Impact of Invasive Weeds on the Diversity and Dissimilarity of Bird Communities in Forested Areas
Abstract
:1. Introduction
2. Materials and Methods
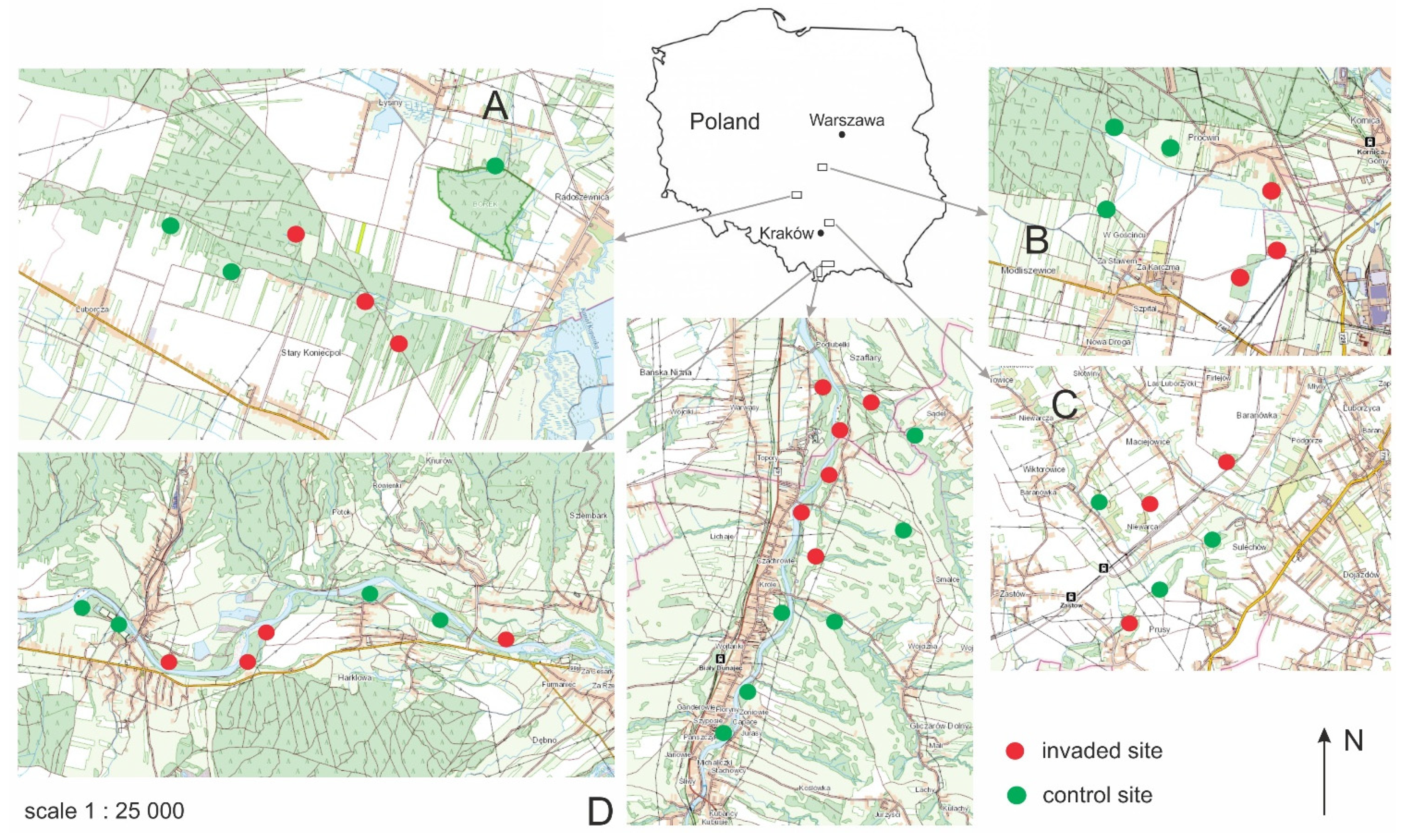
2.1. Study Sites
2.2. Bird Survey
2.3. Habitat Measurements
2.4. Data Analyses
3. Results
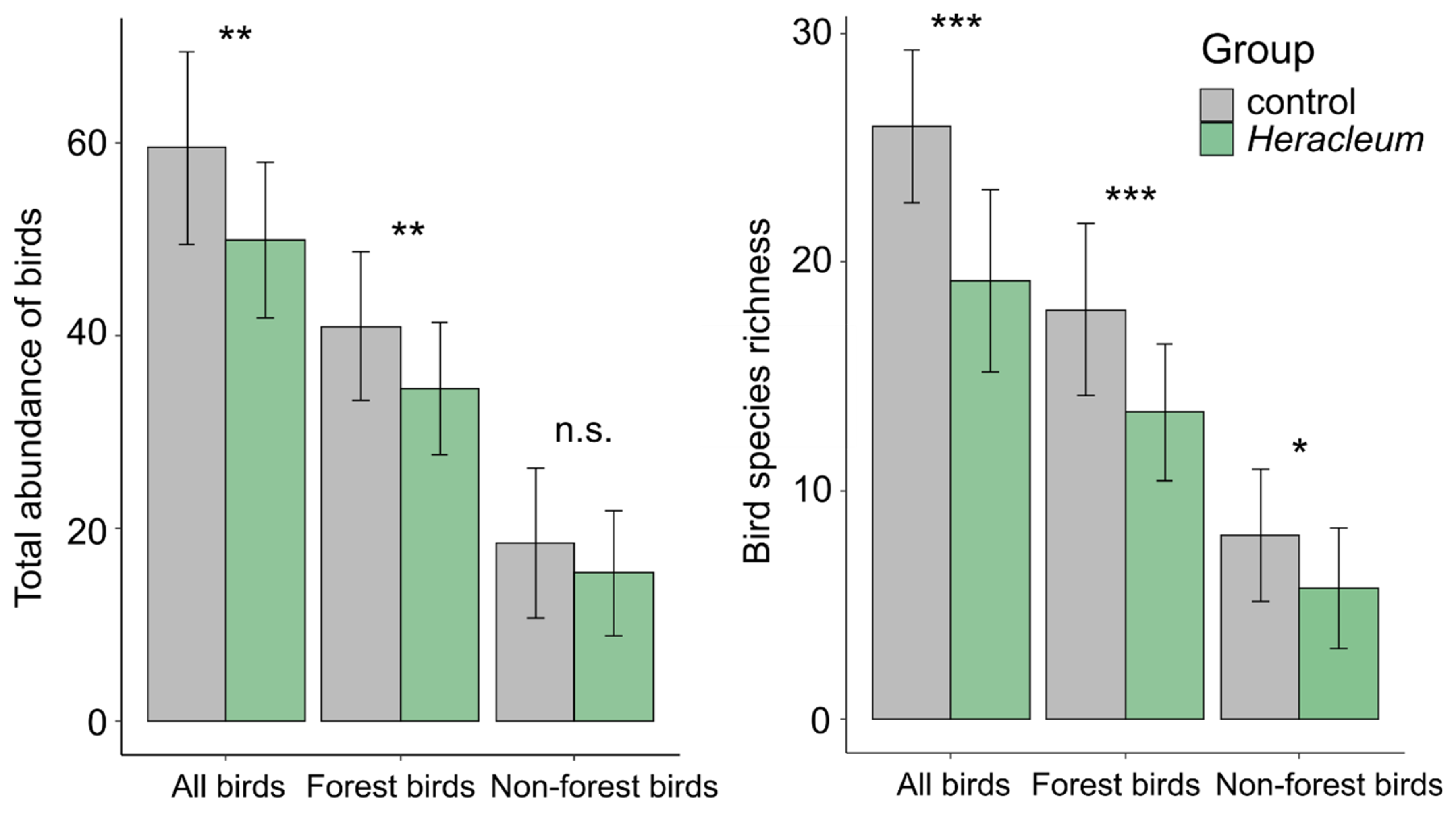
3.1. Impact of the Invading Weed Presence on the Bird Abundance and Species Richness
3.2. Impact of Invaded Area, Forest Area and Anthropogenic Area on Forest Birds
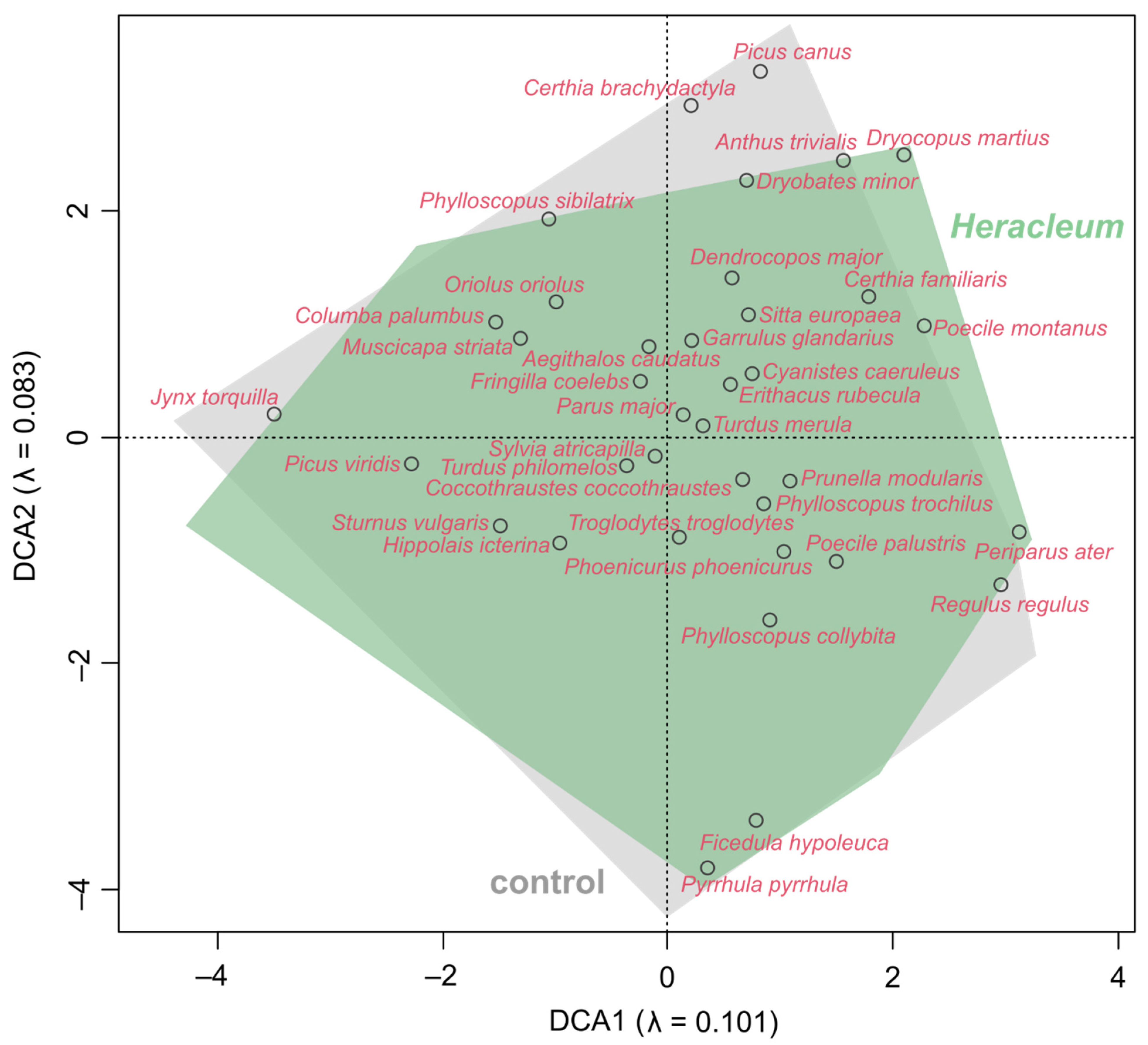
3.3. Impact of Invading Weeds on the Bird Communities’ Diversity and Dissimilarity
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Species | Bird Abundance | Species Habitat Traits, CWMs | Community | |||
|---|---|---|---|---|---|---|
| Total | Control Sites | Heracleum Sites | Control Sites | Heracleum Sites | ||
| Sturnus vulgaris | 162 | 86 | 76 | 3.79 | 3.79 | forest |
| Parus major | 139 | 79 | 60 | 4.00 | 4.00 | forest |
| Turdus pilaris | 125 | 73 | 52 | 1.68 | 1.37 | non-forest |
| Turdus merula | 139 | 70 | 69 | 3.00 | 2.84 | forest |
| Sylvia atricapilla | 150 | 69 | 81 | 3.58 | 3.63 | forest |
| Fringilla coelebs | 142 | 69 | 73 | 3.84 | 3.37 | forest |
| Turdus philomelos | 111 | 60 | 51 | 3.00 | 2.68 | forest |
| Sylvia communis | 139 | 52 | 87 | 1.00 | 1.00 | non-forest |
| Columba palumbus | 60 | 42 | 18 | 3.26 | 2.05 | forest |
| Carduelis carduelis | 87 | 42 | 45 | 1.00 | 1.00 | non-forest |
| Erithacus rubecula | 59 | 36 | 23 | 3.05 | 1.79 | forest |
| Dendrocopos major | 56 | 35 | 21 | 2.95 | 2.26 | forest |
| Phylloscopus collybita | 63 | 34 | 29 | 2.05 | 1.89 | forest |
| Garrulus glandarius | 59 | 33 | 26 | 3.58 | 2.95 | forest |
| Cyanistes caeruleus | 48 | 32 | 16 | 2.53 | 1.26 | forest |
| Alauda arvensis | 53 | 32 | 21 | 0.00 | 0.00 | non-forest |
| Coccothraustes coccothraustes | 49 | 29 | 20 | 2.21 | 1.58 | forest |
| Emberiza calandra | 38 | 29 | 9 | 0.00 | 0.00 | non-forest |
| Hippolais icterina | 51 | 26 | 25 | 1.00 | 0.00 | forest |
| Phasianus colchicus | 43 | 26 | 17 | 3.00 | 2.68 | non-forest |
| Troglodytes troglodytes | 38 | 24 | 14 | 3.21 | 1.68 | forest |
| Phylloscopus trochilus | 52 | 23 | 29 | 2.05 | 2.21 | forest |
| Sitta europaea | 26 | 19 | 7 | 0.58 | 0.53 | forest |
| Lanius collurio | 36 | 19 | 17 | 3.16 | 1.26 | non-forest |
| Saxicola rubetra | 33 | 19 | 14 | 0.00 | 0.00 | non-forest |
| Emberiza citrinella | 33 | 19 | 14 | 1.00 | 1.00 | non-forest |
| Oriolus oriolus | 34 | 18 | 16 | 1.58 | 1.16 | forest |
| Pica pica | 26 | 17 | 9 | 1.05 | 0.42 | non-forest |
| Streptopelia decaocto | 17 | 15 | 2 | 0.84 | 0.21 | non-forest |
| Cuculus canorus | 18 | 15 | 3 | 1.37 | 0.32 | non-forest |
| Phylloscopus sibilatrix | 24 | 14 | 10 | 2.05 | 1.26 | forest |
| Aegithalos caudatus | 19 | 13 | 6 | 0.74 | 0.32 | forest |
| Motacilla cinerea | 19 | 12 | 7 | 0.74 | 0.42 | non-forest |
| Corvus cornix | 13 | 11 | 2 | 0.74 | 0.10 | non-forest |
| Motacilla alba | 21 | 11 | 10 | 0.00 | 0.00 | non-forest |
| Sylvia borin | 14 | 9 | 5 | 0.63 | 0.32 | non-forest |
| Jynx torquilla | 12 | 8 | 4 | 1.68 | 0.84 | forest |
| Picus viridis | 11 | 8 | 3 | 1.68 | 0.63 | forest |
| Dryobates minor | 11 | 8 | 3 | 1.26 | 0.47 | forest |
| Passer montanus | 8 | 8 | 0 | 0.32 | 0.00 | non-forest |
| Linaria cannabina | 10 | 8 | 2 | 0.42 | 0.10 | non-forest |
| Ficedula hypoleuca | 11 | 7 | 4 | 0.84 | 0.84 | forest |
| Anthus trivialis | 9 | 7 | 2 | 0.79 | 0.32 | forest |
| Pyrrhula pyrrhula | 9 | 7 | 2 | 0.63 | 0.16 | forest |
| Muscicapa striata | 10 | 6 | 4 | 0.21 | 0.10 | forest |
| Phoenicurus phoenicurus | 9 | 6 | 3 | 0.00 | 0.00 | forest |
| Prunella modularis | 8 | 6 | 2 | 0.21 | 0.42 | forest |
| Regulus regulus | 12 | 6 | 6 | 0.00 | 0.00 | forest |
| Corvus corax | 7 | 6 | 1 | 0.84 | 0.63 | non-forest |
| Lullula arborea | 7 | 6 | 1 | 0.63 | 0.47 | non-forest |
| Luscinia luscinia | 10 | 6 | 4 | 0.00 | 0.00 | non-forest |
| Saxicola rubicola | 9 | 6 | 3 | 1.26 | 0.42 | non-forest |
| Erythrina erythrina | 14 | 6 | 8 | 0.00 | 0.00 | non-forest |
| Poecile montanus | 9 | 5 | 4 | 0.63 | 0.32 | forest |
| Motacilla flava | 5 | 5 | 0 | 0.00 | 0.00 | non-forest |
| Acrocephalus palustris | 13 | 4 | 9 | 0.00 | 0.00 | non-forest |
| Sylvia curruca | 3 | 3 | 0 | 0.32 | 0.00 | non-forest |
| Anthus pratensis | 3 | 3 | 0 | 0.00 | 0.00 | non-forest |
| Serinus serinus | 5 | 3 | 2 | 0.00 | 0.00 | non-forest |
| Picus canus | 2 | 2 | 0 | 0.26 | 0.00 | forest |
| Dryocopus martius | 3 | 2 | 1 | 0.42 | 0.21 | forest |
| Periparus ater | 4 | 2 | 2 | 0.21 | 0.21 | forest |
| Certhia familiaris | 4 | 2 | 2 | 0.00 | 0.00 | forest |
| Acrocephalus scirpaceus | 4 | 2 | 2 | 0.42 | 0.42 | non-forest |
| Chloris chloris | 6 | 2 | 4 | 0.00 | 0.00 | non-forest |
| Poecile palustris | 2 | 1 | 1 | 0.16 | 0.16 | forest |
| Certhia brachydactyla | 1 | 1 | 0 | 0.00 | 0.00 | forest |
| Locustella naevia | 1 | 1 | 0 | 0.21 | 0.00 | non-forest |
| Emberiza hortulana | 1 | 1 | 0 | 0.00 | 0.00 | non-forest |
| Sylvia nisoria | 2 | 0 | 2 | 0.00 | 0.00 | non-forest |
| Habitat Variables | Control | Heracleum | χ2 | df | p |
|---|---|---|---|---|---|
| Mean ± SD, Range (ha *) | Mean ± SD, Range (ha *) | ||||
| 100 m Radius | |||||
| Forest area | 1.360 ± 0.86, 0.405–2.650 | 1.220 ± 0.78, 0.401–2.790 | 0.002 | 1 | 0.961 |
| Number of patches | 8.380 ± 4.41, 3–16.000 | 7.860 ± 3.35, 3–16.000 | 0.015 | 1 | 0.903 |
| Open area | 1.140 ± 0.82, 0–2.290 | 0.639 ± 0.68, 0–2.010 | 2.805 | 1 | 0.094 |
| Overgrown area | 1.680 ± 0.68, 0.850–2.650 | 1.450 ± 0.67, 0.554–2.790 | 0.989 | 1 | 0.319 |
| Anthropogenic area Invasion area | 0.150 ± 0.23, 0–0.672 - | 0.468 ± 0.67, 0–2.070 0.455 ± 0.38, 0.153–1.510 | 2.103 - | 1 | 0.147 - |
Appendix B

References
- Vitousek, P.M.; D’Antonio, C.M.; Loope, L.L.; Westbrooks, R. Biological invasions as global environmental change. Am. Sci. 1996, 84, 468–478. [Google Scholar]
- Ricciardi, A.; Blackburn, T.M.; Carlton, J.T.; Dick, J.T.A.; Hulme, P.E.; Iacarella, J.C.; Jeschke, J.M.; Liebhold, A.M.; Lockwood, J.L.; MacIsaac, H.J.; et al. Invasion Science: A Horizon Scan of Emerging Challenges and Opportunities. Trends Ecol. Evol. 2017, 32, 464–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pyšek, P.; Hulme, P.E.; Simberloff, D.; Bacher, S.; Blackburn, T.M.; Carlton, J.T.; Dawson, W.; Essl, F.; Foxcroft, L.C.; Genovesi, P.; et al. Scientists’ warning on invasive alien species. Biol. Rev. 2020, 95, 1511–1534. [Google Scholar] [CrossRef] [PubMed]
- Ehrenfeld, J.G. Ecosystem consequences of biological invasions. Ann. Rev. Ecol. Evol. Syst. 2010, 41, 59–80. [Google Scholar] [CrossRef] [Green Version]
- Vila, M.; Espinar, J.L.; Hejda, M.; Hulme, P.E.; Jarošík, V.; Maron, J.L.; Pergl, J.; Schaffner, U.; Sun, Y.; Pyšek, P. Ecological impacts of invasive alien plants: A meta-analysis of their effects on species, communities and ecosystems. Ecol. Lett. 2011, 14, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Schirmel, J.; Bundschuh, M.; Entling, M.H.; Kowarik, I.; Buchholz, S. Impacts of invasive plants on resident animals across ecosystems, taxa, and feeding types: A global assessment. Global Chang. Biol. 2016, 22, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Buckley, Y.M. The role of research for integrated management of invasive species, invaded landscapes and communities. J. Appl. Ecol. 2008, 45, 397–402. [Google Scholar] [CrossRef]
- Gaskin, J.F.; Espeland, E.; Johnson, C.D.; Larson, D.L.; Mangold, J.M.; McGee, R.A.; Milner, C.; Paudel, S.; Pearson, D.E.; Perkins, L.B.; et al. Managing invasive plants on Great Plains grasslands: A discussion of current challenges. Rang. Ecol. Manag. 2021, 78, 235–249. [Google Scholar] [CrossRef]
- Elton, C.S. The Ecology of Invasions by Animals and Plants; Springer: Boston, MA, USA, 1958. [Google Scholar]
- Case, T.K. Invasion resistance arises in strongly interacting species-rich model competition communities. Proc. Nat. Acad. Sci. USA 1990, 87, 9610–9614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Law, R.; Morton, R.D. Permanence and the assembly of ecological communities. Ecology 1996, 77, 762–775. [Google Scholar] [CrossRef]
- Rejmánek, M. Species richness and resistance to invasion. In Biodiversity and Ecosystem Processes in Tropical Forests; Orians, G.G., Dirzo, R., Cushman, J.H., Eds.; Springer: Berlin, Germany, 1996; pp. 153–172. [Google Scholar]
- Tilman, D. Community invisibility, recruitment limitation and grassland biodiversity. Ecology 1997, 78, 81–92. [Google Scholar] [CrossRef]
- Firn, J.; Price, J.N.; Whalley, R.D. Using strategically applied grazing to manage invasive alien plants in novel grasslands. Ecol. Process 2013, 2, 26. [Google Scholar] [CrossRef] [Green Version]
- Liebhold, A.M.; Brockerhoff, E.G.; Kalisz, S.; Nuñez, M.A.; Wardle, D.A.; Wingfield, M.J. Biological invasions in forest ecosystems. Biol. Invasions 2017, 19, 3437–3458. [Google Scholar] [CrossRef]
- Hanzelka, J.; Reif, J. Effects of vegetation structure on the diversity of breeding bird communities in forest stands of non-native black pine (Pinus nigra A.) and black locust (Robinia pseudoacacia L.) in the Czech Republic. For. Ecol. Manag. 2016, 379, 102–113. [Google Scholar] [CrossRef]
- Hejda, M.; Hanzelka, J.; Kadlec, T.; Štrobl, M.; Pyšek, P.; Reif, J. Impacts of an invasive tree across trophic levels: Species richness, community composition and resident species’ traits. Divers. Distrib. 2017, 23, 997–1007. [Google Scholar] [CrossRef]
- Kroftová, M.; Reif, J. Management implications of bird responses to variation in non-native/native tree ratios within central European forest stands. For. Ecol. Manag. 2017, 391, 330–337. [Google Scholar] [CrossRef]
- Schneider, S.C.; Miller, J.R. Responses of avian communities to invasive vegetation in urban forest fragments. Condor 2014, 116, 459–471. [Google Scholar] [CrossRef]
- Aravind, N.A.; Rao, D.; Ganeshaiah, K.N.; Shaanker, R.U.; Poulsen, J.G. Impact of the invasive plant, Lantana camara, on bird assemblages at Malé Mahadeshwara Reserve Forest, South India. Trop. Ecol. 2010, 51, 325–338. [Google Scholar]
- Grzędzicka, E.; Reif, J. Impacts of an invasive plant on bird communities differ along a habitat gradient. GECCO 2020, 23, e01150. [Google Scholar] [CrossRef]
- Grzędzicka, E.; Reif, J. The impact of Sosnowsky’s Hogweed on feeding guilds of birds. J. Ornithol. 2021, 162, 1115–1128. [Google Scholar] [CrossRef]
- Wade, T.G.; Riitters, K.H.; Wickham, J.D.; Jones, K.B. Distribution and causes of global forest fragmentation. Conserv. Ecol. 2003, 7, 7. [Google Scholar] [CrossRef]
- Sala, O.E.; Chapin, F.S., III; Armesto, J.J.; Berlow, E.; Bloomfield, J.; Dirzo, R.; Huber-Sanwald, E.; Huenneke, L.F.; Jackson, R.B.; Kinzig, A.; et al. Global biodiversity scenarios for the year 2100. Science 2000, 287, 1770–1774. [Google Scholar] [CrossRef] [PubMed]
- Montanarella, L.; Scholes, R.; Brainich, A. The IPBES Assessment Report on Land Degradation and Restoration; IPBES: Bonn, Germany, 2018. [Google Scholar]
- Nielsen, C.; Ravn, H.P.; Nentwig, W.; Wade, M. (Eds.) The Giant Hogweed Best Practice Manual. Guidelines for the Management and Control of an Invasive Weed in Europe; Forest & Landscape Denmark: Hoersholm, Denmark, 2005; p. 44. [Google Scholar]
- Henry, P.; Le Lay, G.; Goudet, J.; Guisan, A.; Jahodová, S.; Besnard, G. Reduced genetic diversity, increased isolation and multiple introductions of invasive giant hogweed in the western Swiss Alps. Mol Ecol. 2009, 18, 2819–2831. [Google Scholar] [CrossRef] [PubMed]
- Chadin, I.; Dalke, I.; Zakhozhiy, I.; Malyshev, R.; Madi, E.; Kuzivanova, O.; Kirillov, D.; Elsakov, V. Distribution of the invasive plant species Heracleum sosnowskyi Manden. in the Komi Republic (Russia). PhytoKeys 2017, 77, 71–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renčo, M.; Jurová, J.; Gömöryová, E.; Čerevková, A. Long-Term Giant Hogweed Invasion Contributes to the Structural Changes of Soil Nematofauna. Plants 2021, 10, 2103. [Google Scholar] [CrossRef] [PubMed]
- McGill, B.; Enquist, B.; Weiher, E.; Westoby, M. Rebuilding community ecology from functional traits. Trends Ecol. Evol. 2006, 21, 178–185. [Google Scholar] [CrossRef]
- Bellard, C.; Bertelsmeier, C.; Leadley, P.; Thuiller, W.; Courchamp, F. Impacts of climate change on the future of biodiversity. Ecol. Lett. 2012, 15, 365–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devictor, V.; Mouillot, D.; Meynard, C.; Jiguet, F.; Thuiller, W.; Mouquet, N. Spatial mismatch and congruence between taxonomic, phylogenetic and functional diversity: The need for integrative conservation strategies in a changing world. Ecol. Lett. 2010, 13, 1030–1040. [Google Scholar] [CrossRef] [PubMed]
- Cadotte, M.W.; Carscadden, K.; Mirotchnick, N. Beyond species: Functional diversity and the maintenance of ecological processes and services. J. Appl. Ecol. 2011, 48, 1079–1087. [Google Scholar] [CrossRef]
- Carmona, C.P.; de Bello, F.; Mason, N.W.H.; Lepš, J. Traits without borders: Integrating functional diversity across scales. Trends Ecol. Evol. 2016, 31, 382–394. [Google Scholar] [CrossRef]
- Tilman, D.; Knops, J.; Wedin, D.; Reich, P.; Ritchie, M.; Siemann, E. The Influence of functional diversity and composition on ecosystem processes. Science 1997, 277, 1300–1302. [Google Scholar] [CrossRef] [Green Version]
- Newbold, T.; Hudson, L.N.; Arnell, A.P.; Contu, S.; De Palma, A.; Ferrier, S.; Hill, S.L.; Hoskins, A.J.; Lysenko, I.; Phillips, H.R.; et al. Has land use pushed terrestrial biodiversity beyond the planetary boundary? A global assessment. Science 2016, 353, 288–291. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, B.F.; Machac, A.; Costa, G.C.; Brooks, T.M.; Davidson, A.D.; Rondinini, C.; Graham, C.H. Species and functional diversity accumulate differently in mammals. Global Ecol. Biogeogr. 2016, 25, 1119–1130. [Google Scholar] [CrossRef]
- Barnagaud, J.; Kissling, W.D.; Constantinos, T.; Fisikopoulos, V.; Villéger, S.; Sekercioglu, C.H. Biogeographical, environmental and anthropogenic determinants of global patterns in bird taxonomic and trait turnover. Global Ecol. Biogeogr. 2017, 26, 1190–1200. [Google Scholar] [CrossRef]
- Le Provost, G.; Badenhausser, I.; Le Bagousse-Pinguet, Y.; Clough, Y.; Henckel, L.; Violle, C.; Bretagnolle, V.; Roncoroni, M.; Manning, P.; Gross, N. Land-use history impacts functional diversity across multiple trophic groups. Proc. Natl. Acad. Sci. USA 2020, 117, 1573–1579. [Google Scholar] [CrossRef] [PubMed]
- Hanowski, J.M.; Niemi, G.J. Experimental Design Considerations for Establishing an Off-Road, Habitat-specific Bird Monitoring Program Using Point Counts. In USDA Forest Service General Technical Report PSW-GTR-149; US Department of Agriculture, Forest Service, Pacific Southwest Research Station: Albany, NY, USA, 1995. [Google Scholar]
- Jiguet, F. Method-learning caused first-time observer effect in a newly-started breeding bird survey. Hous. Theor. Soc. 2009, 56, 253–258. [Google Scholar] [CrossRef]
- Deikumah, J.P.; Kwafo, R.; Konadu, V.A. Land use types influenced avian assemblage structure in a forest-agriculture landscape in Ghana. Ecol. Evol. 2017, 7, 8685–8697. [Google Scholar] [CrossRef] [PubMed]
- Lüdecke, D.; Makowski, D.; Waggoner, P.; Patil, I. Performance: Assessment of Regression Models Performance. Available online: https://CRAN.R-project.org/package=performance/ (accessed on 13 September 2021).
- Kim, J.H. Multicollinearity and misleading statistical results. Korean J. Anesthesiol. 2019, 72, 558–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wickham, H.; François, R.; Henry, L.; Müller, K. Dplyr: A Grammar of Data Manipulation. R Package Version 1.0.5. Available online: https://CRAN.R-project.org/package=dplyr (accessed on 11 March 2021).
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Soft. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Calcagno, V. Glmulti: Model Selection and Multimodel Inference Made Easy. R Package Version 1.0.8. Available online: https://CRAN.R-project.org/package=glmulti/ (accessed on 7 April 2021).
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.5-7. Available online: https://CRAN.R-project.org/package=vegan (accessed on 8 November 2021).
- Baselga, A.; Orme, D.; Villeger, S.; De Bortoli, J.; Leprieur, F.; Logez, M. Betapart: Partitioning Beta Diversity into Turnover and Nestedness Components. R Package Version 1.5.4. Available online: https://CRAN.R-project.org/package=betapart (accessed on 8 November 2021).
- Gower, J.C. Some distance properties of latent root and vector methods used in multivariate analysis. Biometrika 1966, 53, 325–338. [Google Scholar] [CrossRef]
- Laliberté, E.; Legendre, P. A distance-based framework for measuring functional diversity from multiple traits. Ecology 2010, 91, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Laliberté, E.; Legendre, P.; Shipley, B.; Laliberté, M.E. Package ‘FD’. Measuring Functional Diversity from Multiple Traits, and Other Tools for Functional Ecology. R Package Version 1.0. Available online: https://cran.r-project.org/web/packages/FD/FD.pdf (accessed on 20 December 2021).
- Lavorel, S.; Grigulis, K.; McIntyre, S.; Williams, N.S.G.; Garden, D.; Dorrough, J.; Berman, S.; Quétier, F.; Thébault, A.; Bonis, A. Assessing functional diversity in the field—Methodology matters! Funct. Ecol. 2008, 22, 134–147. [Google Scholar] [CrossRef]
- Mouillot, D.; Mason, W.H.N.; Dumay, O.; Wilson, J.B. Functional regularity: A neglected aspect of functional diversity. Oecologia 2005, 142, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Villéger, S.; Mason, N.W.; Mouillot, D. New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology 2008, 89, 2290–2301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Nelson, S.B.; Coon, J.J.; Duchardt, C.J.; Fischer, J.D.; Halsey, S.J.; Kranz, A.J.; Parker, C.M.; Schneider, S.C.; Swartz, T.M.; Miller, J.M. Patterns and mechanisms of invasive plant impacts on North American birds: A systematic review. Biol. Invasions 2017, 19, 1547–1563. [Google Scholar] [CrossRef]
- Muñoz-Pedreros, A.; González-Urrutia, M.; Encina-Montoya, F.; Norambuena, H.V. Effects of vegetation strata and human disturbance on bird diversity in green areas in a city in southern Chile. Avian Res. 2018, 9, 38. [Google Scholar] [CrossRef] [Green Version]
- Lyashevska, O.; Farnsworth, K.D. How many dimensions of biodiversity do we need? Ecol. Indic. 2012, 18, 485–492. [Google Scholar] [CrossRef]
- Fusco, J.; Walker, E.; Papaïx, J.; Debolini, M.; Bondeau, A.; Barnagaud, J.-Y. Land Use Changes Threaten Bird Taxonomic and Functional Diversity Across the Mediterranean Basin: A Spatial Analysis to Prioritize Monitoring for Conservation. Front. Ecol. Evol. 2021, 9, 612356. [Google Scholar] [CrossRef]
- Dyderski, M.K.; Jagodziński, A.M. Impact of Invasive Tree Species on Natural Regeneration Species Composition, Diversity, and Density. Forests 2020, 11, 456. [Google Scholar] [CrossRef] [Green Version]






| Dependent Variable: Abundance of Forest Birds | |||
|---|---|---|---|
| Habitat Variables | Estimate ± SE | t | p |
| (Intercept) | 33.86 ± 3.46 | 9.78 | <0.001 |
| Forest area | 4.44 ± 1.55 | 2.87 | 0.006 |
| Anthropogenic area | 21.41 ± 6.70 | 3.19 | 0.003 |
| Invasion area | 20.51 ± 7.66 | 2.68 | 0.010 |
| Forest area × anthropogenic area | −19.49 ± 6.12 | −3.19 | 0.003 |
| Forest area × invasion area | −15.79 ± 5.30 | −2.98 | 0.005 |
| Anthropogenic area × invasion area | −38.82 ± 14.52 | −2.67 | 0.010 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grzędzicka, E. Impact of Invasive Weeds on the Diversity and Dissimilarity of Bird Communities in Forested Areas. Diversity 2022, 14, 229. https://doi.org/10.3390/d14030229
Grzędzicka E. Impact of Invasive Weeds on the Diversity and Dissimilarity of Bird Communities in Forested Areas. Diversity. 2022; 14(3):229. https://doi.org/10.3390/d14030229
Chicago/Turabian StyleGrzędzicka, Emilia. 2022. "Impact of Invasive Weeds on the Diversity and Dissimilarity of Bird Communities in Forested Areas" Diversity 14, no. 3: 229. https://doi.org/10.3390/d14030229
APA StyleGrzędzicka, E. (2022). Impact of Invasive Weeds on the Diversity and Dissimilarity of Bird Communities in Forested Areas. Diversity, 14(3), 229. https://doi.org/10.3390/d14030229






