Composition, Distribution, and Factors Affecting Invasive Plants in Grasslands of Guizhou Province of Southwest China
Abstract
:1. Introduction
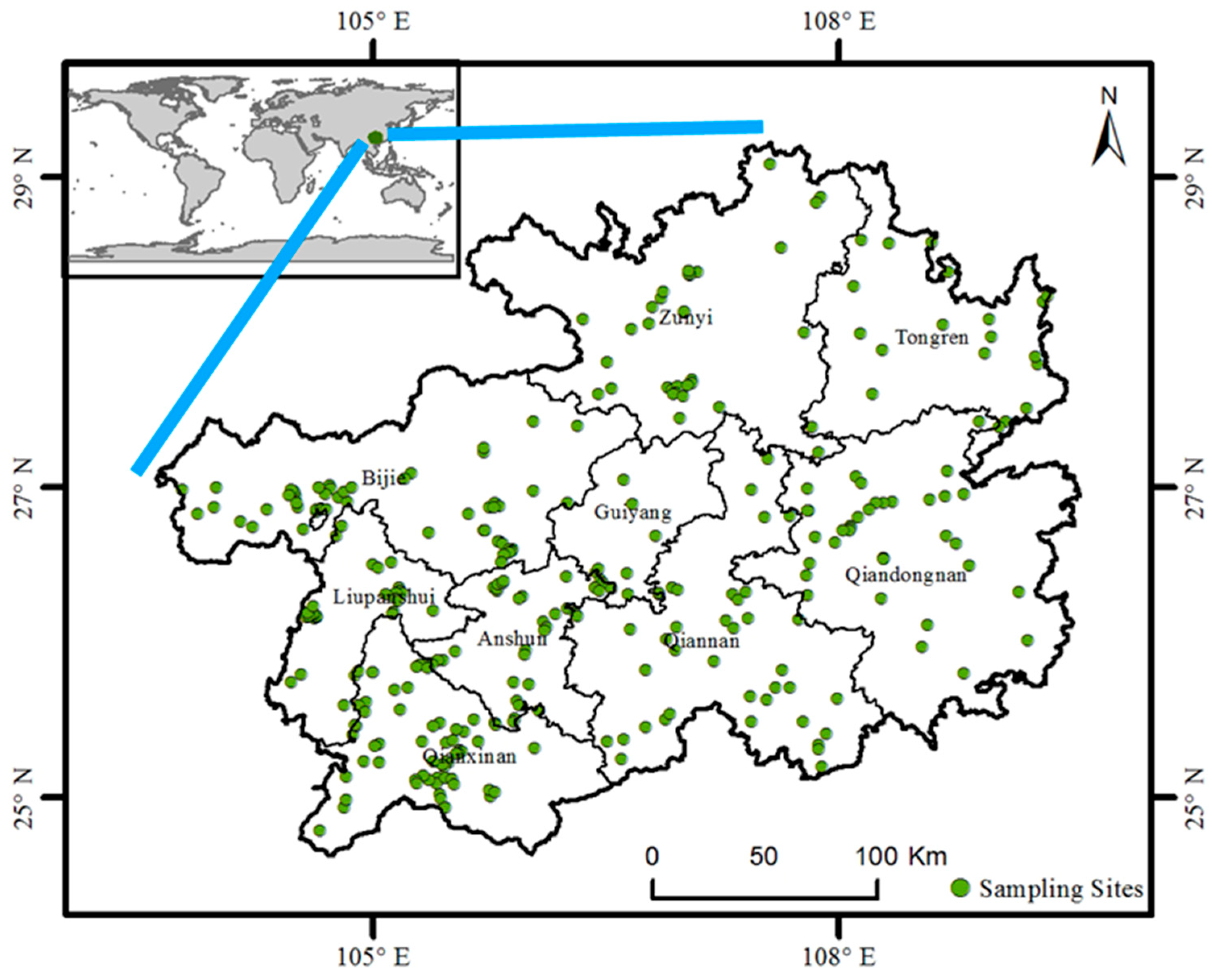
2. Materials and Methods
3. Results
3.1. Catalogue of Invasive Plants
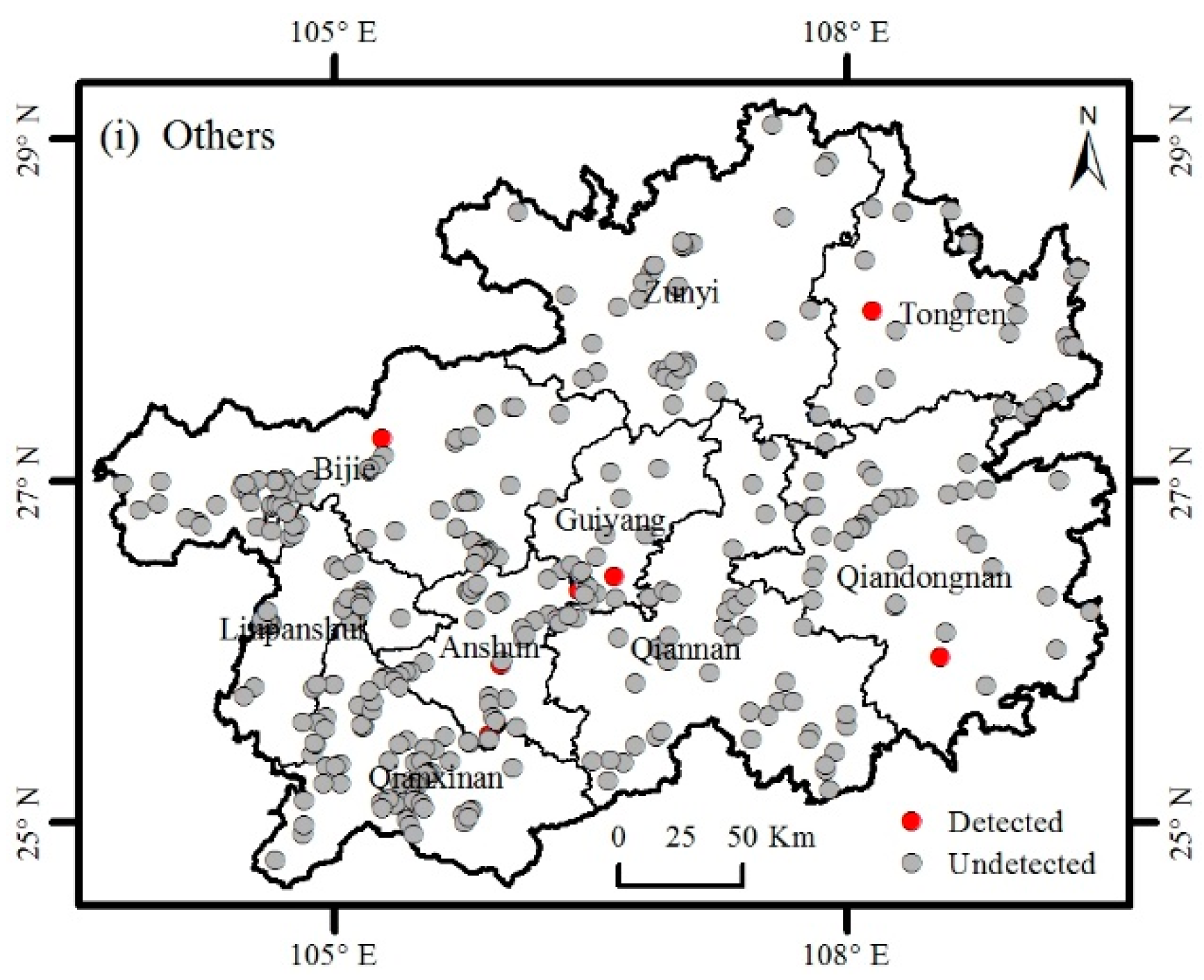
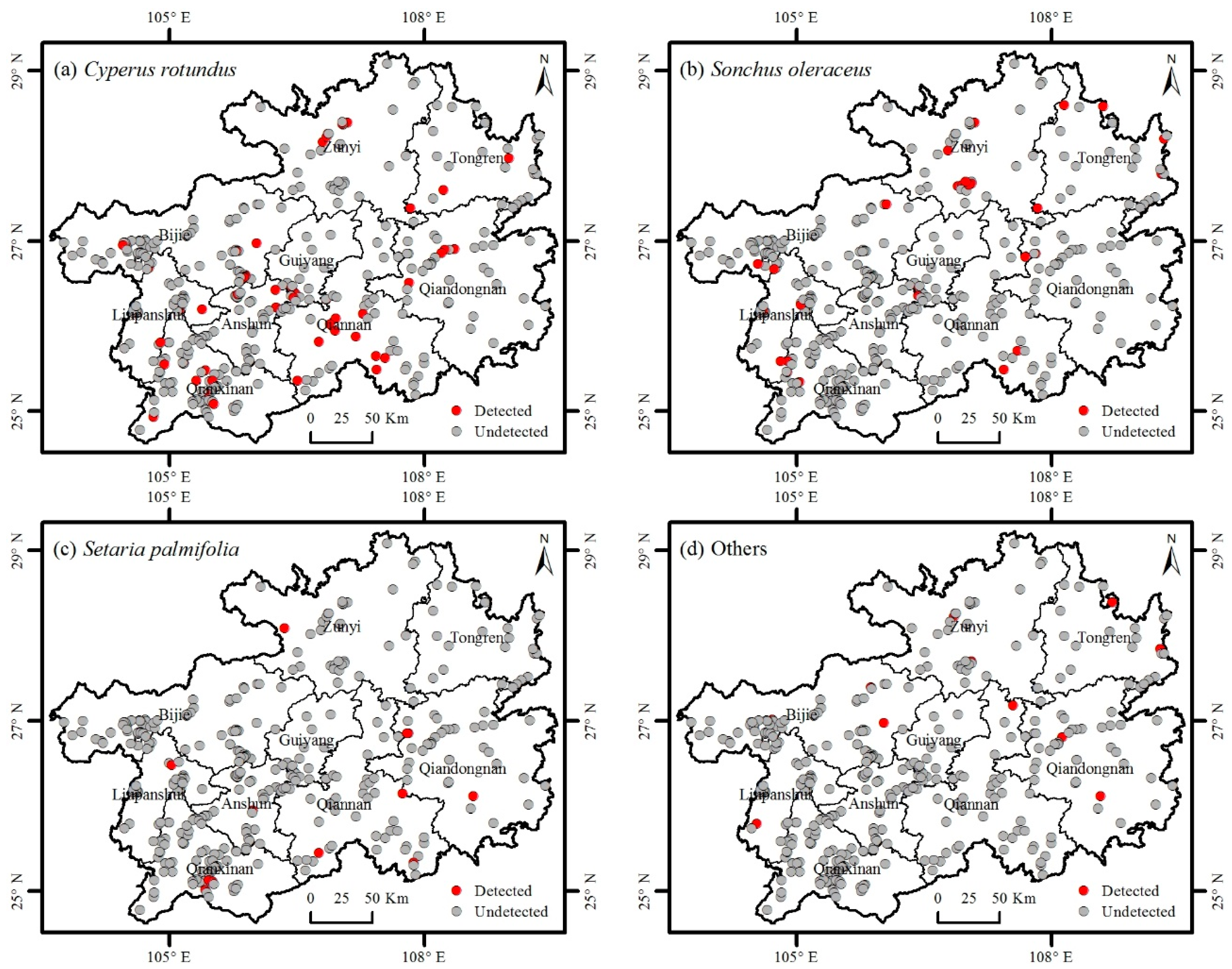
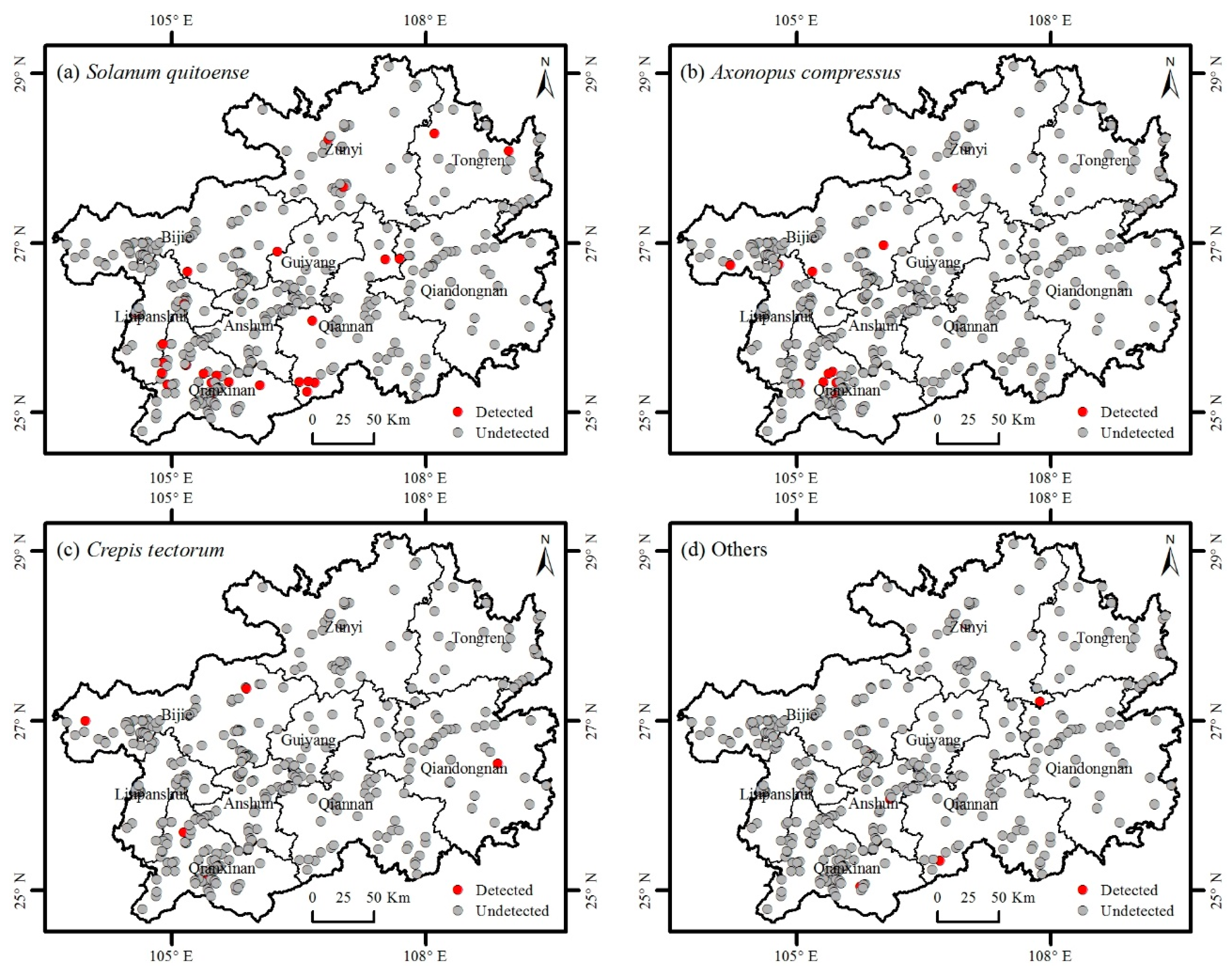
3.2. Distribution of Invasive Plants
3.3. Factors Affecting the Distribution of Invasive Plants
4. Discussion
4.1. Grasslands in Guizhou Province Are Severely Invaded by Non-Native Species
4.2. Distribution Pattern, Affecting Factors, and Management Implications
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elton, C.S.; Simberloff, D.; Ricciardi, A. The Ecology of Invasions by Animals and Plants, 2nd ed.; Springer Nature Switzerland AG: Cham, Switzerland, 2020. [Google Scholar]
- Pejchar, L.; Mooney, H.A. Invasive species, ecosystem services and human well-being. Trends Ecol. Evol. 2009, 24, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.T. Invasive species:“back-seat drivers” of ecosystem change? Biol. Invasions 2012, 14, 1295–1304. [Google Scholar] [CrossRef]
- Walsh, J.R.; Carpenter, S.R.; Vander Zanden, M.J. Invasive species triggers a massive loss of ecosystem services through a trophic cascade. PNAS 2016, 113, 4081–4085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hejda, M.; Pyšek, P.; Jarošík, V. Impact of invasive plants on the species richness, diversity and composition of invaded communities. J. Ecol. 2009, 97, 393–403. [Google Scholar] [CrossRef]
- McNeely, J. Invasive species: A costly catastrophe for native biodiversity. Land Use Water Resour. Res. 2001, 2, 1–10. [Google Scholar] [CrossRef]
- Hejda, M.; Sadlo, J.; Kutlvasr, J.; Petrik, P.; Vitkova, M.; Vojik, M.; Pysek, P.; Pergl, J. Impact of invasive and native dominants on species richness and diversity of plant communities. Preslia 2021, 93, 181–201. [Google Scholar] [CrossRef]
- Morelli, T.L.; Brown-Lima, C.J.; Allen, J.M.; Beaury, E.M.; Fusco, E.J.; Barker-Plotkin, A.; Laginhas, B.B.; Quirion, B.R.; Griffin, B.; McLaughlin, B.; et al. Translational invasion ecology: Bridging research and practice to address one of the greatest threats to biodiversity. Biol. Invasions 2021, 23, 3323–3335. [Google Scholar] [CrossRef]
- Perrino, E.V.; Wagensommer, R.P. Crop Wild Relatives (CWRs) threatened and endemic to Italy: Urgent actions for protection and use. Biology 2022, 11, 193. [Google Scholar] [CrossRef]
- Hoffmann, B.D.; Broadhurst, L.M. The economic cost of managing invasive species in Australia. NeoBiota 2016, 31, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Epanchin-Niell, R.S. Economics of invasive species policy and management. Biol. Invasions 2017, 19, 3333–3354. [Google Scholar] [CrossRef] [Green Version]
- Mehta, S.V.; Haight, R.G.; Homans, F.R.; Polasky, S.; Venette, R.C. Optimal detection and control strategies for invasive species management. Ecol. Econ. 2007, 61, 237–245. [Google Scholar] [CrossRef]
- Larson, D.L.; Phillips-Mao, L.; Quiram, G.; Sharpe, L.; Stark, R.; Sugita, S.; Weiler, A. A framework for sustainable invasive species management: Environmental, social, and economic objectives. J. Environ. Manag. 2011, 92, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Moon, K.; Blackman, D.A.; Brewer, T.D. Understanding and integrating knowledge to improve invasive species management. Biol. Invasions 2015, 17, 2675–2689. [Google Scholar] [CrossRef]
- Stinca, A.; Musarella, C.M.; Rosati, L.; Laface, V.L.A.; Licht, W.; Fanfarillo, E.; Wagensommer, R.P.; Galasso, G.; Fascetti, S.; Esposito, A.; et al. Italian vascular flora: New findings, updates and exploration of floristic similarities between regions. Diversity 2021, 13, 600. [Google Scholar] [CrossRef]
- Asner, G.P.; Elmore, A.J.; Olander, L.P.; Martin, R.E.; Harris, A.T. Grazing systems, ecosystem responses, and global change. Annu. Rev. Env. Resour. 2004, 29, 261–299. [Google Scholar] [CrossRef]
- Gong, P.; Wang, J.; Yu, L.; Zhao, Y.; Zhao, Y.; Liang, L.; Niu, Z.; Huang, X.; Fu, H.; Liu, S.; et al. Finer resolution observation and monitoring of global land cover: First mapping results with Landsat TM and ETM+ data. Int. J. Remote Sens. 2013, 34, 2607–2654. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Wang, J.; Gong, P. Improving 30 m global land-cover map FROM-GLC with time series MODIS and auxiliary data sets: A segmentation-based approach. Int. J. Remote Sens. 2013, 34, 5851–5867. [Google Scholar] [CrossRef]
- Funk, J.L.; Vitousek, P.M. Resource-use efficiency and plant invasion in low-resource systems. Nature 2007, 446, 1079–1081. [Google Scholar] [CrossRef]
- Muirhead, J.R.; Minton, M.S.; Miller, W.A.; Ruiz, G.M. Projected effects of the Panama Canal expansion on shipping traffic and biological invasions. Divers. Distrib. 2015, 21, 75–87. [Google Scholar] [CrossRef]
- Yan, X.; Zhenyu, L.; Gregg, W.P.; Dianmo, L. Invasive species in China—an overview. Biodivers. Conserv. 2001, 10, 1317–1341. [Google Scholar] [CrossRef]
- Gross, K.L.; Mittelbach, G.G.; Reynolds, H.L. Grassland invasibility and diversity: Responses to nutrients, seed input, and disturbance. Ecology 2005, 86, 476–486. [Google Scholar] [CrossRef]
- Rickey, M.A.; Anderson, R.C. Effects of nitrogen addition on the invasive grass Phragmites australis and a native competitor Spartina pectinata. J. Appl. Ecol. 2004, 41, 888–896. [Google Scholar] [CrossRef]
- Chytrý, M.; Jarošík, V.; Pyšek, P.; Hájek, O.; Knollová, I.; Tichý, L.; Danihelka, J. Separating habitat invasibility by alien plants from the actual level of invasion. Ecology 2008, 89, 1541–1553. [Google Scholar] [CrossRef] [Green Version]
- Lemke, A.; Kowarik, I.; von der Lippe, M. How traffic facilitates population expansion of invasive species along roads: The case of common ragweed in Germany. J. Appl. Ecol. 2019, 56, 413–422. [Google Scholar] [CrossRef]
- Jenkins, P.T.; Mooney, H.A. The United States, China, and invasive species: Present status and future prospects. Biol. Invasions 2006, 8, 1589–1593. [Google Scholar] [CrossRef]
- Weber, E.; Sun, S.-G.; Li, B. Invasive alien plants in China: Diversity and ecological insights. Biol. Invasions 2008, 10, 1411–1429. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Liang, S.; Liu, F.; Wang, R.; Dong, M. Invasive alien plant species in China: Regional distribution patterns. Divers. Distrib. 2005, 11, 341–347. [Google Scholar] [CrossRef]
- Ding, J.Q.; Mack, R.N.; Lu, P.; Ren, M.X.; Huang, H.W. China’s booming economy is sparking and accelerating biological invasions. Bioscience 2008, 58, 317–324. [Google Scholar] [CrossRef]
- Yan, X.; Liu, Q.; Shou, H.; Zeng, X.; Zhang, Y.; Chen, L.; Liu, Y.; Ma, H.; Qi, S.; Ma, J. The categorization and analysis on the geographic distribution patterns of Chinese alien invasive plants. Biodivers. Sci. 2014, 22, 667–676. [Google Scholar] [CrossRef]
- Ma, J. The Checklist of the Chinese Invasive Plants; Higher Education Press: Beijing, China, 2013. [Google Scholar]
- Cao, J.; Xu, H.; Pan, X.; Rong, Y. Study on the status of invasive plants in Chinese grassland. Acta Agrestia Sin. 2020, 28, 1–11. [Google Scholar] [CrossRef]
- Bai, Y.; Cao, X.; Chen, C.; Hu, B.; Liu, F. Potential distribution areas of alien invasive plant Flaveria bidentis (Asteraceae) in China. Chin. J. Appl. Ecol. 2009, 20, 2377–2383. [Google Scholar]
- Wang, R.; Wang, J.-F.; Qiu, Z.-J.; Meng, B.; Wan, F.-H.; Wang, Y.-Z. Multiple mechanisms underlie rapid expansion of an invasive alien plant. New Phytol. 2011, 191, 828–839. [Google Scholar] [CrossRef] [PubMed]
- Sang, W.G.; Zhu, L.; Axmacher, J.C. Invasion pattern of Eupatorium adenophorum Spreng in southern China. Biol. Invasions 2010, 12, 1721–1730. [Google Scholar] [CrossRef]
- Chen, J.; Ma, F.; Zhang, Y.; Wang, C.; Xu, H. Spatial distribution patterns of invasive alien species in China. Glob. Ecol. Conserv. 2021, 26, e01432. [Google Scholar] [CrossRef]
- Tu, Y. Biological invasion—Alien invasive plants in Guizhou. Environ. Prot. Technol. 2002, 8, 1–4, 10. [Google Scholar] [CrossRef]
- Liu, M.; Chen, M.; Gao, J.; Zheng, Y. Composition and source of alien plant species in Tongren city. Guizhou Agric. Sci. 2021, 49, 96–103. [Google Scholar]
- Shi, D.; Li, C. Alien plant invasion situation in “two lakes and one reservoir” ecological functional area of Guiyang and prevention measures. Guizhou Agric. Sci. 2011, 39, 94–98. [Google Scholar]
- Guo, C.; Zhu, J.; Liu, X.; Zhao, C.; Li, J. Contrasting biodiversity of invasive herbs inside and outside nature reserves in Guizhou. Biodivers. Sci. 2021, 29, 596–604. [Google Scholar] [CrossRef]
- Lin, M.; Liu, Z.; Han, F.; Ren, M.; Zhang, J. The investigation of invasive plants in Guizhou Dashahe nature reserve. J. Weed Sci. 2008, 1, 31–32, 51. [Google Scholar] [CrossRef]
- Zhou, Y.; Wei, Z.; Zhang, R.; Deng, T. Current situation and investigation of invasive plants in Zhaozishan nature reserve. Guizhou Agric. Sci. 2019, 47, 36–42. [Google Scholar]
- Yan, X.; Zhang, B.; Liu, B.; Wu, J.; Yang, H. Investigation and control measures of foreign pests in Guizhou Bijiang national wetland park. Anhui Agric. Sci. Bull. 2021, 27, 146–149. [Google Scholar] [CrossRef]
- Yang, C.; Li, F.; Yu, D.; Liang, Y. A preliminary study on alien invasive plants in Leigong mountain national nature reserve of Guizhou province. For. By-Prod. Spec. China 2017, 3, 79–84. [Google Scholar] [CrossRef]
- Yu, S.; Tian, M.; Zhang, N.; Qiu, J.; Zhou, Q.; Ban, Q. Prediction of suitable area of invasive plant Eupatorium adenophorum in Guizhou. For. Pest Dis. 2022, 41, 1–7. [Google Scholar] [CrossRef]
- Huang, J.; Wan, Y.; Liu, Z. The damage situation and control countermeasures for Eupatorium adenophorum Spreng in Guizhou province. Guizhou For. Sci. Technol. 2007, 52–56. [Google Scholar]
- Li, R.; Zha, L.; Wu, C.; Qin, L. Three alien invasive plants in Bijie City: Their outbreaks and control measures. Mod. Agric. Sci. Technol. 2015, 209–210. [Google Scholar]
- Yu, S.; Tian, M.; Zhang, N.; Qiu, J.; Zhou, Q.; Ban, Q.; Chen, Y. Prediction and risk analysis of suitable growth area of invasive plant Chromolaena odorata in Guizhou. J. West China For. Sci. 2021, 50, 125–130. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, X.; Wei, H.; Wang, D.; Chen, R.; Wang, L.; Gu, W. Predicting the invasive trend of exotic plants in China based on the ensemble model under climate change: A case for three invasive plants of Asteraceae. Sci. Total Environ. 2021, 756, 143841. [Google Scholar] [CrossRef]
- Sun, Q.; He, S.; Yang, X. Invasion status and countermeasures of two new exotic species in Guizhou. Guizhou Agric. Sci. 2010, 38, 90–92. [Google Scholar]
- Sun, Q.; He, S.; Yang, L.; Zhou, H.; Xu, W.; Wang, Y. Xanthium mongolicum, a northeastern species is spreading rapidly in Guizhou and several southeastern provinces in China. Chin. Wild Plant Resour. 2010, 29, 21–22. [Google Scholar]
- Kou, D.; Zhang, J.; Liu, X.; Li, Z.; Wang, P.; Sun, S. The invasion status and effect on plant diversity of Alternanthera philoxeroides in Aha reservoir in Guizhou. Guizhou Agric. Sci. 2012, 40, 129–132. [Google Scholar]
- Zhang, J.; Cai, G.; Wu, D.; Li, Z.; Wang, J.; Yi, W.; Tu, S.; Tao, H. The plant community structure and quantitative features after the invasion of Alternanthera philoxeroides in Caohai Wetland of Guizhou Province. Ecol. Environ. Sci. 2018, 27, 827–833. [Google Scholar] [CrossRef]
- Yang, Z.; Hu, Q.; Li, X.; Liu, T.; Zhang, N.; Qiu, J. Risk evaluation of Ambrosia trifida in Guizhou province. Guizhou For. Sci. Technol. 2017, 45, 1–5. [Google Scholar] [CrossRef]
- Chen, S.; Ye, Z.; Ran, H.; Lan, X.; Zhou, P.; He, Y. Effect of different environmental condition on biological characteristics of Ageratum conyzoides, an exotic invasion weed. Guizhou Agric. Sci. 2018, 46, 64–66. [Google Scholar]
- Tan, G.; Luo, Q.; Zhong, X.; Wang, T. The distribution and control measures of Cyclospermum leptophyllum in Guizhou. Agric. Technol. Serv. 2019, 36, 71–72. [Google Scholar]
- Ban, Q.; Tian, M.; Yu, S.; Zhang, N.; Qiu, J.; Luo, N. Risk analysis of Mikania micrantha in Guizhou. Guizhou For. Sci. Technol. 2020, 48, 9–12. [Google Scholar] [CrossRef]
- FAO; ISRIC; ISSCAS; JRC. Harmonized World Soil Database (Version 1.2); FAO: Rome, Italy; IIASA: Laxenburg, Austria, 2012. [Google Scholar]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Shi, H.; Cao, W.; Gao, Y.; Zhong, Q. Situation and control strategies of alien invasive plants in Northeast China grasslands. Pratacult. Sci. 2016, 33, 2485–2493. [Google Scholar] [CrossRef]
- Witt, A.B.R.; Kiambi, S.; Beale, T.; Van Wilgen, B.W. A preliminary assessment of the extent and potential impacts of alien plant invasions in the Serengeti-Mara ecosystem, East Africa. Koedoe 2017, 59, a1426. [Google Scholar] [CrossRef]
- Martín-Forés, I.; Castro, I.; Acosta-Gallo, B.; del Pozo, A.; Sánchez-Jardón, L.; de Miguel, J.M.; Ovalle, C.; Casado, M.A. Alien plant species coexist over time with native ones in Chilean Mediterranean grasslands. J. Plant Ecol. 2016, 9, 682–691. [Google Scholar] [CrossRef] [Green Version]
- Guido, A.; Vélez-Martin, E.; Overbeck, G.E.; Pillar, V.D. Landscape structure and climate affect plant invasion in subtropical grasslands. Appl. Veg. Sci. 2016, 19, 600–610. [Google Scholar] [CrossRef]
- Datta, A.; Kuhn, I.; Ahmad, M.; Michalski, S.; Auge, H. Processes affecting altitudinal distribution of invasive Ageratina adenophora in western Himalaya: The role of local adaptation and the importance of different life-cycle stages. PLoS ONE 2017, 12, e0187708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, F.H.; Liu, W.X.; Guo, J.Y.; Qiang, S.; Li, B.P.; Wang, J.J.; Yang, G.Q.; Niu, H.B.; Gui, F.R.; Huang, W.K.; et al. Invasive mechanism and control strategy of Ageratina adenophora (Sprengel). Sci. China Life Sci. 2010, 53, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.G.; He, W.M. Soil biota, but not soil nutrients, facilitate the invasion of Bidens pilosa relative to a native species Saussurea deltoidea. Weed Res. 2009, 49, 201–206. [Google Scholar] [CrossRef]
- Blumenthal, D. Interrelated Causes of Plant Invasion. Science 2005, 310, 243–244. [Google Scholar] [CrossRef] [Green Version]
- Burns, J.H. A comparison of invasive and non-invasive dayflowers (Commelinaceae) across experimental nutrient and water gradients. Divers. Distrib. 2010, 10, 387–397. [Google Scholar] [CrossRef]
- Chen, M.; Jia, S. China Forage Plants; China Agriculture Press: Beijing, China, 2002. [Google Scholar]
- Norris, I.B. Temperature response and flowering of white clover (Trifolium repens) varieties in controlled environments and the field. Ann. Appl. Biol. 1986, 108, 659–665. [Google Scholar] [CrossRef]
- Britten, E.J. The influence of genotype and temperature on flowering in Trifolium repens. Agron. J. 1961, 53, 11–14. [Google Scholar] [CrossRef]




| Item | Source |
|---|---|
| Mean annual temperature Mean annual precipitation | WorldClim Data version 2.1 (https://www.worldclim.org/, accessed on 1 January 2022) |
| Elevation | ASTER GDEM (https://www.gscloud.cn/, accessed on 1 January 2022) |
| SOC | Harmonized World Soil Database version 1.21 (http://webarchive.iiasa.ac.at/Research/LUC/External-World-soil-database/, accessed on 1 January 2022) |
| Traffic Network | Geographical Information Monitoring Cloud Platform (http://www.dsac.cn/, accessed on 1 January 2022) |
| Rank | Family | Name | Invasive Level | Source Area | Frequency (%) |
|---|---|---|---|---|---|
| 1 | Asteraceae | Bidens pilosa L. | 1 | America | 39.14 |
| 2 | Asteraceae | Ageratina adenophora (Spreng.) R.King & H.Rob | 1 | Mexico | 23.06 |
| 3 | Asteraceae | Praxelis clematidea (Griseb.) R.King & H.Rob | 1 | South America | 14.75 |
| 4 | Asteraceae | Erigeron sumatrensis Retz. | 1 | South America | 9.65 |
| 5 | Asteraceae | Chromolaena odorata (L.) R.King & H.Rob | 1 | Mexico | 6.97 |
| 6 | Amaranthaceae | Alternanthera philoxeroides (Mart.) Griseb | 1 | Brazil | 4.02 |
| 7 | Asteraceae | Ageratum conyzoides L. | 1 | Tropical America | 3.75 |
| 8 | Convolvulaceae | Ipomoea purpurea (L.) Roth | 1 | America | 2.95 |
| 9 | Amaranthaceae | Dysphania ambrosioides (L.) Mosyakin & Clemants | 1 | Tropical America | 0.8 |
| 10 | Convolvulaceae | Ipomoea cairica (L.) Sweet | 1 | America | 0.27 |
| 11 | Asteraceae | Ambrosia artemisiifolia L. | 1 | Central America, North America | 0.27 |
| 12 | Asteraceae | Ambrosia trifida L. | 1 | North America | 0.27 |
| 13 | Asteraceae | Parthenium hysterophorus L. | 1 | Tropical America | 0.27 |
| 14 | Asteraceae | Solidago canadensis L. | 1 | North America | 0.27 |
| 15 | Amaranthaceae | Amaranthus spinosus L. | 1 | Tropical America | 0.27 |
| 16 | Fabaceae | Trifolium repens L. | 2 | North Africa, Central Asia, West Asia, Europe | 41.29 |
| 17 | Asteraceae | Crassocephalum crepidioides (Benth.) S.Moore | 2 | Africa | 7.51 |
| 18 | Poaceae | Avena fatua L. | 2 | South Europe, Mediterranean | 6.17 |
| 19 | Fabaceae | Trifolium pratense L. | 2 | North Africa, Central Asia, Europe | 4.83 |
| 20 | Apiaceae | Daucus carota L. | 2 | Europe | 2.68 |
| 21 | Solanaceae | Solanum aculeatissimum Jacq. | 2 | Brazil | 0.8 |
| 22 | Amaranthaceae | Celosia argentea L. | 2 | India | 0.8 |
| 23 | Nyctaginaceae | Mirabilis jalapa L. | 2 | Tropical America | 0.54 |
| 24 | Amaranthaceae | Gomphrena celosioides L. | 2 | Tropical America | 0.54 |
| 25 | Convolvulaceae | Ipomoea triloba L. | 2 | India | 0.27 |
| 26 | Cactaceae | Opuntia dillenii (Ker Gawl.) Haw. | 2 | Caribbean | 0.27 |
| 27 | Asteraceae | Tridax procumbens L. | 2 | Tropical America | 0.27 |
| 28 | Poaceae | Paspalum dilatatum Poir. | 3 | South America | 3.22 |
| 29 | Poaceae | Pennisetum purpureum Schumach. | 3 | Africa | 2.68 |
| 30 | Euphorbiaceae | Euphorbia dentata Michx. | 3 | North America | 0.8 |
| 31 | Scrophulariaceae | Veronica persica Poir. | 3 | West Asia | 0.54 |
| 32 | Onagraceae | Oenothera rosea Aiton | 3 | Tropical America | 0.54 |
| 33 | Cyperaceae | Cyperus rotundus L. | 4 | India | 15.01 |
| 34 | Asteraceae | Sonchus oleraceus L. | 4 | Europe, Mediterranean | 7.77 |
| 35 | Poaceae | Setaria palmifolia (J. Koenig) Stapf | 4 | Africa | 3.22 |
| 36 | Asteraceae | Sonchus asper (L.) Hill | 4 | Europe, Mediterranean | 1.34 |
| 37 | Fabaceae | Medicago sativa L. | 4 | West Asia | 1.07 |
| 38 | Malvaceae | Sida acuta Burm. fil. | 4 | Tropical America | 0.54 |
| 39 | Scrophulariaceae | Veronica arvensis L. | 4 | South Europe, West Asia | 0.27 |
| 40 | Asteraceae | Cichorium intybus L. | 4 | Europe, Central Asia, West Asia, North Africa | 0.27 |
| 41 | Asteraceae | Helianthus tuberosus L. | 4 | North America | 0.27 |
| 42 | Asteraceae | Senecio vulgaris L. | 4 | Europe | 0.27 |
| 43 | Solanaceae | Solanum quitoense Lam. | 5 | Asia | 7.51 |
| 44 | Poaceae | Axonopus compressus (Sw.) P.Beauv. | 5 | Tropical America | 3.49 |
| 45 | Asteraceae | Crepis tectorum L. | 5 | Europe | 1.34 |
| 46 | Iridaceae | Sisyrinchium rosulatum E.P.Bicknell | 5 | North America | 0.54 |
| 47 | Asteraceae | Crassocephalum rubens (Juss. ex Jacq.) S.Moore | 5 | Tropical Africa | 0.54 |
| 48 | Poaceae | Chrysopogon zizanioides (L.) Roberty | 5 | India | 0.27 |
| 49 | Fabaceae | Amorpha fruticosa L. | 5 | America | 0.27 |
| Invasive Level | Elevation | Precipitation | Temperature | SOC | Distance to Railways | Distance to Expressways | Distance to National Roads | Distance to Country Roads | |
|---|---|---|---|---|---|---|---|---|---|
| Number of Invasive Species | 1 | −0.24 * | 0.30 * | 0.39 * | 0.18 * | 0.14 * | 0.03 | 0.18 * | 0.01 |
| 2 | 0.38 * | −0.33 * | −0.44 * | −0.11 * | −0.06 | 0.16 * | 0.01 | −0.16 * | |
| 3 | 0.11 * | −0.02 | −0.08 | 0.00 | 0.00 | −0.01 | 0.21 * | −0.08 | |
| 4 | −0.01 | 0.02 | 0.02 | 0.00 | −0.10 | −0.07 | −0.04 | 0.03 | |
| 5 | 0.05 | 0.04 | −0.01 | −0.06 | 0.01 | −0.08 | −0.01 | 0.04 | |
| Biomass of Invasive Species | 1 | −0.15 * | 0.22 * | 0.26 * | 0.14 * | 0.09 | 0.00 | 0.08 | 0.06 |
| 2 | 0.23 * | −0.23 * | −0.25 * | −0.07 | 0.04 | 0.05 | −0.04 | −0.11 * | |
| 3 | 0.03 | −0.03 | −0.02 | −0.03 | −0.04 | 0.03 | 0.10 | −0.03 | |
| 4 | 0.00 | 0.01 | 0.00 | 0.09 | −0.09 | −0.08 | −0.08 | 0.03 | |
| 5 | 0.04 | 0.07 | 0.02 | −0.05 | −0.02 | −0.07 | 0.02 | −0.06 |
| Invasion Level | Number of Species | Height | Plant Cover | Biomass | |
|---|---|---|---|---|---|
| Number of Invasive Species | 1 | −0.09 | 0.08 | −0.13 * | −0.15 * |
| 2 | 0.33 * | −0.46 * | 0.09 | −0.05 | |
| 3 | 0.06 | −0.10 * | −0.02 | −0.07 | |
| 4 | −0.01 | −0.07 | 0.07 | −0.04 | |
| 5 | −0.01 | −0.06 | −0.14 * | −0.11 * | |
| Biomass of Invasive Species | 1 | −0.07 | 0.05 | −0.13 * | −0.10 |
| 2 | 0.10 * | −0.32 * | 0.08 | −0.05 | |
| 3 | −0.01 | −0.06 | −0.01 | −0.02 | |
| 4 | −0.06 | −0.06 | 0.13 * | 0.07 | |
| 5 | 0.00 | −0.08 | −0.12 * | −0.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Q.; Jin, B.; Zhao, X.; Chen, C.; Cheng, H.; Wang, H.; He, D.; Zhang, Y.; Peng, J.; Li, Z.; et al. Composition, Distribution, and Factors Affecting Invasive Plants in Grasslands of Guizhou Province of Southwest China. Diversity 2022, 14, 167. https://doi.org/10.3390/d14030167
Yang Q, Jin B, Zhao X, Chen C, Cheng H, Wang H, He D, Zhang Y, Peng J, Li Z, et al. Composition, Distribution, and Factors Affecting Invasive Plants in Grasslands of Guizhou Province of Southwest China. Diversity. 2022; 14(3):167. https://doi.org/10.3390/d14030167
Chicago/Turabian StyleYang, Qin, Baocheng Jin, Xuechun Zhao, Chao Chen, Hua Cheng, Huanhuan Wang, Dengming He, Yaoyao Zhang, Jing Peng, Zhongcai Li, and et al. 2022. "Composition, Distribution, and Factors Affecting Invasive Plants in Grasslands of Guizhou Province of Southwest China" Diversity 14, no. 3: 167. https://doi.org/10.3390/d14030167
APA StyleYang, Q., Jin, B., Zhao, X., Chen, C., Cheng, H., Wang, H., He, D., Zhang, Y., Peng, J., Li, Z., & Han, M. (2022). Composition, Distribution, and Factors Affecting Invasive Plants in Grasslands of Guizhou Province of Southwest China. Diversity, 14(3), 167. https://doi.org/10.3390/d14030167






