Abstract
Understanding the mechanisms that generate and maintain diversity in marine prokaryotic communities is one of the main challenges for contemporary marine microbiology. We here review how observational, experimental, and theoretical evidence converge on the conclusion that the marine pelagic community of heterotrophic prokaryotes consists of organisms with two main types of life strategies. We illustrate this dichotomy by SAR11 and Vibrio spp. as typical representatives of the two strategies. A theory for life strategy dichotomy exists in classical r/K-selection. We here discuss an additional dichotomy introduced by what we term S/L-selection (for Small and Large, respectively). While r/K-selection focuses on the role of environmental disturbances, steady-state models suggest that high abundance at species level should be closely related to a low trade-off between competition and defense. We summarize literature indicating that the high availability of organic C is an essential environmental factor favoring Vibrio spp. and suggest that the essence of the generalized L-strategy is to reduce the competition-predator defense trade-off by using non-limiting organic C to increase size. The “streamlining” theory that has been suggested for the S-strategist SAR11 proposes the opposite: that low trade-off is achieved by a reduction in size. We show how this apparent contradiction disappears when the basic assumptions of diffusion-limited uptake are considered. We propose a classification scheme that combines S/L and r/K-selection using the two dimensions of organic C availability and environmental disturbance. As organic C in terrestrial runoff and size of the oligotrophic oceanic gyres are both changing, habitat size for both S- and L-strategists are affected by global change. A theory capturing the main aspects of prokaryote life strategies is therefore crucial for predicting responses of the marine microbial food web to climate change and other anthropogenic influences.
1. Introduction
The sequencing revolution has brought a dramatic increase in our observational knowledge of diversity in natural microbial communities. This calls for a deeper theoretical understanding of the mechanisms that generate patterns in this diversity. A focus in much of the studies of marine prokaryotes has been the huge richness of species (as defined by their 16 rRNA gene amplicons) [1]. Rank-abundance curves for these communities are, however, highly skewed, with a few dominant and a long tail of rare species [2]. Typical in the dominant part of the community are members of the SAR11 (Pelagibacter) clade, possibly making this the most abundant organism on earth [3]. As discussed later, this pattern sometimes shifts, and community members normally in the rare tail of the distribution become dominant. Whole-genome sequencing of diverse isolates from ocean surface samples around the world has suggested that this reflects two main life strategies [4]: (1) a group with SAR11 and a few other species with small genomes, nearly always dominating in abundance, and (2) a diverse group of organisms with larger genomes, rarely dominating in abundance, but capable of rapid growth in energy-rich situations. For reasons that will become clear, we will term these “S” and “L“ strategists for “Small” and “Large”, respectively. With its SAR11 dominance, the group of S-strategists is dominated by α-proteobacteria. Examples of L-strategists suggested later in this review include both γ-proteobacteria and Bacteriodota, corroborating the finding of a larger diversity in the usually rare part of the community [4]. The difference in genome size can be illustrated by SAR11′s 1.34 Mb average genome size as compared to Vibrio spp., which may have a total genome size >5 Mb [5]. The aim of this review is to explore the mechanisms behind S/L-selection and the ecological conditions that make each of them successful in terms of abundance.
Whole-genome sequencing of isolates from the same species has revealed that their genomes have large variable regions [6]. Each species can thus be seen as a collection of strains. With this, there are two ways for a species to become abundant, either by (1) establishing many strains (requiring competitive abilities) or (2) having many individuals within each strain (requiring defensive abilities). The success recipe for high abundance at the species level is, therefore, to combine highly competitive with highly defensive abilities, possible only if there is a low trade-off between competition and defense. Relative differences in the trade-offs associated with their defense mechanisms have thus been suggested to determine the relative differences in the abundance of species [7]. Following this logic, the environmental conditions that determine whether S- or L-strategists dominate should somehow affect the relative value of trade-offs between the two groups.
There has been a considerable debate on what mechanisms make SAR11 so successful [3,8,9,10]. Important in the present context is the focus on whether this success is based on competitive or on defensive traits [11]. It is intriguing that the free-living organism (parasites, symbionts, and viruses excluded) with the smallest genome probably also is the earth’s most abundant [3]. The prevailing hypothesis is “streamlining” [10], whereby the small genome reduces resource requirements, allows the production of more cells per unit of resources, and allows for a size small enough to reduce protozoan predation. Since this simultaneously would increase competitiveness and reduce predatory loss, streamlining fits the hypothesis that a low trade-off between competition and predator defense promotes high abundance. While this gives a plausible explanation for the success of S-strategists, it leaves the important and largely unanswered question: How can the L-strategists with their “opposite” strategy of larger cell sizes and more complex genomes sometimes replace the S-strategists? I.e., what environmental conditions gives the L-strategy a lower trade-off between competition and defense than the S-strategy? Understanding the L-strategy and its implications is, therefore, a main goal of this review.
Ten years ago, Pedrós-Alió [12] pointed out the importance of understanding also the rare biosphere in the ocean. Yet, with a large species diversity in this group, the incentives to look for common properties within this group seem to have been less than for the dominant SAR11 clade. To understand the L-strategy, we here focus on the genus Vibrio as one suggested sub-group. Vibrio is chosen here not only because it illustrates the applied importance of understanding the L-strategy but also because its applied importance has made it one of the few relatively well-studied members of the (usually) rare part of the marine heterotrophic prokaryote community. We summarize some of this literature, together with experimental work on adding or removing organic C (OC) from seawater communities. Based on this information, we propose that the common trait shared by L-strategists is the use of non-limiting OC to lower the trade-off between competition and defense. Importantly, this use of excess OC in predator defense also suggests why Vibrio and possibly also some other L-strategists have developed pathogenicity.
The steady-state arguments used for such S- vs. L-selection distinguishes this theory from classical r- vs. K-selection theory, which emphasizes the role of non-steady-states in disturbed systems [13]. We believe the proposed mechanistic framework for S- and L-selection may be helpful in interpreting data on prokaryote community composition and will return to how these two classifications can be combined.
2. Connections between Non-Limiting Organic C, Predation Resistance, and Pathogenicity
The applied importance of marine Vibrio comes primarily from their role as pathogens. Infections occur in both shellfish [14] and fish [15], with Vibrio splendidus and Vibrio anguillarum as examples of causative agents. Vibrio also includes human pathogens such as Vibrio cholerae [16] and Vibrio vulnificus [17], responsible for severe gastroenteritis and skin and muscle tissue infections, respectively. Human food poisoning from seafood is another challenging problem, with raw oysters infected with Vibrio parahaemolyticus as an example [18]. As opposed to many of the other members of the usually rare marine prokaryotes, this has led to considerable literature on Vibrio in natural marine environments. Of particular importance in our context is the question of which factor(s) promote increased abundance of Vibrio spp.
By comparing wild-type with mutants deficient in glycogen storage, Bourassa and Camilli [19] concluded that glycogen storage contributes not only to survival in the natural environment but also to the pathogenicity of V. cholerae. Interestingly, this is consistent with suggestions that pathogenicity is a case of “coincidental evolution” where the original fitness gain was in the evolution of defenses against natural protozoan predators; coincidentally providing resistance also to macrophage phagocytosis [20,21,22]. Understanding the ecological conditions that select for predation-resistant bacteria may therefore provide direct clues to the mechanisms that select for pathogenic bacteria in natural waters.
Several investigations have shown how exposure of natural aquatic bacterial communities to increased protozoan predation leads to a change in community composition of prey toward predation-resistant morphologies and taxa [23,24,25,26,27,28]. Important in the present context, Matz and Jürgens [24] found that predation-resistant forms increased more in cultures limited by phosphate and replete in glucose than in cultures limited by glucose and replete in phosphate. Matz et al. [26] found that large bacterial size, high swimming speed, and strong surface charge were important phenotypical bacterial traits for defense against the model predator Spumella (a 2–5 µm flagellated protist). In addition, morphological features such as capsules, slime, and associated clumping likely contribute to defense. Most of these defense strategies have costs in terms of energy and/or in terms of carbon used for structural purposes. Environments rich in organic C (OC) thus offer better opportunities for the selection of predation-resistant heterotrophic prokaryotes than those where carbon and energy are limiting.
It is a classical observation that many bacteria, when limited by mineral nutrients such as nitrogen or phosphorous, accumulate C-rich material in granules as, e.g., poly-β-hydroxybutyrate or glycogen [29,30]. The fitness advantage of such storage is traditionally assumed to be in fluctuating environments where OC may become growth limiting in the near future (e.g., the work of [31]). If this was the whole explanation, such organisms should only become abundant in fluctuating environments since stored carbon would not provide any fitness gain in environments permanently enriched in OC. Importantly, the granules also fill a large part of the cell volume, and the consequence is often a considerable increase in cell volume (See, e.g., Figure 2 in the work of [32]). In cells depending on uptake of dissolved nutrients, size is not only important for predation loss but also for diffusion-limited nutrient uptake and thus for competitive ability. For a spherical cell, diffusion transport to the cell surface increases linearly with cell radius (r) [33]. Competitive ability, however, depends on the ratio between acquisition rate and requirement. If the amount of limiting substrate required to build a new cell is proportional to the cell volume, the competitive ability for a spherical cell, therefore, scales as r1/r3 = r−2. This is why small cell size is usually thought to be an important competitive trait under nutrient-limiting conditions [33]. In the present context, this is consistent with the streamlining hypothesis for S-strategists. If, however, size can be increased without an increase in the need for the limiting element, the competitive ability will scale as r1, and the competitive advantage will be in increasing, rather than in decreasing size (See Box 1 for detailed argument). When combined with the defense advantage of a size large enough to avoid predation by heterotrophic flagellates [26], this means that cells that can use a non-limiting substrate to increase size can reduce the trade-off between competition and defense [32]. Our L-strategists are thus a subclass of the larger class of “Winnie-the-Pooh” strategists, previously defined as organisms able to use a non-limiting resource to reduce the trade-off between competition and defense [32] (referring to A.A. Milne’s story: “… and when the Rabbit said, ‘Honey or condensed milk with your bread?’ he was so excited that he said, ‘Both’.” Milne A.A. (1926) Winnie-the-Pooh, Methuen & Co., London, UK, p. 37).
Both increasing (L-strategists) and decreasing (S-strategists) size can thus reduce trade-off, as illustrated in Figure 1. Since L-strategists replace S-strategists given access to sufficient OC, it follows that the L-strategy, when feasible, is more efficient (gives a larger reduction in trade-off) than the S-strategy.
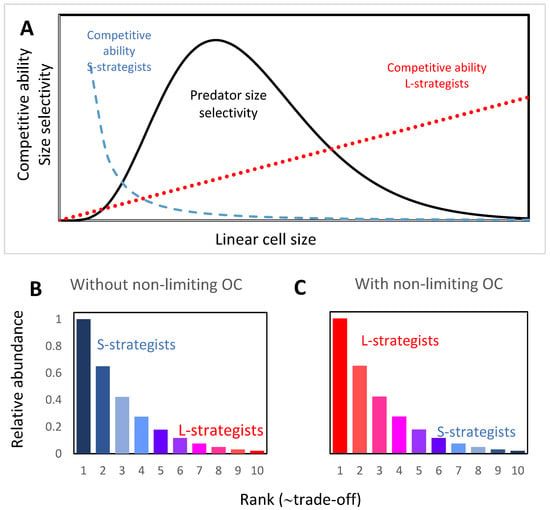
Figure 1.
(A) Schematic illustration of the proposed mechanism whereby S- and L-strategists decrease the trade-off between competition and defense by reducing or increasing size, respectively. The size-increase of L-strategists requires access to sufficient non-limiting organic C (OC). For S-strategists, competitive ability is assumed to scale with r−2. With a size below the peak in prey selectivity, a further decrease in size will increase competitiveness and simultaneously reduce predator loss. For L-strategists where competitiveness scales with r1, a reduction in trade-off is obtained if size increases above the peak in predator selectivity. With rank (in abundance) dependent on trade-off, the usually (B) S-dominated (blue) rank-abundance curves shift to (C) L-dominated (red) in environments with sufficient non-limiting OC.
Additional negative effects of OC on bacterial predation loss come from the possibility that C-rich food may cause food quality limitation in their protozoan predators [34,35], thus potentially reducing growth and activity of the predator population.
Box 1. Summary of the theoretical arguments for why both increasing and decreasing cell size can increase a cell’s competitive ability. Adapted from the work of [32].
At sufficiently low concentrations of the limiting nutrient, transport to the cell by molecular diffusion is assumed to be the growth-limiting process. For a spherical cell of radius r, the maximum diffusive flux is:
where D is the diffusion constant in water for the substrate molecules, S is the substrate concentration at infinite distance from the cell, and all molecules reaching the cell surface are captured (). Assume for simplicity a non-respired substrate being the only molecule containing the limiting element (e.g., phosphate). The cell has to sequester an amount equal to the cell quota before it can divide. Growth rate μ is, therefore:
where Q is the cell quota of the limiting element. Competitive ability at permanently low concentrations is then defined by the maximum affinity α:
If r can be increased without affecting Q (by, e.g., C-storage), competitive ability thus increases linearly with r as hypothesized for L-strategists (Figure 1).
If, however volume-specific content ρ of the limiting element is constant, α will scale as r−2:
Both a reduction in size (r) and a reduction in the volume-specific requirement for limiting element (ρ) (by, e.g., reduction in genome size) will then increase competitive ability as hypothesized for S-strategists (Figure 1).
The simple physiological hypothesis behind the two strategies is thus that either cell quota Q (L-strategists) or volume-specific content ρ (S-strategists) of the limiting element is independent of size. The validity of this either-or assumption is an obvious issue for further research.
3. Observational Studies of Marine Vibrios
There are two main environmental factors recurring in the literature on the occurrence of Vibrio in natural systems: increased temperature and/or the presence of OC.
A strong direct indication of the role of OC in favoring Vibrio under natural conditions comes from a phenomenon called “milky seas”. This is attributed to the high abundances of the luminescent bacterium Vibrio harveyi living in association with carbohydrate-rich colonies of the microalgae Phaeocystis [36]. The large-scale “blooming” capabilities of Vibrio are illustrated by observations from both ship and satellite of one such bloom extending over ca. 14,500 km2 in the Indian Ocean (Miller et al., 2005). Enrichment of V. anguillarum-like organisms has also been found in water enriched in carbohydrates from sugar and cellulose industries [37], leading the author to suggest that such pollution increases the exposure of fish to vibriosis. Saharan dust events also induce rapid growth of Vibrio spp. such as V. cholerae and Vibrio alginolyticus [38] and is suggested to be caused by the high iron content of the dust. Experimental Fe-addition was, however, not found to stimulate the growth of Vibrio in oligotrophic waters [39]. As Saharan dust contains bioavailable forms also of nitrogen, phosphorous, and organic C [40,41], it can be difficult to identify one single growth-stimulating factor in the dust.
Correlation between the occurrence of Vibrio spp. and the concentration of organic C in particulate and dissolved forms has been shown in the Bengal delta [42]. Another environment of interest in this context is the Adriatic Sea, as it is known to have states with extreme accumulation of organic C as mucus of probably mainly diatom origin [43]. A study in Italian coastal water detected both V. alginolyticus and V. vulnificus from July to September 1997, although this study focused on Vibrios attached to zooplankton rather than on any potential relationships with the organic content of the water masses. Live copepods are known to function as a reservoir of V. cholerae [44].
Interesting evidence of a correlation between increasing temperature and increasing abundances of Vibrio in the North Atlantic comes from the continuous plankton recorder [45,46]. There has also been an unprecedented occurrence of environmentally acquired Vibrio infections in the human population of Northern Europe and the Atlantic coast of the United States in recent years [46]. Outbreaks of V. parahaemolyticus have been reported in association with El Niño Southern Oscillation events [47], where cold upwelling water on the Pacific coast of South America is replaced by warm surface water. Increased ocean surface temperatures also correlated with increased numbers of V. cholerae infections in Bangladesh and increased numbers of infections with human pathogenic Vibrio spp. in the Baltic region, the North Atlantic, and the North Sea [48]. Many Vibrio infections seem to be correlated with warm water conditions, and this has been used to argue that Vibrio and associated problems may increase at high latitudes as climate change leads to increasing water temperatures [49].
The evidence for the role of temperature in stimulating Vibrios is thus strong, and clear and consistent statistical correlations with environmental variables other than salinity and temperature have been difficult to establish in observational data sets [50]. Temperature, however, affects many important phenomena in pelagic ecosystems. It can therefore be difficult to separate direct temperature effects on Vibrio spp. occurrence and/or pathogenicity from indirect effects via, e.g., increased water column stability or changes in the autochthonous supply of organic matter from phytoplankton or allochthonous supply from rivers. The possibility also remains that OC-rich situations at lower temperatures are better exploited by L-strategists other than Vibrio (see next section on mesocosm experiments). From studies of V. harveyi in microcosms at elevated temperatures, Montánche et al. [51] suggested the alternative explanation that the increase in Vibrio infections at a higher temperature more likely is caused by an increase in virulence, giving them access to the nutrient-rich environment of the infected hosts, rather than an increase in fitness in seawater itself.
4. Bacterial Community Shifts in Mesocosms Amended with Glucose
Changes in community composition toward dominance of previously rare bacterial species seem to be a highly reproducible phenomenon in mesocosm experiments where glucose is added as an N- and P-free source of OC. The outcomes include a Vibrio-dominated community in a Danish fjord [52]; a community dominated by V. splendidus, also in a Danish fjord [53]; and one dominated by Psychromonadaceae in an Arctic location in Spitsbergen [54].
Since these shifts occur under conditions with non-limiting glucose, the organisms were not selected because they are superior competitors for glucose. They are selected over periods of days with permanent glucose excess [54], making it also unlikely that OC stored as reserve material provided them a fitness advantage in periods with C-limitation. The previously discussed use of OC by L-strategist, here as glucose, to simultaneously reduce predation and increase diffusion-limited uptake of limiting mineral nutrients has therefore been suggested as the mechanism behind the glucose-induced shifts in bacterial community composition [32].
As V. splendidus is a shellfish pathogen [14], its dominance in glucose amended mesocosms serves as a case study demonstrating how experimental conditions can select for pathogens, independent of the presence of any infectees. Like the Vibrionaceae, the Psychromonadaceae also belong to the γ-Proteobacteria [55]. It is interesting to note that Psychromonas spp. were also enriched in experiments with detritus degradation in the Arctic [56]. In addition, several Psychromonas spp. (P. aquamarine, P. japonica, and P. macrocephali) have been isolated from samples taken in the vicinity of whale carcasses [55], indicating that they may share with Vibrios the ability to proliferate in environments rich in organic matter. Psychrophilic Psychromonas spp. thus possibly fill an ecological niche as L-strategists in cold waters, similar to that of Vibrio spp. in warmer environments. Pathogenic members of the Psychromonodaceae seem, however, not to have been reported.
Microbial population dynamics in experimentally perturbed mesocosms have been successfully described using a mathematical “minimum” model [57] where the community of heterotrophic bacteria is one single plankton functional type (PFT). Its functional role in the microbial food web is defined by its ability to compete with phytoplankton for mineral nutrients and its defensive properties against predation by heterotrophic flagellates. However, to obtain the response to glucose additions correct, the prokaryote community had to be divided into two groups, essentially corresponding to the S- and L-strategists discussed here [54]. For models aspiring to accurately reproduce shifts between situations with heterotrophic bacteria limited by OC and by mineral nutrients, some kind of representation, either of L- and S-strategists as two separate PFTs, or as one PFT with adaptive strategy, seems to be required.
5. Viability of Marine Larvae
In aquaculture of larvae of marine fish and crustacea, the mortality at the early stage of industrialization is highly variable and can be >90%. The reason has been attributed to various problems, including egg/brood-stock quality, nutrition, and dysbiosis due to negative interactions between the host and its microbiota [58,59]. The high variability between replicates is only consistent with a microbial problem, although the other factors also are important [60]. In many cases, specific pathogens are not detected, but the situation is often referred to as a “Vibrio-problem” [61,62].
To solve this problem has been an active field of research, and several countermeasures have been tested, including disinfection, prophylactic use of antibiotics, pro- and pre-biotics, and setting up selection regimes against detrimental bacteria [59]. The outcome is variable, but an approach with consistently strong positive effects has been described in terms of r- vs. K-selection: K-selection is sought within the rearing system to avoid blooms by opportunistic r-strategist (reviewed by the authors of [60]). Initial work suggested that the problem with poor and variable performance was due to blooms of fast-growing opportunists (=r-strategists), and a solution to the problem was achieved by setting up K-selection within the system [58]. K-selection is stimulated by securing competition for resources, and this is achieved by introducing a biofilter within the system. Biofilms serve as a protection against protist predators [63] and enable the build-up of high biomass of non-pathogenic K-strategists consuming organic matter. With high bacterial biomass on the biofilter, a low supply rate of resources per bacterium is secured. This K-selected bacterial community will release bacteria into the water. The K-selection approach has been tested with a considerable number of cultivated host species, including Atlantic cod, and in different types of cultivation systems (traditional flow-through and recirculating aquaculture systems). The K-selection excludes Vibrio species and other fast-growing γ-proteobacteria, and when comparing the performance of the cultivated species with and without the biofilter, significant differences in the microbiota of the larvae are obtained. Typically a 50% to 100% improvement in appetite, growth rate, survival, and robustness to stress is obtained when larvae are grown with the K-selected bacterial community (reviewed by the authors of [60]).
Whereas manipulation of bacterial communities in aquaculture has received considerable attention [64], the role of bacterial infections in population control in natural fish stock is less known but could be substantial [65]. It is an important question whether bacterial infection is an important mortality factor in natural populations of fish larvae and whether environments selecting for Vibrio and other pathogenic bacteria, therefore, can lead to recruitment problems in local fish stocks. A potentially interesting case is the Oslo fjord (Norway) which has experienced a largely unexplained collapse in the local fish stocks [66]. With its recurrent bathing season problems of human infections by the “flesh-eating” V. vulnificus [67], it is tempting to speculate whether the two phenomena are connected through environmental changes leading to enrichment in OC with subsequent stimulation of L-strategist bacteria.
6. What Drives Natural Environments toward Enrichment in Organic C?
With an important role in the global carbon cycle, the ecological conditions that produce environments with sustained excess of bioavailable OC have been given considerable experimental (e.g., the work of [68]), observational (e.g., the work of [69]), and theoretical (e.g., the work of [70]) consideration.
Accumulation of OC to levels not limiting bacterial growth occurs in ecological situations where supply (allochthonous + autochthonous) exceeds losses due to consumption and export. OC accumulation is thus a combined result of the (allochthonous and autochthonous) production processes and the ecological processes regulating bacterial carbon demand. Bacterial consumption of OC is the product of per capita consumption and abundance. Theoretically, constraining bacterial consumption, therefore, requires mechanisms that keep both activity per cell and number of cells low. This has been demonstrated in laboratory experiments where bacterial abundance was controlled by protozoan predators, while the bacterial growth rate was controlled by competition with phytoplankton for phosphate. This combination of top-down and bottom-up control strongly reduced bacterial glucose consumption, not only relative to a situation with bacteria alone but also to the two situations where bacteria were exposed to either predation or competition separately [71]. Mineral nutrient limitation of bacterial growth (and therefore a potential for OC accumulation) has been found in both limnic [72,73], brackish [74], and marine waters [75,76].
Elevated autochthonous OC production is connected to species composition of the phytoplankton community. One example is blooms of carbohydrate-producing Phaeocystis sp., in extreme cases leading to foam formation to an extent representing a nuisance phenomenon on North Sea beaches [77]. Diatoms can also produce OC far above what one would predict if linking photosynthesis to mineral nutrient uptake with a Redfield stoichiometry [78]. Mesocosm experiments have also demonstrated how large diatoms, when not limited by silicate, consume the limiting mineral nutrients and therefore stop bacterial consumption of glucose [79]. The combination of glucan production from diatoms and low mineral nutrient availability for heterotrophic prokaryotes may favor L-strategists such as Polaribacter spp. (Bacteriodota), which have the complex enzyme machinery needed for glycan degradation [80].
In coastal zones, important allochthonous sources add to this and include organic pollution sources such as pulp industry (e.g., the work of [37]) and natural OC content in freshwater discharges. As the OC content in land runoff is influenced by precipitation, permafrost thawing [81], and ice melting [82], OC supply to coastal regions is believed to increase with climate change.
7. Are the Flow-Cytometer Groups of LNA and HNA Populations Reflecting S- and L-Strategists, Respectively?
Further studies on the ecological role of shifts between S- and L-strategists would benefit greatly from any easily obtained indicator of the two populations. An obviously interesting candidate is the LNA/HNA balance frequently recorded in flow-cytometric analysis of picoplanktonic communities. Using the fluorescent DNA stain Cyber Green I usually reveals two distinct populations, separated by the fluorescent signal into a high nucleic acid (HNA) and a low nucleic acid (LNA) population. As expected, considering its small genome, SAR11 is a dominant member of the LNA community [83]. With our “large or small” hypothesis (Figure 1) in mind, L-strategists with their larger genomes would be expected to be members of the HNA community. In Arctic samples, the HNA community has been found to be dominated by members of the Bacteriodota phylum [83], an interesting phylum in the present context since it has pathogenic members [84]. With our hypothesis that the L-strategy is successful when non-limiting OC is available, a dominance of L-strategists in the HNA community should lead to high HNA/LNA ratios in OC-enriched environments. This prediction does, however, not immediately fit observations, as low ratios have been associated with OC-enriched situations in upwelling gyres in the North Atlantic [85], and high ratios have been observed in deep water samples in the Mediterranean [86]. Inferring the S/L-balance from HNA/LNA ratios would be blurred if the L-strategist is a variable fraction within the HNA community. The possibility is illustrated by the observation that photosynthetic members of the picoplankton community may dominate the HNA community in some environments [83]. An interesting parallel would be if chemolithotrophs make a substantial contribution to the HNA population in deep water samples.
8. Relating S- vs. L- to r- vs. K-Selection
The fish larvae literature has linked infections and pathogenicity to the classical r- vs. K-strategies, while results from the mesocosm studies have been explained in terms of the S- and L-strategies described above. As noted in the introduction, the foci of the two frameworks are different, with r- vs. K-selection focusing on the effect of environmental disturbances, while the S- vs. L-selection focus on the importance of trade-offs between competitive and defensive traits. The two frameworks are thus not in conflict but can be seen as supplementing each other with S- and L-strategists as subgroups of K-strategists (Figure 2). Importantly, however, the mechanisms whereby C-storage is suggested to lower trade-off in L-strategists also provide them with the additional advantage of stored C and energy that can be exploited in environments fluctuating between mineral nutrient and OC limitation. With its <10 min minimum recorded generation time [87], Vibrio natriegens serves as a demonstration of how at least some L-strategists may be capable of rapid growth when conditions allow. The traditional idea has been that many of the traits defining r- and K-strategies would be either-or, defining two distinct groups of bacteria [13]. The suggested L-strategists do not fit this dichotomy since they combine K-defining traits (high defensive and competitive abilities at low concentration of limiting nutrient) with r-defining traits (i.e., C-storage and rapid growth) (Figure 2). Whether the best interpretation of their dominance is as r- strategists (as used in the aquaculture literature) or as L-type K-strategists (in the mesocosm literature) thus depends on the stability of the actual OC-enriched environment. There may also be a division within the L-strategist group with organisms with either more r- or more K-defining traits. Experimental evidence for such a division within the Vibrio genus has been reported [88]. The streamlining of the genome needed for the S-strategy likely necessitates abandoning the more complicated genetic machinery required to cope with fluctuating environments [10], preventing S-strategists from such a combination of r- and K-strategies.
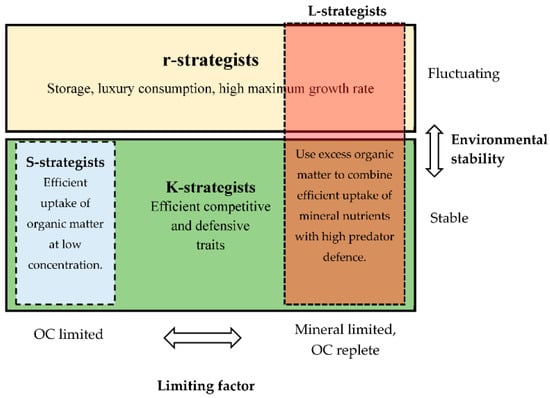
Figure 2.
Summary of strategies discussed (S (blue), L (red), r (yellow), and K (green)), arranged in the two dimensions of (y-axis) environmental disturbances and (x-axis) growth rate limitation by mineral nutrient vs. organic C (OC). Fluctuating environments select for r-strategists with characteristic traits such as high maximum growth rate, rapid luxury consumption, and storage of presently non-limiting nutrients. Stable environments create climax communities consisting of K-strategists, where competitive ability at low nutrient concentrations and defense mechanisms against predation and viral infections are assumed to be important traits. In this scheme, the S-strategists, with their “streamlining” strategy, dominate when OC/energy is limiting. L-strategists dominate when there is sufficient non-limiting OC to allow a reduction in their competition-defense trade-off below that of the S-strategists. As the S-strategy is suggested to involve storage of OC, they have traits classifying them as both r- and K-strategists.
The literature also uses the terminology of oligotrophs and copiotrophs to describe the dichotomy observed in marine prokaryote strategies (e.g., the work of [89]). Like S/L-selection, this emphasizes the role of OC rather than the role of environmental stability but does not explicitly include the suggested role of OC in reducing competition-predation trade-off.
Relevant to Figure 2 is a recent meta-analysis of the correlation between maximum growth rate, cell size, and genome size in cultured prokaryotes [90]. Their finding of little correlation between these traits over all clades and habitats may seem to weaken the case for a fundamental role of cell size, also reflected in genome size, as argued here. The S/L-selection proposed here does, however, explain why organisms with both large and small size and large and small genomes may be K-strategists and grow slowly. It also explains how large size–large genome L-strategists may grow both slowly (as K-strategists) and rapidly (as r-strategists). The size flexibility of L-strategists with or without access to excess OC would also blur cell size-genome size correlations when measured over all habitats. With Figure 2 capturing only two dimensions of a presumably multi-dimensional selecting environment, the possibility for additional selection mechanisms remains open.
9. Final Remarks
We believe that the idealized model in Figure 1 captures essential aspects of how trade-offs between predator defense and competition shape prokaryote community structure. However, other mechanisms clearly add detail to this picture. One important candidate for such an additional mechanism is how host-specific viruses and the associated trade-offs split the S- and L-strategists into species and strains [7]. Another is the chemical composition of the OC-pool and how this presumably selects for different L-strategists based on differences in their substrate specialization [80].
These additional mechanisms may be reflected in different genomic structures for the two types of strategists. One could speculate that, for an abundant organism, viral defense is particularly important. Perhaps the pattern of SAR11 with a small genome per cell but a large pan-genome [91] reflects a dominantly top-down (mortality) controlled splitting of the clade by lytic viruses. The hypothesized L-strategy of residing at low abundance in most of the marine habitat may require less viral defense, whereas the ability to exploit the environments with high OC may require more biochemical pathways. Without the space and resource requirements that constrain evolution in S-strategists, this may have favored diversification of L-strategists at a “species” rather than at a “strain” level. The apparent difference in diversity within the two groups may then be biased by our tendency to associate diversity with the “species” level. Diversification could be large in both groups, but the S- and L-strategies may favor diversification at the “strain” and “species” levels, respectively.
In this framework, the global success of the S-strategist SAR 11 is rooted in the vast size of the oligotrophic marine habitat to which its strategy is adapted. The L-strategy, on the other hand, is “superior” in terms of a potentially lower trade-off between competitive and defensive traits but feasible only in habitats that so far are more restricted in time and space. The oligotrophic oceanic gyres are expanding [92], and terrestrial supply of OC is expected to increase with climate change [81]. Habitat size for S- and L-strategist is, therefore, a feature of the marine microbial ecosystem sensitive to climate change on both local and global scales.
Although simple, the scheme suggested in Figure 2 thus has broad implications, not only in the applied contexts of human health and aquaculture practices but also in basic research questions spanning a broad range from genome organization, via diversification processes at different levels, to ecosystem responses to global change.
Author Contributions
T.F.T., L.Ø. and O.V. all contributed to previously published experimental work leading up to the ideas presented here. T.F.T., L.Ø. and O.V. all participated in writing the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Research Council of Norway through the project The Nansen Legacy (RCN # 276730).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sunagawa, S.; Coelho, L.P.; Chaffron, S.; Kultima, J.R.; Labadie, K.; Salazar, G.; Djahanschiri, B.; Zeller, G.; Mende, D.R.; Alberti, A.; et al. Structure and function of the global ocean microbiome. Science 2015, 348, 1261359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eguíluz, V.M.; Salazar, G.; Fernández-Gracia, J.; Pearman, J.K.; Gasol, J.M.; Acinas, S.G.; Sunagawa, S.; Irigoien, X.; Duarte, C.M. Scaling of species distribution explains the vast potential marine prokaryote diversity. Sci. Rep. 2019, 9, 18710. [Google Scholar] [CrossRef] [PubMed]
- Giovannoni, S.J. SAR11 Bacteria: The Most Abundant Plankton in the Oceans. Annu. Rev. Mar. Sci. 2017, 9, 231–255. [Google Scholar] [CrossRef] [PubMed]
- Yooseph, S.; Nealson, K.H.; Rusch, D.B.; McCrow, J.P.; Dupont, C.L.; Kim, M.; Johnson, J.; Montgomery, R.; Ferriera, S.; Beeson, K.; et al. Genomic and functional adaptation in surface ocean planktonic prokaryotes. Nature 2010, 468, 60–66. [Google Scholar] [CrossRef] [Green Version]
- Tagomori, K.; Iida, T.; Honda, T. Comparison of genome structures of vibrios, bacteria possessing two chromosomes. J. Bacteriol. 2002, 184, 4351–4358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Valera, F.; Martin-Cuadrado, A.-B.; Rodriguez-Brito, B.; Pasic, L.; Thingstad, T.F.; Rohwer, F.; Mira, A. OPINION Explaining microbial population genomics through phage predation. Nat. Rev. Microbiol. 2009, 7, 828–836. [Google Scholar] [CrossRef] [PubMed]
- Thingstad, T.F.; Våge, S.; Storesund, J.E.; Sandaa, R.-A.; Giske, J. A theoretical analysis of how strain-specific viruses can control microbial species diversity. Proc. Natl. Acad. Sci. USA 2014, 111, 7813–7818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giovannoni, S. Streamlining theory in microbial evolution. FEBS J. 2014, 281, 44. [Google Scholar]
- Morris, R.M.; Rappe, M.S.; Connon, S.A.; Vergin, K.L.; Siebold, W.A.; Carlson, C.A.; Giovannoni, S.J. SAR11 clade dominates ocean surface bacterioplankton communities. Nature 2002, 420, 806–810. [Google Scholar] [CrossRef]
- Giovannoni, S.J.; Tripp, H.J.; Givan, S.; Podar, M.; Vergin, K.L.; Baptista, D.; Bibbs, L.; Eads, J.; Richardson, T.H.; Noordewier, M.; et al. Genome Streamlining in a Cosmopolitan Oceanic Bacterium. Science 2005, 309, 1242–1245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.L.; Temperton, B.; Thrash, J.C.; Schwalbach, M.S.; Vergin, K.L.; Landry, Z.C.; Ellisman, M.; Deerinck, T.; Sullivan, M.B.; Giovannoni, S.J. Abundant SAR11 viruses in the ocean. Nature 2013, 494, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Pedrós-Alió, C. The rare bacterial biosphere. Ann. Rev. Mar. Sci. 2012, 4, 449–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrews, J.H.; Harris, R.F. R-Selection and K-Selection and Microbial Ecology. Adv. Microb. Ecol. 1986, 9, 99–147. [Google Scholar]
- Vezzulli, L.; Pezzati, E.; Stauder, M.; Stagnaro, L.; Venier, P.; Pruzzo, C. Aquatic Ecology of the Oyster Pathogens Vibrio splendidus and Vibrio aestuarianus. Environ. Microbiol. 2014, 17, 1065–1080. [Google Scholar] [CrossRef] [PubMed]
- Frans, I.; Michiels, C.W.; Bossier, P.; Willems, K.A.; Lievens, B.; Rediers, H. Vibrio anguillarum as a fish pathogen: Virulence factors, diagnosis and prevention. J. Fish Dis. 2011, 34, 643–661. [Google Scholar] [CrossRef] [PubMed]
- Colwell, R.R. Global climate and infectious disease: The cholera paradigm. Science 1996, 274, 2025–2031. [Google Scholar] [CrossRef] [Green Version]
- Jones, M.K.; Oliver, J.D. Vibrio vulnificus: Disease and Pathogenesis. Infect. Immun. 2009, 77, 1723–1733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, Y.-C.; Liu, C. Vibrio parahaemolyticus: A concern of seafood safety. Food Microbiol. 2007, 24, 549–558. [Google Scholar] [CrossRef]
- Bourassa, L.; Camilli, A. Glycogen contributes to the environmental persistence and transmission of Vibrio cholerae. Mol. Microbiol. 2009, 72, 124–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adiba, S.; Nizak, C.; van Baalen, M.; Denamur, E.; Depaulis, F. From grazing resistance to pathogenesis: The coincidental evolution of virulence factors. PLoS ONE 2010, 5, e11882. [Google Scholar] [CrossRef]
- Hilbi, H.; Weber, S.S.; Ragaz, C.; Nyfeler, Y.; Urwyler, S. Environmental predators as models for bacterial pathogenesis. Environ. Microbiol. 2007, 9, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Erken, M.; Lutz, C.; McDougald, D. The Rise of Pathogens: Predation as a Factor Driving the Evolution of Human Pathogens in the Environment. Microb. Ecol. 2013, 65, 860–868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, R.; Kjelleberg, S.; McDougald, D.; Jurgens, K. Species-specific patterns in the vulnerability of carbon-starved bacteria to protist grazing. Aquat. Microb. Ecol. 2011, 64, 105–116. [Google Scholar] [CrossRef] [Green Version]
- Matz, C.; Jürgens, K. Interaction of nutrient limitation and protozoan grazing determines the phenotypic structure of a bacterial community. Microb. Ecol. 2003, 45, 384–398. [Google Scholar] [CrossRef] [PubMed]
- Jürgens, K.; Matz, C. Predation as a shaping force for the phenotypic and genotypic composition of planktonic bacteria. Antonie Van Leeuwenhoek 2002, 81, 413–434. [Google Scholar] [CrossRef]
- Matz, C.; Boenigk, J.; Arndt, H.; Jürgens, K. Role of bacterial phenotypic traits in selective feeding of the heterotrophic nanoflagellate Spumella sp. Aquat. Microb. Ecol. 2002, 27, 137–148. [Google Scholar] [CrossRef]
- Jürgens, K.; Pernthaler, J.; Schalla, S.; Amann, R. Morphological and compositional changes in a planktonic bacterial community in response to enhanced protozoan grazing. Appl. Environ. Microbiol. 1999, 65, 1241–1250. [Google Scholar] [CrossRef] [Green Version]
- Andersson, A.; Ahlinder, J.; Mathisen, P.; Hagglund, M.; Backman, S.; Nilsson, E.; Sjodin, A.; Thelaus, J. Predators and nutrient availability favor protozoa-resisting bacteria in aquatic systems. Sci. Rep. 2018, 8, 8415. [Google Scholar] [CrossRef] [Green Version]
- Preiss, J.; Romeo, T. Physiology, biochemistry and genetics of bacterial glycogen synthesis. Adv. Microb. Physiol. 1989, 30, 183–238. [Google Scholar]
- Wilkinson, J.F. Carbon and Energy Storage in Bacteria. J. Gen. Microbiol. 1963, 32, 171–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- James, B.W.; Mauchline, W.S.; Dennis, P.J.; Keevil, C.W.; Wait, R. Poly-3-Hydroxybutyrate in Legionella pneumophila, an Energy Source for Survival in Low-Nutrient Environments. Appl. Environ. Microbiol. 1999, 65, 822–827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thingstad, T.F.; Øvreås, L.; Egge, J.K.; Løvdal, T.; Heldal, M. Use of non-limiting substrates to increase size; a generic strategy to simultaneously optimize uptake and minimize predation in pelagic osmotrophs? Ecol. Lett. 2005, 8, 675–682. [Google Scholar] [CrossRef]
- Jumars, P.; Deming, J.; Hill, P.; Karp-Boss, L.; Dade, W. Physical constraints on marine osmotrophy in an optimal foraging context. Mar. Microb. Food Webs 1993, 7, 121–161. [Google Scholar]
- Shannon, S.P.; Chrzanowski, T.H.; Grover, J.P. Prey Food Quality Affects Flagellate Ingestion Rates. Microb. Ecol. 2007, 53, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Olsen, Y.; Andersen, T.; Gismervik, I.; Vadstein, O. Marine heterotrophic bacteria, protozoan and metazoan zooplankton may experience protein N or mineral P limitation in coastal waters. Mar. Ecol. Prog. Ser. 2011, 436, 81–100. [Google Scholar] [CrossRef]
- Lapota, D.; Galt, C.; Losee, J.R.; Huddell, H.D.; Orzech, J.K.; Nealson, K.H. Observations and measurements of planktonic bioluminescence in and around a milky sea. J. Exp. Mar. Biol. Ecol. 1988, 119, 55–81. [Google Scholar] [CrossRef]
- Larsen, J.L. Vibrio anguillarum: Prevalence in three carbohydrate loaded marine recipients and a control. Zentralblatt für Bakteriologie Mikrobiologie und Hygiene: I. Abt. Originale C: Allgemeine, angewandte und ökologische Mikrobiologie 1982, 3, 519–530. [Google Scholar] [CrossRef]
- Westrich, J.R.; Ebling, A.M.; Landing, W.M.; Joyner, J.L.; Kemp, K.M.; Griffin, D.W.; Lipp, E.K. Saharan dust nutrients promote Vibrio bloom formation in marine surface waters. Proc. Natl. Acad. Sci. USA 2016, 113, 5964–5969. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.F.; Kelly, R.L.; Kauffman, K.M.; Reid, A.K.; Lauderdale, J.M.; Follows, M.J.; John, S.G. Growth of marine Vibrio in oligotrophic environments is not stimulated by the addition of inorganic iron. Earth Planet. Sci. Lett. 2019, 516, 148–155. [Google Scholar] [CrossRef]
- Djaoudi, K.; Wambeke, F.; Barani, A.; Bhairy, N.; Chevaillier, S.; Desboeufs, K.; Nunige, S.; Labiadh, M.; Tureaux, T.; Lefèvre, D.; et al. Potential bioavailability of organic matter from atmospheric particles to marine heterotrophic bacteria. Biogeosciences 2020, 17, 6271–6285. [Google Scholar] [CrossRef]
- Herut, B.; Rahav, E.; Tsagaraki, T.M.; Giannakourou, A.; Tsiola, A.; Psarra, S.; Lagaria, A.; Papageorgiou, N.; Mihalopoulos, N.; Theodosi, C.N.; et al. The Potential Impact of Saharan Dust and Polluted Aerosols on Microbial Populations in the East Mediterranean Sea, an Overview of a Mesocosm Experimental Approach. Front. Mar. Sci. 2016, 3, 226. [Google Scholar] [CrossRef] [Green Version]
- Neogi, S.B.; Lara, R.; Alam, M.; Harder, J.; Yamasaki, S.; Colwell, R.R. Environmental and hydroclimatic factors influencing Vibrio populations in the estuarine zone of the Bengal delta. Environ. Monit. Assess. 2018, 190, 565. [Google Scholar] [CrossRef] [PubMed]
- Stachowitsch, M.; Fanuko, N.; Richter, M. Mucus Aggregates in the Adriatic Sea: An Overview of Stages and Occurrences. Mar. Ecol. 1990, 11, 327–350. [Google Scholar] [CrossRef]
- Huq, A.; Small, E.B.; West, P.A.; Huq, M.I.; Rahman, R.; Colwell, R.R. Ecological relationships between Vibrio cholerae and planktonic crustacean copepods. Appl. Environ. Microbiol. 1983, 45, 275–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vezzulli, L.; Brettar, I.; Pezzati, E.; Reid, P.C.; Colwell, R.R.; Höfle, M.G.; Pruzzo, C. Long-term effects of ocean warming on the prokaryotic community: Evidence from the vibrios. ISME J. 2012, 6, 21–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vezzulli, L.; Grande, C.; Reid, P.C.; Hélaouët, P.; Edwards, M.; Höfle, M.G.; Brettar, I.; Colwell, R.R.; Pruzzo, C. Climate influence on Vibrio and associated human diseases during the past half-century in the coastal North Atlantic. Proc. Natl. Acad. Sci. USA 2016, 113, E5062–E5071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raszl, S.M.; Froelich, B.A.; Vieira, C.R.; Blackwood, A.D.; Noble, R.T. Vibrio parahaemolyticus and Vibrio vulnificus in South America: Water, seafood and human infections. J. Appl. Microbiol. 2016, 121, 1201–1222. [Google Scholar] [CrossRef] [PubMed]
- Cavicchioli, R.; Ripple, W.J.; Timmis, K.N.; Azam, F.; Bakken, L.R.; Baylis, M.; Behrenfeld, M.J.; Boetius, A.; Boyd, P.W.; Classen, A.T.; et al. Scientists’ warning to humanity: Microorganisms and climate change. Nat. Rev. Microbiol. 2019, 17, 569–586. [Google Scholar] [CrossRef] [Green Version]
- Baker-Austin, C.; Trinanes, J.A.; Taylor, N.G.H.; Hartnell, R.; Siitonen, A.; Martinez-Urtaza, J. Emerging Vibrio risk at high latitudes in response to ocean warming. Nat. Clim. Chang. 2013, 3, 73–77. [Google Scholar] [CrossRef]
- Takemura, A.; Chien, D.; Polz, M. Associations and dynamics of Vibrionaceae in the environment, from the genus to the population level. Front. Microbiol. 2014, 5, 38. [Google Scholar] [CrossRef] [Green Version]
- Montánchez, I.; Ogayar, E.; Plágaro, A.H.; Esteve-Codina, A.; Gómez-Garrido, J.; Orruño, M.; Arana, I.; Kaberdin, V.R. Analysis of Vibrio harveyi adaptation in sea water microcosms at elevated temperature provides insights into the putative mechanisms of its persistence and spread in the time of global warming. Sci. Rep. 2019, 9, 289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joint, I.; Henriksen, P.; Fonnes, G.A.; Bourne, D.; Thingstad, T.F.; Riemann, B. Competition for inorganic nutrients between phytoplankton and bacterioplankton in nutrient manipulated mesocosms. Aquat. Microb. Ecol. 2002, 29, 145–159. [Google Scholar] [CrossRef]
- Øvreås, L.; Bourne, D.; Sandaa, R.A.; Casamayor, E.O.; Benlloch, S.; Goddard, V.; Smerdon, G.; Heldal, M.; Thingstad, T.F. Response of bacterial and viral communities to nutrient manipulations in seawater mesocosms. Aquat. Microb. Ecol. 2003, 31, 109–121. [Google Scholar] [CrossRef] [Green Version]
- Tsagaraki, T.M.; Pree, B.; Leiknes, O.; Larsen, A.; Bratbak, G.; Øvreås, L.; Egge, J.K.; Spanek, R.; Paulsen, M.L.; Olsen, Y.; et al. Bacterial community composition responds to changes in copepod abundance and alters ecosystem function in an Arctic mesocosm study. ISME J. 2018, 12, 2694–2705. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, M.; Nogi, Y. The Family Psychromonadaceae. In The Prokaryotes: Gammaproteobacteria; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 583–590. [Google Scholar] [CrossRef]
- Hoffmann, K.; Hassenruck, C.; Salman-Carvalho, V.; Holtappels, M.; Bienhold, C. Response of Bacterial Communities to Different Detritus Compositions in Arctic Deep-Sea Sediments. Front. Microbiol. 2017, 8, 266. [Google Scholar] [CrossRef] [Green Version]
- Larsen, A.; Egge, J.K.; Nejstgaard, J.C.; Di Capua, I.; Thyrhaug, R.; Bratbak, G.; Thingstad, T.F. Contrasting response to nutrient manipulation in Arctic mesocosms are reproduced by a minimum microbial food web model. Limnol. Oceanogr. 2015, 60, 360–374. [Google Scholar] [CrossRef] [Green Version]
- Vadstein, O.; Øie, G.; Olsen, Y.; Salvesen, I.; Skjermo, J.; Skjåk-Bræk, G. A strategy to obtain microbial control during larval development of marine fish. In Fish Farming Technology; Reinertsen, H., Dahle, L.A., Jørgensen, L., Tvinnereim, K., Eds.; A.A. Balkema Publishers: Amsterdam, The Netherlands, 1993; pp. 69–75. [Google Scholar]
- Vadstein, O.; Attramadal, K.J.K.; Bakke, I.; Forberg, T.; Olsen, Y.; Verdegem, M.; Giatsis, C.; Skjermo, J.; Aasen, I.M.; Gatesoupe, F.J.; et al. Managing the Microbial Community of Marine Fish Larvae: A Holistic Perspective for Larviculture. Front. Microbiol. 2018, 9, 1820. [Google Scholar] [CrossRef]
- Vadstein, O.; Attramadal, K.J.K.; Bakke, I.; Olsen, Y. K-Selection as Microbial Community Management Strategy: A Method for Improved Viability of Larvae in Aquaculture. Front. Microbiol. 2018, 9, 2730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Schryver, P.; Defoirdt, T.; Sorgeloos, P. Early Mortality Syndrome Outbreaks: A Microbial Management Issue in Shrimp Farming? PLoS Path. 2014, 10, e1003919. [Google Scholar] [CrossRef]
- Kumar, T.S.; Vidya, R.; Kumar, S.; Alavandi, S.V.; Vijayan, K.K. Zoea-2 syndrome of Penaeus vannamei in shrimp hatcheries. Aquaculture 2017, 479, 759–767. [Google Scholar] [CrossRef]
- Matz, C.; Webb, J.S.; Schupp, P.J.; Phang, S.Y.; Penesyan, A.; Egan, S.; Steinberg, P.; Kjelleberg, S. Marine Biofilm Bacteria Evade Eukaryotic Predation by Targeted Chemical Defense. PLoS ONE 2008, 3, e2744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olafsen, J. Interaction between fish larvae and bacteria in marine aquaculture. Aquaculture 2001, 200, 223–247. [Google Scholar] [CrossRef]
- Bergh, O. The dual myths of the healthy wild fish and the unhealthy farmed fish. Dis. Aquat. Org. 2007, 75, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Moland, E.; Synnes, A.-E.; Naustvoll, L.-J.; Brandt, C.; Norderhaug, K.; Thormar, J.; Biuw, M.; Jorde, P.; Knutsen, H.; Dahle, G.; et al. Krafttak for Kysttorsken_ Kunnskap for Stedstilpasset Gjenoppbygging av Bestander, Naturtyper og økosystem i Færder- og Ytre Hvaler Nasjonalparker; Institute of Marine Research: Bergen, Norway, 2021. (In Norwegian) [Google Scholar]
- Norwegian Institute of Public Health. Bakterier i Sjøvann kan gi Infeksjoner. Available online: https://www.fhi.no/ml/badevann/bakterier-i-sjovann-kan-gi-infeksjoner/ (accessed on 9 March 2022).
- Olsen, L.M.; Reinertsen, H.; Vadstein, O. Can phosphorus limitation inhibit dissolved organic carbon consumption in aquatic microbial food webs? A study of three food web structures in microcosms. Microb. Ecol. 2002, 43, 353–366. [Google Scholar] [CrossRef] [PubMed]
- Carlson, C.; Ducklow, H. Growth of bacterioplankton and consumption of dissolved organic carbon in the Sargasso Sea. Aquat. Microb. Ecol. 1996, 10, 69–85. [Google Scholar] [CrossRef] [Green Version]
- Thingstad, T.F.; Hagstrom, A.; Rassoulzadegan, F. Accumulation of degradable DOC in surface waters: Is it caused by a malfunctioning microbial loop? Limnol. Oceanogr. 1997, 42, 398–404. [Google Scholar] [CrossRef]
- Pengerud, B.; Skjoldal, E.F.; Thingstad, T.F. The reciprocal interaction between degradation of glucose and ecosystem—Studies in mixed chemostat cultures of marine-bacteria, algae, and bacterivorous nanoflagellates. Mar. Ecol. Prog. Ser. 1987, 35, 111–117. [Google Scholar] [CrossRef]
- Vadstein, O.; Jensen, A.; Olsen, Y.; Reinertsen, H. Growth and Phosphorus Status of Limnetic Phytoplankton and Bacteria. Limnol. Oceanogr. 1988, 33, 489–503. [Google Scholar] [CrossRef]
- Vadstein, O. Heterotrophic, planktonic bacteria and cycling of phosphorus—Phosphorus requirements, competitive ability, and food web interactions. Adv. Microb. Ecol. 2000, 16, 115–167. [Google Scholar]
- Thingstad, T.F.; Skjoldal, E.F.; Bohne, R.A. Phosphorus cycling and algal-bacterial competition in Sandsfjord, western Norway. Mar. Ecol. Prog. Ser. 1993, 99, 239–259. [Google Scholar] [CrossRef]
- Rivkin, R.B.; Anderson, M.R. Inorganic nutrient limitation of oceanic bacterioplankton. Limnol. Oceanogr. 1997, 42, 730–740. [Google Scholar] [CrossRef] [Green Version]
- Zohary, T.; Robarts, R.D. Experimental study of microbial P limitation in the eastern Mediterranean. Limnol. Oceanogr. 1998, 43, 387–395. [Google Scholar] [CrossRef]
- Lancelot, C.; Billen, G.; Sournia, A.; Weisse, T.; Coljin, F.; Veldhuis, M.; Davies, A.; Wassman, P. Phaeocystis blooms and nutrient enrichment in the continental coastal zones of the North Sea. Ambio 1987, 16, 38–46. [Google Scholar]
- Thingstad, T.F.; Bellerby, R.G.J.; Bratbak, G.; Borsheim, K.Y.; Egge, J.K.; Heldal, M.; Larsen, A.; Neill, C.; Nejstgaard, J.; Norland, S.; et al. Counterintuitive carbon-to-nutrient coupling in an Arctic pelagic ecosystem. Nature 2008, 455, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Thingstad, T.F.; Havskum, H.; Zweifel, U.L.; Berdalet, E.; Sala, M.M.; Peters, F.; Alcaraz, M.; Scharek, R.; Perez, M.; Jacquet, S.; et al. Ability of a “minimum” microbial food web model to reproduce response patterns observed in mesocosms manipulated with N and P, glucose, and Si. J. Mar. Syst. 2007, 64, 15–34. [Google Scholar] [CrossRef] [Green Version]
- Avcı, B.; Krüger, K.; Fuchs, B.M.; Teeling, H.; Amann, R.I. Polysaccharide niche partitioning of distinct Polaribacter clades during North Sea spring algal blooms. ISME J. 2020, 14, 1369–1383. [Google Scholar] [CrossRef]
- Delpech, L.M.; Vonnahme, T.R.; McGovern, M.; Gradinger, R.; Praebel, K.; Poste, A.E. Terrestrial Inputs Shape Coastal Bacterial and Archaeal Communities in a High Arctic Fjord (Isfjorden, Svalbard). Front. Microbiol. 2021, 12, 614634. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, M.L.; Nielsen, S.E.B.; Müller, O.; Møller, E.F.; Stedmon, C.A.; Juul-Pedersen, T.; Markager, S.; Sejr, M.K.; Delgado Huertas, A.; Larsen, A.; et al. Carbon Bioavailability in a High Arctic Fjord Influenced by Glacial Meltwater, NE Greenland. Front. Mar. Sci. 2017, 4, 176. [Google Scholar] [CrossRef] [Green Version]
- Schattenhofer, M.; Wulf, J.; Kostadinov, I.; Glöckner, F.O.; Zubkov, M.V.; Fuchs, B.M. Phylogenetic characterisation of picoplanktonic populations with high and low nucleic acid content in the North Atlantic Ocean. Syst. Appl. Microbiol. 2011, 34, 470–475. [Google Scholar] [CrossRef]
- Wexler, H.M. Bacteroides: The good, the bad, and the nitty-gritty. Clin. Microbiol. Rev. 2007, 20, 593–621. [Google Scholar] [CrossRef] [Green Version]
- Mojica, K.D.A.; Carlson, C.A.; Behrenfeld, M.J. Regulation of Low and High Nucleic Acid Fluorescent Heterotrophic Prokaryote Subpopulations and Links to Viral-Induced Mortality Within Natural Prokaryote-Virus Communities. Microb. Ecol. 2020, 79, 213–230. [Google Scholar] [CrossRef]
- Van Wambeke, F.; Catala, P.; Pujo-Pay, M.; Lebaron, P. Vertical and longitudinal gradients in HNA-LNA cell abundances and cytometric characteristics in the Mediterranean Sea. Biogeosciences 2011, 8, 1853–1863. [Google Scholar] [CrossRef] [Green Version]
- Eagon, R.G. Pseudomonas natriegens, a marine bacterium with a generation time of less than 10 minutes. J. Bacteriol. 1962, 83, 736–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, Y.Y.; Lee, C.W.; Bong, C.W.; Lim, J.H.; Narayanan, K.; Sim, E.U.H. Environmental control of Vibrio spp. abundance and community structure in tropical waters. FEMS Microbiol. Ecol. 2019, 95, fiz176. [Google Scholar] [CrossRef]
- Lauro, F.M.; McDougald, D.; Thomas, T.; Williams, T.J.; Egan, S.; Rice, S.; DeMaere, M.Z.; Ting, L.; Ertan, H.; Johnson, J.; et al. The genomic basis of trophic strategy in marine bacteria. Proc. Natl. Acad. Sci. USA 2009, 106, 15527–15533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westoby, M.; Nielsen, D.A.; Gillings, M.R.; Litchman, E.; Madin, J.S.; Paulsen, I.T.; Tetu, S.G. Cell size, genome size, and maximum growth rate are near-independent dimensions of ecological variation across bacteria and archaea. Ecol. Evol. 2021, 11, 3956–3976. [Google Scholar] [CrossRef]
- Lopez-Perez, M.; Haro-Moreno, J.M.; Coutinho, F.H.; Martinez-Garcia, M.; Rodriguez-Valera, F. The Evolutionary Success of the Marine Bacterium SAR11 Analyzed through a Metagenomic Perspective. Msystems 2020, 5, e00605-20. [Google Scholar] [CrossRef]
- Polovina, J.J.; Howell, E.A.; Abecassis, M. Ocean’s least productive waters are expanding. Geophys. Res. Lett. 2008, 35, L031745. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).