li, P.; Zhang, W.; Wang, Y.; Moller, B.L.; Zhang, P.; et al. Cassava genome from a wild ancestor to cultivated varieties. Nat. Commun. 2014, 5, 5110. [Google Scholar] [CrossRef] [PubMed]
- Bredeson, J.V.; Lyons, J.B.; Prochnik, S.E.; Wu, G.A.; Ha, C.M.; Edsinger-Gonzales, E.; Grimwood, J.; Schmutz, J.; Rabbi, I.Y.; Egesi, C.; et al. Sequencing wild and cultivated cassava and related species reveals extensive interspecific hybridization and genetic diversity. Nat. Biotechnol. 2016, 34, 562–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceballos, N.; Morante, N.; Calle, F.; Lenis, J.; Salazar, S. Developing new cassava varieties: Tools, techniques, and strategies. In Achieving Sustainable Cultivation of Cassava. Volume 2: Genetics, Breeding, Pests and Diseases; Hershey, C., Ed.; Burleigh Dodds Science Publishing: London, UK, 2017; pp. 49–90. [Google Scholar]
- Olsen, K.; Schaal, B. Microsatellite variation in cassava (Manihot esculenta, Euphorbiaceae) and its wild relatives: Further evidence for a southern Amazonian origin of domestication. Am. J. Bot. 2001, 88, 131–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isendahl, C. The domestication and early spread of manioc (Manihot esculenta Crantz): A brief synthesis. Lat. Am. Antiq. 2011, 22, 452–468. [Google Scholar] [CrossRef]
- Jones, W. Manioc in Africa; Stanford University Press: Stanford, CT, USA, 1959. [Google Scholar]
- Hillocks, R.J. Cassava in Africa. In Cassava: Biology, Production, and Utilization; Hillocks, R.J., Thresh, J.M., Bellotti, A.C., Eds.; CAB International: Wallingford, UK, 2002; pp. 41–54. [Google Scholar]
- Boster, J. Classification, cultivation and selection of Aguaruna varieties of Manihot esculenta (Euphorbiaceae). Adv. Econ. Bot. 1984, 1, 34–47. [Google Scholar]
- Boster, J. Selection for perceptual distinctiveness: Evidence from Aguaruna Jívaro varieties of Manihot esculenta. Econ. Bot. 1984, 39, 310–325. [Google Scholar] [CrossRef]
- Nye, M.M. The mis-measure of manioc (Manihot esculenta, Euphorbiaceae). Econ. Bot. 1990, 45, 47–57. [Google Scholar] [CrossRef]
- Peroni, N.; Kageyama, P.Y.; Begossi, A. Molecular differentiation, diversity, and folk classification of “sweet” and “bitter” cassava (Manihot esculenta) in Caiçara and Caboclo management systems (Brazil). Genet. Resour. Crop Evol. 2007, 54, 1333–1349. [Google Scholar] [CrossRef]
- Bradbury, E.J.; Duputié, A.; Delêtre, M.; Roullier, C.; Narváez-Trujillo, A.; Manu-Aduening, J.A.; Emshwiller, E.; McKey, D. Geographic differences in patterns of genetic differentiation among bitter and sweet manioc (Manihot esculenta subsp. esculenta; Euphorbiaceae). Am. J. Bot. 2013, 100, 857–866. [Google Scholar] [CrossRef]
- Grunhert, C.; Biehl, B.; Selmar, D. Compartmentation of cyanogenic glucosides and their degrading enzymes. Planta 1994, 195, 36–42. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2010. [Google Scholar]
- Venables, W.; Ripley, B. MASS: Modern Applied Statistics with S; Springer: Berlin, Germany, 2002. [Google Scholar]
- Dunn, O. Multiple comparisons among means. J. Am. Stat. Assoc. 1961, 56, 52–64. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Society. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Elias, M.; Panaud, O.; Robert, T. Assessment of genetic variability in a traditional cassava (Manihot esculenta Crantz) farming system, using AFLP markers. Heredity 2000, 85, 219–230. [Google Scholar] [CrossRef] [Green Version]
- Elias, M.; Penet, L.; Vindry, P.; McKey, D.; Panaud, O.; Robert, T. Unmanaged sexual reproduction and the dynamics of genetic diversity of a vegetatively propagated crop plant, cassava (Manihot esculenta Crantz), in a traditional farming system. Mol. Ecol. 2001, 10, 1895–1907. [Google Scholar] [CrossRef] [Green Version]
- Pujol, B.; David, P.; McKey, D. Microevolution in agricultural environments: How a traditional Amerindian farming practice favours heterozygosity in cassava (Manihot esculenta Crantz, Euphorbiaceae). Ecol. Lett. 2004, 8, 138–147. [Google Scholar] [CrossRef]
- Yeoh, H.H.; Sanchez, T.; Iglesias, C. Large-scale screening of cyanogenic potential in cassava roots using the enzyme-based dipsticks. J. Food Compos. Anal. 1998, 11, 2–10. [Google Scholar] [CrossRef]
- King, N.L.R.; Bradbury, J.H. Bitterness of Cassava: Identification of a new apiosyl glucoside and other compounds that affect its bitter taste. J. Sci. Food Agric. 1995, 68, 223–230. [Google Scholar] [CrossRef]
- Delêtre, M.; McKey, D.B.; Hodkinson, T.R. Marriage exchanges, seed exchanges, and the dynamics of manioc diversity. Proc. Natl. Acad. Sci. USA 2011, 108, 18249–18254. [Google Scholar] [CrossRef] [Green Version]
- Charlesworth, B.; Charlesworth, D.; Barton, N. The effects of genetic and geographic structure on neutral variation. Annu. Rev. Ecol. Syst. 2003, 34, 99–125. [Google Scholar] [CrossRef]
- Olsen, K.M.; Schaal, B.A. Insights on the evolution of a vegetatively propagated crop species. Mol. Ecol. 2007, 16, 2838–2840. [Google Scholar] [CrossRef]
- Olsen, K.M.; Schaal, B.A. Evidence on the origin of cassava: Phylogeography of Manihot esculenta. Proc. Natl. Acad. Sci. USA 1999, 96, 5586–5591. [Google Scholar] [CrossRef] [Green Version]
- Alves-Pereira, A.; Zucchi, M.I.; Clement, C.R.; Viana, J.P.G.; Pinheiro, J.B.; Veasey, E.A.; de Souza, A.P. Selective signatures and high genome-wide diversity in traditional Brazilian manioc (Manihot esculenta Crantz) varieties. Sci. Rep. 2022, 12, 1268. [Google Scholar] [CrossRef]




| Cultivar Base Term | Interpretation | |
|---|---|---|
| amarilla | yellow | |
| andioca | manioc | |
| añera | year-old | |
| arpón | harpoon | |
| blanca | white | |
| blanca (dark stem) | white with dark stem | |
| blanca (light stem) | white with light stem | |
| brava (cultivated) | “wild”cassava being cultivated | |
| brava (feral) | “wild” cassava in abandoned chacra | |
| bufeo | Amazon river dolphin (Inia geoffrensis) | |
| cerveza | beer | |
| cogollo colorado | red bud | |
| cogollo morado | purple bud | |
| colorada | red | |
| crema | cream | |
| gallinazo | vulture (black vulture, Coragyps atratus, is locally common) | |
| iguano | iguano tree (Dilodendron costaricense) | |
| indianino | from Indiana (a town in the area) | |
| inviernino | winter type | |
| lobera | sword of Saint Ferdinand | |
| lobera colorada | red sword of Saint Ferdinand | |
| lobera negra | black sword of Saint Ferdinand | |
| lupuna | lupuna tree (Ceiba pentandra) | |
| lupunillo | little lupuna tree (Ceiba pentandra) | |
| mandioca amarilla | yellow manioc | |
| mano de tunche | hand of tunche (a forest spirit) | |
| morada | purple | |
| morada amarilla | purple yellow | |
| motelillo | little tortoise | |
| motelo | tortoise | |
| napino | from the Napo river (a large river in the area) | |
| palmera | palm tree | |
| palo blanco | white stem | |
| palo negro Antonio (narrow leaf) | black stem from Antonio (a neighbor), narrow leaf morph | |
| palo negro Antonio (wide leaf) | black stem from Antonio (a neighbor), wide leaf morph | |
| palo negro Arimuya | black stem from Arimuya (a neighbor) | |
| piririca | rough, like sandpaper | |
| posheco enano | pale dwarf | |
| posheco gigante | pale giant | |
| señorita | lady | |
| umishina | umisha-like (an umisha is a tree decorated for carnival) | |
| ungurahui | ungurahui palm tree (Oenocarpus bataua) | |
| vidrio | glass | |
| viejillo | old man | |
| virginia | Virginia is a nearby town |
| Plant Part | Measure | Min. | Mean | Max. | Std. Dev. |
|---|---|---|---|---|---|
| Main structure | Height (cm) | 140 | 311.8 | 497 | 90.9 |
| Crown width (cm) | 90 | 235.8 | 490 | 114.2 | |
| Height of lowest branch (cm) | 30 | 148.8 | 435 | 81.4 | |
| Stem diameter (mm) | 17 | 25.8 | 39 | 6.1 | |
| Leaf | Number of lobes | 3 | 6.0 | 9 | 2.0 |
| Lobe length (mm) | 94 | 137.7 | 205 | 36.7 | |
| Lobe width (mm) | 17 | 37.6 | 62 | 14.1 | |
| Area (cm2) | 44 | 156.8 | 365 | 87.2 | |
| Tuber | Number of tubers | 3 | 8.6 | 14 | 2.97 |
| Total tuber mass (kg) | 0.5 | 2.4 | 5.0 | 1.2 | |
| Tuber length (mm) | 120 | 292.5 | 462 | 97.3 | |
| Tuber width (mm) | 15 | 43.6 | 77 | 14.5 | |
| Nutrition | Cyanide content (ppm fresh mass) | 1 | 11.7 | 25 | 7.6 |
| Starch (% dry mass) | 33 | 70.7 | 96 | 16.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wooding, S.P.; Payahua, C.N. Ethnobotanical Diversity of Cassava (Manihot esculenta Crantz) in the Peruvian Amazon. Diversity 2022, 14, 252. https://doi.org/10.3390/d14040252
Wooding SP, Payahua CN. Ethnobotanical Diversity of Cassava (Manihot esculenta Crantz) in the Peruvian Amazon. Diversity. 2022; 14(4):252. https://doi.org/10.3390/d14040252
Chicago/Turabian StyleWooding, Stephen P., and Christian Nolorbe Payahua. 2022. "Ethnobotanical Diversity of Cassava (Manihot esculenta Crantz) in the Peruvian Amazon" Diversity 14, no. 4: 252. https://doi.org/10.3390/d14040252
APA StyleWooding, S. P., & Payahua, C. N. (2022). Ethnobotanical Diversity of Cassava (Manihot esculenta Crantz) in the Peruvian Amazon. Diversity, 14(4), 252. https://doi.org/10.3390/d14040252






