Volatile Organic Compounds (VOCs) Diversity in the Orchid Himantoglossum robertianum (Loisel.) P. Delforge from Sardinia (Italy)
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material
2.3. Extraction of Essential Oils
2.4. GC-FID and GC-Ms Analysis
2.5. Identification of the Components of the Volatile Fractions
2.6. Statistical Analysis
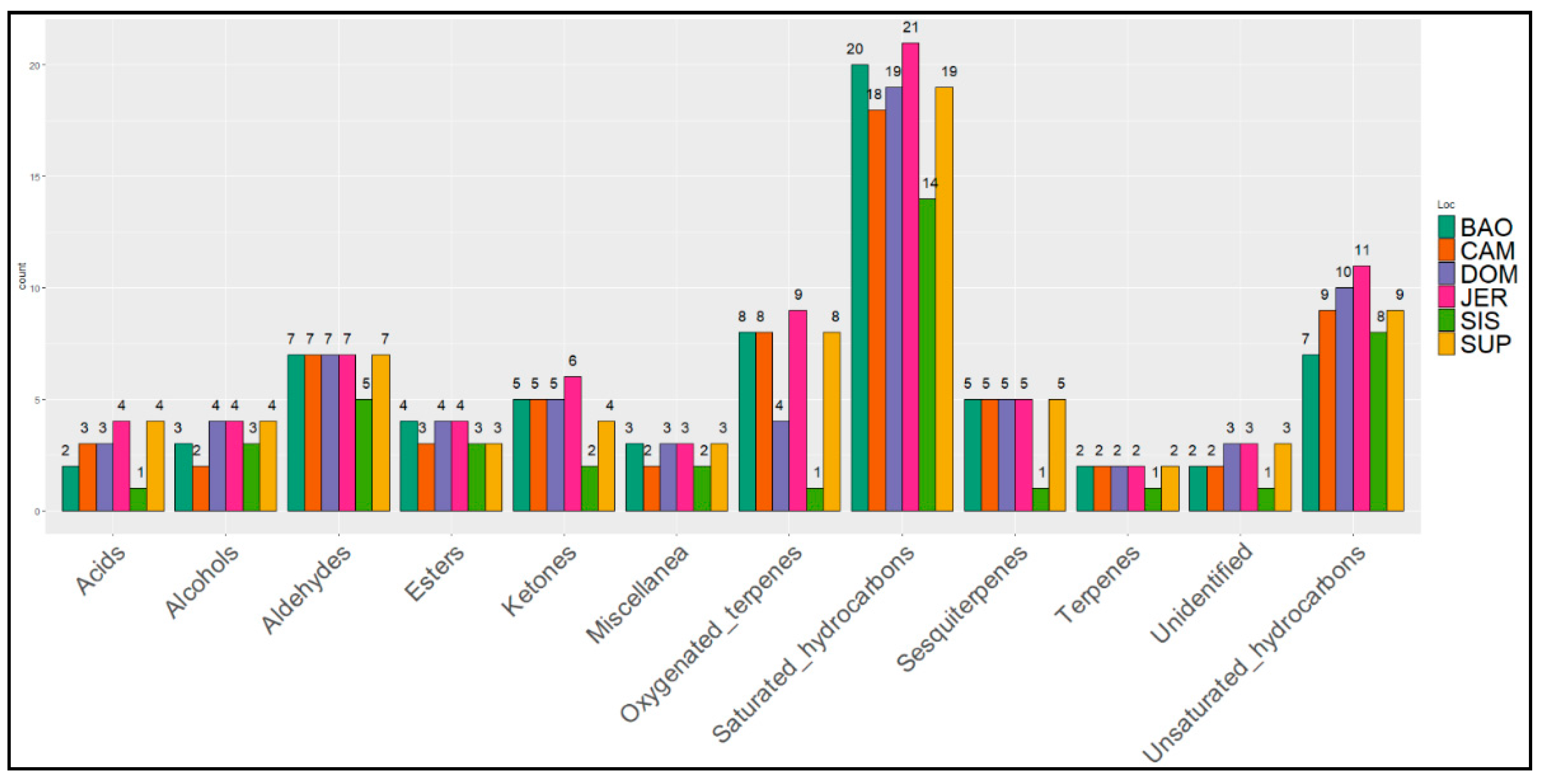
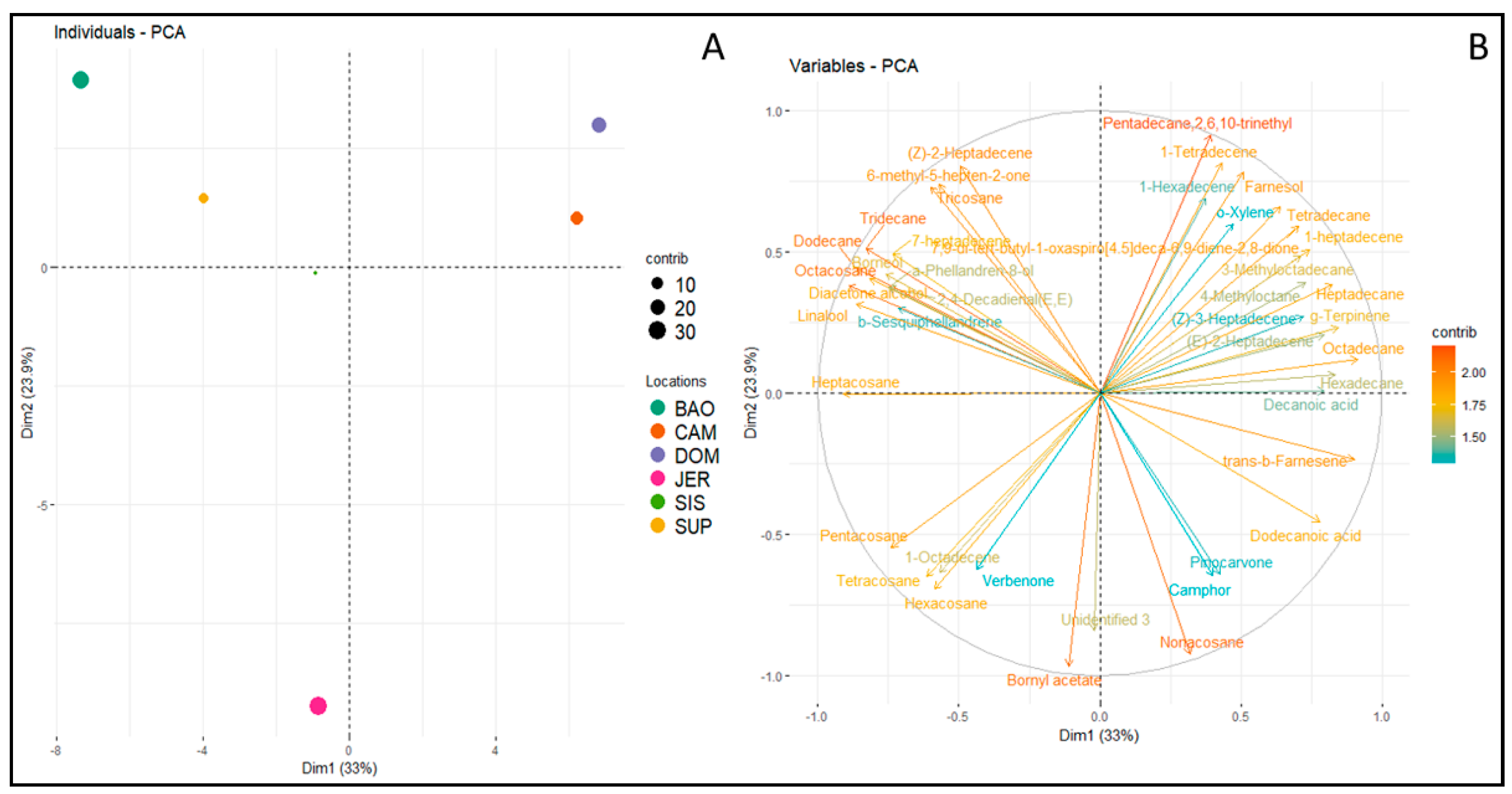
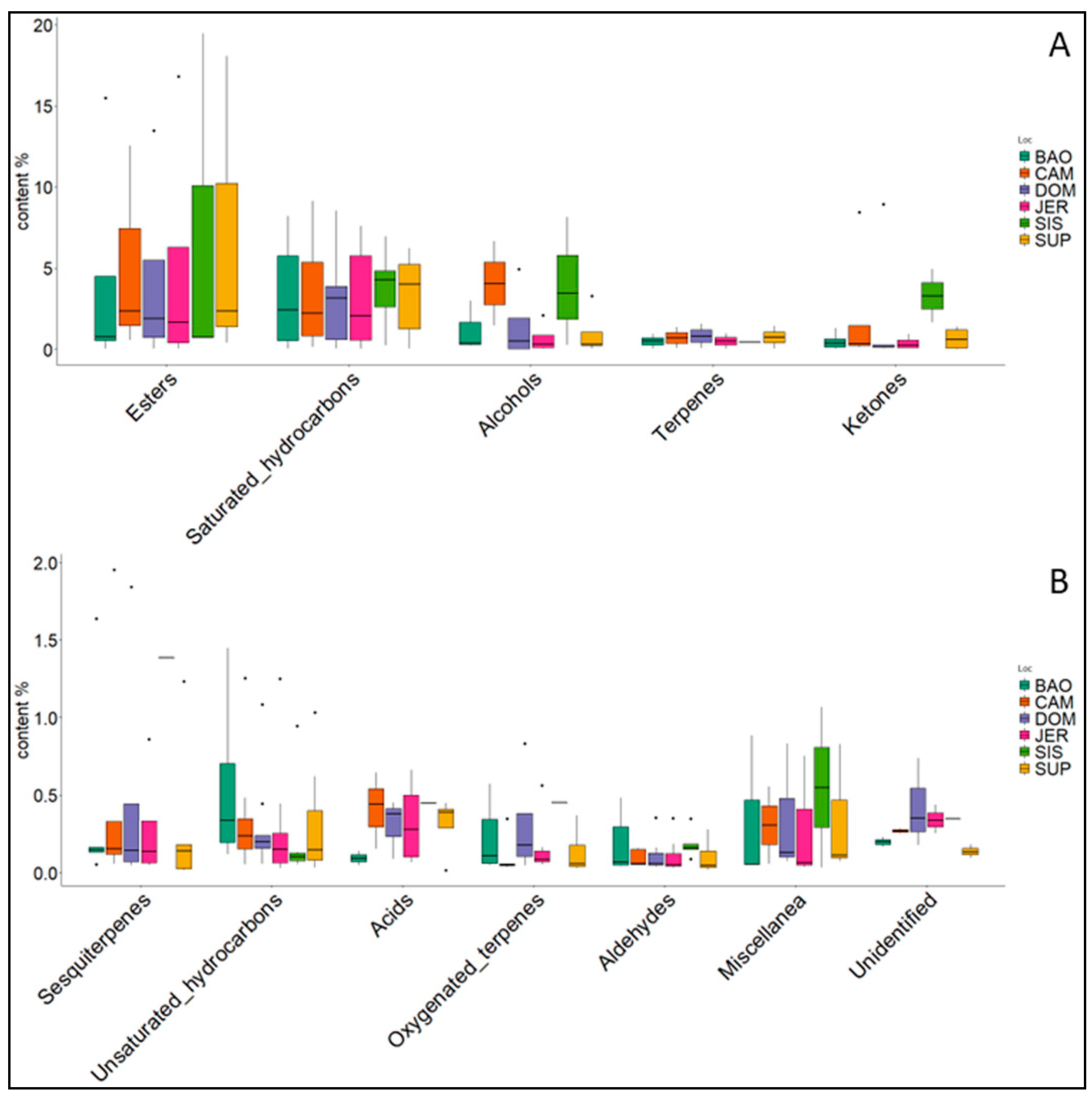
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loreto, F.; Dicke, M.; Schnitzler, J.P.; Turlings, T.C.J. Plant volatiles and the environment. Plant Cell Environ. 2014, 37, 1905–1908. [Google Scholar] [CrossRef]
- Raguso, R.A. Wake up and smell the roses: The ecology and evolution of floral scent. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 549–569. [Google Scholar] [CrossRef]
- Ramya, M.; Kwon, O.K.; An, H.R.; Park, P.M.; Baek, Y.S.; Park, P.H. Floral scent: Regulation and role of MYB transcription factors. Phytochem. Lett. 2017, 19, 114–120. [Google Scholar] [CrossRef]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Scheffer, J.J.C. Factors affecting secondary metabolite production in plants: Volatile components and essential oils. Flavour Fragr. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Loreto, F.; Bagnoli, F.; Fineschi, S. One species, many terpenes: Matching chemical and biological diversity. Trends Plant Sci. 2009, 14, 416–420. [Google Scholar] [CrossRef]
- Vickers, C.E.; Gershenzon, J.; Lerdau, M.T.; Loreto, F. A unified mechanism of action for volatile isoprenoids in plant abiotic stress. Nat. Chem. Biol. 2009, 5, 283–291. [Google Scholar] [CrossRef]
- Vivaldo, G.; Masi, E.; Taiti, C.; Caldarelli, G.; Mancuso, S. The network of plants volatile organic compounds. Sci. Rep. 2017, 7, 11050. [Google Scholar] [CrossRef]
- Givnish, T.J.; Spalink, D.; Ames, M.; Lyon, S.P.; Hunter, S.J.; Zuluaga, A.; Iles, W.J.D.; Clements, M.A.; Arroyo, M.T.K.; Leebens-Mack, J.; et al. Orchid phylogenomics and multiple drivers of their extraordinary diversification. Proc. R. Soc. B Biol. Sci. 2015, 282, 20151553. [Google Scholar] [CrossRef]
- Cozzolino, S.; Widmer, A. Orchid diversity: An evolutionary consequence of deception? Trends Ecol. Evol. 2005, 20, 487–494. [Google Scholar] [CrossRef]
- Cortis, P.; Vereecken, N.J.; Schiestl, F.P.; Barone Lumaga, M.R.; Scrugli, A.; Cozzolino, S. Pollinator convergence and the nature of species’ boundaries in sympatric Sardinian Ophrys (Orchidaceae). Ann. Bot. 2009, 104, 497–506. [Google Scholar] [CrossRef]
- Lussu, M.; De Agostini, A.; Marignani, M.; Cogoni, A.; Cortis, P. Ophrys annae and Ophrys chestermanii: An impossible love between two orchid sister species. Nord. J. Bot. 2018, 36, e01798. [Google Scholar] [CrossRef]
- Bazzicalupo, M.; Burlando, B.; Denaro, M.; Barreca, D.; Trombetta, D.; Smeriglio, A.; Cornara, L. Polyphenol characterization and skin-preserving properties of hydroalcoholic flower extract from Himantoglossum robertianum (Orchidaceae). Plants 2019, 8, 502. [Google Scholar] [CrossRef]
- Delforge, P. Orchids of Europe, North Africa and the Middle East; Timber Press: Portland, OR, USA, 2006. [Google Scholar]
- Lussu, M.; Marignani, M.; Lai, R.; Loi, M.C.; Cogoni, A.; Cortis, P. A synopsis of Sardinian studies: Why is it important to work on island orchids? Plants 2020, 9, 853. [Google Scholar] [CrossRef]
- Agenzia Regionale Per La Protezione Dell’ambiente Della Sardegna (Arpas)—Dipartimento Specialistico Regionale Meteoclimatico. Available online: http://www.sar.sardegna.it/pubblicazioni/riepiloghimensili/mensili.asp (accessed on 3 March 2022).
- Aru, A.; Baldaccini, P.; Vacca, A.; Delogu, G.; Dessena, M.A.; Madrau, S.; Melis, R.T.; Vacca, S. Nota illustrativa alla Carta dei Suoli della Sardegna. In Dipartimento Scienze Della Terra Università di Cagliari, Assessorato Regionale alla Programmazione Bilancio ed Assetto del Territorio; UniCa—Università degli Studi di Cagliari: Cagliari, Italy, 1991. [Google Scholar]
- Aru, A.; Baldaccini, P.; Vacca, A.; Delogu, G.; Dessena, M.A.; Madrau, S.; Melis, R.T.; Vacca, S. Carta dei suoli della Sardegna, in scala 1:250.000. In Dipartimento Scienze Della Terra Università di Cagliari, Assessorato Regionale alla Programmazione Bilancio ed Assetto del Territorio; UniCa—Università degli Studi di Cagliari: Cagliari, Italy, 1990. [Google Scholar]
- Robustelli della Cuna, F.S.; Calevo, J.; Bari, E.; Giovannini, A.; Boselli, C.; Tava, A. Characterization and antioxidant activity of essential oil of four sympatric orchid species. Molecules 2019, 24, 3878. [Google Scholar] [CrossRef]
- Robustelli della Cuna, F.S.; Cortis, P.; Esposito, F.; De Agostini, A.; Sottani, C.; Sanna, C. Chemical composition of essential oil from four sympatric orchids in NW-Italy. Plants 2022, 11, 826. [Google Scholar] [CrossRef]
- Adams, R. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Pub Corp: Carol Stream, IL, USA, 2007. [Google Scholar]
- Stein, S.E. NIST/EPA/NIH Mass Spectral Database, Version 2.1; Perkin-Elmer Instrument LLC: Waltham, MA, USA, 2000. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. 2022. Available online: https://www.R-project.org/ (accessed on 7 December 2022).
- Fois, M.; Farris, E.; Calvia, G.; Campus, G.; Fenu, G.; Porceddu, M.; Bacchetta, G. The endemic vascular flora of Sardinia: A dynamic checklist with an overview of biogeography and conservation status. Plants 2022, 11, 601. [Google Scholar] [CrossRef]
- Cañadas, E.M.; Fenu, G.; Peñas, J.; Lorite, J.; Mattana, E.; Bacchetta, G. Hotspots within hotspots: Endemic plant richness, environmental drivers, and implications for conservation. Biol. Conserv. 2014, 170, 282–291. [Google Scholar] [CrossRef]
- Sanna, C.; Maxia, A.; Fenu, G.; Loi, M.C. So uncommon and so singular, but underexplored: An updated overview on ethnobotanical uses, biological properties and phytoconstituents of Sardinian endemic plants. Plants 2020, 9, 958. [Google Scholar] [CrossRef]
- Guzzo, F.; Durán, A.G.; Sanna, C.; Marasco, R.; Molfetta, N.; Buommino, E.; Fiorentino, A.; D’Abrosca, B. Gallomyrtucommulones g and h, new phloroglucinol glycosides, from bioactive fractions of Myrtus communis against Staphylococcus species. Molecules 2022, 27, 7109. [Google Scholar] [CrossRef]
- Venditti, A.; Lattanzi, C.; Ornano, L.; Maggi, F.; Sanna, C.; Ballero, M.; Alvino, A.; Serafini, M.; Bianco, A. A new glucosidic phthalide from Helichrysum microphyllum subsp. tyrrhenicum from La Maddalena Island (Sardinia, Italy). Nat. Prod. Res. 2016, 30, 789–795. [Google Scholar] [CrossRef]
- Ongaro, S.; Martellos, S.; Bacaro, G.; De Agostini, A.; Cogoni, A.; Cortis, P. Distributional pattern of Sardinian orchids under a climate change scenario. Community Ecol. 2018, 19, 223–232. [Google Scholar] [CrossRef]
- De Agostini, A.; Caltagirone, C.; Caredda, A.; Cicatelli, A.; Cogoni, A.; Farci, D.; Guarino, F.; Garau, A.; Labra, M.; Lussu, M.; et al. Heavy metal tolerance of orchid populations growing on abandoned mine tailings: A case study in Sardinia Island (Italy). Ecotoxicol. Environ. Saf. 2020, 189, 110018. [Google Scholar] [CrossRef] [PubMed]
- De Agostini, A.; Cortis, P.; Cogoni, A.; Gargiulo, R.; Fenu, G. Epipactis tremolsii seed diversity in two close but extremely different populations: Just a case of intraspecific variability? Plants 2020, 9, 625. [Google Scholar] [CrossRef] [PubMed]
- De Agostini, A.; Cogoni, D.; Cogoni, A.; Vacca, A.; Fenu, G.; Cortis, P. Seed bank conservation and incipient seed development in orchids colonizing mining wastes: Results of a field pilot experiment. Plants 2022, 11, 3315. [Google Scholar] [CrossRef] [PubMed]
- Gallego, E.; Gelabert, A.; Roca, F.J.; Perales, J.F.; Guardino, X. Identification of volatile organic compounds (VOC) emitted from three European orchid species with different pollination strategies: Two deceptive orchids (Himantoglossum robertianum and Ophrys apifera) and a rewarding orchid (Gymnadenia conopsea). J. Bio. Environ. Sci. 2012, 2, 18–29. [Google Scholar]
- Romano, V.A.; Rosati, L.; Fascetti, S.; Cittadini, A.M.R.; Racioppi, R.; Lorenz, R.; D’Auria, M. Spatial and temporal variability of the floral scent emitted by Barlia robertiana (Loisel.) Greuter, a Mediterranean food-deceptive orchid. Compounds 2022, 2, 37–53. [Google Scholar] [CrossRef]
- Mecca, M.; Racioppi, R.; Romano, V.A.; Viggiani, L.; Lorenz, R.; D’Auria, M. The scent of Himantoglossum species found in Basilicata (Southern Italy). Compounds 2021, 1, 164–173. [Google Scholar] [CrossRef]
- Possell, M.; Loreto, F. The role of volatile organic compounds in plant resistance to abiotic stresses: Responses and mechanisms. In Biology, Control and Models of Tree Volatile Organic Compound Emissions; Niinemets, Ü., Monson, R., Eds.; Springer: Dordrecht, The Netherlands, 2013; Volume 5, pp. 209–235. [Google Scholar] [CrossRef]
- Holopainen, J.K.; Gershenzon, J. Multiple stress factors and the emission of plant VOCs. Trends Plant Sci. 2010, 15, 176–184. [Google Scholar] [CrossRef]
- Jakobsen, H.B.; Olsen, C.E. Influence of climatic factors on emission of flower volatiles in situ. Planta 1994, 192, 365–371. [Google Scholar] [CrossRef]
- Yatagai, M.; Ohira, M.; Ohira, T.; Nagai, S. Seasonal variations of terpene emission from trees and influence of temperature, light and contact stimulation on terpene emission. Chemosphere 1995, 30, 1137–1149. [Google Scholar] [CrossRef]
- Rivoal, A.; Fernandez, C.; Lavoir, A.V.; Olivier, R.; Lecareux, C.; Greff, S.; Roche, P.; Vila, B. Environmental control of terpene emissions from Cistus monspeliensis L. in natural Mediterranean shrublands. Chemosphere 2010, 78, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Schiestl, F.P.; Ayasse, M.; Paulus, H.F.; Löfstedt, C.; Hansson, B.S.; Ibarra, F.; Francke, W. Sex pheromone mimicry in the early spider orchid (Ophrys sphegodes): Patters of hydrocarbons as the key mechanism for pollination by sexual deception. J. Comp. Physiol. A 2000, 186, 567–574. [Google Scholar] [CrossRef] [PubMed]

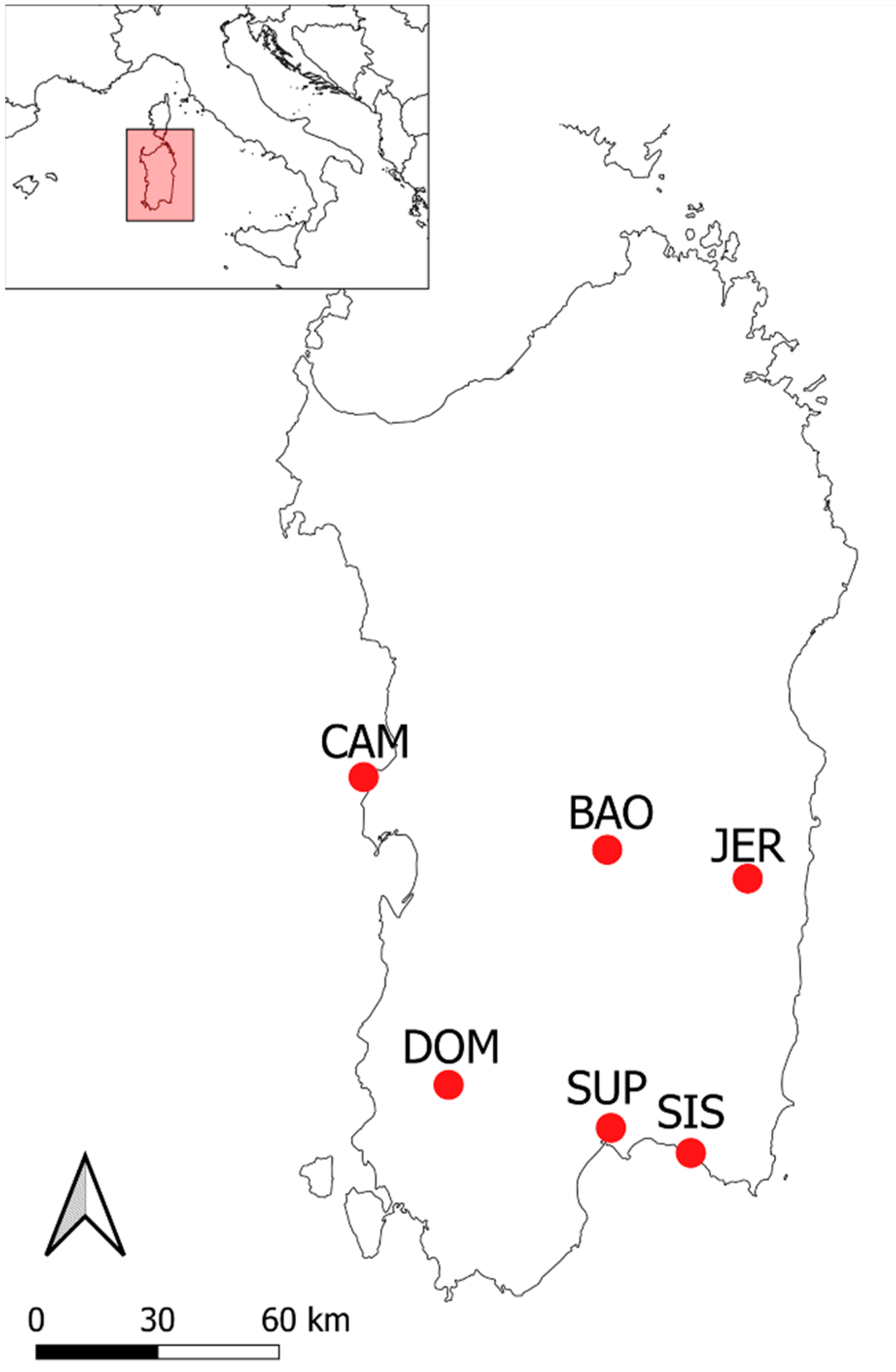

| Label a | Site b | Municipality c | Coord. d | Alt. e | Date f | Voucher g |
|---|---|---|---|---|---|---|
| BAO | Rio Bau Onu | Laconi | 39°52′31.36″ N 9°5′36.38″ E | 719 m | 16 March 2021 | CAG1305/V1e |
| CAM | Capo Mannu | San Vero Milis | 40° 2′35.60″ N 8°23′15.46″ E | 48 m | 23 February 2021 | CAG1305/V1f |
| DOM | Domusnovas | Domusnovas | 39°21′30.13″ N 8°37′4.20″ E | 258 m | 2 March 2021 | CAG1305/V1c |
| JER | Jerzu | Jerzu | 39°48′20.6″ N 9°30′46.3″ E | 550 m | 25 February 2021 | CAG1305/V1b |
| SIS | Sant’Isidoro | Quartucciu | 39°14′43.03″ N 9°18′3.31″ E | 54 m | 1 March 2021 | CAG1305/V1d |
| SUP | Su Planu | Selargius | 39°15′27.62″ N 9°6′28.60″ E | 41 m | 17 February 2021 | CAG1305/V1a |
| Compound a | RI Tab b | RI mean c | BAO | CAM | DOM | JER | SIS | SUP | Identification e |
|---|---|---|---|---|---|---|---|---|---|
| % d | % | % | % | % | % | ||||
| 2-Methyl-2-pentenal | 821 | 828 | 0.16 ± 0.03 | 0.15 ± 0.04 | 0.35 ± 0.04 | 0.35 ± 0.04 | 0.15 ± 0.03 | 0.28 ± 0.04 | NIST. RI |
| Diacetone alcohol | 841 | 836 | 0.35 ± 0.04 | 0.14 ± 0.05 | 0.12 ± 0.03 | 0.15 ± 0.04 | - | - | NIST. RI |
| 4-Methyloctane | 862 | 861 | 0.05 ± 0.04 | - | 0.10 ± 0.04 | 0.06 ± 0.03 | - | - | NIST. RI |
| o-Xylene | 867 | 869 | 0.05 ± 0.03 | - | 0.13 ± 0.05 | 0.03 ± 0.02 | - | 0.11 ± 0.03 | NIST. RI |
| Heptanal | 901 | 902 | 0.43 ± 0.03 | 0.05 ± 0.03 | 0.09 ± 0.04 | 0.05 ± 0.02 | 0.35 ± 0.04 | 0.04 ± 0.03 | NIST. RI |
| Anisole | 916 | 916 | 0.05 ± 0.03 | 0.06 ± 0.04 | 0.07 ± 0.04 | 0.06 ± 0.05 | 0.03 ± 0.02 | 0.07 ± 0.02 | NIST. RI |
| 6-methyl-5-hepten-2-one | 986 | 985 | 0.64 ± 0.04 | 0.24 ± 0.04 | 0.26 ± 0.03 | 0.06 ± 0.03 | - | - | NIST. RI |
| Decane | 1000 | 1000 | 0.04 ± 0.04 | - | 0.04 ± 0.03 | 0.03 ± 0.02 | - | 0.02 ± 0.01 | NIST. RI |
| Octanal | 1007 | 1004 | 0.05 ± 0.03 | 0.05 ± 0.02 | 0.06 ± 0.03 | 0.04 ± 0.04 | 0.16 ± 0.05 | 0.03 ± 0.02 | NIST. RI |
| 2-Ethylhexanol | 1029 | 1029 | - | - | 0.03 ± 0.02 | 0.03 ± 0.02 | - | 0.05 ± 0.04 | NIST. RI |
| β-Phorone | 1044 | 1044 | 1.30 ± 0.11 | 1.45 ± 0.04 | - | 0.63 ± 0.04 | 1.66 ± 0.05 | 1.13 ± 0.04 | NIST. RI |
| γ-Terpinene | 1059 | 1060 | 0.04 ± 0.03 | 0.05 ± 0.03 | 0.07 ± 0.02 | 0.04 ± 0.04 | - | 0.04 ± 0.02 | NIST. RI |
| 1-Octanol | 1074 | 1071 | 0.25 ± 0.05 | - | 0.05 ± 0.04 | 0.13 ± 0.03 | 0.27 ± 0.05 | 0.28 ± 0.05 | NIST. RI |
| p-Cresol | 1076 | 1077 | 2.99 ± 0.10 | 6.65 ± 0.03 | 4.94 ± 0.04 | 2.08 ± 0.04 | 8.13 ± 0.03 | 3.29 ± 0.02 | NIST. RI |
| Linalool | 1097 | 1099 | 0.34 ± 0.03 | 0.06 ± 0.05 | 0.12 ± 0.03 | 0.14 ± 0.04 | - | 0.18 ± 0.04 | NIST. RI |
| 2-Phenyl ethanol | 1102 | 1107 | 0.35 ± 0.04 | 1.46 ± 0.04 | 0.93 ± 0.03 | 0.48 ± 0.03 | 3.45 ± 0.04 | 0.33 ± 0.01 | NIST. RI |
| cis-Verbenol | 1141 | 1142 | 0.06 ± 0.04 | 0.04 ± 0.04 | 0.05 ± 0.04 | 0.05 ± 0.04 | - | 0.03 ± 0.03 | NIST. RI |
| trans-Verbenol | 1145 | 1148 | 0.05 ± 0.04 | 0.05 ± 0.03 | - | 0.07 ± 0.04 | - | 0.03 ± 0.02 | NIST. RI |
| Camphor | 1150 | 1154 | 0.04 ± 0.04 | - | 0.06 ± 0.03 | 0.07 ± 0.03 | - | 0.02 ± 0.01 | NIST. RI |
| β-Phellandren-8-ol | 1154 | 1156 | - | - | - | 0.06 ± 0.04 | - | 0.05 ± 0.03 | NIST. RI |
| Pinocarvone | 1165 | 1162 | 0.15 ± 0.03 | 0.35 ± 0.04 | 0.15 ± 0.04 | 0.34 ± 0.05 | - | 0.09 ± 0.04 | NIST. RI |
| α-Phellandren-8-ol | 1170 | 1176 | 0.3 4 ± 0.05 | 0.05 ± 0.02 | - | 0.14 ± 0.04 | - | 0.18 ± 0.07 | NIST. RI |
| Borneol | 1173 | 1179 | 0.57 ± 0.04 | 0.03 ± 0.03 | 0.23 ± 0.04 | 0.16 ± 0.04 | - | - | NIST. RI |
| Terpinen-4-ol | 1182 | 1186 | 0.07 ± 0.04 | 0.06 ± 0.03 | - | 0.08 ± 0.03 | - | 0.04 ± 0.01 | NIST. RI |
| Dodecane | 1200 | 1200 | 0.53 ± 0.04 | 0.15 ± 0.03 | 0.16 ± 0.04 | 0.18 ± 0.04 | 0.26 ± 0.03 | 0.32 ± 0.03 | NIST. RI |
| Decanal | 1204 | 1207 | 0.04 ± 0.04 | 0.15 ± 0.04 | 0.05 ± 0.03 | 0.03 ± 0.02 | - | 0.07 ± 0.03 | NIST. RI |
| Verbenone | 1208 | 1213 | 0.06 ± 0.04 | 0.04 ± 0.04 | - | 0.07 ± 0.03 | - | 0.06 ± 0.02 | NIST. RI |
| Geraniol | 1253 | 1252 | 0.15 ± 0.03 | 0.35 ± 0.04 | 0.83 ± 0.02 | 0.56 ± 0.03 | 0.45 ± 0.04 | 0.37 ± 0.04 | NIST. RI |
| 2-Decenal. (E) | 1264 | 1264 | 0.05 ± 0.04 | 0.06 ± 0.03 | 0.05 ± 0.03 | 0.06 ± 0.03 | - | 0.02 ± 0.01 | NIST. RI |
| Nonanoic acid | 1271 | 1270 | - | - | - | 0.11 ± 0.03 | - | 0.40 ± 0.54 | NIST. RI |
| Bornyl acetate | 1289 | 1288 | 0.04 ± 0.03 | - | 0.03 ± 0.03 | 0.05 ± 0.03 | - | - | NIST. RI |
| Tridecane | 1300 | 1300 | 0.55 ± 0.04 | 0.15 ± 0.02 | 0.18 ± 0.04 | 0.15 ± 0.04 | 0.24 ± 0.02 | 0.46 ± 0.02 | NIST. RI |
| 2.4-Decadienal (E.Z) | 1302 | 1308 | 0.07 ± 0.05 | 0.05 ± 0.04 | 0.04 ± 0.03 | 0.05 ± 0.03 | 0.09 ± 0.05 | 0.03 ± 0.03 | NIST. RI |
| 2.4-Decadienal (E.E) | 1319 | 1321 | 0.48 ± 0.07 | 0.16 ± 0.02 | 0.16 ± 0.03 | 0.18 ± 0.02 | 0.18 ± 0.05 | 0.21 ± 0.03 | NIST. RI |
| Decanoic acid | 1372 | 1366 | 0.05 ± 0.04 | 0.15 ± 0.05 | 0.09 ± 0.04 | 0.06 ± 0.02 | - | 0.01 ± 0.01 | NIST. RI |
| 1-Tetradecene | 1390 | 1392 | - | - | 0.06 ± 0.03 | 0.03 ± 0.02 | - | - | MS. RI |
| Tetradecane | 1400 | 1401 | 1.62 ± 0.03 | 1.83 ± 0.03 | 2.07 ± 0.02 | 1.14 ± 0.02 | - | 1.24 ± 0.02 | NIST. RI |
| β-Caryophyllene | 1417 | 1428 | 0.05 ± 0.04 | 0.15 ± 0.03 | 0.07 ± 0.04 | 0.06 ± 0.03 | - | 0.01 ± 0.01 | NIST. RI |
| Unidentified | - | 1433 | 0.23 ± 0.04 | 0.25 ± 0.04 | 0.74 ± 0.03 | 0.44 ± 0.04 | - | 0.18 ± 0.04 | - |
| trans-b-Farnesene | 1452 | 1459 | 0.15 ± 0.04 | 0.33 ± 0.03 | 0.44 ± 0.04 | 0.33 ± 0.04 | - | 0.18 ± 0.03 | NIST. RI |
| 2,5-Cyclohexadiene-1,4-dione, 2,6-bis(1.1-dimethylethyl) | 1469 | 1466 | 0.88 ± 0.12 | 0.55 ± 0.03 | 0.83 ± 0.03 | 0.75 ± 0.03 | 1.07 ± 0.02 | 0.83 ± 0.03 | NIST. RI |
| β-Sesquiphellandrene | 1537 | 1530 | 0.16 ± 0.02 | 0.12 ± 0.03 | 0.14 ± 0.03 | 0.14 ± 0.04 | - | 0.14 ± 0.05 | NIST. RI |
| Unidentified | - | 1533 | 0.17 ± 0.07 | 0.28 ± 0.02 | 0.35 ± 0.03 | 0.25 ± 0.04 | 0.34 ± 0.04 | 0.09 ± 0.04 | - |
| Dodecanoic acid | 1566 | 1565 | 0.14 ± 0.03 | 0.44 ± 0.04 | 0.45 ± 0.04 | 0.44 ± 0.04 | - | 0.38 ± 0.04 | |
| 1-Hexadecene | 1591 | 1590 | 0.12 ± 0.01 | 0.15 ± 0.03 | 0.15 ± 0.04 | 0.05 ± 0.04 | 0.05 ± 0.04 | 0.14 ± 0.04 | MS. RI |
| 2-Hexadecene | 1598 | 1596 | 0.15 ± 0.04 | 0.05 ± 0.03 | 0.16 ± 0.03 | 0.07 ± 0.04 | 0.09 ± 0.04 | 0.07 ± 0.02 | MS. RI |
| Hexadecane | 1600 | 1601 | 0.38 ± 0.08 | 0.64 ± 0.05 | 0.85 ± 0.04 | 0.57 ± 0.03 | - | 0.61 ± 0.03 | NIST. RI |
| γ-eudesmol | 1630 | 1634 | 0.13 ± 0.03 | 0.06 ± 0.04 | 0.05 ± 0.04 | 0.05 ± 0.04 | - | 0.02 ± 0.01 | NIST. RI |
| Pentadecane. 2.6.10-trinethyl | 1647 | 1647 | 0.32 ± 0.02 | 0.34 ± 0.05 | 0.35 ± 0.04 | 0.26 ± 0.03 | - | - | NIST. RI |
| 7-heptadecene | 1673 | 1674 | 0.86 ± 0.03 | 0.13 ± 0.03 | 0.24 ± 0.05 | 0.15 ± 0.02 | 0.08 ± 0.05 | 0.62 ± 0.03 | MS. RI |
| 1-heptadecene | 1680 | 1679 | - | 0.15 ± 0.04 | 0.15 ± 0.04 | 0.03 ± 0.02 | 0.07 ± 0.04 | 0.03 ± 0.01 | MS. RI |
| (E)-3-Heptadecene | 1686 | 1683 | 0.33 ± 0.03 | 0.24 ± 0.05 | 0.24 ± 0.04 | 0.12 ± 0.03 | 0.11 ± 0.02 | 0.08 ± 0.01 | MS. RI |
| (Z)-3-Heptadecene | 1692 | 1693 | - | 0.25 ± 0.04 | 0.24 ± 0.03 | 0.17 ± 0.03 | 0.13 ± 0.04 | 0.14 ± 0.02 | MS. RI |
| Heptadecane | 1700 | 1703 | 2.05 ± 0.03 | 2.59 ± 0.02 | 3.35 ± 0.04 | 2.04 ± 0.03 | 2.25 ± 0.04 | 2.15 ± 0.04 | NIST. RI |
| (E)-2-Heptadecene | 1702 | 1709 | 0.24 ± 0.04 | 0.48 ± 0.04 | 0.44 ± 0.04 | 0.25 ± 0.03 | 0.12 ± 0.04 | 0.21 ± 0.02 | MS. RI |
| (Z)-2-Heptadecene | 1716 | 1713 | 0.55 ± 0.04 | 0.35 ± 0.03 | - | 0.25 ± 0.03 | - | - | MS. RI |
| Farnesol | 1718 | 1720 | 1.64 ± 0.09 | 1.95 ± 0.04 | 1.84 ± 0.03 | 0.86 ± 0.04 | 1.38 ± 0.08 | 1.23 ± 0.03 | NIST. RI |
| Methyl tetradecanoate | 1722 | 1725 | 0.85 ± 0.04 | 0.56 ± 0.02 | 0.94 ± 0.14 | 0.54 ± 0.04 | 0.70 ± 0.04 | 0.40 ± 0.01 | NIST. RI |
| 1-Octadecene | 1791 | 1793 | - | - | 0.16 ± 0.04 | 0.44 ± 0.15 | - | 0.40 ± 0.54 | MS. RI |
| Octadecane | 1800 | 1806 | 2.79 ± 0.12 | 5.85 ± 0.04 | 6.75 ± 0.04 | 4.26 ± 0.04 | 5.25 ± 0.04 | 4.75 ± 0.43 | STD. RI |
| Phytane | 1811 | 1810 | 0.93 ± 0.04 | 1.36 ± 0.03 | 1.56 ± 0.03 | 0.95 ± 0.04 | 0.44 ± 0.04 | 1.42 ± 0.02 | NIST. RI |
| 3-Methyloctadecane | 1874 | 1874 | 0.75 ± 0.03 | 1.36 ± 0.03 | 1.74 ± 0.04 | 0.74 ± 0.03 | - | 1.36 ± 0.02 | NIST. RI |
| Nonadecane | 1900 | 1903 | - | 6.15 ± 0.04 | - | 5.74 ± 0.05 | 6.94 ± 0.02 | 5.70 ± 0.03 | STD. RI |
| 7.9-di-tert-butyl-1-oxaspiro[4.5]deca-6.9-diene-2.8-dione | 1916 | 1910 | - | 8.45 ± 0.04 | 8.94 ± 0.04 | 0.93 ± 0.03 | 4.93 ± 0.03 | 1.39 ± 0.04 | NIST. RI |
| Hexadecanoic acid methyl ester | 1926 | 1934 | 15.50 ± 0.45 | 12.55 ± 0.04 | 13.47 ± 0.14 | 16.81 ± 0.05 | 19.46 ± 0.15 | 18.08 ± 0.05 | NIST. RI |
| Hexadecanoic acid | 1942 | 1939 | - | 0.64 ± 0.03 | 0.38 ± 0.05 | 0.66 ± 0.04 | 0.45 ± 0.04 | 0.45 ± 0.04 | NIST. RI |
| Eicosane | 2000 | 2006 | 8.22 ± 0.07 | 9.15 ± 0.04 | 8.55 ± 0.04 | 6.14 ± 0.19 | 6.45 ± 0.04 | 5.22 ± 0.03 | STD. RI |
| Heneicosane | 2100 | 2105 | 6.73 ± 0.04 | 7.73 ± 0.05 | 6.65 ± 0.06 | 5.84 ± 0.04 | 4.81 ± 0.06 | 5.77 ± 0.15 | STD. RI |
| Methyl octadecanoate | 2127 | 2124 | 0.68 ± 0.06 | 2.37 ± 0.08 | 2.85 ± 0.05 | 2.78 ± 0.03 | 0.74 ± 0.03 | 2.35 ± 0.03 | NIST. RI |
| Docosane | 2200 | 2203 | 5.76 ± 0.02 | 5.76 ± 0.17 | 5.05 ± 0.04 | 5.75 ± 0.04 | 4.48 ± 0.05 | 4.02 ± 0.03 | STD. RI |
| 9-Tricosene | 2279 | 2279 | 1.45 ± 0.04 | 1.20 ± 0.02 | 1.08 ± 0.05 | 1.25 ± 0.03 | 0.95 ± 0.04 | 1.03 ± 0.04 | MS. RI |
| Tricosane | 2300 | 2303 | 6.25 ± 0.04 | 4.13 ± 0.04 | 3.84 ± 0.04 | 2.73 ± 0.03 | 3.74 ± 0.03 | 5.61 ± 0.36 | STD. RI |
| Tetracosane | 2400 | 2402 | 4.94 ± 0.04 | 2.80 ± 0.04 | 3.44 ± 0.12 | 5.94 ± 0.03 | 3.84 ± 0.04 | 3.93 ± 0.05 | STD. RI |
| Pentacosane | 2500 | 2503 | 5.75 ± 0.03 | 2.96 ± 0.04 | 3.15 ± 0.05 | 6.25 ± 0.04 | 4.84 ± 0.04 | 4.04 ± 0.03 | STD. RI |
| Unidentified | - | 2590 | - | - | 0.18 ± 0.04 | 0.34 ± 0.04 | - | 0.13 ± 0.06 | - |
| Hexacosane | 2600 | 2602 | 4.64 ± 0.03 | 1.33 ± 0.04 | 3.24 ± 0.03 | 7.59 ± 0.08 | 4.06 ± 0.04 | 5.24 ± 0.04 | STD. RI |
| Heptacosane | 2700 | 2702 | 7.65 ± 0.05 | 1.76 ± 0.03 | 3.94 ± 0.04 | 5.63 ± 0.04 | 4.55 ± 0.04 | 6.22 ± 0.02 | STD. RI |
| Octacosane | 2800 | 2801 | 5.30 ± 0.03 | 0.35 ± 0.04 | 1.44 ± 0.05 | 1.54 ± 0.04 | 2.26 ± 0.08 | 4.62 ± 0.04 | STD. RI |
| Nonacosane | 2900 | 2900 | 1.24 ± 0.05 | - | - | 1.84 ± 0.05 | - | 1.27 ± 0.07 | STD. RI |
| Class | BAO | CAM | DOM | JER | SUP | SIS |
|---|---|---|---|---|---|---|
| Acids | 0.18 | 1.23 | 0.91 | 1.28 | 1.23 | 0.45 |
| Alcohols | 3.59 | 8.11 | 5.96 | 2.72 | 3.95 | 11.85 |
| Aldeydes | 1.27 | 0.67 | 0.79 | 0.75 | 0.68 | 0.93 |
| Esters | 17.07 | 15.47 | 17.30 | 20.18 | 20.83 | 20.91 |
| Ketones | 2.48 | 10.63 | 9.52 | 2.19 | 2.63 | 6.59 |
| Saturated hydrocarbons | 65.58 | 55.02 | 54.91 | 64.41 | 62.55 | 53.96 |
| Unsaturated hydrocarbons | 3.69 | 3.00 | 2.91 | 2.83 | 2.72 | 1.61 |
| Terpenes | 0.98 | 1.42 | 1.62 | 0.99 | 1.46 | 0.44 |
| Oxygenated terpenes | 1.64 | 0.68 | 1.23 | 1.33 | 0.94 | 0.45 |
| Sesquiterpenes | 2.13 | 2.61 | 2.54 | 1.44 | 1.59 | 1.38 |
| Miscellanea | 0.99 | 0.61 | 1.03 | 0.85 | 1.01 | 1.10 |
| Unidentified | 0.39 | 0.54 | 1.26 | 1.02 | 0.41 | 0.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Agostini, A.; Robustelli della Cuna, F.S.; Cortis, P.; Cogoni, A.; Sottani, C.; Soddu, F.; Sanna, C. Volatile Organic Compounds (VOCs) Diversity in the Orchid Himantoglossum robertianum (Loisel.) P. Delforge from Sardinia (Italy). Diversity 2022, 14, 1125. https://doi.org/10.3390/d14121125
De Agostini A, Robustelli della Cuna FS, Cortis P, Cogoni A, Sottani C, Soddu F, Sanna C. Volatile Organic Compounds (VOCs) Diversity in the Orchid Himantoglossum robertianum (Loisel.) P. Delforge from Sardinia (Italy). Diversity. 2022; 14(12):1125. https://doi.org/10.3390/d14121125
Chicago/Turabian StyleDe Agostini, Antonio, Francesco Saverio Robustelli della Cuna, Pierluigi Cortis, Annalena Cogoni, Cristina Sottani, Francesca Soddu, and Cinzia Sanna. 2022. "Volatile Organic Compounds (VOCs) Diversity in the Orchid Himantoglossum robertianum (Loisel.) P. Delforge from Sardinia (Italy)" Diversity 14, no. 12: 1125. https://doi.org/10.3390/d14121125
APA StyleDe Agostini, A., Robustelli della Cuna, F. S., Cortis, P., Cogoni, A., Sottani, C., Soddu, F., & Sanna, C. (2022). Volatile Organic Compounds (VOCs) Diversity in the Orchid Himantoglossum robertianum (Loisel.) P. Delforge from Sardinia (Italy). Diversity, 14(12), 1125. https://doi.org/10.3390/d14121125












