Contrasting Impact of Viral Activity on Prokaryotic Populations in the Coastal and Offshore Regions of the Eastern Arabian Sea
Abstract
:1. Introduction
2. Materials and Methods
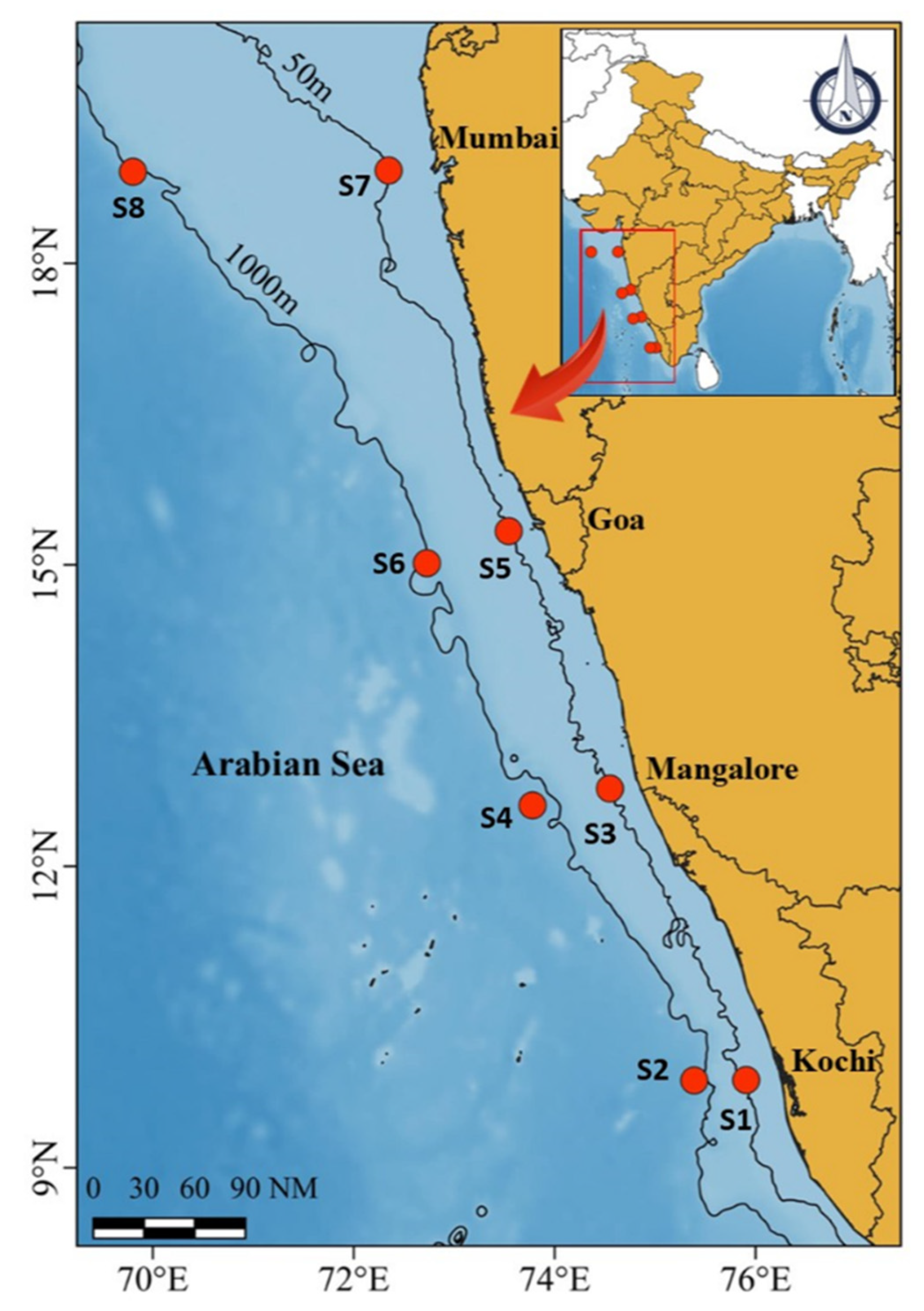
2.1. Study Location and Sample Collection
2.2. Estimation of Physicochemical Parameters
2.3. Viral Abundance (VA), Prokaryotic Abundance (PA), and Total Viable Prokaryotic Count (TVC)
2.4. Viral Production (VP) and Viral-Mediated Mortality of Prokaryotes (VMM)
2.5. Induction Assays for Lysogenic Prokaryotes
2.6. Viral Lytic Infection and Burst Size Estimates (BS)
2.7. Statistical Analysis
3. Results
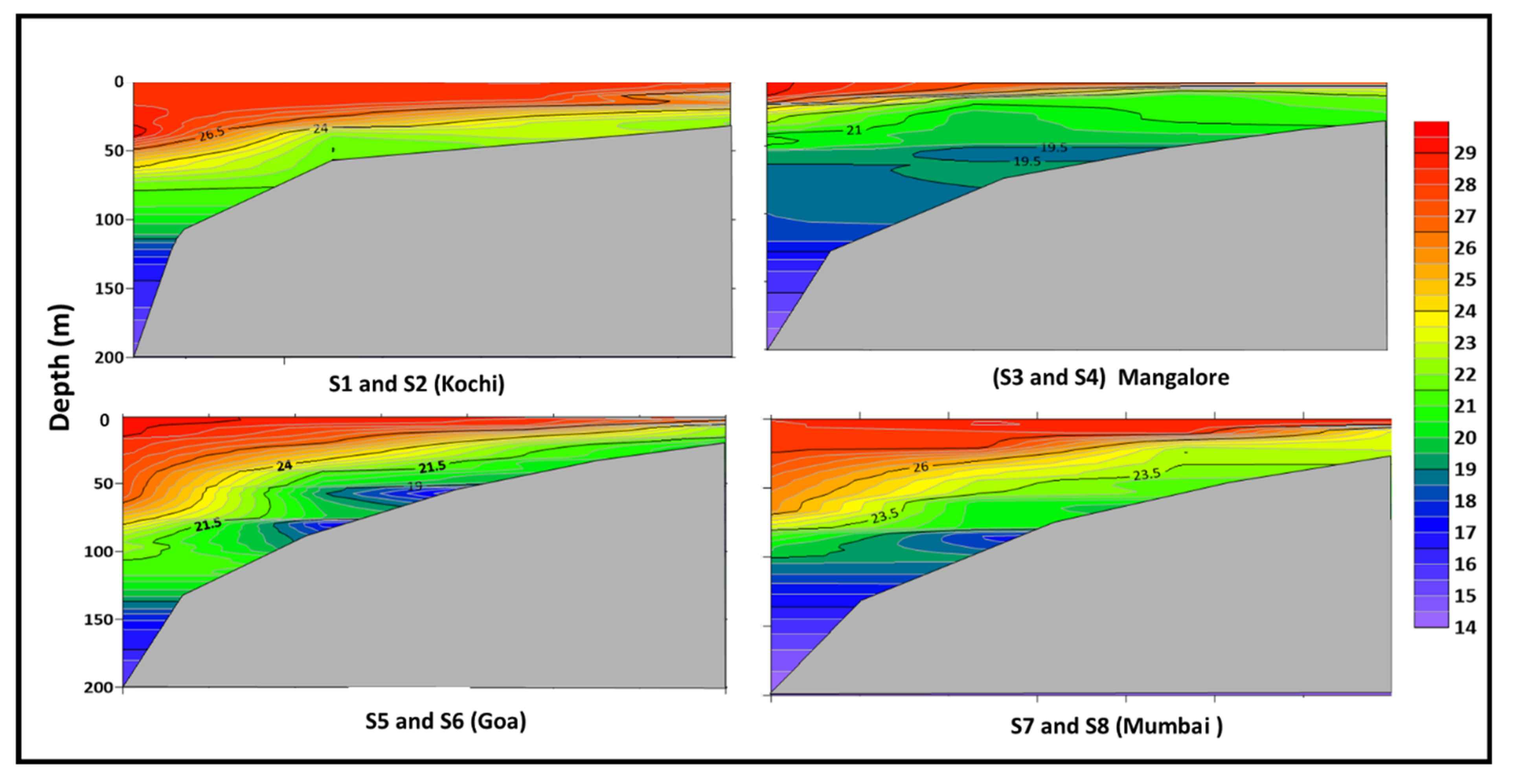
3.1. Prevailing Environmental Conditions in The Eastern Arabian Sea
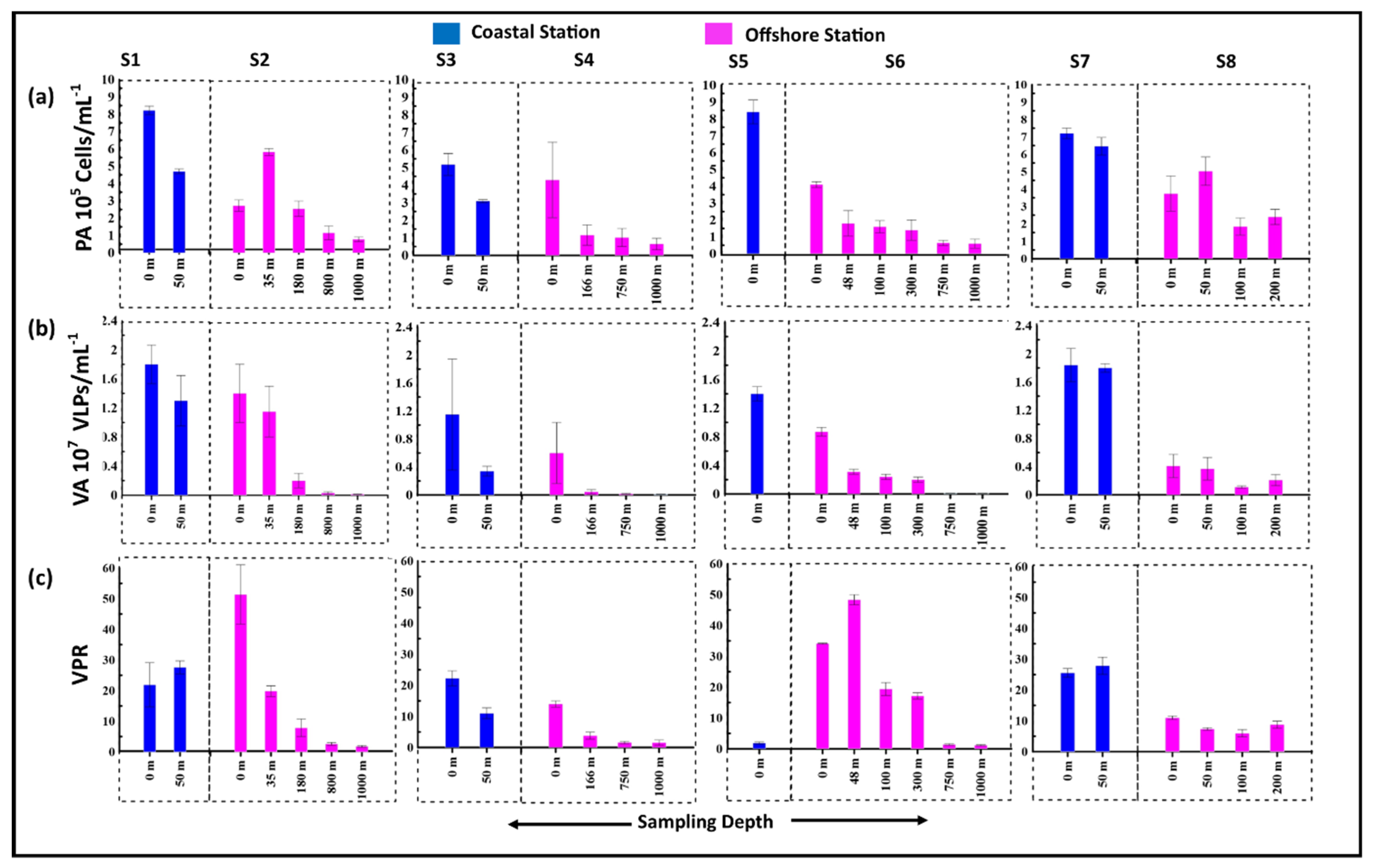
3.2. Standing Stocks of Viruses and Prokaryotes
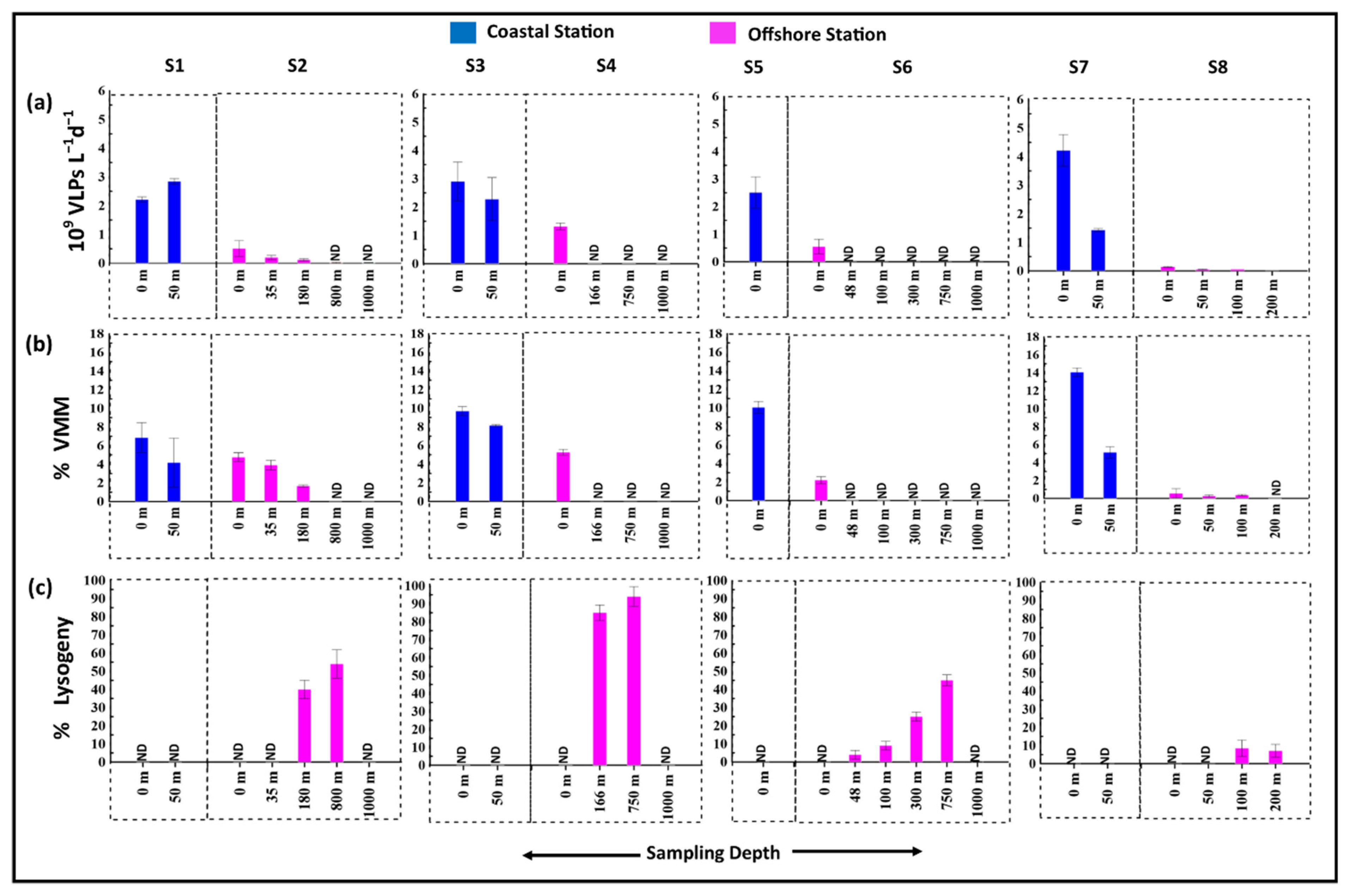
3.3. Viral Production and Viral Mediated Prokaryotic Mortality
3.4. Lysogenic Induction
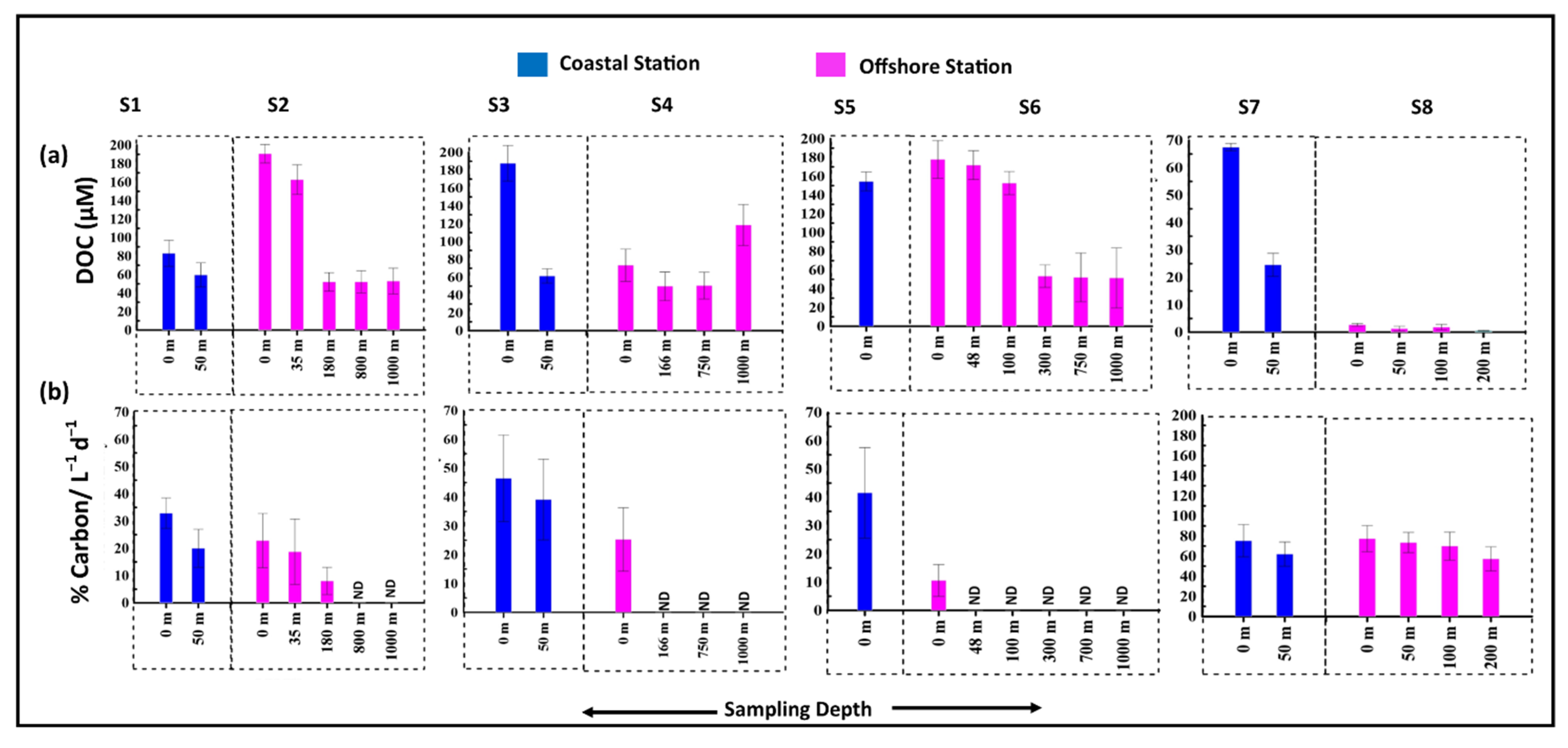
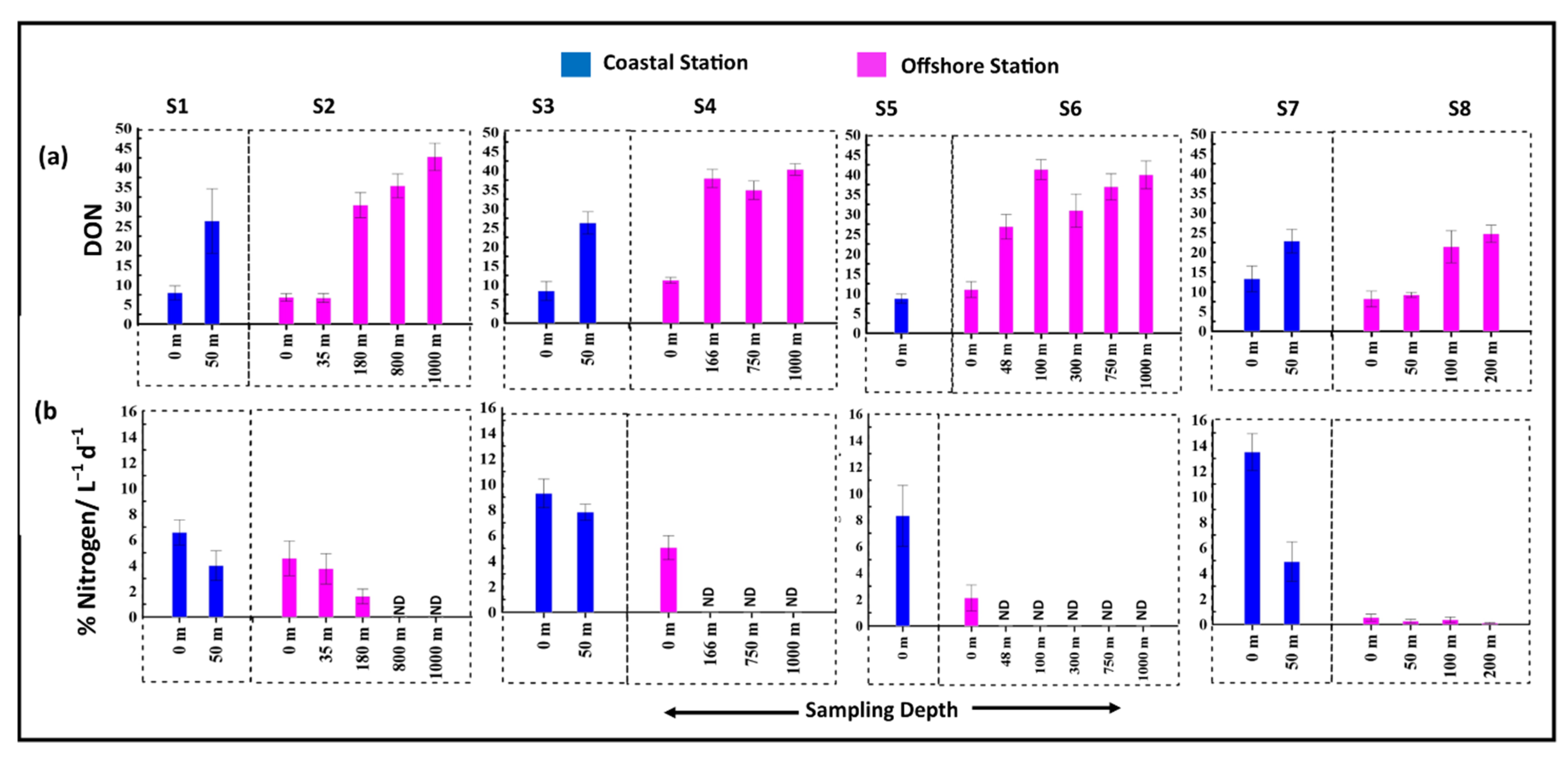
3.5. Release of Organic Carbon and Nitrogen Due to Viral Mediated Prokaryotic Mortality
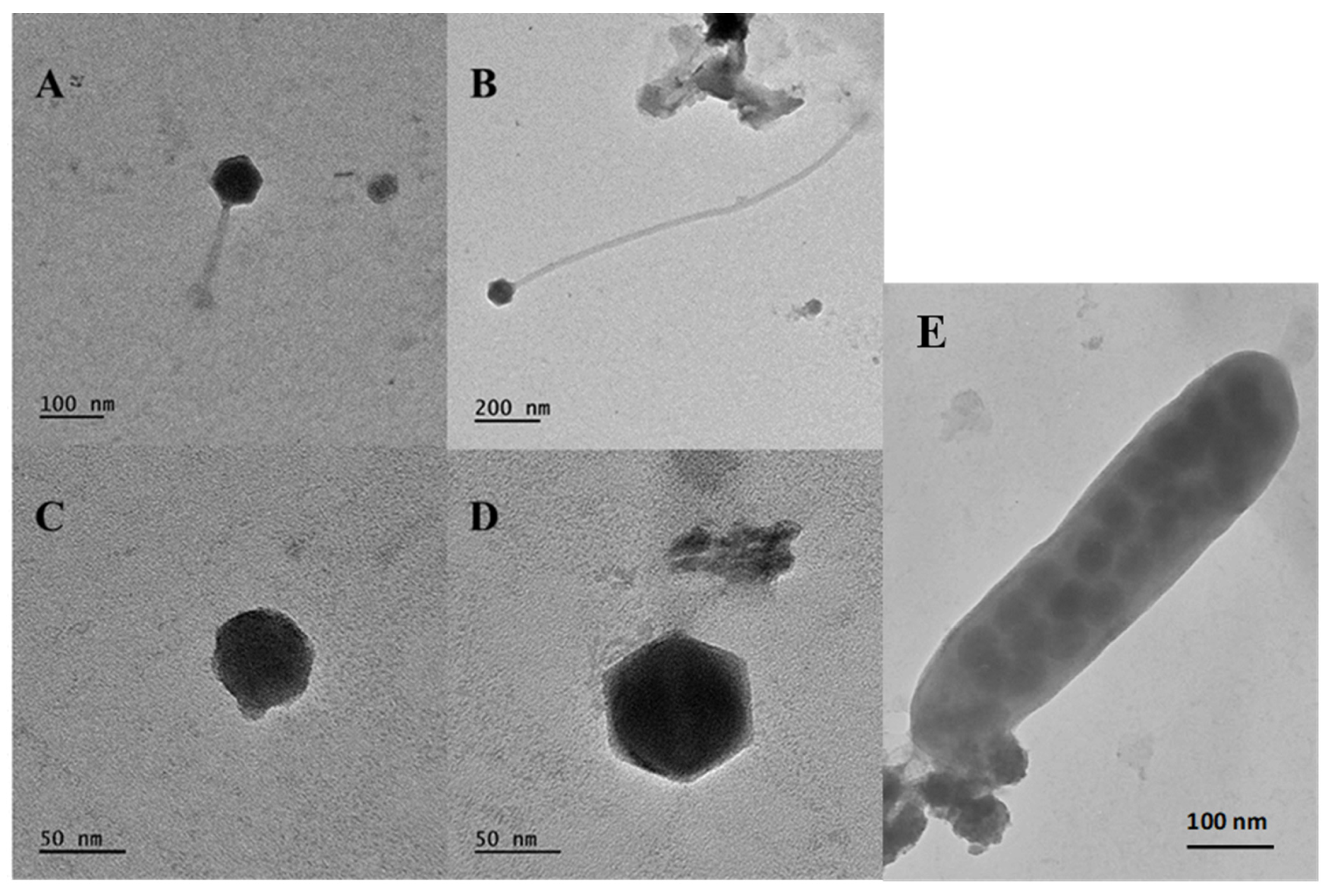
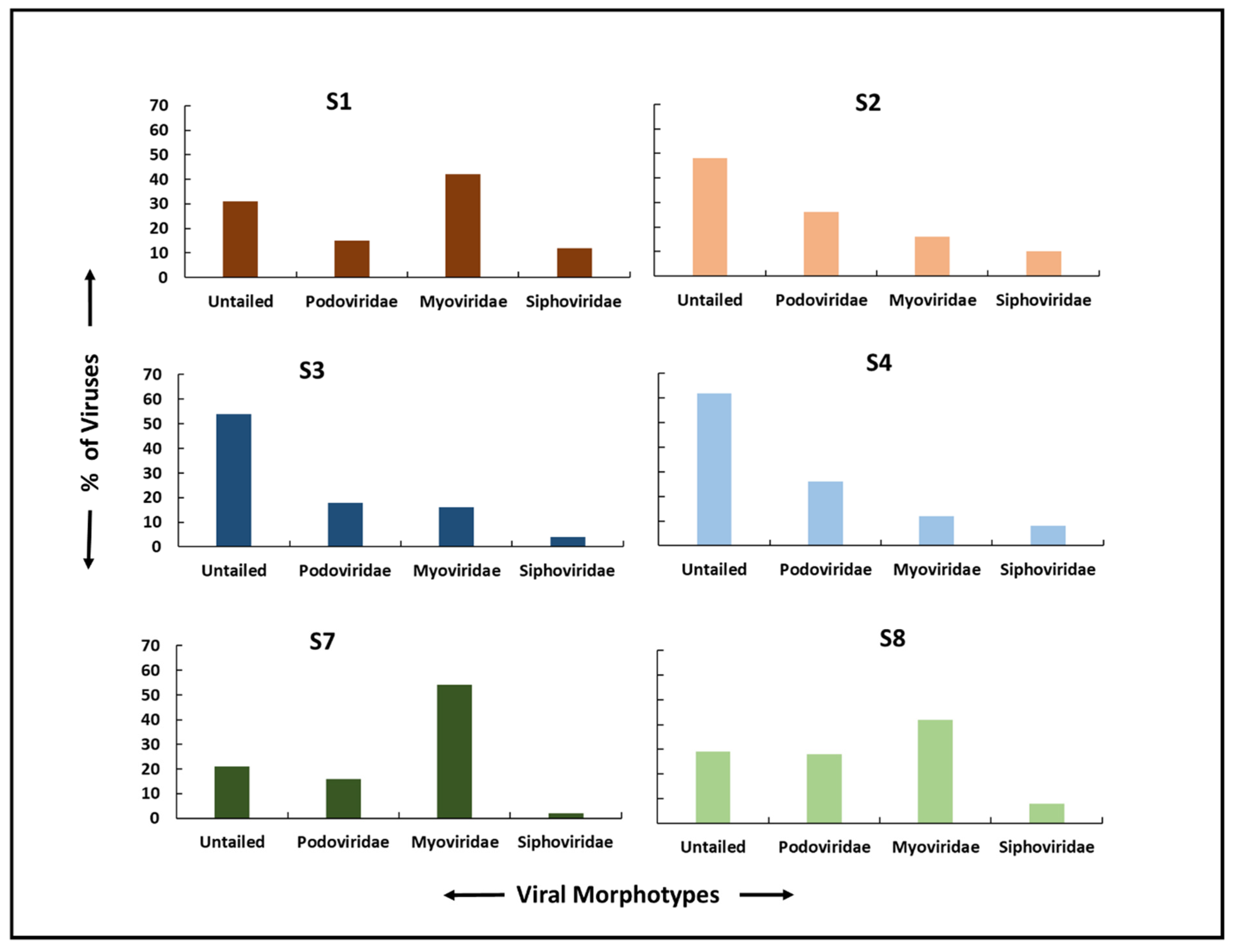
3.6. Viral Morphotypes and Size Classification
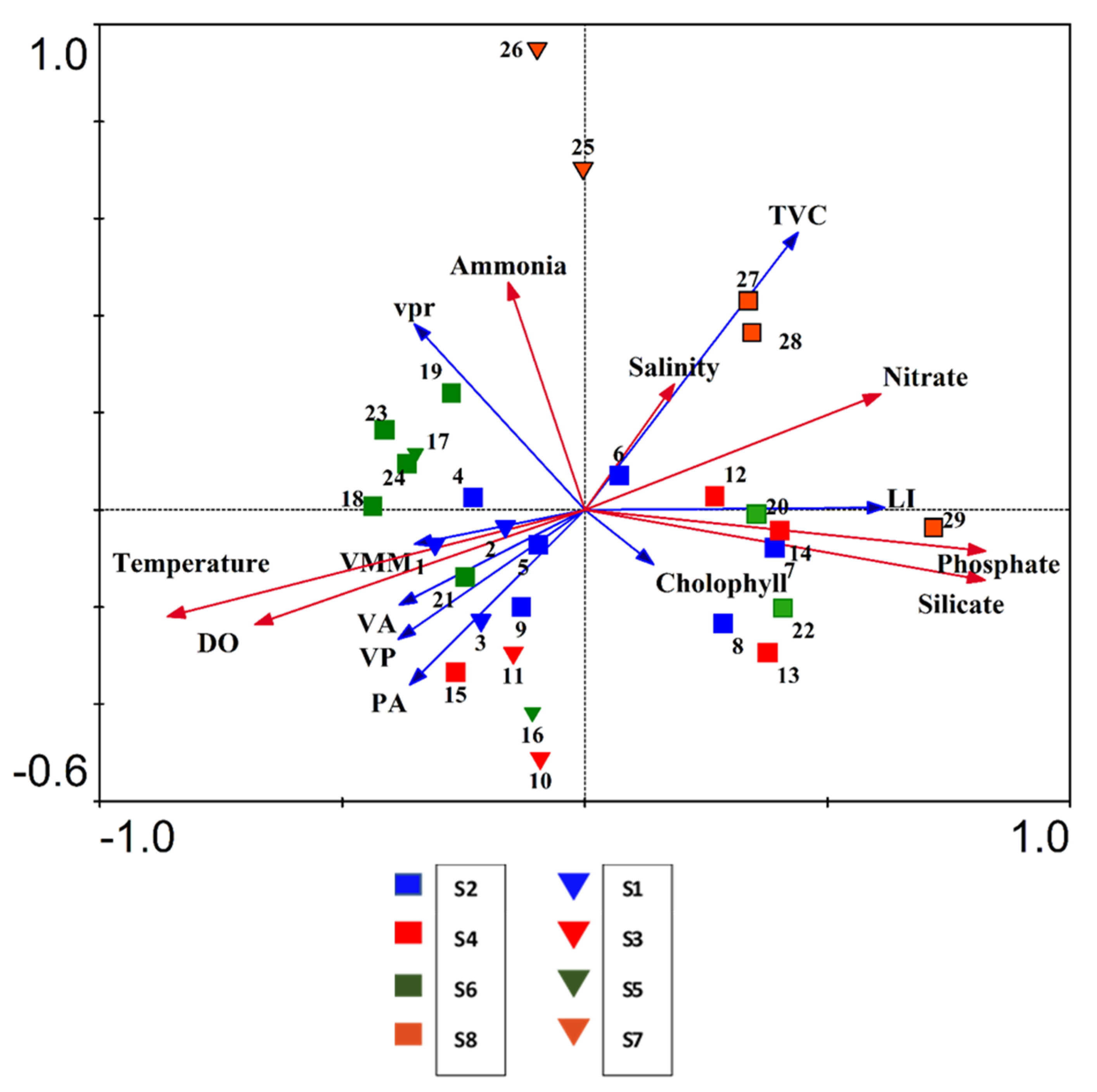
3.7. Impact of Physico—Chemical Factors on Viral Activity
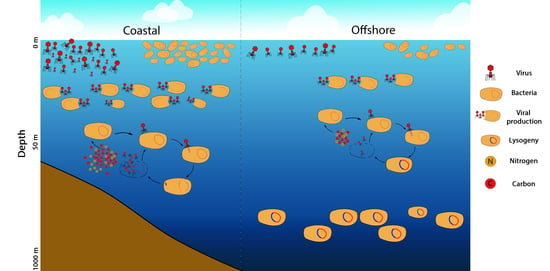
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Middelboe, M.; Brussaard, C.P. Marine Viruses: Key Players in Marine Ecosystems. Viruses 2017, 9, 302. [Google Scholar] [CrossRef] [Green Version]
- Suttle, C.A. Marine Viruses—Major Players in the Global Ecosystem. Nat. Rev. Microbiol. 2007, 5, 801–812. [Google Scholar] [CrossRef]
- Wigington, C.H.; Sonderegger, D.; Brussaard, C.P.D.; Buchan, A.; Finke, J.F.; Fuhrman, J.A.; Lennon, J.T.; Middelboe, M.; Suttle, C.A.; Stock, C.; et al. Re-Examination of the Relationship between Marine Virus and Microbial Cell Abundances. Nat. Microbiol. 2016, 1, 15024. [Google Scholar] [CrossRef] [Green Version]
- Johannessen, T.V.; Larsen, A.; Bratbak, G.; Pagarete, A.; Edvardsen, B.; Egge, E.D.; Sandaa, R.-A. Seasonal Dynamics of Haptophytes and DsDNA Algal Viruses Suggest Complex Virus-Host Relationship. Viruses 2017, 9, 84. [Google Scholar] [CrossRef]
- Thingstad, T.; Heldal, M.; Bratbak, G.; Dundas, I. Are Viruses Important Partners in Pelagic Fend Webs? Trends Ecol. Evol. 1993, 8, 209–213. [Google Scholar] [CrossRef]
- Gerson, V.J.; Madhu, N.; Jyothibabu, R.; Balachandran, K.; Nair, M.; Revichandran, C. Oscillating Environmental Responses of the Eastern Arabian Sea. Indian J. Geo-Mar. Sci. 2014, 43, 67–75. [Google Scholar]
- Naqvi, S.; Noronha, R.; Somasundar, K.; Gupta, R.S. Seasonal Changes in the Denitrification Regime of the Arabian Sea. Deep Sea Res. Part Oceanogr. Res. Pap. 1990, 37, 593–611. [Google Scholar] [CrossRef]
- Wright, J.J.; Konwar, K.M.; Hallam, S.J. Microbial Ecology of Expanding Oxygen Minimum Zones. Nat. Rev. Microbiol. 2012, 10, 381–394. [Google Scholar] [CrossRef]
- Naqvi, S.; Moffett, J.; Gauns, M.; Narvekar, P.; Pratihary, A.; Naik, H.; Shenoy, D.; Jayakumar, D.; Goepfert, T.; Patra, P. The Arabian Sea as a High-Nutrient, Low-Chlorophyll Region during the Late Southwest Monsoon. Biogeosciences 2010, 7, 2091–2100. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.P.; Madhupratap, M.; Kumar, M.D.; Gauns, M.; Muraleedharan, P.M.; Sarma, V.V.S.S.; De Souza, S.N. Physical Control of Primary Productivity on a Seasonal Scale in Central and Eastern Arabian Sea. J. Earth Syst. Sci. 2000, 109, 433–441. [Google Scholar] [CrossRef]
- Sabbagh, E.I.; Huete-Stauffer, T.M.; Calleja, M.L.L.; Silva, L.; Viegas, M.; Morán, X.A.G. Weekly Variations of Viruses and Heterotrophic Nanoflagellates and Their Potential Impact on Bacterioplankton in Shallow Waters of the Central Red Sea. FEMS Microbiol. Ecol. 2020, 96, fiaa033. [Google Scholar] [CrossRef]
- Aparna, S.; Parvathi, A.; Jasna, V.; Ram, A.S.P.; Sime-Ngando, T. Seasonal Variations in Viral Distribution, Dynamics, and Viral-Mediated Host Mortality in the Arabian Sea. Mar. Biol. 2021, 168, 28. [Google Scholar] [CrossRef]
- Ordulj, M.; Krstulović, N.; Šantić, D.; Jozić, S.; Šolić, M. Viral Dynamics in Two Trophically Different Areas in the Central Adriatic Sea. Helgol. Mar. Res. 2017, 71, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Long, A.; McDaniel, L.D.; Mobberley, J.; Paul, J.H. Comparison of Lysogeny (Prophage Induction) in Heterotrophic Bacterial and Synechococcus Populations in the Gulf of Mexico and Mississippi River Plume. ISME J. 2008, 2, 132–144. [Google Scholar] [CrossRef] [Green Version]
- Zeigler Allen, L.; McCrow, J.P.; Ininbergs, K.; Dupont, C.L.; Badger, J.H.; Hoffman, J.M.; Ekman, M.; Allen, A.E.; Bergman, B.; Venter, J.C. The Baltic Sea Virome: Diversity and Transcriptional Activity of DNA and RNA Viruses. MSystems 2017, 2, e00125-16. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Wang, L.; Wang, Z.; Zhao, J.; Yang, Q.; Wang, M.; Yang, K.; Zhang, L.; Jiao, N.; Zhang, Y. Metagenomic Analysis of the Diversity of DNA Viruses in the Surface and Deep Sea of the South China Sea. Front. Microbiol. 2019, 10, 1951. [Google Scholar] [CrossRef] [Green Version]
- Hurwitz, B.L.; Sullivan, M.B. The Pacific Ocean Virome (POV): A Marine Viral Metagenomic Dataset and Associated Protein Clusters for Quantitative Viral Ecology. PLoS ONE 2013, 8, e57355. [Google Scholar] [CrossRef]
- Brum, J.R.; Schenck, R.O.; Sullivan, M.B. Global Morphological Analysis of Marine Viruses Shows Minimal Regional Variation and Dominance of Non-Tailed Viruses. ISME J. 2013, 7, 1738–1751. [Google Scholar] [CrossRef] [Green Version]
- Jasna, V.; Parvathi, A.; Pradeep Ram, A.S.; Balachandran, K.K.; Madhu, N.V.; Nair, M.; Jyothibabu, R.; Jayalakshmy, K.V.; Revichandran, C.; Sime-Ngando, T. Viral-Induced Mortality of Prokaryotes in a Tropical Monsoonal Estuary. Front. Microbiol. 2017, 8, 895. [Google Scholar] [CrossRef]
- Jasna, V.; Parvathi, A.; Aswathy, V.; Aparna, S.; Dayana, M.; Aswathy, A.; Madhu, N. Factors Determining Variations in Viral Abundance and Viral Production in a Tropical Estuary Influenced by Monsoonal Cycles. Reg. Stud. Mar. Sci. 2019, 28, 100589. [Google Scholar]
- Grasshoff, K.; Kremling, K.; Ehrhardt, M. Methods of Seawater Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2009; ISBN 3-527-61399-4. [Google Scholar]
- Grasshoff, K. Determination of Nitrite. Methods Sea Water Anal. 1983, 1, 139–142. [Google Scholar]
- Gupta, G.V.M.; Thottathil, S.D.; Balachandran, K.K.; Madhu, N.V.; Madeswaran, P.; Nair, S. CO2 Supersaturation and Net Heterotrophy in a Tropical Estuary (Cochin, India): Influence of Anthropogenic Effect: Carbon Dynamics in Tropical Estuary. Ecosystems 2009, 12, 1145–1157. [Google Scholar] [CrossRef]
- Patel, A.; Noble, R.T.; Steele, J.A.; Schwalbach, M.S.; Hewson, I.; Fuhrman, J.A. Virus and Prokaryote Enumeration from Planktonic Aquatic Environments by Epifluorescence Microscopy with SYBR Green I. Nat. Protoc. 2007, 2, 269–276. [Google Scholar] [CrossRef] [Green Version]
- Joux, F.; Lebaron, P. Ecological Implications of an Improved Direct Viable Count Method for Aquatic Bacteria. Appl. Environ. Microbiol. 1997, 63, 3643–3647. [Google Scholar] [CrossRef] [Green Version]
- Wilhelm, S.W.; Brigden, S.M.; Suttle, C.A. A Dilution Technique for the Direct Measurement of Viral Production: A Comparison in Stratified and Tidally Mixed Coastal Waters. Microb. Ecol. 2002, 43, 168–173. [Google Scholar] [CrossRef]
- Hewson, I.; Fuhrman, J.A. Covariation of Viral Parameters with Bacterial Assemblage Richness and Diversity in the Water Column and Sediments. Deep Sea Res. Part Oceanogr. Res. Pap. 2007, 54, 811–830. [Google Scholar] [CrossRef]
- Evans, C.; Archer, S.D.; Jacquet, S.; Wilson, W.H. Direct Estimates of the Contribution of Viral Lysis and Microzooplankton Grazing to the Decline of a Micromonas spp. Population. Aquat. Microb. Ecol. 2003, 30, 207–219. [Google Scholar] [CrossRef] [Green Version]
- Weinbauer, M.G.; Suttle, C.A. Lysogeny and Prophage Induction in Coastal and Offshore Bacterial Communities. Aquat. Microb. Ecol. 1999, 18, 217–225. [Google Scholar] [CrossRef] [Green Version]
- Fukuda, R.; Ogawa, H.; Nagata, T.; Koike, I. Direct Determination of Carbon and Nitrogen Contents of Natural Bacterial Assemblages in Marine Environments. Appl. Environ. Microbiol. 1998, 64, 3352–3358. [Google Scholar] [CrossRef] [Green Version]
- Pradeep Ram, A.; Arnous, B.; Danger, M.; Carrias, J.-F.; Lacroix, G.; Sime-Ngando, T. High and Differential Viral Infection Rates within Bacterial ‘Morphopopulations’ in a Shallow Sand Pit Lake (Lac de Créteil, France). FEMS Microbiol. Ecol. 2010, 74, 83–92. [Google Scholar] [CrossRef] [Green Version]
- Weinbauer, M.G.; Winter, C.; Höfle, M.G. Reconsidering Transmission Electron Microscopy Based Estimates of Viral Infection of Bacterio-Plankton Using Conversion Factors Derived from Natural Communities. Aquat. Microb. Ecol. 2002, 27, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Brum, J.R.; Ignacio-Espinoza, J.C.; Roux, S.; Doulcier, G.; Acinas, S.G.; Alberti, A.; Chaffron, S.; Cruaud, C.; De Vargas, C.; Gasol, J.M. Patterns and Ecological Drivers of Ocean Viral Communities. Science 2015, 348, 1261498. [Google Scholar] [CrossRef] [Green Version]
- Barth, O.M. Estudos Sobre a Contrastacao Negativa de Suspensoes Virais. Rev. Bras. Biol. 1984, 44, 71–80. [Google Scholar]
- Karnan, C.; Jyothibabu, R.; Arunpandi, N.; Albin, K.J.; Parthasarathi, S.; Krishnan, S.S. Response of Microplankton Size Structure to Summer Stratification, Freshwater Influx and Coastal Upwelling in the Southeastern Arabian Sea. Cont. Shelf Res. 2020, 193, 104038. [Google Scholar] [CrossRef]
- Habeebrehman, H.; Prabhakaran, M.; Jacob, J.; Sabu, P.; Jayalakshmi, K.; Achuthankutty, C.; Revichandran, C. Variability in Biological Responses Influenced by Upwelling Events in the Eastern Arabian Sea. J. Mar. Syst. 2008, 74, 545–560. [Google Scholar] [CrossRef]
- Sharma, G.S. Upwelling off the Southwest Coast of India. Indian J. Mar. Sci. 1978, 7, 209–218. [Google Scholar]
- Gupta, G.; Sudheesh, V.; Sudharma, K.; Saravanane, N.; Dhanya, V.; Dhanya, K.; Lakshmi, G.; Sudhakar, M.; Naqvi, S. Evolution to Decay of Upwelling and Associated Biogeochemistry over the Southeastern Arabian Sea Shelf. J. Geophys. Res. Biogeosci. 2016, 121, 159–175. [Google Scholar] [CrossRef] [Green Version]
- Eissler, Y.; Letelier, J.; Cuevas, L.A.; Morales, C.E.; Escribano, R. The Microbial Community in the Coastal Upwelling System off Concepción, Chile, 36 S, 2002–2003 Period. Rev. Biol. Mar. Oceanogr. 2010, 45, 1–18. [Google Scholar] [CrossRef]
- Corinaldesi, C.; Crevatin, E.; Del Negro, P.; Marini, M.; Russo, A.; Fonda-Umani, S.; Danovaro, R. Large-Scale Spatial Distribution of Virioplankton in the Adriatic Sea: Testing the Trophic State Control Hypothesis. Appl. Environ. Microbiol. 2003, 69, 2664–2673. [Google Scholar] [CrossRef] [Green Version]
- Bongiorni, L.; Magagnini, M.; Armeni, M.; Noble, R.; Danovaro, R. Viral Production, Decay Rates, and Life Strategies along a Trophic Gradient in the North Adriatic Sea. Appl. Environ. Microbiol. 2005, 71, 6644–6650. [Google Scholar] [CrossRef] [Green Version]
- Weinbauer, M.G.; Brettar, I.; Höfle, M.G. Lysogeny and Virus-induced Mortality of Bacterioplankton in Surface, Deep, and Anoxic Marine Waters. Limnol. Oceanogr. 2003, 48, 1457–1465. [Google Scholar] [CrossRef] [Green Version]
- Cochlan, W.P.; Wikner, J.; Steward, G.F.; Smith, D.C.; Azam, F. Spatial Distribution of Viruses, Bacteria and Chlorophyll a in Neritic, Oceanic and Estuarine Environments. Mar. Ecol. Prog. Ser. 1993, 92, 77. [Google Scholar] [CrossRef]
- Weinbauer, M.G.; Fuks, D.; Peduzzi, P. Distribution of Viruses and Dissolved DNA along a Coastal Trophic Gradient in the Northern Adriatic Sea. Appl. Environ. Microbiol. 1993, 59, 4074–4082. [Google Scholar] [CrossRef] [Green Version]
- He, L.; Yin, K.; Yuan, X.; Li, D.; Zhang, D.; Harrison, P.J. Spatial Distribution of Viruses, Bacteria and Chlorophyll in the Northern South China Sea. Aquat. Microb. Ecol. 2009, 54, 153–162. [Google Scholar] [CrossRef] [Green Version]
- Xie, L.; Wei, W.; Cai, L.; Chen, X.; Huang, Y.; Jiao, N.; Zhang, R.; Luo, Y.-W. A Global Viral Oceanography Database (GVOD). Earth Syst. Sci. Data 2021, 13, 1251–1271. [Google Scholar] [CrossRef]
- Bettarel, Y.; Sime-Ngando, T.; Bouvy, M.; Arfi, R.; Amblard, C. Low Consumption of Virus-Sized Particles by Heterotrophic Nanoflagellates in Two Lakes of the French Massif Central. Aquat. Microb. Ecol. 2005, 39, 205–209. [Google Scholar] [CrossRef] [Green Version]
- Garza, D.; Suttle, C.A. The Effect of Cyanophages on the Mortality of Synechococcus spp. and Selection for UV Resistant Viral Communities. Microb. Ecol. 1998, 36, 281–292. [Google Scholar] [CrossRef]
- Noble, R.T.; Fuhrman, J. Virus Decay and Its Causes in Coastal Waters. Appl. Environ. Microbiol. 1997, 63, 77–83. [Google Scholar] [CrossRef] [Green Version]
- Clasen, J.L.; Brigden, S.M.; Payet, J.P.; Suttle, C.A. Evidence That Viral Abundance across Oceans and Lakes Is Driven by Different Biological Factors. Freshw. Biol. 2008, 53, 1090–1100. [Google Scholar] [CrossRef]
- Parvathi, A.; Jasna, V.; Aparna, S.; Pradeep Ram, A.S.; Aswathy, V.K.; Balachandran, K.K.; Muraleedharan, K.R.; Mathew, D.; Sime-Ngando, T. High Incidence of Lysogeny in the Oxygen Minimum Zones of the Arabian Sea (Southwest Coast of India). Viruses 2018, 10, 588. [Google Scholar] [CrossRef] [Green Version]
- Beman, J.M.; Shih, J.L.; Popp, B.N. Nitrite Oxidation in the Upper Water Column and Oxygen Minimum Zone of the Eastern Tropical North Pacific Ocean. ISME J. 2013, 7, 2192–2205. [Google Scholar] [CrossRef] [Green Version]
- Wilhelm, S.W.; Suttle, C.A. Viruses and Nutrient Cycles in the Sea: Viruses Play Critical Roles in the Structure and Function of Aquatic Food Webs. BioScience 1999, 49, 781–788. [Google Scholar] [CrossRef] [Green Version]
- Boras, J.A.; Sala, M.M.; Vázquez-Domínguez, E.; Weinbauer, M.G.; Vaqué, D. Annual Changes of Bacterial Mortality Due to Viruses and Protists in an Oligotrophic Coastal Environment (NW Mediterranean). Environ. Microbiol. 2009, 11, 1181–1193. [Google Scholar] [CrossRef]
- Karuza, A.; Del Negro, P.; Crevatin, E.; Umani, S.F. Viral Production in the Gulf of Trieste (Northern Adriatic Sea): Preliminary Results Using Different Methodological Approaches. J. Exp. Mar. Biol. Ecol. 2010, 383, 96–104. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shruthi, P.; Parvathi, A.; Pradeep Ram, A.S.; Hafza, S.; Albin, J.K.; Vignesh, E.R.; Abdul, J.; Sime-Ngando, T. Contrasting Impact of Viral Activity on Prokaryotic Populations in the Coastal and Offshore Regions of the Eastern Arabian Sea. Diversity 2022, 14, 230. https://doi.org/10.3390/d14030230
Shruthi P, Parvathi A, Pradeep Ram AS, Hafza S, Albin JK, Vignesh ER, Abdul J, Sime-Ngando T. Contrasting Impact of Viral Activity on Prokaryotic Populations in the Coastal and Offshore Regions of the Eastern Arabian Sea. Diversity. 2022; 14(3):230. https://doi.org/10.3390/d14030230
Chicago/Turabian StyleShruthi, PK, Ammini Parvathi, Angia Sriram Pradeep Ram, Shyla Hafza, Jose K. Albin, Erathodi Rajagopalan Vignesh, Jaleel Abdul, and Telesphore Sime-Ngando. 2022. "Contrasting Impact of Viral Activity on Prokaryotic Populations in the Coastal and Offshore Regions of the Eastern Arabian Sea" Diversity 14, no. 3: 230. https://doi.org/10.3390/d14030230
APA StyleShruthi, P., Parvathi, A., Pradeep Ram, A. S., Hafza, S., Albin, J. K., Vignesh, E. R., Abdul, J., & Sime-Ngando, T. (2022). Contrasting Impact of Viral Activity on Prokaryotic Populations in the Coastal and Offshore Regions of the Eastern Arabian Sea. Diversity, 14(3), 230. https://doi.org/10.3390/d14030230