Diversity of Endophytic Fungi in Annual Shoots of Prunus mandshurica (Rosaceae) in the South of Amur Region, Russia
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material, Sampling, and Fungal Isolation
2.2. Morphological Characterization of Fungi
2.3. Molecular Characterization and Phylogenetic Analysis of Pycnidial Fungi
3. Results
3.1. Sanitary Conditions of the Apricot Trees
3.2. Identification of Isolates by Morphological Features
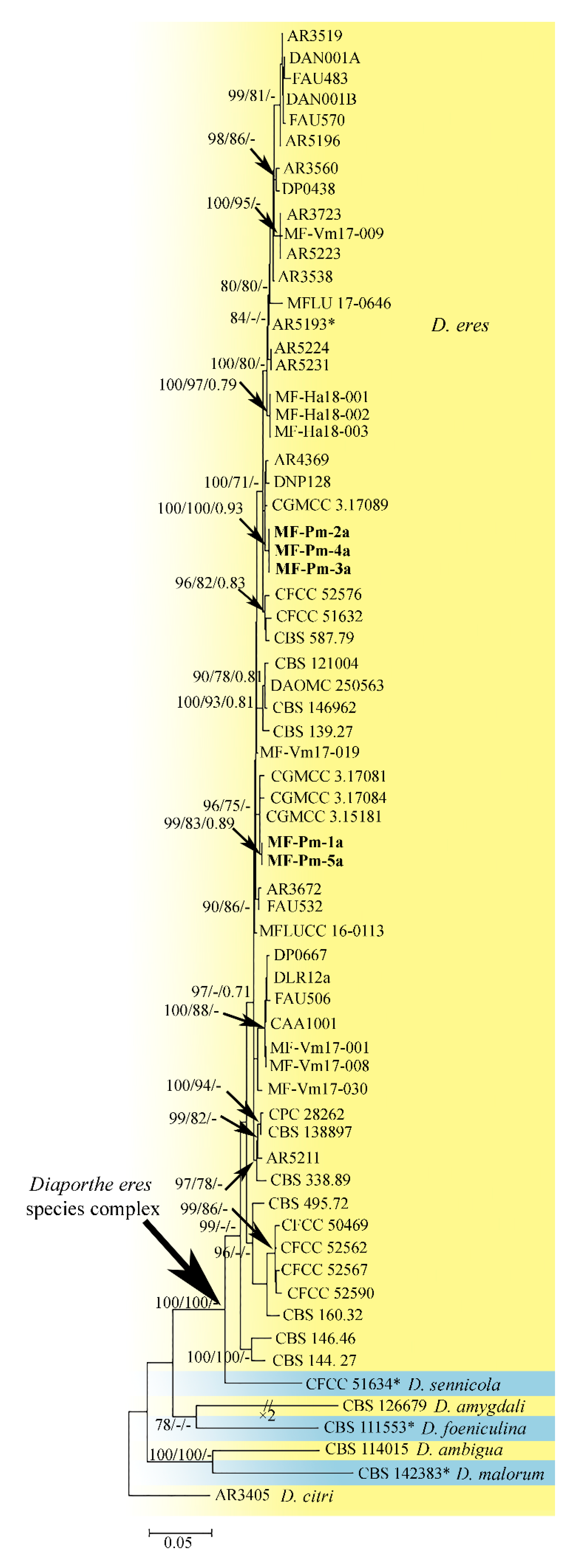
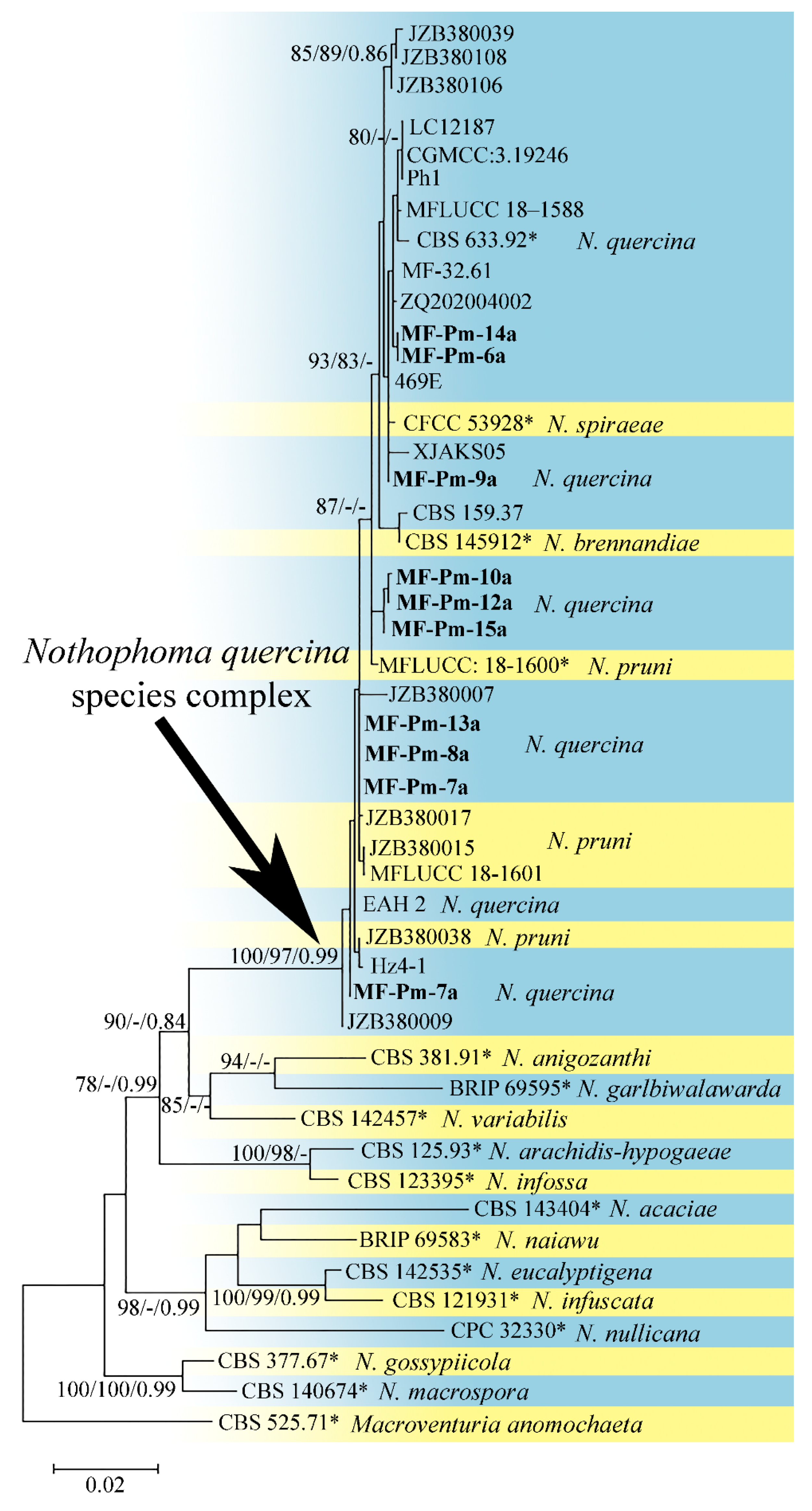
3.3. Molecular Characterization and Phylogenetic Analysis of Didymellaceae sp. and Diaporthe sp. Isolates
3.4. Distribution of Fungi in the Apricot Shoots
4. Discussion
4.1. Diversity of Fungi Associated with Apricot Species
4.2. Ecological Features, Distribution, and Potential Pathogenicity of the Fungi Associated with the Manchurian Apricot
4.3. Phylogenetic Analysis of the Pycnidial Fungi
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Saikkonen, K.; Faeth, S.H.; Helander, M.; Sullivan, T.J. Fungal endophytes: A continuum of interactions with host plants. Ann. Rev. Ecol. Syst. 1998, 29, 319–343. [Google Scholar] [CrossRef]
- Zeilinger, S.; Gupta, V.K.; Dahms, T.E.S.; Silva, R.N.; Singh, H.B.; Upadhyay, R.S.; Gomes, E.V.; Tsui, C.K.M.; Nayak, S.C. Friends or foes? Emerging insights from fungal interactions with plants. FEMS Microb. Rev. 2016, 40, 182–207. [Google Scholar] [CrossRef] [PubMed]
- Naranjo-Ortiz, M.A.; Gabaldon, T. Fungal evolution: Major ecological adaptations and evolutionary transitions. Biol. Rev. 2019, 94, 1443–1476. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Singh, D.; Ghosh, P.; Kumar, A. Endophytic and epiphytic modes of microbial interactions and benefits. In Plant-Microbe Interactions in Agro-Ecological Perspectives; Singh, D., Singh, H., Prabha, R., Eds.; Springer: Singapore, 2017; pp. 227–253. [Google Scholar] [CrossRef]
- Zabalgogeazcoa, I. Fungal endophytes and their interaction with plant pathogens. Span. J. Agric. Res. 2008, 6, 138–146. [Google Scholar] [CrossRef]
- Hardoim, P.R.; van Overbeek, L.S.; Berg, G.; Pirttila, A.M.; Compant, S.; Campisano, A.; Doring, M.; Sessitsch, A. The hidden world within plants: Ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol. Mol. Biol. Rev. 2015, 79, 293–320. [Google Scholar] [CrossRef]
- Khan, A.L.; Hussain, J.; Al-Harrasi, A.; Al-Rawahi, A.; Lee, I.-J. Endophytic fungi: Resource for gibberellins and crop abiotic stress resistance. Crit. Rev. Biotech. 2015, 35, 62–74. [Google Scholar] [CrossRef]
- Rodriguez, R.J.; White, J.F.; Arnold, A.E.; Redman, R.S. Fungal endophytes: Diversity and functional roles. N. Phytol. 2009, 182, 314–330. [Google Scholar] [CrossRef]
- Promputtha, I.; Lumyong, S.; Dhanasekaran, V.; McKenzie, E.H.C.; Hyde, K.D.; Jeewon, R. A phylogenetic evaluation of whether endophytes become saprotrophs at host senescence. Microb. Ecol. 2007, 53, 579–590. [Google Scholar] [CrossRef]
- Kazmin, G.T. AbrikosnaDal’nemVostoke (Apricot in the Far East); Khabarovskoeknizhnoeizdatelstvo: Khabarovsk, Russia, 1973. (In Russian) [Google Scholar]
- Bardunov, L.V.; Novikov, V.S. (Eds.) Krasnaya Kniga Rossijskoj Federacii (Rasteniya i Griby) (Red Book of Russian Federation (Plants and Fungi)); Tovarishchestvo nauchnyh izdanij KMK: Moscow, Russia, 2008; pp. 488–490. (In Russian) [Google Scholar]
- Damm, U.; Fourie, P.H.; Crous, P.W. Coniochaeta (Lecythophora), Collophora gen. nov. and Phaeomoniella species associated with wood necroses of Prunus trees. Persoonia 2010, 24, 60–80. [Google Scholar] [CrossRef]
- Bernadovikova, S.; Ivanova, H. Hyphomycetes and coelomycetes fungi isolated from affected leaves and twigs of cherry laurel trees. Folia Oecol. 2011, 38, 137–145. [Google Scholar]
- Ko, Y.; Liu, C.W.; Chen, S.S.; Chiu, K.Y.; Sun, Y.W.; Maruthasalam, S. First report of gummosis disease of Japanese apricot caused by Botryosphaeria dothidea in Taiwan. Plant Dis. 2011, 95, 77. [Google Scholar] [CrossRef]
- Olmo, D.; Gramaje, D.; Agustí-Brisach, C.; Leon, M.; Armengol, J. First report of Phaeoacremonium venezuelense associated with wood decay of apricot trees in Spain. Plant Dis. 2014, 98, 1001–1002. [Google Scholar] [CrossRef]
- Lawrence, D.P.; Travadon, R.; Baumgartner, K. Diversity of Diaporthe species associated with wood cankers of fruit and nut crops in northern California. Mycologia 2015, 107, 926–940. [Google Scholar] [CrossRef]
- Agustí-Brisach, C.; Moldero, D.; Raya, M.C.; Lorite, I.J.; Orgaz, F.; Trapero, A. Water stress enhances the progression of branch dieback and almond decline under field conditions. Plants 2020, 9, 1213. [Google Scholar] [CrossRef]
- Soltaninejad, N.; Mohammadi, H.; Massumi, H. Isolation, identification and pathogenicity of Botryosphaeriaceae and Phaeoacremonium species associated with decline of Prunus species in Iran. J. Plant Pathol. 2017, 99, 571–581. [Google Scholar] [CrossRef]
- Pan, M.; Zhu, H.; Bonthond, G.; Tian, C.; Fan, X. High diversity of Cytospora associated with canker and dieback of Rosaceae in China, with 10 new species described. Front. Plant Sci. 2020, 11, 690. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Y.; Du, Y.; Yang, Z.; Huang, X.; Hong, N.; Xu, W.; Wang, G. Characterization of Diaporthe species associated with peach constriction canker, with two novel species from China. MycoKeys 2021, 80, 77–90. [Google Scholar] [CrossRef]
- Ntuba-Jua, G.M.; Mih, A.M.; Bechem, E.E.T. Diversity and distribution of endophytic fungi in different Prunus africana (Hook. f.) Kalkman provenances in Cameroon. Int. J. Curr. Res. Biosci. Plant Biol. 2017, 4, 7–23. [Google Scholar] [CrossRef]
- Aghdam, S.A.; Fotouhifar, K.B. New reports of endophytic fungi associated with cherry (Prunus avium) and sour cherry (Prunus cerasus) trees in Iran. Mycol. Iran. 2016, 3, 75–85. [Google Scholar] [CrossRef]
- Aghdam, S.A.; Fotouhifar, K.-B. Identification of some endophytic fungi of cherry trees (Prunus avium) in Iran. Iran. J. Plant Prot. Sci. 2017, 48, 43–57. [Google Scholar] [CrossRef]
- Haddadderafshi, N.; Posa, T.B.; Peter, G.; Gaspar, L.; Ladanyi, M.; Hrotko, K.; Lukacs, N.; Halasz, K. Characterization of community structure of culturable endophytic fungi in sweet cherry composite trees and their growthretarding effect against pathogens. Acta Biol. Hung. 2016, 67, 269–285. [Google Scholar] [CrossRef] [PubMed]
- Rezvani, V.; Taheri, P.; Pourianfar, H.R.; Drakhshan, A. Biocontrol and plant growth promotion activities of endophytic and rhizospheric fungi from almond trees (Prunus dulcis) indigenous in the northeast of Iran. Curr. Res. Environ. Appl. Mycol. 2020, 10, 50–62. [Google Scholar] [CrossRef]
- Sessa, L.; Abreo, E.; Lupo, S. Diversity of fungal latent pathogens and true endophytes associated with fruit trees in Uruguay. J. Phytopathol. 2018, 166, 633–647. [Google Scholar] [CrossRef]
- Soltani Nejad, M.; Samandari Najafabadi, N.; Aghighi, S.; Pakina, E.; Zargar, M. Evaluation of Phoma sp. biomass as an endophytic fungus for synthesis of extracellular gold nanoparticles with antibacterial and antifungal properties. Molecules 2022, 27, 1181. [Google Scholar] [CrossRef]
- Krol, E.D.; Abramczyk, B.A.; Zalewska, E.D.; Zimowska, B. Fungi inhabiting fruit tree shoots with special reference to the Diaporthe (Phomopsis) genus. Acta Sci. Pol. Hortorum Cultus 2017, 16, 113–126. [Google Scholar] [CrossRef]
- Nekrasov, E.V. In vitro propagation of Armeniaca mandshurica (Rosaceae). Bull. Bot. Gard. Inst. 2017, 18, 81–88. (In Russian) [Google Scholar] [CrossRef]
- SanitarnyepravilavlesahRossijskojfederacii (Sanitary Regulations in the Forests of the Russian Federation). 2006. Available online: https://docs.cntd.ru/document/901965290?marker=6540IN (accessed on 12 August 2022). (In Russian).
- Dhingra, O.B.; Sinclair, J.B. Basic Plant Pathology Methods; CRC Press: Boca Raton, FL, USA, 1995. [Google Scholar]
- Mirchink, T.G.; Stepanova, L.N.; Marfenina, O.E.; Ozerskaya, S.M. Harakteristika kompleksov gribov mikromicetov nekotoryh pochv Sovetskogo Soyuza (Characteristics of the micromycetic fungi complexes of certain soil types of the Soviet Union). Vestn. Mosk. Univ. Seriya Pochvovedenie 1981, 1, 35–39. (In Russian) [Google Scholar]
- Crous, P.W.; Verkley, G.J.M.; Groenewald, J.Z.; Samson, R.A. Fungal Biodiversity. CBS Laboratory Manual Series; Westerdijk Fungal Biodiversity Institute: Utrecht, The Netherlands, 2009. [Google Scholar]
- Ingold, C.T.; Cox, V.J. On Tripospermum and Campylospora. Trans. Brit. Mycol. Soc. 1957, 40, 317–321. [Google Scholar] [CrossRef]
- Egorova, L.N. Pochvennye griby Dal’nego Vostoka: Gifomitsety (Soil Fungi of the Russian Far East: Hyphomycetes); Nauka: Leningrad, Russia, 1986. (In Russian) [Google Scholar]
- Domsch, K.H.; Gams, W.; Anderson, T.-H. Compendium of Soil Fungi; IHW-Verlag: Eching, Germany, 2007. [Google Scholar]
- Shipilova, N.P.; Ivashchenko, V.G. Systematics and Diagnostics of Fungi of the Genus Fusarium in Common Crops; VIZR: Sankt-Peterburg, Russia, 2008. (In Russian) [Google Scholar]
- Bensch, K.; Braun, U.; Groenewald, J.Z.; Crous, P.W. The genus Cladosporium. Stud. Mycol. 2012, 72, 1–401. [Google Scholar] [CrossRef]
- Lawrence, D.P.; Rotondo, F.; Gannibal, P.B. Biodiversity and taxonomy of the pleomorphic genus Alternaria. Mycol. Prog. 2016, 15, 3. [Google Scholar] [CrossRef]
- Boerema, G.H.; de Gruyter, J.; Noordeloos, M.E.; Hamers, M.E.C. Phoma Identification Manual. Differentiation of Specific and Infra-specific Taxa in Culture; CABI Publishing: Wallingford, UK, 2004. [Google Scholar]
- Species Fungorum. Available online: http://www.speciesfungorum.org/Names/Names.asp (accessed on 2 August 2022).
- MycoBank. Available online: http://www.mycobank.org/ (accessed on 27 February 2020).
- Doyle, J.J.; Doyle, J.L. Isolation of plant DNA from fresh tissue. Focus 1990, 12, 13–15. [Google Scholar]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes—Application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky., J.J., White, T.J., Eds.; Academic Press: London, UK, 1990; pp. 315–322. [Google Scholar]
- Udayanga, D.; Castlebury, L.A.; Rossman, A.Y.; Chukeatirote, E.; Hyde, K.D. Insights into the genus Diaporthe: Phylogenetic species delimitation in the D. eres species complex. Fungal Div. 2014, 67, 203–229. [Google Scholar] [CrossRef]
- Carbone, I.; Kohn, L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- Aveskamp, M.M.; Verkley, G.J.M.; de Gruyter, J.; Murace, M.A.; Perello, A.; Woudenberg, J.H.C.; Groenewald, J.Z.; Crous, P.W. DNA phylogeny reveals polyphyly of Phoma section Peyronellaea and multiple taxonomic novelties. Mycologia 2009, 101, 363–382. [Google Scholar] [CrossRef]
- Sung, G.-H.; Sung, J.-M.; Hywel-Jones, N.L.; Spatafora, J.W. A multi-gene phylogeny of Clavicipitaceae (Ascomycota, Fungi): Identification of localized incongruence using a combinational bootstrap approach. Mol. Phylogenet. Evol. 2007, 31, 1204–1223. [Google Scholar] [CrossRef]
- Liu, Y.J.; Whelen, S.; Hall, B.D. Phylogenetic relationships among ascomycetes: Evidence from an RNA polymerase II subunit. Mol. Biol. Evol. 1999, 16, 1799–1808. [Google Scholar] [CrossRef]
- Boyle, J.S.; Lew, A.M. An inexpensive alternative to glassmilk for DNA purification. Trends Gen. 1995, 11, 8. [Google Scholar] [CrossRef]
- Sanger, F.; Nicklen, S.; Coulson, A.R. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 1977, 74, 5463–5467. [Google Scholar] [CrossRef]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The Clustal_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 24, 4876–4882. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Vaydia, G.; Lohman, D.J.; Meier, R. SequenceMatrix: Concatenation software for the fast assembly of multi-gene datasets with character set and codon information. Cladistics 2011, 27, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Lord, E.; Leclercq, M.; Boc, A.; Diallo, A.B.; Makarenkov, V. Armadillo 1.1: An original workflow platform for designing and conducting phylogenetic analysis and simulations. PLoS ONE 2012, 7, e29903. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Quoirin, M.; Lepoivre, P. Etude de milieux adaptes aux cultures in vitro de Prunus. Acta Hort. 1977, 78, 437–442. [Google Scholar] [CrossRef]
- Perez-Tornero, O.; Burgos, L. Apricot micropropagation. In Protocols for Micropropagation of Woody Trees and Fruits; Jain, S.M., Häggman, H., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 267–278. [Google Scholar]
- Farr, D.F.; Rossman, A.Y. Fungal Databases, U.S. National Fungus Collections, ARS, USDA. Available online: https://nt.ars-grin.gov/fungaldatabases/fungushost/fungushost.cfm (accessed on 2 August 2022).
- Shumilova, L.P.; Kuimova, N.G.; Terekhova, V.A.; Aleksandrova, A.A. Diversity and structure of fungal communities in soils of Blagoveshchensk city. Mycol. Phytopath. 2014, 48, 238–245. (In Russian) [Google Scholar] [CrossRef]
- Melgarejo, P.; Carrillo, R.; Sagasta, E.M. Mycoflora of peach twigs and flowers and its possible significance in biological control of Monilinia laxa. Trans. Br. Mycol. Soc. 1985, 85, 313–317. [Google Scholar] [CrossRef]
- Sanz-Ros, A.V.; Muller, M.M.; San Martin, R.; Diez, J.J. Fungal endophytic communities on twigs of fast and slow growing Scots pine (Pinus sylvestris L.) in northern Spain. Fung. Biol. 2015, 119, 870–883. [Google Scholar] [CrossRef]
- Rashmi, M.; Kushveer, J.S.; Sarma, V.V. A worldwide list of endophytic fungi with notes on ecology and diversity. Mycosphere 2019, 10, 798–1079. [Google Scholar] [CrossRef]
- Kowalski, T.; Kehr, R.D. Endophytic fungal colonization of branch bases in several forest tree species. Sydowia 1992, 44, 137–168. [Google Scholar]
- Bissegger, M.; Sieber, T.N. Assemblages of endophytic fungi in coppice shoots of Castanea sativa. Mycologia 1994, 86, 648–655. [Google Scholar] [CrossRef]
- Pirttila, A.M.; Pospiech, H.; Laukkanen, H.; Myllyla, R.; Hohtola, A. Two endophytic fungi in different tissues of Scots pine buds (Pinus sylvestris L.). Microb. Ecol. 2003, 45, 53–62. [Google Scholar] [CrossRef]
- Thomma, B.P.H.J. Alternaria spp.: From general saprophyte to specific parasite. Mol. Plant Pathol. 2003, 4, 225–236. [Google Scholar] [CrossRef]
- Smith, S.N. An overview of ecological and habitat aspects in the genus Fusarium with special emphasis on the soil-borne pathogenic forms. Plant Pathol. Bull. 2007, 16, 97–120. [Google Scholar]
- Demers, J.E.; Gugino, B.K.; Jimenez-Gasco, M.M. Highly diverse endophytic and soil Fusarium oxysporum populations associated with field-grown tomato plants. Appl. Environ. Microbiol. 2015, 81, 81–90. [Google Scholar] [CrossRef]
- Goswami, R.S.; Kistler, H.C. Heading for disaster: Fusarium graminearum on cereal crops. Mol. Plant Pathol. 2004, 5, 515–525. [Google Scholar] [CrossRef]
- Lofgren, L.A.; LeBlanc, N.R.; Certano, A.K.; Nachtigall, J.; LaBine, K.M.; Riddle, J.; Broz, K.; Dong, Y.; Bethan, B.; Kafer, C.W.; et al. Fusarium graminearum: Pathogen or endophyte of North American grasses? N. Phytol. 2018, 217, 1203–1212. [Google Scholar] [CrossRef]
- Chethana, K.W.T.; Jayawardene, R.S.; Zhang, W.; Zhou, Y.Y.; Liu, M.; Hyde, K.D.; Li, X.H.; Wang, J.; Zhang, K.C.; Yan, J.Y. Molecular characterization and pathogenicity of fungal taxa associated with cherry leaf spot disease. Mycosphere 2019, 10, 490–530. [Google Scholar] [CrossRef]
- Vettraino, A.M.; Li, H.-M.; Eschen, R.; Morales-Rodriguez, C.; Vannini, A. The sentinel tree nursery as an early warning system for pathway risk assessment: Fungal pathogens associated with Chinese woody plants commonly shipped to Europe. PLoS ONE 2017, 12, e0188800. [Google Scholar] [CrossRef]
- Mahadevakumar, S.; Jayaramaiah, K.M.; Janardhana, G.R. First report of leaf spot disease caused by Epicoccum nigrum on Lablab purpureus in India. Plant Dis. 2014, 98, 284. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, D.H.; Timko, M.P.; Li, M.Y.; Liang, G.L. First report of Epicoccum nigrum causing brown leaf spot of Loquat in southwestern China. Plant Dis. 2017, 101, 1553. [Google Scholar] [CrossRef]
- Summerbell, R.C.; Gueidan, C.; Schroers, H.J.; de Hoog, G.S.; Starink, M.; Rosete, Y.A.; Guarro, J.; Scott, J.A. Acremonium phylogenetic overview and revision of Gliomastix, Sarocladium, and Trichothecium. Stud. Mycol. 2011, 68, 139–162. [Google Scholar] [CrossRef] [PubMed]
- Racedo, J.; Salazar, S.M.; Castagnaro, A.P.; Diaz Ricci, J.C. A strawberry disease caused by Acremonium strictum. Eur. J. Plant Pathol. 2013, 137, 649–654. [Google Scholar] [CrossRef]
- Favaro, L.C.; de Melo, F.L.; Aguilar-Vildoso, C.I.; Araujo, W.L. Polyphasic analysis of intraspecific diversity in Epicoccum nigrum warrants reclassification into separate species. PLoS ONE 2011, 6, e14828. [Google Scholar] [CrossRef] [PubMed]
- Dissanayake, A.J.; Zhang, W.; Liu, M.; Hyde, K.D.; Zhao, W.S.; Li, X.H.; Yan, J.Y. Diaporthe species associated with peach tree dieback in Hubei, China. Mycosphere 2017, 8, 533–549. [Google Scholar] [CrossRef]
- Egorova, L.N. Redkie vidy anamorfnyh gribov na ohranyaemyh prirodnyh territoriyah bassejna reki Amur (yug Dal’nego Vostoka) (Rare species of anamorphic fungi in protected natural areas of the Amur river basin (the south of Far East)). In Proceedings of the XI International Scientific and Practical Ecological Conference, Belgorod, Russia, 20–25 September 2010. (In Russian). [Google Scholar]
- Gonczol, J.; Revay, A. Fungal spores in rainwater: Stemflow, throughfall and gutter conidial assemblages. Fungal Div. 2004, 16, 67–86. [Google Scholar]
- Revay, A.; Gonczol, J. Canopy fungi (“terrestrial aquatic hyphomycetes”) from twigs of living evergreen and deciduous trees in Hungary. Nova Hedwig. 2011, 92, 303–316. [Google Scholar] [CrossRef]
- Sokolski, S.; Piche, Y.; Chauvet, E.; Berube, J.A. A fungal endophyte of black spruce (Picea mariana) needles is also an aquatic hyphomycete. Mol. Ecol. 2006, 15, 1955–1962. [Google Scholar] [CrossRef]
- Grabowski, M. The study of new fungus species causing apple sooty blotch. Folia Hortic. 2007, 19, 89–97. [Google Scholar]
- Schena, L.; Nigro, F.; Pentimone, I.; Ligorio, A.; Ippolito, A. Control of postharvest rots of sweet cherries and table grapes with endophytic isolates of Aureobasidium pullulans. Postharv. Biol. Technol. 2003, 30, 209–220. [Google Scholar] [CrossRef]
- Choi, G.J.; Kim, J.C.; Jang, K.S.; Cho, K.Y.; Kim, H.T. Mycoparasitism of Acremonium strictum BCP on Botrytis cinerea, the gray mold pathogen. J. Microbiol. Biotech. 2008, 18, 167–170. [Google Scholar]
- De Cal, A.; Larena, I.; Linan, M.; Torres, R.; Lamarca, N.; Usall, J.; Domenichini, P.; Bellini, A.; De Eribe, X.O.; Melgarejo, P. Population dynamics of Epicoccum nigrum, a biocontrol agent against brown rot in stone fruit. J. Appl. Microbiol. 2009, 106, 592–605. [Google Scholar] [CrossRef]
- Martini, M.; Musetti, R.; Grisan, S.; Polizzotto, R.; Borselli, S.; Pavan, F.; Osler, R. DNA-dependent detection of the grapevine fungal endophytes Aureobasidium pullulans and Epicoccum nigrum. Plant Dis. 2009, 93, 993–998. [Google Scholar] [CrossRef]
- Alabouvette, C.; Olivain, C.; L–Haridon, F.; Aime, S.; Steinberg, C. Using strains of Fusarium oxysporum to control fusarium wilts: Dream or reality? In Novel Biotechnologies for Biocontrol Agent Enhancement and Management; NATO Security through Science Series; Vurro, M., Gressel, J., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 157–177. [Google Scholar] [CrossRef]
- Sanchez, C.; Graca Barreiro, M.; Ferreira-Pinto, M.M. Characterization of Aureobasidium pullulans, a promising biocontrol agent for postharvest diseases. ISHS Acta Hortic. 2012, 934, 335–342. [Google Scholar] [CrossRef]
- Torres, D.E.; Rojas-Martinez, R.I.; Zavaleta-Mejia, E.; Guevara-Fefer, P.; Marquez-Guzman, G.J.; Perez-Martinez, C. Cladosporium cladosporioides and Cladosporium pseudocladosporioides as potential new fungal antagonists of Puccinia horiana Henn., the causal agent of chrysanthemum white rust. PLoS ONE 2017, 12, e0170782. [Google Scholar] [CrossRef]
- Farr, D.F.; Castlebury, L.A.; Rossman, A.Y. Morphological and molecular characterization of Phomopsis vaccinii and additional isolates of Phomopsis from blueberry and cranberry in the eastern United States. Mycologia 2002, 94, 494–504. [Google Scholar] [CrossRef]
- Udayanga, D.; Castlebury, L.A.; Rossman, A.Y.; Hyde, K.D. Species limits in Diaporthe: Molecular re-assessment of D. citri, D. cytosporella, D. foeniculina and D. rudis. Persoonia 2014, 32, 83–101. [Google Scholar] [CrossRef]
- Fan, X.; Yang, Q.; Bezerra, J.D.P.; Alvarez, L.V.; Tian, C. Diaporthe from walnut tree (Juglans regia) in China, with insight of the Diaporthe eres complex. Mycol. Prog. 2018, 17, 841–853. [Google Scholar] [CrossRef]
- Yang, Q.; Du, Z.; Tian, C.M. Phylogeny and morphology reveal two new species of Diaporthe from Traditional Chinese Medicine in Northeast China. Phytotaxa 2018, 336, 159–170. [Google Scholar] [CrossRef]
- Hilario, S.; Gonçalves, M.F.M.; Alves, A. Using genealogical concordance and coalescent-based species delimitation to assess species boundaries in the Diaporthe eres complex. J. Fungi 2021, 7, 507. [Google Scholar] [CrossRef]
- Gomzhina, M.M.; Gasich, E.L.; Gagkaeva, T.Y.; Gannibal, P.B. Biodiversity of fungi inhabiting blueberry growing in North-West Russia and Finland. Mycol. Phytopath. 2021, 55, 353–370. (In Russian) [Google Scholar] [CrossRef]
- Chaisiri, C.; Liu, X.; Lin, Y.; Fu, Y.; Zhu, F.; Luo, C. Phylogenetic and haplotype network analyses of Diaporthe eres species in China based on sequences of multiple loci. Biology 2021, 10, 179. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.X.; Yin, T.; Pan, M.; Tian, C.M.; Fan, X.L. Occurrence and identification of Nothophoma spiraeae sp. nov. in China. Phytotaxa 2020, 430, 147–156. [Google Scholar] [CrossRef]
- Hou, L.W.; Hernández-Restrepo, M.; Groenewald, J.Z.; Cai, L.; Crous, P.W. Citizen science project reveals high diversity in Didymellaceae (Pleosporales, Dothideomycetes). MycoKeys 2020, 65, 49–99. [Google Scholar] [CrossRef]
- Chen, Q.; Jiang, J.R.; Zhang, G.Z.; Cai, L.; Crous, P.W. Resolving the Phoma enigma. Stud. Mycol. 2015, 82, 137–217. [Google Scholar] [CrossRef]
- Aveskamp, M.M.; de Gruyter, J.; Woudenberg, J.H.; Verkley, G.J.; Crous, P.W. Highlights of the Didymellaceae: A polyphasic approach to characterise Phoma and related pleosporalean genera. Stud. Mycol. 2010, 65, 1–60. [Google Scholar] [CrossRef]
| Species | StrainNumber | GenBank Accession Number | |||||
|---|---|---|---|---|---|---|---|
| tub2 | tef1-α | cal | apn2 | rpb2 | ITS | ||
| Diaportheambigua | CBS 114015 | KC343978 | KC343736 | KC343252 | - | - | - |
| D.amygdali | CBS 126679 | KC343990 | KC343748 | KC343264 | - | - | - |
| D. citri | AR3405 = CBS 135422 | KC344020 | KC343778 | KC843157 | KJ380981 | - | - |
| D. eres | MF-Pm-1a | MZ671975 | MZ671970 | MZ671923 | MZ671918 | - | MZ646151 |
| D. eres | MF-Pm-2a | MZ671976 | MZ671971 | MZ671924 | MZ671919 | - | MZ646152 |
| D. eres | MF-Pm-3a | MZ671977 | MZ671972 | MZ671925 | MZ671920 | - | MZ646153 |
| D. eres | MF-Pm-4a | MZ671978 | MZ671973 | MZ671926 | MZ671921 | - | MZ646154 |
| D. eres | MF-Pm-5a | MZ671979 | MZ671974 | MZ671927 | MZ671922 | - | MZ646155 |
| D. eres | MF-Ha18-001 | MK033490 | MK039422 | MZ671934 | MZ671915 | - | - |
| D. eres | MF-Ha18-002 | MK033491 | MK039423 | MZ671935 | MZ671916 | - | - |
| D. eres | MF-Ha18-003 | MW008495 | MW008506 | MZ671931 | MZ671917 | - | - |
| D. eres | CBS 495.72 | KC343975 | KC343733 | KC343249 | KJ380963 | - | - |
| D. eres | CBS 146.46 | KC343976 | KC343734 | KC343250 | KJ380969 | - | - |
| D. eres | CFCC 50469 | KT733020 | KT733016 | KT732997 | - | - | - |
| D. eres | CFCC 52562 | MH121579 | MH121539 | MH121421 | - | - | - |
| D. eres | CBS 121004 | KC344102 | KC343860 | KC343376 | KJ380976 | - | - |
| D. eres | CGMCC 3.17081 | KF576306 | KF576257 | - | - | - | - |
| D. eres | CBS 146962 | MN136190 | MN136153 | MN136129 | MN136122 | - | - |
| D. eres | CBS 587.79 | KC344121 | KC343879 | KC343395 | KJ380975 | - | - |
| D. eres | CFCC 51632 | KY228893 | KY228887 | KY228877 | - | - | - |
| D. eres | DNP128 | JX275438 | JX275401 | JX197430 | - | - | - |
| D. eres | CBS 139.27 | KC344015 | KC343773 | KC343289 | KJ380974 | - | - |
| D. eres | CPC 28262 | MG281190 | MG281538 | MG281712 | - | - | - |
| D. eres | CFCC 52567 | MH121584 | MH121544 | MH121426 | - | - | - |
| D. eres | DP0667 | KC843229 | KJ210548 | KC843155 | KJ380923 | - | - |
| D. eres | CGMCC 3.17084 | KF576291 | KF576245 | - | - | - | - |
| D. eres | AR3672 = MAFF625034 = CBS 116964 | KJ420819 | JQ807418 | KJ435023 | KJ380937 | - | - |
| D. eres | AR5211 = CBS 138596 | KJ420828 | KJ210559 | KJ435043 | KJ380977 | - | - |
| D. eres | CGMCC 3.17089 | KF576291 | KF576242 | - | - | - | - |
| D. eres | CGMCC 3.15181 | KF576312 | KC153087 | KT459461 | - | - | - |
| D. eres | DAOMC 250563 | KU574616 | KU552022 | - | KU552020 | - | - |
| D. eres | MFLUCC 16-0113 | KU557587 | KU557631 | KU557611 | - | - | - |
| D. eres | CBS 144. 27 | KC344112 | KC343870 | KC343386 | KJ380973 | - | - |
| D. eres | CFCC 52590 | MH121604 | MH121567 | MH121443 | - | - | - |
| D. eres | CBS 138897 | KP004507 | - | - | - | - | - |
| D. eres | CBS 338.89 | KC344120 | KC343878 | KC343394 | KJ380978 | - | - |
| D. eres | MFLU 17-0646 | MG843877 | MG829270 | MG829274 | - | - | - |
| D. eres | CBS 160.32 | KC344196 | KC343954 | KC343470 | KJ380968 | - | - |
| D. eres | CAA1001 | MT309458 | MT309432 | MT309449 | - | - | - |
| D. eres | AR5193 * | KJ420799 | KJ210550 | KJ434999 | KJ380958 | - | - |
| D. eres | AR5196 | KJ420817 | KJ210554 | KJ435006 | KJ380932 | - | - |
| D. eres | DP0438 | KJ420816 | KJ210553 | KJ435016 | KJ380935 | - | - |
| D. eres | FAU483 | KJ420827 | JQ807422 | KJ435022 | KJ380933 | - | - |
| D. eres | DAN001A | KJ420781 | KJ210540 | KJ434994 | KJ380914 | - | - |
| D. eres | DAN001B | KJ420782 | KJ210541 | KJ434995 | KJ380915 | - | - |
| D. eres | AR3519 | KJ420789 | KJ210547 | KJ435008 | KJ380922 | - | - |
| D. eres | FAU570 | KJ420794 | JQ807410 | KJ435025 | KJ380926 | - | - |
| D. eres | AR3723 | KJ420793 | JQ807351 | KJ435024 | KJ380941 | - | - |
| D. eres | AR3560 | KJ420795 | KJ210551 | KJ435011 | KJ380939 | - | - |
| D. eres | AR5224 | KJ420802 | KJ210555 | KJ435036 | KJ380961 | - | - |
| D. eres | AR5231 | KJ420818 | KJ210549 | KJ435038 | KJ380936 | - | - |
| D. eres | AR5223 | KJ420830 | KJ210549 | KJ435000 | KJ380938 | - | - |
| D. eres | DLR12a | KJ420783 | KJ210542 | KJ434996 | KJ380916 | - | - |
| D. eres | AR4369 | KJ420813 | JQ807366 | KJ435005 | KJ380953 | - | - |
| D. eres | MF-Vm17-001 | MZ054675 | MZ054665 | - | MZ054647 | - | - |
| D. eres | MF-Vm17-008 | MZ054676 | MZ054666 | - | MZ054648 | - | - |
| D. eres | MF-Vm17-009 | MZ054677 | MZ054667 | - | MZ054649 | - | - |
| D. eres | MF-Vm17-019 | MZ054678 | MZ054669 | - | MZ054650 | - | - |
| D. eres | MF-Vm17-030 | MZ054679 | MZ054670 | - | MZ054651 | - | - |
| D. eres | CFCC 52576 | MH121593 | MH121553 | MH121432 | - | - | - |
| D. eres | AR3538 = CBS 109767 | KC344043 | KC343801 | KC343317 | KJ380940 | - | - |
| D. eres | FAU506 | KJ420792 | JQ807403 | KJ435012 | KJ380925 | - | - |
| D. eres | FAU532 | KJ420815 | JQ807408 | KJ435015 | KJ380934 | - | - |
| D. foeniculina | CBS 111553 * | KC344069 | KC343827 | KC343343 | - | - | - |
| D. malorum | CBS 142383 = CAA734 * | KY435668 | KY435627 | KY435658 | - | - | - |
| D. sennicola | CFCC 51634 * | KY228889 | KY228883 | KY228873 | - | - | - |
| Macroventuria anomochaeta | CBS 525.71 * | GU237545 | - | - | - | GU456346 | MH860249 |
| Nothophoma acaciae | CBS 143404 * | MG386167 | - | - | - | MG386144 | MG386056 |
| N. anigozanthi | CBS 381.91 * | GU237580 | - | - | - | KT389655 | GU237852 |
| N. arachidis-hypogaeae | CBS 125.93 * | GU237583 | - | - | - | KT389656 | GU237771 |
| N. brennandiae | CBS 145912 * | MN824753 | - | - | - | MN824604 | MN823579 |
| N. garlbiwalawarda | BRIP 69595 * | - | - | - | - | MN604937 | MN5676786 |
| N. eucalyptigena | CBS 142535 * | KY979935 | - | - | - | KY979852 | KY979771 |
| N. gossypiicola | CBS 377.67 | GU237611 | - | - | - | KT389658 | GU237845 |
| N. infossa | CBS 123395 * | FJ427135 | - | - | - | KT389659 | FJ427025 |
| N. infuscata | CBS 121931 * | MT005662 | - | - | - | MT018203 | MN973559 |
| N. macrospora | CBS 140674 * | LN880539 | - | - | - | LT593073 | LN880536 |
| N. naiawu | BRIP 69583 * | - | - | - | - | MN604938 | MN5676787 |
| N. nullicana | CPC 32330 * | MG386165 | - | - | - | MG386143 | NR_156665 |
| N. pruni | JZB380017 | MH853670 | - | - | - | MH853663 | MH827006 |
| N. pruni | JZB380015 | MH853668 | - | - | - | MH853661 | MH827004 |
| N. pruni | MFLUCC 18-1601 | MH853669 | - | - | - | MH853662 | MH827005 |
| N. pruni | JZB380038 | MN991303 | - | - | - | MN991306 | MN533798 |
| N. pruni | MFLUCC: 18-1600 * | MH853671 | - | - | - | MH853664 | MH827007 |
| N. quercina | CBS 633.92 * | GU237609 | - | - | - | KT389657 | GU237900 |
| N. quercina | MF-Pm-6a | MZ671980 | - | - | - | MZ671944 | MZ646156 |
| N. quercina | MF-Pm-7a | MZ671981 | - | - | - | MZ671945 | MZ646157 |
| N. quercina | MF-Pm-8a | MZ671982 | - | - | - | MZ671946 | MZ646158 |
| N. quercina | MF-Pm-9a | MZ671983 | - | - | - | MZ671947 | MZ646159 |
| N. quercina | MF-Pm-10a | MZ671984 | - | - | - | MZ671948 | MZ646160 |
| N. quercina | MF-Pm-12a | MZ671986 | - | - | - | MZ671950 | MZ646162 |
| N. quercina | MF-Pm-13a | MZ671987 | - | - | - | MZ671951 | MZ646163 |
| N. quercina | MF-Pm-14a | MZ671988 | - | - | - | MZ671952 | MZ646164 |
| N. quercina | MF-Pm-15a | MZ671989 | MZ671953 | MZ646165 | |||
| N. quercina | JZB380007 | MZ646165 | - | MZ671989 | - | - | MZ671953 |
| N. quercina | JZB380009 | KY887673.1 | - | KY887679 | - | - | KY887677 |
| N. quercina | MFLUCC 18–1588 | MH827008 | - | MH853672 | - | - | MH853665 |
| N. quercina | CGMCC:3.19246 | MK088574 | - | MK088595 | - | - | MK088588 |
| N. quercina | JZB380039 | MN533799 | - | MN537423 | - | - | MN537426 |
| N. quercina | LC12187 | MK088575 | - | MK088596 | - | - | MK088589 |
| N. quercina | XJAKS05 | KX225387 | KX645664 | ||||
| N. quercina | ZQ202004002 | MW883394 | - | - | - | MW883395 | MW541930 |
| N. quercina | EAH 2 | MW330391 | - | - | - | MW330390 | MW325676 |
| N. quercina | Ph1 | MK522081 | - | - | - | - | MK522080 |
| N. quercina | JZB380108 | ON351014 | - | - | - | ON350993 | ON316870 |
| N. quercina | JZB380106 | ON351012 | - | - | - | ON350991 | ON316868 |
| N. quercina | 469E | - | - | - | - | - | MZ078709 |
| N. quercina | Hz4-1 | ON961030 | - | - | - | ON996909 | ON429028 |
| N. quercina | CBS 159.37 | MN984016 | - | - | - | - | MN973004 |
| N. quercina | MF-32.61 | - | - | - | - | - | KY552963 |
| N. spiraeae | CFCC 53928 * | MN879295 | - | - | - | MN879292 | MN737833 |
| N. variabilis | CBS 142457 * | LT593008 | - | - | - | LT593078 | LT592939 |
| Date of Collection | ||||
|---|---|---|---|---|
| 8 November 2013 | 10 January 2014 | 25 March 2014 | 10 June 2014 | |
| Number of tested trees/shoots | 2/13 + 3 1 | 3/10 + 10 1 | 5/13 2 | 5/21 3 |
| Total infected shoots, % | 100 | 100 | 84.6 | 100 |
| Infected upper sections | ||||
| shoots, % | 46.2 | 90 | 61.5 | 85.7 |
| buds, % | 23.1 | 70 | 61.5 | n.d. |
| nodes, % | 15.4 | 90 | n.d. | 31.6 |
| internodes, % | 7.7 | 20 | 15.4 | n.d. |
| Infected middle sections | ||||
| shoots, % | 84.6 | 50 | 69.2 | 100 |
| buds, % | 69.2 | 40 | 61.5 | n.d. |
| nodes, % | 30.8 | 30 | n.d. | 36 |
| internodes, % | 0 | 10 | 30.8 | n.d. |
| Infected basal sections | ||||
| shoots, % | 61.5 | 70 | 38.5 | 100 |
| buds, % | 30.8 | 70 | 30.8 | n.d. |
| nodes, % | 46.2 | 40 | n.d. | 49.4 |
| internodes, % | 0 | 40 | 30.8 | n.d. |
| Infected inner parts of buds (without scales), % | 0 | 10 | 5.1 | n.d. |
| Infected bud scales, % | n.d. | 100 | 46.2 | n.d. |
| Fungi | Date of Collection | |||
|---|---|---|---|---|
| 8 November 2013 | 10 January 2014 | 25 March 2014 | 10 June 2014 | |
| Surface-sterilized shoots | ||||
| Alternaria alternata | F | D | F | O |
| A. tenuissima | − | − | F | F |
| Cladosporium cladosporioides | R | O | − | O |
| Diaporthe eres | D | F | F | R |
| Epicoccum nigrum | − | R | − | R |
| Fusarium oxysporum | F | − | − | O |
| Nothophoma quercina | F | − | D | F |
| Unsterilized shoots | ||||
| A. alternata | + | + | + | + |
| A. tenuissima | − | − | + | + |
| C. cladosporioides | + | + | − | + |
| D. eres | − | − | + | − |
| E. nigrum | − | + | − | + |
| Fusarium graminearum | − | − | − | + |
| F. oxysporum | + | + | + | − |
| N. quercina | + | − | + | + |
| Light-colored sterile mycelium | − | − | − | + |
| Dark-colored sterile mycelium | + | − | − | − |
| Phenological Stage (Date of Collection) 1 | ||||
|---|---|---|---|---|
| Beginning of Growing Season (30 April) | End of New Shoot Growth (8 June) | End of Summer (28 August) | Dormancy (13 November) | |
| Number of tested trees/shoots | 10/50 | 10/50 | 10/50 | 10/50 |
| Total infected shoots, % | 48 | 8 | 26 | 82 |
| Infected upper sections: | ||||
| shoots, % | 18 | 0 | 10.4 | 46 |
| buds, % | 2.4 | 0 | 2.1 | 14.6 |
| nodes, % | 15 | 0 | 8.5 | 20.8 |
| internodes, % | 5 | 0 | 0 | 16 |
| Infected middle sections: | ||||
| shoots, % | 26 | 2 | 12.5 | 40 |
| buds, % | 5 | 0 | 6.4 | 14 |
| nodes, % | 15 | 2 | 8.3 | 18 |
| internodes, % | 2.5 | 2 | 4.3 | 26 |
| Infected basal sections, shoots % | 26 | 8 | 10.4 | 46 |
| Fungi | Phenological Stage (Date of Collection) | |||
|---|---|---|---|---|
| Beginning of Growing Season (30 April) | End of New Shoot Growth (8 June) | End of Summer (28 August) | Dormancy (13 November) | |
| Alternaria alternata | − | − | F | O |
| A. tenuissima | − | − | − | O |
| Aureobasidium pullulans | − | − | O | R |
| Cladosporium cladosporioides | − | − | − | O |
| C. herbarum | − | − | − | O |
| Diaporthe eres | F | − | − | O |
| Epicoccum nigrum | − | − | − | O |
| Gliocladium sp. | − | − | − | O |
| Sarocladium strictum | − | − | O | − |
| Nothophoma quercina | F | + | D | F |
| Tripospermum myrti | O | − | − | − |
| Phenological Stage (Date of Collection) | ||||||
|---|---|---|---|---|---|---|
| Dormancy (24 February 2015) | Dormancy (25 March 2014) | Beginning of Burst of Vegetative Buds (25 April 2014) | Swelling of Vegetative Buds (30 April 2015) | New Shoot Growth (23 May 2014) | New Shoot Growth (30 May 2014) | |
| Total number of explants/number of infected explants | 21/13 | 40/5 | 40/7 | 40/5 | 45/19 | 10/3 |
| Type of explant | Buds with stem segments (bud scales removed) | Buds without scales | Buds without scales | Buds without scales | Segments of green shoots with nodes | Segments of green shoots with nodes |
| Method of surface sterilization 1 | EtOH (2) + HgCl2 (10) | EtOH (2) + HgCl2 (10) | HgCl2 (10) | EtOH (2) + HgCl2 (10) | HgCl2 (5) | HgCl2 (1) |
| Nutrient medium 2 | Modified QL or 1/2QL | Modified MS | QL | QL or Modified QL | QL | QL |
| Isolated fungal species | N. quercina, A. alternata, C. cladosporioides | A. alternata, C. cladosporioides, sterile mycelium | N. quercina | C. cladosporioides, T. myrti | D. eres, N. quercina, sterile mycelia | N. quercina, sterile mycelium |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nekrasov, E.V.; Shumilova, L.P.; Gomzhina, M.M.; Aleksandrova, A.V.; Kokaeva, L.Y.; Pavlova, L.M. Diversity of Endophytic Fungi in Annual Shoots of Prunus mandshurica (Rosaceae) in the South of Amur Region, Russia. Diversity 2022, 14, 1124. https://doi.org/10.3390/d14121124
Nekrasov EV, Shumilova LP, Gomzhina MM, Aleksandrova AV, Kokaeva LY, Pavlova LM. Diversity of Endophytic Fungi in Annual Shoots of Prunus mandshurica (Rosaceae) in the South of Amur Region, Russia. Diversity. 2022; 14(12):1124. https://doi.org/10.3390/d14121124
Chicago/Turabian StyleNekrasov, Eduard V., Lyudmila P. Shumilova, Maria M. Gomzhina, Alina V. Aleksandrova, Lyudmila Y. Kokaeva, and Lyudmila M. Pavlova. 2022. "Diversity of Endophytic Fungi in Annual Shoots of Prunus mandshurica (Rosaceae) in the South of Amur Region, Russia" Diversity 14, no. 12: 1124. https://doi.org/10.3390/d14121124
APA StyleNekrasov, E. V., Shumilova, L. P., Gomzhina, M. M., Aleksandrova, A. V., Kokaeva, L. Y., & Pavlova, L. M. (2022). Diversity of Endophytic Fungi in Annual Shoots of Prunus mandshurica (Rosaceae) in the South of Amur Region, Russia. Diversity, 14(12), 1124. https://doi.org/10.3390/d14121124






