Status and Trends in the Rate of Introduction of Marine Non-Indigenous Species in European Seas
Abstract
1. Introduction
2. Methodology
2.1. Geographic Coverage
2.2. Data Included
2.3. Detection Year
3. Results
| Group | Species | Pan-European | BAL | NEA | MED | BLK |
|---|---|---|---|---|---|---|
| VER | Ablennes hians (Valenciennes, 1846) | 2018 | 2018 | |||
| VER | Abudefduf sexfasciatus (Lacepède, 1801) | 2017 | 2017 | |||
| VER | Abudefduf vaigiensis (Quoy & Gaimard, 1825) | 2005 | 2005 | |||
| VER | Abudefduf hoefleri (Steindachner, 1881) | 2014 | 2014 | |||
| INV | Acanthaster planci (Linnaeus, 1758) | 2006 | 2006 | |||
| VER | Acanthopagrus bifasciatus (Forsskål, 1775) | 2019 | 2019 | |||
| PP | Acanthosiphonia echinata (Harvey) A.M.Savoie & G.W.Saunders | 2018 | 2018 | |||
| VER | Acanthurus bahianus Castelnau, 1855 | 2013 | 2013 | |||
| VER | Acanthurus cfr gahhm (Forsskål, 1775) | 2019 | 2019 | |||
| VER | Acanthurus coeruleus Bloch & Schneider, 1801 | 2011 | 2013 | 2011 | ||
| VER | Acanthurus sohal (Forsskål, 1775) | 2017 | 2017 | |||
| VER | Acanthurus chirurgus (Bloch, 1787) | 2012 | 2013 | 2012 | ||
| INV | Acartia (Acanthacartia) tonsa Dana, 1849 | 1921 | 1921 | 1921 | 1986 | 1976 |
| INV | Acartia (Acartiura) omorii Bradford, 1976 | 2004 | 2004 | |||
| INV | Achelia sawayai Marcus, 1940 | 2016 | 2016 | |||
| VER | Acipenser baerii Brandt, 1869 | 1960 | 1960 | 1985 | ||
| VER | Acipenser gueldenstaedtii Brandt & Ratzeburg, 1833* | 1962 | 1962 | 2010 | ||
| VER | Acipenser ruthenus Linnaeus, 1758* | 1887 | 1887 | |||
| VER | Acipenser stellatus Pallas, 1771 | 1999 | 1999 | |||
| VER | Acipenser transmontanus Richardson, 1836 | 1999 | 1999 | |||
| PP | Acrochaetium catenulatum M.A.Howe | 1967 | 1967 | |||
| PP | Acrothamnion preissii (Sonder) E.M.Wollaston | 1968 | 2009 | 1968 | ||
| INV | Actaeodes tomentosus (H. Milne Edwards, 1834) | 2013 | 2013 | |||
| INV | Acteocina mucronata (Philippi, 1849) | 1991 | 1991 | |||
| INV | Actumnus globulus Heller, 1861 | 1978 | 1978 | |||
| PP | Adelosina carinatastriata (Wiesner) | 2004 | 2004 | |||
| Pathogen | Aerococcus viridans Williams, Hirch & Cowan | 1961 | 1961 | |||
| PP | Agardhiella subulata (C.Agardh) Kraft & M.J.Wynne | 1984 | 1989 | 1984 | ||
| PP | Agarophyton vermiculophyllum (Ohmi) Gurgel, J.N.Norris & Fredericq | 1989 | 2003 | 1989 | 2008 | |
| PP | Aglaothamnion halliae (Collins) Aponte, D.L.Ballantine & J.N.Norris | 1960 | 1960 | 2016 | ||
| VER | Agonus cataphractus (Linnaeus, 1758) | 2005 | 2005 | |||
| PP | Ahnfeltiopsis flabelliformis (Harvey) Masuda, 1993 | 1994 | 1994 | |||
| PP/micro | Akashiwo sanguinea (K.Hirasaka) G.Hansen & Ø.Moestrup | 1982 | 1982 | |||
| VER | Alepes djedaba (Forsskål, 1775) | 1960 | 1960 | |||
| PP/micro | Alexandrium ostenfeldii (Paulsen) Balech & Tangen | 1986 | 1986 | |||
| PP/micro | Alexandrium affine (H.Inoue & Y.Fukuyo) Balech | 1987 | 1987 | |||
| PP/micro | Alexandrium leei Balech | 1991 | 1991 | |||
| PP/micro | Alexandrium margalefii Balech | 2006 | 2006 | |||
| PP/micro | Alexandrium taylori Balech | 1994 | 1994 | |||
| INV | Aliculastrum cylindricum (Helbling, 1779) | 2020 | 2020 | |||
| INV/par | Allolepidapedon fistulariae Yamaguti, 1940 | 2005 | 2005 | |||
| INV | Alpheus rapacida de Man, 1908 | 1998 | 1998 | |||
| INV | Amathina tricarinata (Linnaeus, 1767) | 2012 | 2012 | |||
| INV | Ammothea hilgendorfi (Böhm, 1879) | 1979 | 2013 | 1979 | ||
| INV | Ampelisca cavicoxa Reid, 1951 | 2005 | 2005 | |||
| INV | Ampelisca heterodactyla Schellenberg, 1925 | 1986 | 1986 | |||
| INV | Amphibalanus eburneus (Gould, 1841) | 1818 | 1872 | 1818 | 1933 | |
| INV | Amphibalanus reticulatus (Utinomi, 1967) | 1977 | 1997 | 1977 | ||
| INV | Amphibalanus variegatus (Darwin, 1854) | 1997 | 1997 | |||
| INV | Amphinome rostrata (Pallas, 1766) | 1900 | 1900 | |||
| PP | Amphistegina cf. papillosa Said, 1949 | 2005 | 2005 | |||
| PP | Amphistegina lessonii d’Orbigny in Guérin-Méneville, 1832 | 2001 | 2001 | |||
| PP | Amphistegina lobifera Larsen, 1976 | 1959 | 1959 | |||
| INV | Ampithoe valida Smith, 1873 | 1985 | 1985 | 2000 | ||
| INV | Anadara kagoshimensis (Tokunaga, 1906) | 1966 | 1993 | 1966 | 1981 | |
| INV | Anadara transversa (Say, 1822) | 1975 | 2016 | 1975 | ||
| INV/par | Anguillicola crassus (Kuwahara, Niimi & Itagaki, 1974) | 1980 | 1988 | 1982 | 1980 | |
| INV | Anomia chinensis Philippi, 1849 | 1974 | 1974 | |||
| INV | Anoplodactylus californicus Hall, 1912 | 1965 | 1965 | |||
| PP | Anotrichium furcellatum (J.Agardh) Baldock | 1950 | 1950 | |||
| PP | Antithamnion densum (Suhr) M.Howe | 1964 | 1964 | |||
| PP | Antithamnion diminuatum Wollaston | 1989 | 1989 | |||
| PP | Antithamnion hubbsii E.Y.Dawson | 1987 | 1989 | 1987 | ||
| PP | Antithamnion amphigeneum A.J.K.Millar | 1992 | 1995 | 1992 | ||
| PP | Antithamnionella ternifolia (Hooker fil. & Harvey) Lyle | 1910 | 2014 | 1910 | 1981 | |
| INV | Aoroides curvipes Ariyama, 2004 | 2009 | 2009 | |||
| INV | Aoroides semicurvatus Ariyama, 2004 | 2009 | 2009 | |||
| INV | Aoroides longimerus Ren & Zheng, 1996 | 2013 | 2013 | 2015 | ||
| INV | Apanthura addui Wägele, 1981 | 1998 | 1998 | |||
| INV | Aplidium antillense (Gravier, 1955) | 2004 | 2004 | |||
| INV | Aplidium accarense (Millar, 1953) | 2012 | 2012 | |||
| VER | Apogonichthyoides pharaonis (Bellotti, 1874) | 1964 | 1964 | |||
| INV | Aquilonastra burtoni (Gray, 1840) | 2003 | 2003 | |||
| INV | Arachnidium lacourti d’Hondt & Faasse, 2006 | 1999 | 2015 | 1999 | ||
| INV | Arachnoidella protecta Harmer, 1915 | 1992 | 1992 | |||
| INV | Arbopercula tenella (Hincks, 1880) | 1990 | 1990 | |||
| INV | Arctapodema australis (Vanhöffen, 1912) | 1967 | 1967 | |||
| INV | Arcuatula senhousia (Benson, 1842) | 1982 | 2002 | 1982 | 2002 | |
| INV | Argopecten gibbus (Linnaeus, 1758) | 2016 | 2016 | |||
| INV | Arhynchite arhynchite (Ikeda, 1924) | 2001 | 2001 | |||
| INV | Arietellus pavoninus Sars G.O., 1905 | 1967 | 1967 | |||
| VER | Arothron hispidus (Linnaeus, 1758) | 2018 | 2018 | |||
| INV | Artemia monica Verrill, 1869 | 1972 | 1987 | 1972 | ||
| INV | Ascidia curvata (Traustedt, 1882) | 2014 | 2014 | |||
| INV | Ascidia interrupta Heller, 1878 | 1990 | 1990 | |||
| INV | Asclerocheilus ashworthi Blake, 1981 | 2005 | 2005 | |||
| PP | Ascophyllum nodosum (Linnaeus) Le Jolis | 2009 | 2009 | |||
| PP | Asparagopsis taxiformis (Delile) Trevisan de Saint-Léon (lineage 2) | 1928 | 1928 | 1992 | ||
| PP | Asparagopsis armata Harvey | 1880 | 1922 | 1880 | ||
| INV | Asterocarpa humilis (Heller, 1878) | 2005 | 2005 | |||
| PP/micro | Asteromphalus sarcophagus Wallich, 1860 | 1993 | 1993 | |||
| INV | Atactodea striata (Gmelin, 1791) | 1977 | 1977 | |||
| INV | Atergatis roseus (Rüppell, 1830) | 2009 | 2009 | |||
| VER | Atherinomorus forskalii (Rüppell, 1838) | 1929 | 1929 | |||
| INV | Atys angustatus E. A. Smith, 1872 | 2017 | 2017 | |||
| INV | Atys ehrenbergi (Issel, 1869) | 2016 | 2016 | |||
| INV | Aurelia coerulea von Lendenfeld, 1884 | 2002 | 2002 | |||
| INV | Aurelia solida Browne, 1905 | 2000 | 2000 | |||
| INV | Austrominius modestus (Darwin, 1854) | 1944 | 1944 | 1990 | ||
| INV | Axionice medusa (Savigny in Lamarck, 1818) | 1976 | 1976 | |||
| INV | Baeolidia moebii Bergh, 1888 | 2017 | 2017 | |||
| INV | Balanus glandula Darwin, 1854 | 2015 | 2015 | |||
| INV | Balanus trigonus Darwin, 1854 | 1887 | 1887 | 1927 | ||
| VER | Balistoides conspicillum (Bloch & Schneider, 1801) | 2012 | 2012 | |||
| INV | Bankia fimbriatula Moll & Roch, 1931 | 1847 | 1847 | |||
| INV | Barentsia ramosa (Robertson, 1900) | 1962 | 1962 | |||
| PP | Batophora occidentalis var. largoensis (Harvey) S.Berger & Kaever ex M.J.Wynne | 2020 | 2020 | |||
| INV | Beania maxilladentata Ramalho, Muricy & Taylor, 2010 | 2013 | 2013 | |||
| INV | Bemlos leptocheirus (Walker, 1909) | 2015 | 2015 | |||
| INV | Beroe ovata Bruguière, 1789 | 1997 | 2011 | 2013 | 2004 | 1997 |
| INV | Berthellina citrina (Rüppell & Leuckart, 1828) | 2019 | 2019 | |||
| PP/micro | Biddulphia rhombus (Ehrenberg) W.Smith | 1983 | 1983 | |||
| PP/micro | Biddulphia sinensis Greville | 1903 | 1904 | 1903 | ||
| INV | Biflustra grandicella (Canu & Bassler, 1929) | 2016 | 2016 | |||
| INV | Bispira polyomma Giangrande & Faasse, 2012 | 2010 | 2010 | 2014 | ||
| INV | Biuve fulvipunctata (Baba, 1938) | 1993 | 1993 | |||
| INV | Boccardia proboscidea Hartman, 1940 | 1996 | 1996 | 2014 | ||
| INV | Boccardia semibranchiata Guérin, 1990 | 1999 | 1999 | |||
| INV | Boccardiella hamata (Webster, 1879) | 2001 | 2001 | |||
| Pathogen | Bonamia exitiosa Hine, Cochennac & Berthe | 2006 | 2006 | 2007 | ||
| Pathogen | Bonamia ostreae Pichot, Comps, Tigé, Grizel & Rabouin | 1978 | 1978 | 1990 | ||
| PP | Bonnemaisonia hamifera Hariot | 1898 | 1900 | 1898 | 1932 | |
| INV | Bostrycapulus odites Collin, 2005 | 1973 | 1973 | |||
| INV | Botrylloides diegensis Ritter & Forsyth, 1917 | 1999 | 1999 | 2004 | ||
| INV | Botrylloides giganteum (Pérès, 1949) | 2003 | 2003 | |||
| INV | Botrylloides niger Herdman, 1886 | 2013 | 2013 | 2014 | ||
| INV | Botrylloides violaceus Oka, 1927 | 1991 | 1999 | 1991 | ||
| PP | Botryocladia wrightii (Harvey) W.E.Schmidt, D.L.Ballantine & Fredericq | 1978 | 2005 | 1978 | ||
| PP | Botryocladia madagascariensis G.Feldmann | 1978 | 1978 | |||
| PP | Botrytella parva (Takamatsu) H.S.Kim | 1996 | 1996 | |||
| INV | Bougainvillia macloviana Lesson, 1830 | 1895 | 1895 | |||
| INV | Brachidontes exustus (Linnaeus, 1758) | 1977 | 1977 | |||
| INV | Brachidontes pharaonis (P. Fischer, 1870) | 1960 | 1960 | |||
| INV | Branchiomma bairdi (McIntsosh, 1885) | 1998 | 2012 | 1998 | ||
| INV | Branchiomma boholense (Grube, 1878) | 2004 | 2004 | |||
| INV | Branchiomma luctuosum (Grube, 1870) | 1978 | 2015 | 1978 | ||
| VER | Bregmaceros nectabanus Whitley, 1941 | 2014 | 2014 | |||
| INV | Bugulina simplex (Hincks, 1886) | 1982 | 1982 | |||
| INV | Bugulina stolonifera (Ryland, 1960) | 1976 | 1976 | |||
| INV | Bulla arabica Malaquias & Reid, 2008 | 1998 | 1998 | |||
| INV | Bursatella leachii Blainville, 1817 | 1969 | 1969 | |||
| INV | Calanopia elliptica (Dana, 1849) | 1891 | 1891 | |||
| INV | Callinectes danae Smith, 1869 | 1981 | 1981 | |||
| INV | Callinectes pallidus (de Rochebrune, 1883) | 2013 | 2013 | |||
| INV | Callinectes sapidus Rathbun, 1896 | 1901 | 1951 | 1901 | 1947 | 1967 |
| VER | Callionymus filamentosus Valenciennes, 1837 | 2003 | 2003 | |||
| INV | Calyptospadix cerulea Clarke, 1882 | 1940 | 2014 | 1978 | 1940 | |
| VER | Cantherhines pullus (Ranzani, 1842) | 2015 | 2015 | |||
| INV | Caprella mutica Schurin, 1935 | 1985 | 2017 | 1985 | ||
| INV | Caprella scaura Templeton, 1836 | 1985 | 1985 | 1994 | ||
| VER | Carassius auratus (Linnaeus, 1758) | 2012 | 2012 | |||
| VER | Carassius gibelio (Bloch, 1782)* | 1800 | 1800 | |||
| INV | Carijoa riisei (Duchassaing & Michelotti, 1860) | 2016 | 2016 | |||
| INV | Carupa tenuipes Dana, 1852 | 2009 | 2009 | |||
| INV | Cassiopea andromeda (Forsskål, 1775) | 1903 | 1903 | |||
| PP | Caulacanthus okamurae Yamada | 1999 | 1999 | 2002 | ||
| PP | Caulerpa cylindracea Sonder | 1991 | 1997 | 1991 | ||
| PP | Caulerpa lamourouxii (Turner) C.Agardh | 1956 | 1956 | |||
| PP | Caulerpa taxifolia (M.Vahl) C.Agardh | 1984 | 1984 | |||
| PP | Caulerpa taxifolia var. distichophylla (Sonder) Verlaque, Huisman & Procaccini | 2007 | 2007 | |||
| PP | Caulerpa webbiana Montagne | 2002 | 2002 | |||
| INV | Caulibugula zanzibariensis (Waters, 1913) | 2003 | 2003 | |||
| INV | Cellana rota (Gmelin, 1791) | 2007 | 2007 | |||
| INV | Celleporaria inaudita Tilbrook, Hayward & Gordon, 2001 | 2007 | 2007 | |||
| INV | Celleporaria aperta (Hincks, 1882) | 1975 | 1975 | |||
| INV | Celleporaria brunnea (Hincks, 1884) | 2007 | 2007 | 2010 | ||
| INV | Celleporaria vermiformis (Waters, 1909) | 2015 | 2015 | |||
| INV | Celleporella carolinensis Ryland, 1979 | 1993 | 1993 | |||
| INV | Celtodoryx ciocalyptoides (Burton, 1935) | 1996 | 1996 | |||
| INV | Centropages furcatus (Dana, 1849) | 1988 | 1988 | |||
| VER | Cephalopholis hemistiktos (Rüppell, 1830) | 2009 | 2009 | |||
| VER | Cephalopholis taeniops (Valenciennes, 1828) | 2009 | 2009 | |||
| VER | Cephalopholis nigri (Günther, 1859) | 2016 | 2016 | |||
| INV | Cephalothrix simula Iwata, 1952 | 2012 | 2012 | |||
| PP | Ceramium atrorubescens Kylin | 1988 | 1988 | |||
| PP | Ceramium sungminbooi Hughey & Boo | 2018 | 2018 | |||
| PP | Ceramium tenuicorne (Kützing) Waern | 2011 | 2011 | |||
| PP | Ceramium bisporum D.L.Ballantine | 1980 | 1980 | |||
| PP | Ceramium strobiliforme G.W.Lawson & D.M.John | 1991 | 1991 | |||
| INV | Ceratonereis mirabilis Kinberg, 1865 | 1997 | 1997 | |||
| INV | Cerithidium perparvulum (Watson, 1886) | 1995 | 1995 | |||
| INV | Cerithiopsis pulvis (Issel, 1869) | 1985 | 1985 | |||
| INV | Cerithiopsis tenthrenois (Melvill, 1896) | 1985 | 1985 | |||
| INV | Cerithium scabridum Philippi, 1848 | 1972 | 1972 | |||
| PP/micro | Chaetoceros peruvianus Brightwell | 1981 | 1981 | |||
| PP/micro | Chaetoceros rostratus Ralfs | 2003 | 2003 | |||
| PP/micro | Chaetoceros bacteriastroides G.H.H.Karsten | 1996 | 1996 | |||
| PP/micro | Chaetoceros concavicornis Mangin | 2011 | 2011 | |||
| PP/micro | Chaetoceros pseudosymmetricus Nielsen | 2015 | 2015 | |||
| VER | Chaetodipterus faber (Broussonet, 1782) | 2019 | 2019 | |||
| VER | Chaetodon sanctaehelenae Günther, 1868 | 1993 | 1993 | |||
| VER | Chaetodon auriga Forsskål, 1775 | 2015 | 2015 | |||
| VER | Chaetodontoplus septentrionalis (Temminck & Schlegel, 1844) | 2015 | 2015 | |||
| INV | Chaetopleura angulata (Spengler, 1797) | 1850 | 1850 | |||
| INV | Chaetozone corona Berkeley & Berkeley, 1941 | 1982 | 1996 | 1982 | ||
| INV | Chama asperella Lamarck, 1819 | 2007 | 2007 | |||
| INV | Chama pacifica Broderip, 1835 | 1998 | 1998 | |||
| VER | Champsodon nudivittis (Ogilby, 1895) | 2012 | 2012 | |||
| INV | Charybdis (Charybdis) japonica (A. Milne-Edwards, 1861) | 2006 | 2006 | |||
| INV | Charybdis (Charybdis) feriata (Linnaeus, 1758) | 2004 | 2004 | |||
| INV | Charybdis (Charybdis) hellerii (A. Milne-Edwards, 1867) | 1998 | 1998 | |||
| INV | Charybdis (Charybdis) lucifera (Fabricius, 1798) | 2006 | 2006 | |||
| INV | Charybdis (Goniohellenus) longicollis Leene, 1938 | 1969 | 1969 | |||
| PP/micro | Chattonella marina (Subrahmanyan) Hara & Chihara | 1974 | 1974 | |||
| VER | Cheilodipterus novemstriatus (Rüppell, 1838) | 2015 | 2015 | |||
| INV | Chelicorophium robustum (G.O. Sars, 1895) | 2018 | 2018 | |||
| INV | Chelicorophium curvispinum (G.O. Sars, 1895) | 1912 | 1921 | 1912 | ||
| VER | Chlorurus rhakoura Randall & Anderson, 1997 | 2017 | 2017 | |||
| PP | Chondria pygmaea Garbary & Vandermeulen | 1974 | 1974 | |||
| PP | Chondria curvilineata F.S.Collins & Hervey | 1981 | 1981 | |||
| PP | Chondrus giganteus f. flabellatus Mikami | 1994 | 1994 | |||
| VER | Chromis multilineata (Guichenot, 1853) | 2015 | 2015 | |||
| INV | Chromodoris quadricolor (Rüppell & Leuckart, 1830) | 1982 | 1982 | |||
| INV | Chrysaora achlyos Martin, Gershwin, Burnett, Cargo & Bloom, 1997 | 2018 | 2018 | |||
| VER | Chrysiptera cyanea (Quoy & Gaimard, 1825) | 2013 | 2013 | |||
| VER | Chrysiptera hemicyanea (Weber, 1913) | 2017 | 2017 | |||
| PP | Chrysonephos lewisii (W.R.Taylor) W.R.Taylor | 1988 | 1988 | |||
| INV | Cingulina isseli (Tryon, 1886) | 1998 | 1998 | |||
| INV | Ciona robusta Hoshino & Tokioka, 1967 | 1901 | 2007 | 1901 | ||
| VER | Cirrhitus atlanticus Osório, 1893 | 2018 | 2018 | |||
| PP | Cladophora patentiramea (Montagne) Kützing | 1991 | 1991 | |||
| INV | Clavelina oblonga Herdman, 1880 | 1929 | 1971 | 1929 | ||
| Pathogen | Claviceps purpurea (Fr.:Fr.)Tul. | 1960 | 1960 | |||
| PP | Clavulina cf. multicamerata Chapman, 1907 | 2012 | 2012 | |||
| INV | Clementia papyracea (Gmelin, 1791) | 1985 | 1985 | |||
| INV | Clymenella torquata (Leidy, 1855) | 1977 | 1977 | |||
| INV | Clytia gregaria (Agassiz, 1862) | 2017 | 2017 | |||
| INV | Clytia hummelincki (Leloup, 1935) | 1996 | 1996 | |||
| INV | Clytia linearis (Thorneley, 1900) | 1951 | 1983 | 1951 | ||
| PP | Codium arabicum Kützing | 2006 | 2006 | |||
| PP | Codium fragile subsp. fragile (Suringar) Hariot | 1895 | 1919 | 1895 | 1946 | |
| PP | Colaconema codicola (Børgesen) H.Stegenga, J.J. Bolton & R.J.Anderson | 1926 | 1926 | 1952 | ||
| PP | Colaconema dasyae (F.S.Collins) Stegenga, I.Mol, Prud’homme van Reine & Lokhorst | 1951 | 1951 | |||
| INV | Coleusia signata (Paul’son, 1875) | 2005 | 2005 | |||
| PP | Colpomenia peregrina Sauvageau | 1905 | 1905 | 1918 | ||
| INV | Conomurex persicus (Swainson, 1821) | 1983 | 1983 | |||
| INV | Corambe obscura (A.E. Verrill, 1870) | 1879 | 1879 | 1986 | ||
| INV | Corbicula fluminea (O. F. Müller, 1774) | 1978 | 1978 | |||
| INV | Corella eumyota Traustedt, 1882 | 2002 | 2002 | |||
| PP/micro | Corymbellus aureus J.C.Green | 1992 | 1992 | |||
| PP | Corynomorpha prismatica (J.Agardh) J.Agardh | 1990 | 1990 | |||
| PP | Corynophlaea verruculiformis (Y.-P.Lee & I.K.Lee) Y.-P.Lee | 1994 | 1994 | |||
| INV | Coryphellina rubrolineata O’Donoghue, 1929 | 2008 | 2008 | |||
| INV | Crassostrea rhizophorae (Guilding, 1828) | 1976 | 1976 | |||
| INV | Crassostrea virginica (Gmelin, 1791) | 1861 | 1861 | 1974 | ||
| INV | Crepidacantha poissonii (Audouin, 1826) | 1982 | 1982 | |||
| INV | Crepidula fornicata (Linnaeus, 1758) | 1902 | 1902 | 1957 | ||
| INV | Crepipatella dilatata (Lamarck, 1822) | 2005 | 2005 | 2014 | ||
| INV | Crisularia plumosa (Pallas, 1766) | 1937 | 1937 | |||
| INV | Crisularia serrata (Lamarck, 1816) | 1902 | 1902 | |||
| PP | Cryptonemia hibernica Guiry & L.M.Irvine | 1911 | 1911 | |||
| PP | Cushmanina striatopunctata (Parker & Jones, 1865) | 1913 | 1913 | |||
| INV | Cuthona perca (Er. Marcus, 1958) | 1976 | 1976 | |||
| INV | Cycloscala hyalina (G. B. Sowerby II, 1844) | 1992 | 1992 | |||
| INV | Cymodoce fuscina Schotte & Kensley, 2005 | 2015 | 2015 | |||
| VER | Cynoscion regalis (Bloch & Schneider, 1801) | 2009 | 2009 | |||
| VER | Cyprinus carpio (Linnaeus, 1758)* | 1200 | 1200 | 1879 | ||
| PP | Dasya sessilis Yamada | 1984 | 1989 | 1984 | ||
| PP | Dasysiphonia japonica (Yendo) H.-S.Kim | 1984 | 1984 | 1998 | ||
| INV | Dendostrea frons (Linnaeus, 1758) | 1983 | 1983 | |||
| INV | Dendostrea folium (Linnaeus, 1758) | 2005 | 2005 | |||
| PP | Derbesia rhizophora Yamada | 1984 | 1984 | |||
| INV | Desdemona ornata Banse, 1957 | 1983 | 1993 | 1983 | ||
| INV | Diadema setosum (Leske, 1778) | 2010 | 2010 | |||
| INV | Diadumene lineata (Verrill, 1869) | 1925 | 2011 | 1963 | 1925 | 1945 |
| PP/micro | Dicroerisma psilonereiella F.J.R.Taylor & S.A. Cattell | 1998 | 1998 | |||
| PP | Dictyota cyanoloma Tronholm, De Clerck, A.Gómez-Garreta & Rull Lluch in Tronholm et al. | 1935 | 2006 | 1935 | ||
| INV | Didemnum perlucidum Monniot F., 1983 | 2006 | 2006 | |||
| INV | Didemnum vexillum Kott, 2002 | 1968 | 1968 | 2007 | ||
| INV | Dikerogammarus villosus (Sowinsky, 1894) | 2015 | 2015 | |||
| INV | Dikoleps micalii Agamennone, Sbrana, Nardi, Siragusa & Germanà, 2020 | 2016 | 2016 | |||
| PP/micro | Dinophysis sacculus Stein | 2004 | 2004 | |||
| INV | Diodora funiculata (Reeve, 1850) | 2013 | 2013 | |||
| INV | Diplosoma listerianum (Milne Edwards, 1841) | 1877 | 1877 | |||
| INV | Dipolydora quadrilobata (Jacobi, 1883) | 2003 | 2003 | |||
| INV | Dipolydora socialis (Schmarda, 1861) | 2006 | 2006 | |||
| INV | Dipolydora tentaculata (Blake & Kudenov, 1978) | 2005 | 2005 | |||
| PP | Dipterosiphonia dendritica (C.Agardh) F.Schmitz | 1961 | 1961 | |||
| INV | Dispio magna (Day, 1955) | 1982 | 1982 | |||
| PP/micro | Dissodinium pseudocalani (Gonnert) Drebes ex Elbrachter & Drebes | 2003 | 2003 | |||
| INV | Distaplia magnilarva (Della Valle, 1881) | 1929 | 1929 | |||
| INV | Distaplia bermudensis Van Name, 1902 | 1953 | 2006 | 1953 | ||
| INV | Distaplia corolla Monniot F., 1974 | 1971 | 1971 | |||
| INV | Dodecaceria capensis Day, 1961 | 1976 | 1976 | |||
| INV | Dorvillea similis (Crossland, 1924) | 2014 | 2014 | |||
| INV | Dreissena rostriformis bugensis (Andrusov, 1897) | 2014 | 2014 | |||
| VER | Dussumieria elopsoides Bleeker, 1849 | 2005 | 2005 | |||
| INV | Dyspanopeus texanus (Stimpson, 1859) | 2015 | 2015 | |||
| INV | Dyspanopeus sayi (Smith, 1869) | 1992 | 2007 | 1992 | ||
| INV | Echinogammarus trichiatus (Martynov, 1932) | 2014 | 2014 | |||
| INV | Ecteinascidia styeloides (Traustedt, 1882) | 1983 | 1983 | |||
| INV | Ectopleura crocea (Agassiz, 1862) | 1895 | 1989 | 1895 | ||
| INV | Edwardsiella lineata (Verrill in Baird, 1873) | 2010 | 2010 | |||
| PP | Elachista spp mentioned as E. flaccida | 1993 | 1993 | |||
| VER | Elates ransonnettii (Steindachner, 1876) | 2005 | 2005 | |||
| PP | Elodea canadensis Michx.* | 1873 | 1873 | |||
| PP | Elodea nuttallii (Planch.) H.St.John | 1991 | 1991 | 2006 | ||
| PP | Elphidium striatopunctatum (Fichtel & Moll, 1798) | 1911 | 1911 | |||
| INV | Elysia nealae (Ostergaard, 1955) | 2018 | 2018 | |||
| PP/micro | Emiliania huxleyi (Lohmann) W.W.Hay & H.P.Mohler | 1989 | 1989 | |||
| INV | Endeis biseriata Stock, 1968 | 1979 | 1979 | |||
| INV | Ensis leei M. Huber, 2015 | 1978 | 1991 | 1978 | ||
| INV | Eocuma dimorphum Fage, 1928 | 1992 | 1992 | |||
| INV | Eocuma sarsii (Kossmann), 1880 | 1901 | 1901 | |||
| VER | Epinephelus fasciatus (Forsskål, 1775) | 2018 | 2018 | |||
| VER | Epinephelus coioides (Hamilton, 1822) | 1998 | 1998 | |||
| VER | Epinephelus malabaricus (Bloch & Schneider, 1801) | 2011 | 2011 | |||
| VER | Epinephelus merra Bloch, 1793 | 2004 | 2004 | |||
| VER | Equulites klunzingeri (Steindachner, 1898) | 1955 | 1955 | |||
| INV | Ergalatax junionae Houart, 2008 | 1993 | 1993 | |||
| INV | Eriocheir sinensis H. Milne Edwards, 1853* | 1912 | 1921 | 1912 | 1959 | 1997 |
| INV | Erugosquilla massavensis (Kossmann, 1880) | 1956 | 1956 | |||
| PP/micro | Ethmodiscus punctiger Castracane | 1800 | 1979 | 1800 | ||
| VER | Etrumeus golanii DiBattista, Randall & Bowen, 2012 | 1999 | 1999 | |||
| INV | Euchaeta concinna Dana, 1849 | 1987 | 1987 | |||
| INV | Eucheilota paradoxica Mayer, 1900 | 1967 | 1967 | |||
| INV | Euchone limnicola Reish, 1959 | 2015 | 2015 | |||
| INV | Eucidaris tribuloides (Lamarck, 1816) | 1998 | 1998 | |||
| INV | Eudendrium carneum Clarke, 1882 | 1950 | 1950 | |||
| INV | Eudendrium merulum Watson, 1985 | 1969 | 1969 | |||
| INV | Eunaticina papilla (Gmelin, 1791) | 2020 | 2020 | |||
| INV | Euplana gracilis Girard, 1853 | 2002 | 2002 | |||
| INV | Euplokamis dunlapae Mills, 1987 | 2011 | 2011 | |||
| PP/micro | Eupyxidicula turris (Greville) S.Blanco & C.E. Wetzel | 1983 | 1983 | |||
| INV | Eurypanopeus depressus (Smith, 1869) | 2009 | 2009 | |||
| INV | Eurytemora americana Williams, 1906 | 1938 | 1938 | |||
| INV | Eurytemora carolleeae Alekseev & Souissi, 2011 | 2011 | 2012 | 2011 | ||
| INV | Eurytemora pacifica Sato, 1913 | 2014 | 2014 | |||
| INV | Eurythoe laevisetis Fauvel, 1914 | 2011 | 2011 | |||
| INV | Eusarsiella zostericola (Cushman, 1906) | 2012 | 2012 | |||
| INV | Eusyllis kupfferi Langerhans, 1879 | 1998 | 1998 | |||
| INV | Euthymella colzumensis (Jousseaume, 1898) | 2017 | 2017 | |||
| PP/micro | Eutintinnus lusus-undae (Entz) | 2001 | 2001 | |||
| INV | Fauveliopsis glabra (Hartman, 1960) | 2007 | 2007 | |||
| INV | Favorinus ghanensis Edmunds, 1968 | 2020 | 2020 | |||
| INV | Faxonius limosus (Rafinesque, 1817) | 2015 | 2015 | |||
| INV | Fenestrulina malusii (Audouin, 1826) | 2011 | 2011 | |||
| INV | Fenestrulina delicia Winston, Hayward & Craig, 2000 | 2002 | 2002 | |||
| INV | Ferosagitta galerita (Dallot, 1971) | 2011 | 2011 | |||
| PP/micro | Fibrocapsa japonica S.Toriumi & H.Takano | 1924 | 1924 | |||
| INV | Ficopomatus enigmaticus (Fauvel, 1923) | 1919 | 1939 | 1921 | 1919 | 1935 |
| INV | Finella pupoides A. Adams, 1860 | 1996 | 1996 | |||
| VER | Fistularia petimba Lacepède, 1803 | 2018 | 2018 | |||
| VER | Fistularia commersonii Rüppell, 1838 | 1999 | 1999 | |||
| INV | Fistulobalanus albicostatus (Pilsbry, 1916) | 1973 | 1973 | |||
| INV | Fulvia fragilis (Forsskål in Niebuhr, 1775) | 1983 | 1983 | |||
| VER | Fundulus heteroclitus heteroclitus (Linnaeus, 1766) | 1970 | 1970 | 2005 | ||
| INV | Gafrarium savignyi (Jonas, 1846) | 2005 | 2005 | |||
| INV | Gammarus tigrinus Sexton, 1939 | 1931 | 1975 | 1931 | ||
| PP | Gelidium microdonticum W.R.Taylor | 2017 | 2017 | |||
| PP | Gelidium vagum Okamura | 2010 | 2010 | |||
| VER | Genyatremus cavifrons (Cuvier, 1830) | 2015 | 2015 | |||
| INV | Glabropilumnus laevis (Dana, 1852) | 1956 | 1956 | |||
| INV | Glycinde bonhourei Gravier, 1904 | 2007 | 2007 | |||
| VER | Gobiosoma bosc (Lacepède, 1800) | 2009 | 2009 | |||
| INV | Godiva quadricolor (Barnard, 1927) | 1985 | 1985 | |||
| INV | Goniadella gracilis (Verrill, 1873) | 1968 | 1968 | |||
| INV | Goniobranchus annulatus (Eliot, 1904) | 2004 | 2004 | |||
| INV | Goniobranchus obsoletus (Rüppell & Leuckart, 1830) | 2018 | 2018 | |||
| INV | Gonioinfradens giardi (Nobili, 1905) | 2010 | 2010 | |||
| INV | Gonionemus vertens A. Agassiz, 1862 | 1700 | 1700 | 1918 | ||
| PP | Goniotrichopsis sublittoralis G.M.Smith | 1975 | 1975 | 1989 | ||
| PP | Gracilariopsis chorda (Holmes) Ohmi | 2010 | 2010 | |||
| INV | Grandidierella japonica Stephensen, 1938 | 2010 | 2010 | 2010 | 2013 | |
| PP | Grateloupia imbricata Holmes | 2005 | 2005 | |||
| PP | Grateloupia asiatica S.Kawaguchi & H.W.Wang | 1984 | 1984 | |||
| PP | Grateloupia patens (Okamura) S.Kawaguchi & H.W.Wang | 1994 | 1994 | |||
| PP | Grateloupia subpectinata Holmes | 1978 | 1978 | 1990 | ||
| PP | Grateloupia turuturu Yamada | 1982 | 1989 | 1982 | ||
| PP | Grateloupia yinggehaiensis H.W.Wang & R.X.Luan | 2008 | 2008 | |||
| INV | Guinearma alberti (Rathbun, 1921) | 2016 | 2016 | |||
| VER | Gymnomuraena zebra (Shaw, 1797) | 2002 | 2002 | |||
| PP | Gymnophycus hapsiphorus Huisman & Kraft | 2011 | 2011 | |||
| INV/par | Gyrodactylus salaris Malmberg, 1957 | 1975 | 1975 | |||
| PP/micro | Gyrodinium corallinum Kofoid & Swezy | 2001 | 2001 | |||
| INV | Halgerda willeyi Eliot, 1904 | 1988 | 1988 | |||
| INV | Haliclona (Halichoclona) vansoesti de Weerdt, de Kluijver & Gómez, 1999 | 2019 | 2019 | |||
| INV | Haliclystus tenuis Kishinouye, 1910 | 2010 | 2010 | |||
| PP | Halimeda incrassata (J.Ellis) J.V.Lamouroux | 2011 | 2011 | |||
| INV | Haliotis discus hannai Ino, 1953 | 1985 | 1985 | |||
| INV | Haloa japonica (Pilsbry, 1895) | 1992 | 1992 | 1992 | ||
| PP | Halophila stipulacea (Forsskål) Ascherson | 1894 | 1894 | |||
| INV | Haminella solitaria (Say, 1822) | 2016 | 2016 | 2020 | ||
| Pathogen | Haplosporidium nelsoni Haskin, Stauber & Mackin | 1975 | 1975 | |||
| INV | Heleobia charruana (d’Orbigny, 1841) | 2014 | 2014 | |||
| INV | Heliacus implexus (Mighels, 1845) | 2019 | 2019 | |||
| INV | Hemigrapsus sanguineus (De Haan, 1835) | 1999 | 1999 | 1999 | 2008 | |
| INV | Hemigrapsus takanoi Asakura & Watanabe, 2005 | 1993 | 2014 | 1993 | ||
| INV | Hemimysis anomala G.O. Sars, 1907* | 1962 | 1962 | 1999 | 2007 | |
| VER | Hemiramphus far (Forsskål, 1775) | 1943 | 1943 | |||
| VER | Heniochus acuminatus (Linnaeus, 1758) | 2014 | 2014 | |||
| VER | Heniochus intermedius Steindachner, 1893 | 2013 | 2013 | 2014 | ||
| INV | Herbstia nitida Manning & Holthuis, 1981 | 2002 | 2002 | |||
| INV | Herdmania momus (Savigny, 1816) | 1998 | 1998 | |||
| PP | Herposiphonia parca Setchell | 1997 | 2006 | 1997 | ||
| INV | Hesperibalanus fallax (Broch, 1927) | 1976 | 1976 | 1976 | ||
| PP | Heterostegina depressa d’Orbigny, 1826 | 1988 | 1988 | |||
| INV | Heterotentacula mirabilis (Kramp, 1957) | 1997 | 1997 | |||
| PP | Hildenbrandia occidentalis Setch. | 2011 | 2011 | |||
| VER | Hippocampus kuda Bleeker, 1852 | 2014 | 2014 | |||
| INV | Hippopodina feegeensis (Busk, 1884) | 1996 | 1996 | |||
| VER | Holacanthus africanus Cadenat, 1951 | 2017 | 2018 | 2017 | ||
| VER | Holacanthus ciliaris (Linnaeus, 1758) | 2011 | 2011 | |||
| VER | Holocentrus adscensionis (Osbeck, 1765) | 2016 | 2016 | |||
| INV | Homarus americanus H. Milne Edwards, 1837 | 1961 | 2007 | 1961 | 2018 | |
| VER | Huso huso (Linnaeus, 1758)* | 1962 | 1962 | |||
| PP | Hydroclathrus tilesii (Endlicher) Santiañez & M.J.Wynne | 2006 | 2006 | |||
| INV | Hydroides brachyacantha Rioja, 1941 | 2015 | 2015 | |||
| INV | Hydroides dirampha Mörch, 1863 | 1981 | 1982 | 1981 | ||
| INV | Hydroides elegans (Haswell, 1883) | 1868 | 1973 | 1868 | ||
| INV | Hydroides ezoensis Okuda, 1934 | 1968 | 1968 | |||
| INV | Hydroides heterocera (Grube, 1868) | 1998 | 1998 | |||
| INV | Hymeniacidon gracilis (Hentschel, 1912) | 2017 | 2017 | |||
| INV | Hypania invalida (Grube, 1860) | 1995 | 1995 | |||
| INV | Hypereteone heteropoda (Hartman, 1951) | 2017 | 2017 | |||
| PP | Hypnea musciformis (Wulfen) J.V.Lamouroux | 2005 | 2005 | |||
| PP | Hypnea anastomosans Papenfuss, Lipkin & P.C.Silva | 2008 | 2008 | |||
| PP | Hypnea cervicornis J.Agardh | 2009 | 2009 | |||
| PP | Hypnea cornuta (Kützing) J.Agardh | 1894 | 1894 | |||
| PP | Hypnea spinella (C.Agardh) Kützing | 1977 | 1977 | |||
| PP | Hypnea valentiae (Turner) Montagne | 1996 | 2006 | 1996 | ||
| INV | Hypselodoris infucata (Rüppell & Leuckart, 1830) | 2002 | 2002 | |||
| INV | Ianiropsis serricaudis Gurjanova, 1936 | 2000 | 2000 | 2012 | ||
| INV | Incisocalliope aestuarius (Watling & Maurer, 1973) | 1975 | 1975 | |||
| INV | Indothais lacera (Born, 1778) | 1983 | 1983 | |||
| INV | Isognomon aff. australicus (Reeve, 1858) | 2016 | 2016 | |||
| INV | Isognomon legumen (Gmelin, 1791) | 2016 | 2016 | |||
| INV | Isognomon radiatus (Anton, 1838) | 1996 | 1996 | |||
| INV | Isolda pulchella Müller in Grube, 1858 | 1994 | 1994 | |||
| INV | Ixa monodi Holthuis & Gottlieb, 1956 | 1999 | 1999 | |||
| INV | Jasus lalandii (H. Milne Edwards, 1837) | 1980 | 1980 | |||
| PP | Kapraunia schneideri (Stuercke & Freshwater) A.M.Savoie & G.W.Saunders | 2010 | 2010 | 2016 | ||
| PP/micro | Karenia longicanalis Z.B.Yang, I.J.Hodgkiss & Gerd Hansen | 2008 | 2008 | |||
| PP/micro | Karenia mikimotoi (Miyake & Kominami ex Oda) Gert Hansen & Ø.Moestrup | 1968 | 1980 | 1968 | ||
| PP/micro | Karenia papilionacea A.J.Haywood & K.A.Steidinger | 1994 | 1994 | |||
| INV | Koinostylochus ostreophagus (Hyman, 1955) | 1970 | 1970 | |||
| Pathogen | Labyrinthula zosterae D. Porter & Muehlst. in Muehlstein & Short | 1930 | 1930 | |||
| VER | Lactophrys triqueter (Linnaeus, 1758) | 1909 | 1909 | |||
| VER | Lagocephalus guentheri Miranda Ribeiro, 1915 | 1952 | 1952 | |||
| VER | Lagocephalus sceleratus (Gmelin, 1789) | 2004 | 2004 | |||
| VER | Lagocephalus suezensis Clark & Gohar, 1953 | 2003 | 2003 | |||
| INV | Lamprohaminoea ovalis (Pease, 1868) | 2001 | 2001 | |||
| INV | Laonome xeprovala Bick & Bastrop, in Bick et al., 2018 | 2012 | 2012 | 2016 | 2018 | |
| INV | Latopilumnus malardi (De Man, 1914) | 1910 | 1910 | |||
| PP/micro | Lauderia pumila Castracane | 1995 | 1995 | |||
| PP | Laurencia brongniartii J.Agardh | 1989 | 1989 | |||
| PP | Laurencia caduciramulosa Masuda & Kawaguchi | 1991 | 1991 | |||
| PP | Laurencia okamurae Yamada | 1984 | 1984 | |||
| PP | Leathesia marina (Lyngbye) Decaisne | 1905 | 1905 | |||
| INV | Leiocapitellides analis Hartmann-Schröder, 1960 | 2000 | 2000 | |||
| INV | Leiochrides australis Augener, 1914 | 2002 | 2002 | |||
| PP/micro | Lennoxia faveolata H.A.Thomsen & K.R.Buck | 2007 | 2007 | |||
| INV | Leonnates persicus Wesenberg-Lund, 1949 | 2013 | 2013 | |||
| INV | Lepidonotus tenuisetosus (Gravier, 1902) | 2007 | 2007 | |||
| INV | Lepidonotus carinulatus (Grube, 1870) | 1984 | 1984 | |||
| INV | Leucotina natalensis E. A. Smith, 1910 | 1996 | 1996 | |||
| INV | Limnodrilus profundicola (Verrill, 1871) | 2014 | 2014 | |||
| INV | Limulus polyphemus (Linnaeus, 1758) | 1866 | 1866 | |||
| INV | Linguimaera caesaris Krapp-Schickel, 2003 | 1997 | 1997 | |||
| INV | Linopherus canariensis Langerhans, 1881 | 1997 | 1997 | |||
| INV | Lioberus ligneus (Reeve, 1858) | 2019 | 2019 | |||
| PP | Lithophyllum yessoense Foslie | 1994 | 1994 | |||
| PP | Lomentaria flaccida Tanaka | 2002 | 2002 | |||
| PP | Lomentaria hakodatensis Yendo | 1978 | 1984 | 1978 | ||
| PP | Lophocladia lallemandii (Montagne) F.Schmitz | 1908 | 1908 | |||
| INV | Lottia sp. | 2015 | 2015 | |||
| INV | Lovenella assimilis (Browne, 1905) | 2007 | 2007 | |||
| INV | Lumbrinerides crassicephala (Hartman, 1965) | 1994 | 1994 | |||
| INV | Lumbrinerides neogesae Miura, 1981 | 2002 | 2002 | |||
| INV | Lumbrineris perkinsi Carrera-Parra, 2001 | 1973 | 1973 | |||
| VER | Lutjanus argentimaculatus (Forsskål, 1775) | 2019 | 2019 | |||
| VER | Lutjanus griseus (Linnaeus, 1758) | 2018 | 2018 | |||
| VER | Lutjanus jocu (Bloch & Schneider, 1801) | 2005 | 2005 | |||
| VER | Lutjanus sebae (Cuvier, 1816) | 2010 | 2010 | |||
| VER | Lutjanus fulviflamma (Forsskål, 1775) | 2013 | 2013 | |||
| INV | Lysidice collaris Grube, 1870 | 1961 | 1961 | |||
| PP | Macrocystis pyrifera (Linnaeus) C.Agardh | 1972 | 1972 | |||
| INV | Macromedaeus voeltzkowi (Lenz, 1905) | 1910 | 1910 | |||
| INV | Macrophthalmus (Macrophthalmus) indicus Davie, 2012 | 2009 | 2009 | |||
| INV | Macrorhynchia philippina Kirchenpauer, 1872 | 1982 | 1982 | |||
| INV | Magallana angulata (Lamarck, 1819) | 1700 | 1700 | |||
| INV | Magallana gigas (Thunberg, 1793) | 1700 | 2019 | 1700 | 1850 | 2010 |
| INV | Magallana rivularis (Gould, 1861) | 1994 | 1994 | |||
| INV | Magallana sikamea (Amemiya, 1928) | 1994 | 1994 | |||
| INV | Malleus regula (Forsskål in Niebuhr, 1775) | 1970 | 1970 | |||
| INV | Marenzelleria arctia (Chamberlin, 1920) | 2004 | 2004 | |||
| INV | Marenzelleria neglecta Sikorski & Bick, 2004 | 1983 | 1983 | 1985 | ||
| INV | Marenzelleria viridis (Verrill, 1873) | 1983 | 1985 | 1983 | ||
| INV | Marginella glabella (Linnaeus, 1758) | 2009 | 2009 | |||
| INV | Maritigrella fuscopunctata (Prudhoe, 1978) | 2014 | 2014 | |||
| INV | Marivagia stellata Galil & Gershwin, 2010 | 2019 | 2019 | |||
| INV | Marphysa victori Lavesque, Daffe, Bonifácio & Hutchings, 2017 | 1975 | 1975 | |||
| Pathogen | Marteilia refringens Grizel, Comps, Bonami, Cousserans, Duthoit & Le Pennec | 1975 | 1975 | 1992 | ||
| INV | Matuta victor (J.C. Fabricius, 1781) | 2018 | 2018 | |||
| PP/micro | Mediopyxis helysia Kühn, Hargreaves & Halliger | 2003 | 2003 | |||
| INV | Megabalanus tintinnabulum (Linnaeus, 1758) | 1764 | 1764 | 1971 | ||
| INV | Megabalanus coccopoma (Darwin, 1854) | 1851 | 1851 | |||
| INV | Melanella orientalis Agamennone, Micali & Siragusa, 2020 | 2016 | 2016 | |||
| PP | Melanothamnus flavimarinus (M.-S.Kim & I.K.Lee) Díaz-Tapia & Maggs | 2010 | 2010 | |||
| PP | Melanothamnus harveyi (Bailey) Díaz-Tapia & Maggs | 1958 | 1982 | 2015 | 1958 | |
| PP | Melanothamnus japonicus (Harvey) Díaz-Tapia & Maggs | 2016 | 2016 | |||
| INV | Melibe viridis (Kelaart, 1858) | 1970 | 1970 | |||
| INV | Melita nitida S.I. Smith in Verrill, 1873 | 1996 | 2010 | 1996 | ||
| INV | Menaethius monoceros (Latreille, 1825) | 1978 | 1978 | |||
| INV | Mercenaria mercenaria (Linnaeus, 1758) | 1861 | 1861 | 1964 | ||
| INV | Mesanthura cfr. romulea Poore & Lew Ton, 1986 | 2000 | 2000 | |||
| INV | Metacalanus acutioperculum Ohtsuka, 1984 | 1995 | 1995 | |||
| INV | Metacirolana rotunda (Bruce & Jones, 1978) | 1998 | 1998 | |||
| INV | Metapenaeopsis aegyptia Galil & Golani, 1990 | 1996 | 1996 | |||
| INV | Metapenaeopsis mogiensis consobrina (Nobili, 1904) | 1995 | 1995 | |||
| INV | Metapenaeus monoceros (Fabricius, 1798) | 1961 | 1961 | |||
| INV | Metaxia bacillum (Issel, 1869) | 1995 | 1995 | |||
| INV | Microcosmus anchylodeirus Traustedt, 1883 | 1980 | 1980 | |||
| INV | Microcosmus squamiger Michaelsen, 1927 | 1971 | 1992 | 1971 | ||
| INV | Microcosmus exasperatus Heller, 1878 | 2005 | 2005 | 2014 | ||
| VER | Micropogonias undulatus (Linnaeus, 1766) | 1998 | 1998 | |||
| PP | Miliolinella fichteliana (d’Orbigny, 1839) | 1911 | 1911 | |||
| INV | Millepora alcicornis Linnaeus, 1758 | 2004 | 2004 | |||
| PP | Mimosina affinis Millett, 1900 | 2012 | 2012 | |||
| INV | Mitrella psilla (Duclos, 1846) | 2016 | 2016 | |||
| INV | Mizuhopecten yessoensis (Jay, 1857) | 1979 | 1979 | |||
| INV | Mnemiopsis leidyi A. Agassiz, 1865 | 1986 | 2006 | 2001 | 1990 | 1986 |
| INV | Mnestia girardi (Audouin, 1826) | 1990 | 1990 | |||
| INV | Moerisia inkermanica Paltschikowa-Ostroumowa | 1959 | 2018 | 1959 | ||
| INV | Molgula occidentalis Traustedt, 1883 | 2010 | 2010 | |||
| INV | Monocorophium uenoi (Stephensen, 1932) | 2007 | 2007 | |||
| VER | Morone saxatilis x Morone chrysops | 2019 | 2019 | |||
| INV | Mulinia lateralis (Say, 1822) | 2017 | 2017 | |||
| INV | Murchisonellidae T. L. Casey, 1904 | 2013 | 2013 | |||
| INV | Mycale (Carmia) senegalensis Lévi, 1952 | 2002 | 2002 | |||
| VER | Mycteroperca tigris (Valenciennes, 1833) | 2018 | 2018 | |||
| INV/par | Myicola ostreae Hoshina & Sugiura, 1953 | 1972 | 1972 | 1972 | ||
| INV | Myra subgranulata Kossmann, 1877 | 2004 | 2004 | |||
| INV/par | Mytilicola orientalis Mori, 1935 | 1977 | 2018 | 1977 | 1977 | |
| INV | Mytilopsis leucophaeata (Conrad, 1831) | 1835 | 1928 | 1835 | ||
| INV | Naineris setosa (Verrill, 1900) | 2010 | 2010 | |||
| INV | Namanereis littoralis (Grube, 1872) | 1991 | 1991 | |||
| INV | Neanthes agulhana (Day, 1963) | 2007 | 2007 | |||
| PP | Nemalion vermiculare Suringar | 2005 | 2005 | |||
| VER | Nemipterus randalli Russell, 1986 | 2014 | 2014 | |||
| INV | Nemopsis bachei L. Agassiz, 1849 | 1905 | 1905 | |||
| INV | Neodexiospira brasiliensis (Grube, 1872) | 1982 | 1982 | |||
| PP | Neogastroclonium subarticulatum (Turner) L.Le Gall, Dalen & G.W.Saunders | 2017 | 2017 | |||
| VER | Neogobius melanostomus (Pallas, 1814) | 1990 | 1990 | 2004 | ||
| PP | Neoizziella divaricata (C.K.Tseng) S.-M.Lin, S.-Y.Yang & Huisman | 1989 | 1989 | |||
| INV | Neomysis americana (S.I. Smith, 1873) | 2010 | 2010 | |||
| INV | Nereis jacksoni Kinberg, 1865 | 1964 | 1964 | |||
| INV | Nerita sanguinolenta Menke, 1829 | 1969 | 1969 | |||
| INV | Nippoleucon hinumensis (Gamô, 1967) | 2019 | 2019 | |||
| PP | Nitophyllum stellato-corticatum Okamura | 1984 | 1984 | |||
| PP | Nonionella sp. T1/Nonionella stella | 2012 | 2012 | |||
| INV | Notocochlis gualtieriana (Récluz, 1844) | 1978 | 1978 | |||
| INV | Notomastus aberans Day, 1957 | 1964 | 1964 | |||
| INV | Notomastus mossambicus (Thomassin, 1970) | 1997 | 1997 | |||
| INV | Novafabricia infratorquata (Fitzhugh, 1973) | 1985 | 2013 | 1985 | ||
| INV/par | Nybelinia africana Dollfus, 1960 | 2005 | 2005 | |||
| INV | Obesogammarus crassus (Sars G.O., 1894)* | 1962 | 1962 | 2016 | ||
| INV | Ocinebrellus inornatus (Récluz, 1851) | 1993 | 1993 | |||
| INV | Odontodactylus scyllarus (Linnaeus, 1758) | 2009 | 2009 | |||
| INV | Oithona davisae Ferrari F.D. & Orsi, 1984 | 2000 | 2002 | 2000 | 2009 | |
| VER | Oncorhynchus gorbuscha (Walbaum, 1792) | 1958 | 1958 | 1958 | ||
| VER | Oncorhynchus kisutch (Walbaum, 1792)* | 1905 | 1984 | 1905 | ||
| VER | Oncorhynchus mykiss (Walbaum, 1792)* | 1882 | 1882 | 1899 | ||
| PP | Operculina ammonoides (Gronovius, 1781) | 1911 | 1911 | |||
| INV | Ophiactis macrolepidota Marktanner-Turneretscher, 1887 | 1998 | 1998 | |||
| INV | Ophiactis savignyi (Müller & Troschel, 1842) | 1968 | 1968 | |||
| VER | Ophioblennius atlanticus (Valenciennes, 1836) | 2017 | 2017 | |||
| INV | Ophryotrocha japonica Paxton & Åkesson, 2010 | 1999 | 1999 | |||
| INV | Ophryotrocha diadema Åkesson, 1976 | 2006 | 2006 | |||
| VER | Oplegnathus fasciatus (Temminck & Schlegel, 1844) | 2009 | 2009 | |||
| VER | Orthopristis chrysoptera (Linnaeus, 1766) | 2020 | 2020 | |||
| INV | Oscilla galilae Bogi, Karhan & Yokeş, 2012 | 2016 | 2016 | |||
| VER | Ostorhinchus fasciatus (White, 1790) | 2014 | 2014 | |||
| Pathogen | Ostracoblabe implexa Born & Flahault | 1951 | 1951 | |||
| INV | Ostraea angasi G. B. Sowerby II, 1871 | 1985 | 1985 | |||
| INV | Ostrea equestris Say, 1834 | 1995 | 1995 | |||
| INV | Ostrea denselamellosa Lischke, 1869 | 1982 | 1982 | |||
| INV | Ostrea puelchana d’Orbigny, 1842 | 1989 | 1989 | |||
| INV | Oulastrea crispata (Lamarck, 1816) | 2012 | 2012 | |||
| INV | Oxydromus humesi (Pettibone, 1961) | 2009 | 2009 | |||
| PP/micro | Oxytoxum criophilum Balech | 2003 | 2003 | |||
| VER | Oxyurichthys papuensis (Valenciennes, 1837) | 2010 | 2010 | |||
| INV | Pachygrapsus gracilis (de Saussure, 1857) | 2013 | 2013 | |||
| PP | Pachymeniopsis gargiuli S.Y.Kim, Manghisi, Morabito & S.M.Boo | 1968 | 2001 | 1968 | ||
| PP | Pachymeniopsis lanceolata (K.Okamura) Y.Yamada ex S.Kawabata | 1982 | 2006 | 1982 | ||
| INV | Pacifastacus leniusculus (Dana, 1852) | 2014 | 2014 | |||
| INV | Pacificincola perforata (Okada & Mawatari, 1937) | 2001 | 2001 | |||
| PP | Padina boergesenii Allender & Kraft | 1965 | 1965 | |||
| VER | Pagrus major (Temminck & Schlegel, 1843) | 2004 | 2004 | |||
| INV | Pagurus longicarpus (Say, 1817) | 2020 | 2020 | |||
| INV | Palaemon macrodactylus Rathbun, 1902 | 1998 | 2014 | 1998 | 2005 | 2002 |
| INV | Palola valida (Gravier, 1900) | 2014 | 2014 | |||
| VER | Pampus argenteus (Euphrasen, 1788) | 1896 | 1896 | |||
| INV | Panopeus occidentalis Saussure, 1857 | 2015 | 2015 | |||
| PP | Papenfussiella kuromo (Yendo) Inagaki | 1990 | 1990 | |||
| INV | Paracalanus quasimodo Bowman, 1971 | 2017 | 2017 | |||
| INV | Paracaprella pusilla Mayer, 1890 | 2010 | 2010 | 2011 | ||
| INV | Paracerceis sculpta (Holmes, 1904) | 1981 | 1988 | 1981 | ||
| INV | Paradella dianae (Menzies, 1962) | 1985 | 1988 | 1985 | ||
| INV | Paradyte cf. crinoidicola (Potts, 1910) | 1968 | 1968 | |||
| INV | Paraleucilla magna Klautau, Monteiro & Borojevic, 2004 | 2000 | 2006 | 2000 | ||
| INV | Paralithodes camtschaticus (Tilesius, 1815) | 2008 | 2008 | |||
| INV | Parametopella cypris Holmes, 1905 | 2014 | 2014 | |||
| INV | Paramysis (Mesomysis) intermedia (Czerniavsky, 1882) | 2008 | 2008 | |||
| INV | Paramysis (Serrapalpisis) lacustris (Czerniavsky, 1882) | 1962 | 1962 | |||
| INV | Paranais frici Hrabĕ, 1941 | 1970 | 1970 | |||
| VER | Paranthias furcifer (Valenciennes, 1828) | 2011 | 2014 | 2011 | ||
| INV | Paranthura japonica Richardson, 1909 | 2005 | 2007 | 2005 | ||
| INV | Parasmittina alba Ramalho, Muricy & Taylor, 2011 | 2014 | 2014 | |||
| INV | Parasmittina multiaviculata Souto, Ramalhosa & Canning-Clode, 2016 | 2016 | 2016 | |||
| INV | Parasmittina egyptiaca (Waters, 1909) | 2016 | 2016 | |||
| PP | Parasorites orbitolitoides Hofker, 1930 | 2016 | 2016 | |||
| INV | Paratapes textilis (Gmelin, 1791) | 2004 | 2004 | |||
| INV/par | Paratenuisentis ambiguus (Van Cleave, 1921) | 2001 | 2001 | |||
| VER | Parexocoetus mento (Valenciennes, 1847) | 1955 | 1955 | |||
| VER | Parupeneus forsskali (Fourmanoir & Guézé, 1976) | 2014 | 2014 | |||
| INV | Parvocalanus crassirostris (Dahl F., 1894) | 2009 | 2009 | |||
| PP | Pegidia lacunata McCulloch, 1977 | 2010 | 2010 | |||
| VER | Pempheris rhomboidea Kossmann & Räuber, 1877 | 1983 | 1983 | |||
| INV | Penaeus aztecus Ives, 1891 | 2012 | 2018 | 2012 | ||
| INV | Penaeus hathor (Burkenroad, 1959) | 2012 | 2012 | |||
| INV | Penaeus monodon Fabricius, 1798 | 2011 | 2011 | |||
| INV | Penaeus japonicus Spence Bate, 1888 | 1972 | 1980 | 1972 | ||
| INV | Penaeus pulchricaudatus Stebbing, 1914 | 1961 | 1982 | 1961 | ||
| INV | Penaeus semisulcatus De Haan, 1844 [in De Haan, 1833–1850] | 2016 | 2016 | |||
| PP/micro | Peridiniella catenata (Levander) Balech | 1987 | 1987 | |||
| PP/micro | Peridiniella danica (Paulsen) Y.B.Okolodkov & J.D.Dodge | 1901 | 1901 | |||
| PP/micro | Peridinium quadridentatum (F.Stein) Gert Hansen | 2005 | 2008 | 2005 | ||
| INV | Perinereis linea (Treadwell, 1936) | 2012 | 2012 | |||
| Pathogen | Perkinsus chesapeaki McLaughlin, Tall, Shaheen, El Sayed & Faisal | 1992 | 1992 | 1992 | ||
| Pathogen | Perkinsus olsenii R.J.G.Lester & G.H.G.Davis | 1983 | 1983 | |||
| INV | Perophora multiclathrata (Sluiter, 1904) | 1983 | 1983 | |||
| INV | Perophora viridis Verrill, 1871 | 1971 | 1971 | |||
| INV | Perophora japonica Oka, 1927 | 1982 | 1982 | |||
| PP | Petalonia binghamiseae (J.Agardh) K.L.Vinogradova | 1985 | 1985 | |||
| INV | Petricolaria pholadiformis (Lamarck, 1818) | 1896 | 1927 | 1896 | 1985 | |
| VER | Petroscirtes ancylodon Rüppell, 1835 | 2004 | 2004 | |||
| INV | Phallusia nigra Savigny, 1816 | 2008 | 2008 | |||
| INV | Phascolion convestitum Sluiter, 1902 | 1977 | 1977 | |||
| INV | Phascolosoma (Phascolosoma) scolops (Selenka & de Man, 1883) | 1975 | 1975 | |||
| INV | Photis lamellifera Schellenberg, 1928 | 1990 | 1990 | |||
| Pathogen | Photobacterium damsela Love, Teebken-Fisher, Hose, Farmer III, Hickman & Fanning | 1992 | 1992 | |||
| PP | Phrix spatulata (E.Y.Dawson) M.J.Wynne, M.Kamiya & J.A.West | 1992 | 1992 | |||
| INV | Phyllorhiza punctata Lendenfeld, 1884 | 2005 | 2018 | 2005 | ||
| VER | Piaractus brachypomus (Cuvier, 1818) | 2013 | 2013 | |||
| PP | Pikea californica Harvey | 1991 | 1991 | |||
| INV | Pileolaria berkeleyana (Rioja, 1942) | 1977 | 2007 | 1977 | ||
| INV | Pilumnopeus africanus (de Man, 1902) | 2013 | 2013 | |||
| INV | Pilumnopeus vauquelini (Audouin, 1826) | 1963 | 1963 | |||
| INV | Pilumnus minutus De Haan, 1835 [in De Haan, 1833–1850] | 2017 | 2017 | |||
| INV | Pinctada fucata (A. Gould, 1850) | 2018 | 2018 | |||
| INV | Pinctada radiata (Leach, 1814) | 1899 | 1998 | 1899 | ||
| VER | Pinguipes brasilianus Cuvier, 1829 | 1990 | 1990 | |||
| INV/par | Piscicola pojmanskae Bielecki, 1994 | 2008 | 2008 | |||
| INV | Pista unibranchia Day, 1963 | 1997 | 2005 | 1997 | ||
| INV | Plagusia squamosa (Herbst, 1790) | 1906 | 1906 | |||
| VER | Planiliza haematocheila (Temminck & Schlegel, 1845) | 1972 | 1995 | 1972 | ||
| PP | Planispirinella exigua (Brady, 1879) | 1910 | 1910 | |||
| PP | Planogypsina acervalis (Brady, 1884) | 1909 | 1909 | |||
| VER | Platycephalus indicus (Linnaeus, 1758) | 1978 | 1978 | |||
| PP | Plocamium secundatum (Kützing) Kützing | 1991 | 1991 | |||
| INV | Plocamopherus ocellatus Rüppell & Leuckart, 1828 | 2015 | 2015 | |||
| VER | Poecilopsetta beanii (Goode, 1881) | 1995 | 1995 | |||
| INV | Polyandrocarpa zorritensis (Van Name, 1931) | 1974 | 1974 | |||
| INV | Polycera hedgpethi Er. Marcus, 1964 | 1986 | 2001 | 1986 | ||
| INV | Polycerella emertoni A. E. Verrill, 1880 | 1964 | 1981 | 1964 | ||
| INV | Polycirrus twisti Potts, 1928 | 1983 | 1983 | |||
| INV | Polyclinum constellatum Savigny, 1816 | 2014 | 2014 | |||
| INV | Polydora colonia Moore, 1907 | 1983 | 2018 | 1983 | ||
| INV | Polydora triglanda Radashevsky & Hsieh, 2000 | 2014 | 2014 | |||
| INV | Polydora websteri Hartman in Loosanoff & Engle, 1943 | 2014 | 2014 | |||
| PP | Polyopes lancifolius (Harvey) Kawaguchi & Wang | 2008 | 2008 | |||
| PP | Polysiphonia paniculata Montagne | 1967 | 1967 | |||
| PP | Polysiphonia forfex Harvey | 2011 | 2011 | |||
| PP | Polysiphonia morrowii Harvey | 1975 | 1975 | 1997 | ||
| PP | Polysiphonia senticulosa Harvey | 1993 | 1993 | |||
| VER | Pomacanthus imperator (Bloch, 1787) | 2016 | 2016 | |||
| VER | Pomacanthus paru (Bloch, 1787) | 2015 | 2015 | |||
| VER | Pomacanthus maculosus (Forsskål, 1775) | 1994 | 1994 | 2012 | ||
| VER | Pomadasys stridens (Forsskål, 1775) | 1968 | 1968 | |||
| INV | Pontogammarus robustoides (Sars, 1894)* | 1962 | 1962 | |||
| PP | Porphyra umbilicalis Kützing | 1989 | 1989 | |||
| INV | Portunus segnis (Forskål, 1775) | 1958 | 1958 | |||
| INV | Potamocorbula amurensis (Schrenck, 1862) | 2018 | 2018 | |||
| INV | Potamopyrgus antipodarum (Gray, 1843)* | 1801 | 1801 | 1887 | ||
| INV | Potamothrix moldaviensis Vejdovský & Mrázek, 1903 | 2008 | 2008 | |||
| INV | Potamothrix bavaricus (Oschmann, 1913) | 2015 | 2015 | |||
| INV | Potamothrix bedoti (Piguet, 1913) | 1915 | 1915 | |||
| INV | Potamothrix heuscheri (Bretscher, 1900)* | 1960 | 1960 | |||
| INV | Potamothrix vejdovskyi (Hrabĕ, 1941)* | 1967 | 1967 | |||
| inv | Prionospio aluta Maciolek, 1985 | 1994 | 1994 | |||
| INV | Prionospio depauperata Imajima, 1990 | 2018 | 2018 | |||
| INV | Prionospio pulchra Imajima, 1990 | 1989 | 1989 | 1991 | ||
| INV | Proasellus coxalis (Dollfus, 1892) | 2011 | 2011 | |||
| INV | Procambarus clarkii (Girard, 1852)* | 2000 | 2000 | |||
| INV | Prokelisia marginata (Van Duzee, 1897) | 2011 | 2011 | |||
| PP/micro | Prorocentrum gracile Schütt | 1989 | 1989 | |||
| INV | Prosphaerosyllis longipapillata (Hartmann-Schröder, 1979) | 1997 | 1997 | |||
| VER | Proterorhinus nasalis (De Filippi, 1863) | 2020 | 2020 | |||
| INV | Protodorvillea biarticulata Day, 1963 | 1975 | 1975 | |||
| INV | Protoreaster nodosus (Linnaeus, 1758) | 1981 | 1981 | |||
| INV | Psammacoma gubernaculum (Hanley, 1844) | 2009 | 2009 | |||
| PP/micro | Pseudochattonella farcimen (Riisberg I.) | 1998 | 2001 | 1998 | ||
| PP/micro | Pseudochattonella verruculosa (Y.Hara & M.Chihara) S.Tanabe-Hosoi, D.Honda, S.Fukaya, Y.Inagaki & Y.Sako | 1998 | 2015 | 1998 | ||
| INV/par | Pseudodactylogyrus anguillae (Yin & Sproston, 1948) | 1982 | 1985 | 1982 | ||
| INV/par | Pseudodactylogyrus bini (Kikuchi, 1929) | 1985 | 1985 | 1997 | ||
| INV | Pseudodiaptomus marinus Sato, 1913 | 2007 | 2010 | 2007 | ||
| INV | Pseudonereis anomala Gravier, 1899 | 1969 | 1969 | |||
| PP/micro | Pseudo-nitzschia australis Frenguelli | 1995 | 1995 | 2000 | ||
| PP/micro | Pseudo-nitzschia multistriata (Takano) Takano | 1985 | 1985 | |||
| INV | Pseudopolydora paucibranchiata (Okuda, 1937) | 1977 | 1982 | 1977 | ||
| VER | Pteragogus trispilus Randall, 2013 | 1992 | 1992 | |||
| VER | Pterois miles (Bennett, 1828) | 2009 | 2009 | |||
| INV | Ptilohyale littoralis (Stimpson, 1853) | 2009 | 2009 | |||
| INV | Purpuradusta gracilis notata (Gill, 1858) | 1988 | 1988 | |||
| INV | Pyrgulina pupaeformis (Souverbie, 1865) | 1995 | 1995 | |||
| INV | Pyromaia tuberculata (Lockington, 1877) | 2016 | 2016 | |||
| PP | Pyropia yezoensis (Ueda) M.S.Hwang & H.G.Choi | 1975 | 1984 | 1975 | ||
| PP | Pyropia suborbiculata (Kjellman) J.E.Sutherland, H.G.Choi, M.S.Hwang & W.A.Nelson | 2010 | 2010 | 2014 | ||
| INV | Pyrunculus fourierii (Audouin, 1826) | 1995 | 1995 | |||
| INV | Rangia cuneata (G. B. Sowerby I, 1832) | 1997 | 2011 | 1997 | ||
| INV | Rapana venosa (Valenciennes, 1846) | 1956 | 1997 | 1973 | 1956 | |
| VER | Rastrelliger kanagurta (Cuvier, 1816) | 2018 | 2018 | |||
| INV | Rhinoclavis kochi (Philippi, 1848) | 1976 | 1976 | |||
| INV | Rhithropanopeus harrisii (Gould, 1841) | 1936 | 1936 | 1950 | 1994 | 1948 |
| PP/micro | Rhizosolenia calcar-avis Schultze | 2009 | 2009 | |||
| INV | Rhopilema nomadica Galil, 1990 | 1995 | 1995 | |||
| INV | Ringicula minuta H. Adams, 1872 | 2019 | 2019 | |||
| INV | Rissoina bertholleti Issel, 1869 | 1985 | 1985 | |||
| INV | Ruditapes philippinarum (Adams & Reeve, 1850) | 1973 | 1973 | 1980 | ||
| PP | Rugulopteryx okamurae (E.Y.Dawson) I.K.Hwang, W.J.Lee & H.S.Kim | 2002 | 2015 | 2002 | ||
| PP | Saccharina japonica (J.E. Areschoug) C.E.Lane, C.Mayes, Druehl & G.W.Saunders | 1976 | 1980 | 1976 | ||
| INV | Saccostrea cuccullata (Born, 1778) | 2007 | 2007 | |||
| INV | Saccostrea glomerata (Gould, 1850) | 1984 | 1984 | |||
| VER | Salvelinus fontinalis (Mitchill, 1814)* | 1916 | 1916 | |||
| PP | Sarconema filiforme (Sonder) Kylin | 1990 | 1990 | |||
| PP | Sarconema scinaioides Børgesen | 1980 | 1980 | |||
| PP | Sargassum muticum (Yendo) Fensholt | 1972 | 1972 | 1980 | ||
| VER | Sargocentron rubrum (Forsskål, 1775) | 1943 | 1943 | |||
| VER | Saurida lessepsianus Russell, Golani & Tikochinski, 2015 | 1960 | 1960 | |||
| PP | Scageliopsis patens Wollaston | 1989 | 1989 | |||
| VER | Scarus ghobban Forsskål, 1775 | 2010 | 2010 | |||
| VER | Scatophagus argus (Linnaeus, 1766) | 2007 | 2007 | |||
| INV | Schizoporella japonica Ortmann, 1890 | 1976 | 1976 | |||
| INV | Schizoporella pungens Canu & Bassler, 1928 | 2010 | 2010 | |||
| VER | Sciaenops ocellatus (Linnaeus, 1766) | 2016 | 2016 | |||
| INV | Scolelepis (Parascolelepis) gilchristi (Day, 1961) | 1977 | 1977 | |||
| INV | Scolelepis korsuni Sikorski, 1994 | 1994 | 1994 | |||
| INV | Scolionema suvaense (Agassiz & Mayer, 1899) | 1950 | 1950 | |||
| VER | Scomberomorus commerson (Lacepède, 1800) | 2008 | 2008 | |||
| INV | Scyllarus caparti Holthuis, 1952 | 1977 | 1977 | |||
| PP | Scytosiphon dotyi M.J.Wynne | 1968 | 1991 | 1968 | ||
| VER | Sebastes schlegelii Hilgendorf, 1880 | 2008 | 2008 | |||
| INV | Sebastiscus marmoratus (Cuvier, 1829) | 2016 | 2016 | |||
| INV | Sepioteuthis lessoniana Férussac [in Lesson], 1831 | 2009 | 2009 | |||
| INV | Septifer cumingii Récluz, 1848 | 2005 | 2005 | |||
| VER | Siganus fuscescens (Houttuyn, 1782) | 2020 | 2020 | |||
| VER | Siganus virgatus (Valenciennes, 1835) | 1975 | 1975 | |||
| VER | Siganus luridus (Rüppell, 1829) | 1964 | 1964 | |||
| VER | Siganus rivulatus Forsskål & Niebuhr, 1775 | 1925 | 1925 | |||
| PP | Sigmamiliolinella australis (Parr, 1932) | 2001 | 2001 | |||
| VER | Sillago suezensis Golani, Fricke & Tikochinski, 2013 | 2009 | 2009 | |||
| INV | Sinelobus vanhaareni Bamber, 2014 | 2006 | 2010 | 2006 | ||
| INV | Sinezona plicata (Hedley, 1899) | 2019 | 2019 | |||
| INV | Smaragdia souverbiana (Montrouzier in Souverbie & Montrouzier, 1863) | 1993 | 1993 | |||
| INV | Smittina nitidissima (Hincks, 1880) | 2014 | 2014 | |||
| INV | Smittoidea prolifica Osburn, 1952 | 1995 | 1995 | |||
| PP | Solieria filiformis (Kützing) P.W.Gabrielson | 1922 | 1922 | |||
| PP | Sorites variabilis Lacroix, 1941 | 1996 | 1996 | |||
| PP | Spartina anglica C.E. Hubbard | 1924 | 1924 | |||
| PP | Spartina densiflora Brongn. | 1986 | 1986 | |||
| PP | Spartina patens (Aiton) Muhl. | 1986 | 1986 | |||
| PP | Spartina alterniflora Loisel | 1806 | 1806 | |||
| PP | Spermothamnion cymosum (Harvey) De Toni | 2010 | 2010 | |||
| INV | Sphaeroma walkeri Stebbing, 1905 | 1977 | 2015 | 1977 | 2004 | |
| PP | Sphaerotrichia firma (E.S.Gepp) A.D.Zinova | 1981 | 1981 | |||
| INV | Sphaerozius nitidus Stimpson, 1858 | 2013 | 2013 | |||
| VER | Sphyraena chrysotaenia Klunzinger, 1884 | 1964 | 1964 | |||
| VER | Sphyraena flavicauda Rüppell, 1838 | 2003 | 2003 | |||
| INV | Spirobranchus tetraceros (Schmarda, 1861) | 1970 | 1970 | |||
| PP | Spiroloculina angulata Cushman, 1917 | 1996 | 1996 | |||
| PP | Spiroloculina antillarum d’Orbigny, 1839 | 1911 | 1911 | |||
| INV | Spirorbis (Spirorbis) marioni Caullery & Mesnil, 1897 | 1974 | 1974 | 1977 | ||
| INV | Spondylus spinosus Schreibers, 1793 | 2001 | 2001 | |||
| PP | Spongoclonium caribaeum (Børgesen) M.J.Wynne | 1967 | 1967 | 1974 | ||
| VER | Spratelloides delicatulus (Bennett, 1832) | 2014 | 2014 | |||
| VER | Stegastes variabilis (Castelnau, 1855) | 2014 | 2014 | |||
| INV | Stenothoe georgiana Bynum & Fox, 1977 | 2010 | 2011 | 2010 | ||
| VER | Stephanolepis diaspros Fraser-Brunner, 1940 | 1935 | 1935 | |||
| INV | Sternodromia spinirostris (Miers, 1881) | 1969 | 1969 | |||
| INV | Sticteulima lentiginosa (A. Adams, 1861) | 1995 | 1995 | |||
| INV | Stomatella sp. | 2011 | 2011 | |||
| INV | Streblospio gynobranchiata Rice & Levin, 1998 | 2011 | 2011 | |||
| INV | Streblospio benedicti Webster, 1879 | 1982 | 1982 | |||
| INV | Styela plicata (Lesueur, 1823) | 1877 | 1989 | 1877 | ||
| INV | Styela canopus (Savigny, 1816) | 2006 | 2006 | |||
| INV | Styela clava Herdman, 1881 | 1968 | 2017 | 1968 | 2005 | 2002 |
| PP | Stypopodium schimperi (Kützing) M.Verlaque & Boudouresque | 1990 | 1990 | |||
| INV | Syllis hyllebergi (Licher, 1999) | 1972 | 1972 | |||
| INV | Syllis pectinans Haswell, 1920 | 1982 | 1982 | 2013 | ||
| PP | Symphyocladia marchantioides (Harvey) Falkenberg | 1971 | 1971 | 1984 | ||
| PP | Symphyocladiella dendroidea (Montagne) D.Bustamante, B.Y.Won, S.C.Lindstrom & T.O.Cho | 1993 | 2005 | 1993 | ||
| INV | Symplegma rubra Monniot C., 1972 | 2014 | 2014 | |||
| INV | Symplegma brakenhielmi (Michaelsen, 1904) | 2003 | 2003 | |||
| VER | Synagrops japonicus (Döderlein, 1883) | 1987 | 1987 | |||
| INV | Synaptula reciprocans (Forsskål, 1775) | 1967 | 1967 | |||
| VER | Synchiropus sechellensis Regan, 1908 | 2014 | 2014 | |||
| INV | Synidotea laticauda Benedict, 1897 | 1975 | 1975 | |||
| INV | Syphonota geographica (A. Adams & Reeve, 1850) | 1999 | 1999 | |||
| INV | Syrnola fasciata Jickeli, 1882 | 1995 | 1995 | |||
| INV/par | Taeniastrotos sp. | 1993 | 1993 | |||
| PP/micro | Takayama tasmanica de Salas, Bolch & Hallegraeff | 2008 | 2008 | |||
| INV | Telmatogeton japonicus Tokunaga, 1933 | 1962 | 1962 | 1979 | ||
| INV | Tenellia adspersa (Nordmann, 1845) | 2001 | 2001 | |||
| VER | Terapon theraps (Cuvier, 1829) | 2007 | 2007 | |||
| INV | Terebella ehrenbergi Gravier, 1906 | 1952 | 1952 | |||
| INV | Teredo bartschi Clapp, 1923 | 2003 | 2003 | 2007 | ||
| INV | Thalamita gloriensis Crosnier, 1962 | 1977 | 1977 | |||
| INV | Thalamita poissonii (Audouin, 1826) | 1969 | 1969 | |||
| PP/micro | Thalassiosira nordenskioeldii Cleve | 1967 | 1967 | |||
| PP/micro | Thalassiosira hendeyi Hasle & G.Fryxell | 1978 | 1978 | |||
| PP/micro | Thalassiosira tealata Takano | 1968 | 1968 | |||
| PP/micro | Thecadinium yashimaense S.Yoshimatsu, S.Toriumi & J.D.Dodge | 2002 | 2002 | |||
| INV | Thelepus japonicus Marenzeller, 1884 | 2017 | 2017 | |||
| INV | Theora lubrica Gould, 1861 | 2001 | 2010 | 2001 | ||
| INV | Timarete punctata (Grube, 1859) | 2006 | 2006 | |||
| INV | Tonicia atrata (G.B. Sowerby II, 1840) | 1978 | 1978 | |||
| VER | Torquigener flavimaculosus Hardy & Randall, 1983 | 2006 | 2006 | |||
| INV | Trachysalambria palaestinensis (Steinitz, 1932) | 1995 | 1995 | |||
| INV | Tremoctopus gracilis (Souleyet, 1852) | 1937 | 1937 | |||
| INV | Tricellaria inopinata d’Hondt & Occhipinti Ambrogi, 1985 | 1982 | 1996 | 1982 | ||
| INV | Triconia rufa (Boxshall & Böttger, 1987) | 2004 | 2004 | |||
| INV | Triconia umerus (Böttger-Schnack & Boxshall, 1990) | 2004 | 2004 | |||
| INV | Tridentata marginata (Kirchenpauer, 1864) | 1980 | 1980 | 1990 | ||
| VER | Tridentiger barbatus (Günther, 1861) | 2016 | 2016 | |||
| PP/micro | Trieres mobiliensis (J.W.Bailey) Ashworth & Theriot | 1983 | 1983 | |||
| PP/micro | Trieres regia (M.Schultze) M.P.Ashworth & E.C.Theriot | 1989 | 1989 | |||
| VER | Trinectes maculatus (Bloch & Schneider, 1801) | 1984 | 1984 | |||
| PP/micro | Tripos arietinus (Cleve) F.Gómez | 1992 | 1992 | |||
| PP/micro | Tripos macroceros (Ehrenberg) F.Gómez | 1983 | 1983 | |||
| INV | Trochus erithreus Brocchi, 1821 | 1985 | 1985 | |||
| INV | Tubastraea tagusensis Wells, 1982 | 2017 | 2017 | |||
| INV | Turbonilla edgarii (Melvill, 1896) | 1996 | 1996 | |||
| VER | Tylerius spinosissimus (Regan, 1908) | 2004 | 2004 | |||
| VER | Tylosurus crocodilus crocodilus (Péron & Lesueur, 1821) | 2003 | 2003 | |||
| PP | Ulva australis Areschoug | 1984 | 1990 | 1984 | ||
| PP | Ulva californica Wille | 2006 | 2006 | 2011 | ||
| PP | Ulva gigantea (Kützing) Bliding | 2015 | 2015 | |||
| PP | Ulva ohnoi M.Hiraoka & S.Shimada | 2011 | 2015 | 2011 | ||
| PP | Ulvaria obscura (Kützing) P.Gayral ex C.Bliding | 1985 | 1985 | |||
| PP | Umbraulva dangeardii M.J.Wynne & G.Furnari | 2014 | 2014 | |||
| PP | Undaria pinnatifida (Harvey) Suringar | 1971 | 1975 | 1971 | ||
| PP | Undella hadai Balech | 2004 | 2004 | |||
| VER | Upeneus moluccensis (Bleeker, 1855) | 1947 | 1947 | |||
| VER | Upeneus pori Ben-Tuvia & Golani, 1989 | 2003 | 2003 | |||
| INV | Urocaridella pulchella Yokes & Galil, 2006 | 2018 | 2018 | |||
| PP | Uronema marinum Womersley | 1989 | 1989 | |||
| INV | Urosalpinx cinerea (Say, 1822) | 1960 | 1960 | |||
| INV | Vallicula multiformis Rankin, 1956 | 1998 | 1998 | |||
| VER | Vanderhorstia mertensi Klausewitz, 1974 | 2019 | 2019 | |||
| VER | Variola louti (Forsskål, 1775) | 2018 | 2018 | |||
| PP | Vaucheria longicaulis Hoppaugh | 2020 | 2020 | |||
| INV | Viriola sp.[cf. bayani] Jousseaume, 1884 | 2016 | 2016 | |||
| INV | Watersipora aterrima (Ortmann, 1890) | 1983 | 1983 | |||
| INV | Watersipora subatra (Ortmann, 1890) | 1987 | 1987 | |||
| INV | Watersipora arcuata Banta, 1969 | 1990 | 1990 | 2013 | ||
| PP | Womersleyella setacea (Hollenberg) R.E.Norris | 1986 | 1986 | |||
| INV | Xanthias lamarckii (H. Milne Edwards, 1834) | 2013 | 2013 | |||
| INV | Xenostrobus securis (Lamarck, 1819) | 1991 | 2005 | 1991 | ||
| INV | Yoldia limatula (Say, 1831) | 2019 | 2019 | |||
| INV | Zafra savignyi (Moazzo, 1939) | 1995 | 1995 | |||
| INV | Zafra selasphora (Melvill & Standen, 1901) | 1995 | 1995 | |||
| VER | Zebrasoma flavescens (Bennett, 1828) | 2008 | 2008 | |||
| VER | Zebrasoma xanthurum (Blyth, 1852) | 2015 | 2015 |
4. Discussion
4.1. Validation of European NIS: A Challenging and Dynamic Task
4.2. Issues with Assessing the Spatial Distribution of NIS in Europe’s Seas
4.3. Trends Indicator
4.4. Uncertainties in Trends
4.5. Threshold Values
4.6. Concluding Remarks—The Way forward
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Díaz, S.; Settele, J.; Brondízio, E.S.; Ngo, H.T.; Agard, J.; Arneth, A.; Zayas, C.N. Pervasive human-driven decline of life on Earth points to the need for transformative change. Science 2019, 366, eaax3100. [Google Scholar] [CrossRef] [PubMed]
- Korpinen, S.; Klančnik, K.; Peterlin, M.; Nurmi, M.; Laamanen, L.; Zupančič, G.; Popit, A.; Murray, C.; Harvey, T.; Andersen, J.H.; et al. Multiple Pressures and Their Combined Effects in Europe’s Seas; ETC/ICM Technical Report 4/2019: European Topic Centre on Inland, Coastal and Marine Waters; ETC/ICM: Magdeburg, Germany, 2019. [Google Scholar]
- Simberloff, D.; Martin, J.-L.; Genovesi, P.; Maris, V.; Wardle, D.A.; Aronson, J.; Courchamp, F.; Galil, B.; Garcia-Berthou, E.; Pascal, M.; et al. Impacts of biological invasions: What’s what and the way forward. Trends Ecol. Evol. 2013, 28, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Ojaveer, H.; Galil, B.S.; Campbell, M.L.; Carlton, J.T.; Canning-Clode, J.; Cook, E.J.; Davidson, A.D.; Hewitt, C.L.; Jelmert, A.; Marchini, A.; et al. Classification of Non-Indigenous Species Based on Their Impacts: Considerations for Application in Marine Management. PLoS Biol. 2015, 13, e1002130. [Google Scholar] [CrossRef] [PubMed]
- Anton, A.; Geraldi, N.R.; Lovelock, C.E.; Apostolaki, E.T.; Bennett, S.; Cebrian, J.; Krause-Jensen, D.; Marbà, N.; Martinetto, P.; Pandolfi, J.M. Global ecological impacts of marine exotic species. Nat. Ecol. Evol. 2019, 3, 787–800. [Google Scholar] [CrossRef]
- Viard, F.; Riginos, C.; Bierne, N. Anthropogenic Hybridization at Sea: Three evolutionary questions relevant to invasive species management. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2020, 375, 20190547. [Google Scholar] [CrossRef]
- Tsirintanis, K.; Azzurro, E.; Crocetta, F.; Dimiza, M.; Froglia, C.; Gerovasileiou, V.; Langeneck, J.; Mancinelli, G.; Rosso, A.; Stern, N. Bioinvasion impacts on biodiversity, ecosystem services, and human health in the Mediterranean Sea. Aquat Invasions 2022, 3, 308–352. [Google Scholar] [CrossRef]
- Blackburn, T.M.; Pyšek, P.; Bacher, S.; Carlton, J.T.; Duncan, R.P.; Jarošík, V.; Wilson, J.R.; Richardson, D.M. A proposed unified framework for biological invasions. Trends Ecol. Evol. 2011, 26, 333–339. [Google Scholar] [CrossRef]
- European Commission. Commission Decision (EU) 2017/848 of May 2017 laying down criteria and methodological standards on good environmental status of marine waters and specifications and standardised methods for monitoring and assessment, and repealing Decision 2010/477/EU. Off. J. Eur. Union L 2017, 125, 43–74. [Google Scholar]
- HELCOM. Trends in Arrival of New Non-Indigenous Species. HELCOM Core Indicator Report. Available online: https://helcom.fi/media/core%20indicators/Trends-in-arrival-of-new-non-indigenous-species-HELCOM-core-indicator-2018.pdf (accessed on 10 August 2022).
- OSPAR CEMP Guidelines Common Indicator: Changes to Non-Indigenous Species Communities (NIS3) (OSPAR Agreement2018-04). Available online: https://www.ospar.org/documents?v=38992 (accessed on 10 June 2022).
- UNEP/MED WG. 500/Monitoring and Assessment Scales, Assessment Criteria and Thresholds Values for the IMAP Common Indicator Related to Non-Indigenous Species. In Proceedings of the CORMON Meeting, Online, 10 June 2021. [Google Scholar]
- UNEP First Draft of the Post-Biodiversity Framework. In Proceedings of the Open Ended Working Group on the Post-Global Biodiversity Framework, Online, 23 August–3 September 2021; Convention on Biological Diversity: Montreal, QC, Canada, 2021.
- Tsiamis, K.; Palialexis, A.; Connor, D.; Antoniadis, S.; Bartilotti, C.; Bartolo, G.A.; Berggreen, U.C.; Boschetti, S.; Buschbaum, C.; Canning-Clode, J.; et al. Marine Strategy Framework Directive-Descriptor 2, Non-Indigenous Species, Delivering Solid Recommendations for Setting Threshold Values for Non-Indigenous Species Pressure on European Seas; Publications Office of the European Union: Luxembourg, 2021. [Google Scholar] [CrossRef]
- Streftaris, N.; Zenetos, A.; Papathanassiou, E. Globalisation in marine ecosystems—The story of non indigenous marine species across European Seas. Oceanogr. Mar. Biol. 2005, 43, 419–453. [Google Scholar]
- Zenetos, A.; Streftaris, N.; Micu, D.; Todorova, V.; Joseffson, M.; Gollasch, S.; Zaiko, A.; Olenin, S. Harmonisation of European alien species databases: A 2009 update of marine alien species towards the forthcoming SEBI2010 report. In Proceedings of the Poster Presented at BIOLIEF, World Conference on Biological Invasions and Ecosystem Functioning, Porto, Portugal, 27–30 October 2009. [Google Scholar]
- DAISIE. Handbook on Alien Species in Europe; Springer: Berlin, Germany, 2009; p. 399. [Google Scholar]
- AquaNIS. Editorial Board, Information System on Aquatic Non-Indigenous and Cryptogenic Species. World Wide Web Electronic Publication. Available online: https://www.corpi.ku.lt/databases/aquanis (accessed on 1 June 2021).
- EASIN, European Commission—Joint Research Centre—European Alien Species Information Network (EASIN). 2022. Available online: https://easin.jrc.ec.europa.eu/ (accessed on 16 August 2022).
- Tsiamis, K.; Gervasini, E.; D’Amico, F.; Backeljau, T. The EASIN editorial board: Quality assurance, exchange and sharing of alien species information in Europe. Manag. Biol. Invasions 2016, 7, 321–328. [Google Scholar] [CrossRef]
- Chainho, P.; Fernandes, A.; Amorim, A.; Ávila, S.P.; Canning-Clode, J.; Castro, J.J.; Costa, A.C.; Costa, J.L.; Cruz, T.; Gollasch, S.; et al. Non-indigenous species in Portuguese coastal areas, coastal lagoons, estuaries and islands. Estuar. Coast. Shelf Sci. 2015, 167, 199–211. [Google Scholar] [CrossRef]
- Ojaveer, H.; Olenin, S.; Narščius, A.; Florin, A.-B.; Ezhova, E.; Gollasch, S.; Jensen, K.R.; Lehtiniemi, M.; Minchin, D.; Normant Saremba, M.; et al. Dynamics of Biological Invasions and Pathways over Time: A Case Study of a Temperate Coastal Sea. Biol. Invasions 2017, 19, 799–813. [Google Scholar] [CrossRef]
- Zenetos, A.; Albano, P.G.; Garcia, E.L.; Stern, N.; Tsiamis, K.; Galanidi, M. Established non-indigenous species increased by 40% in 11 years in the Mediterranean Sea. Meditter. Mar. Sci. 2022, 23, 196–212. [Google Scholar] [CrossRef]
- Băncilă, R.I.; Skolka, M.; Ivanova, P.; Surugiu, V.; Stefanova, K.; Todorova, V.; Zenetos, A. Alien species of the Romanian and Bulgarian Black Sea coast: State of knowledge, uncertainties, and needs for future research. Aquat Invasions 2022, 17, 353–373. [Google Scholar] [CrossRef]
- Servello, G.; Andaloro, F.; Azzurro, E.; Castriota, L.; Catra, M.; Chiarore, A.; Crocetta, F.; D’Alessandro, M.; Denitto, F.; Froglia, C.; et al. Marine alien species in Italy: A contribution to the implementation of descriptor D2 of the marine strategy framework directive. Mediterr. Mar. Sci. 2019, 20, 1–48. [Google Scholar] [CrossRef]
- Zenetos, A.; Karachle, P.K.; Corsini-Foka, M.; Gerovasileiou, V.; Simboura, N.; Xentidis, N.J.; Tsiamis, K. Is the trend in new introductions of marine non-indigenous species a reliable criterion for assessing good environmental status? The case study of Greece. Meditter. Mar. Sci. 2020, 21, 775–793. [Google Scholar] [CrossRef]
- Staehr, P.A.; Jakobsen, H.H.; Hansen, J.L.S.; Andersen, P.; Christensen, J.; Göke, C.; Thomsen, M.S.; Stebbing, P.D. Trends in records and contribution of non-indigenous and cryptogenic species to marine communities in Danish waters: Potential indicators for assessing impact. Aquat. Invasions 2020, 15, 217–244. [Google Scholar] [CrossRef]
- Verleye, T.J.; De Raedemaecker, F.; Vandepitte, L.; Fockedey, N.; Lescrauwaet, A.-K.; De Pooter, D.; Mees, J. (Eds.) Niet-inheemse Soorten in Het Belgisch Deel van de Noordzee en Aanpalende Estuaria Anno 2020; VLIZ Special Publication, 86; Vlaams Instituut voor de Zee (VLIZ): Oostende, België, 2020; p. 623. ISBN 9789464206005. [Google Scholar]
- Castro, N.; Carlton, J.T.; Costa, A.C.; Marques, C.; Hewitt, C.L.; Cacabelos, E.; Gizzi, F.; Gestoso, I.; Monteiro, J.G.; Costa, J.L.; et al. Diversity and patterns of marine non-native species in the archipelagos of Macaronesia. Divers. Distrib. 2022, 28, 667–684. [Google Scholar] [CrossRef]
- Galanidi, M.; Zenetos, A. Data-Driven Recommendations for Establishing Threshold Values for the NIS Trend Indicator in the Mediterranean Sea. Diversity 2022, 14, 57. [Google Scholar] [CrossRef]
- Bailey, S.A.; Brown, L.; Campbell, M.L.; Canning-Clode, J.; Carlton, J.T.; Castro, N.; Chainho, P.; Chan, F.T.; Creed, J.C.; Curd, A.; et al. Trends in the Detection of Aquatic Non-indigenous Species across Global Marine, Estuarine and Freshwater Ecosystems: A 50-year Perspective. Divers. Distrib. 2020, 26, 1780–1797. [Google Scholar] [CrossRef]
- Tsiamis, K.; Palialexis, A.; Stefanova, K.; Gladan, Ž.N.; Skejić, S.; Despalatović, M.; Cvitković, I.; Dragičević, B.; Dulčić, J.; Vidjak, O.; et al. Non-indigenous species refined national baseline inventories: A synthesis in the context of the European Union’s Marine Strategy Framework Directive. Mar. Pollut. Bull. 2019, 145, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Tsiamis, K.; Boschetti, S.; Palialexis, A.; Somma, F.; Cardoso, A.C. Marine Strategy Framework Directive—Review and Analysis of EU Member States’ 2018 Reports—Descriptor 2: Non-Indigenous Species; Assessment (Art. 8), Good Environmental Status (Art. 9) and Targets (Art. 10); Publications Office of the European Union: Luxembourg, 2021; EUR EN. [Google Scholar] [CrossRef]
- Jensen, H.M.; Panagiotidis, P.; Reker, J. Delineation of the MSFD Article Marine Regions and Subregions, Version 1.0; European Environment Agency: Kopenhagen, Denmark. Available online: https://data.europa.eu/euodp/data/dataset/data_msfdregions-and-subregions (accessed on 15 September 2021).
- Spalding, M.D.; Fox, H.E.; Allen, G.R.; Davidson, N.; Ferdaña, A.Z.; Finlayson, M.; Halpern, B.S.; Jorge, M.A.; Lombana, A.; Lourie, S.A. Marine ecoregions of the world: A bioregionalization of coastal and shelf areas. BioScience 2007, 57, 573–583. [Google Scholar] [CrossRef]
- Carlton, J.T. Biological invasions and cryptogenic species. Ecology 1996, 77, 1653–1655. [Google Scholar] [CrossRef]
- Bick, A.; Bastrop, R.; Kotta, J.; Meißner, K.; Meyer, M.; Syomin, V. Description of a new species of Sabellidae (Polychaeta, Annelida) from fresh and brackish waters in Europe, with some remarks on the branchial crown of Laonome. Zootaxa 2018, 4483, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Capa, M.; van Moorsel, G.; Tempelman, D. The Australian feather-duster worm Laonome calida Capa, 2007 (Annelida: Sabellidae) introduced into European inland waters? Bioinvasions Rec. 2014, 3, 1–11. [Google Scholar] [CrossRef]
- Tamulyonis, A.Y.; Gagaev, S.Y.; Stratanenko, E.A.; Zuyev, Y.A.; Potin, V.V. Invasion of the Polychaeta Laonome xeprovala Bick & Bastrop, 2018 (Sabellidae, Polychaeta) into the estuary of the Luga and Khabolovka Rivers (Luga Bay, Gulf of Finland). Russ. J. Biol. Invasions 2020, 11, 148–154. [Google Scholar] [CrossRef]
- Gómez, F. Comments on the non-indigenous microalgae in the European seas. Mar Pollut Bull. 2019, 148, 1–2. [Google Scholar] [CrossRef]
- Canning-Clode, J.; Carlton, J.T. Refining and expanding global climate change scenarios in the sea: Poleward creep complexities, range termini, and setbacks and surges. Divers. Distrib. 2017, 23, 463–473. [Google Scholar] [CrossRef]
- Provan, J.; Booth, D.; Todd, N.P.; Beatty, G.E.; Maggs, C.A. Tracking biological invasions in space and time: Elucidating the invasive history of the green alga Codium fragile using old DNA. Divers. Distrib. 2008, 14, 343–354. [Google Scholar] [CrossRef]
- Regan, H.M.; Colyman, M.; Burgman, M.A. A taxonomy and treatment of uncertainty for ecology and conservation biology. Ecol. Appl. 2002, 12, 618–628. [Google Scholar] [CrossRef]
- Latombe, G.; Canavan, S.; Hirsch, H.; Hui, C.; Kumschick, S.; Nsikani, M.M.; Potgieter, L.J.; Robinson, T.B.; Saul, W.-C.; Turner, S.C.; et al. A four-component classification of uncertainties in biological invasions: Implications for management. Ecosphere 2019, 10, e02669. [Google Scholar] [CrossRef]
- Appeltans, W.; Ahyong, S.T.; Anderson, G.; Angel, M.V.; Artois, T.; Bailly, N.; Bamber, R.; Barber, A.; Bartsch, I.; Berta, A. The Magnitude of Global Marine Species Diversity. Curr. Biol. 2012, 22, 2189–2202. [Google Scholar] [CrossRef] [PubMed]
- Bock, D.G.; MacIsaac, H.J.; Cristescu, M.E. Multilocus genetic analyses differentiate between widespread and spatially restricted cryptic species in a model ascidian. Proc. R. Soc. B Biol. Sci. 2012, 279, 2377–2385. [Google Scholar] [CrossRef]
- Gissi, C.; Hastings, K.E.M.; Gasparini, F.; Stach, T.; Pennati, R.; Manni, L. An unprecedented taxonomic revision of a model organism: The paradigmatic case of Ciona robusta and Ciona intestinalis. Zool. Scr. 2017, 46, 521–522. [Google Scholar] [CrossRef]
- Nydam, M.; Harrison, R. Genealogical relationships within and among shallow-water Ciona species (Ascidiacea). Mar. Biol. 2007, 151, 1839–1847. [Google Scholar] [CrossRef]
- Bouchemousse, S.; Bishop, J.; Viard, F. Contrasting global genetic patterns in two biologically similar, widespread and invasive Ciona species (Tunicata, Ascidiacea). Sci. Rep. 2016, 6, 24875. [Google Scholar] [CrossRef] [PubMed]
- Simon, A.; Fraïsse, C.; El Ayari, T.; Liautard-Haag, C.; Strelkov, P.; Welch, J.J.; Bierne, N. How do species barriers decay? Concordance and local introgression in mosaic hybrid zones of mussels. J. Evol. Biol. 2021, 34, 208–223. [Google Scholar] [CrossRef]
- Couton, M.; Lévêque, L.; Daguin-Thiébaut, C.; Comtet, T.; Viard, F. Water eDNA metabarcoding is effective in detecting non-native species in marinas, but detection errors still hinder its use for passive monitoring. Biofouling 2022, 38, 367–383. [Google Scholar] [CrossRef]
- Riquet, F.; Simon, A.; Bierne, N. Weird genotypes? Don’t discard them, transmissible cancer could be an explanation. Evol. Appl. 2017, 10, 140–145. [Google Scholar] [CrossRef]
- Hammel, M.; Simon, A.; Arbiol, C.; Villalba, A.; Burioli, E.A.V.; Pepin, J.F.; Lamy, J.B.; Benabdelmouna, A.; Bernard, I.; Houssin, M.; et al. Prevalence and polymorphism of a mussel transmissible cancer in Europe. Mol. Ecol. 2022, 31, 736–751. [Google Scholar] [CrossRef]
- Gittenberger, A.; Gittenberger, E. Polytypic Mytilus edulis, with a name for the Baltic subspecies. Basteria 2021, 85, 116–125. [Google Scholar]
- Hu, L.; Wang, H.; Zhang, Z.; Li, C.; Guo, X. Classification of small flat oysters of Ostrea stentina species complex and a new specie Ostrea neostentina sp. nov. (Bivalvia: Ostreidae). J. Shellfish Res. 2019, 38, 295–308. [Google Scholar] [CrossRef]
- Lavesque, N.; Daffe, G.; Londono-Mesa, M.H.; Hutchings, P. Revision of the French Terebellidae sensu stricto (Annelida, Terebelliformia), with descriptions of nine new species. Zootaxa 2021, 5038, 1–63. [Google Scholar] [CrossRef] [PubMed]
- Turon, X.; Casso, M.; Pascual, M.; Viard, F. Looks can be deceiving: Didemnum pseudovexillum sp. nov. (Ascidiacea) in European harbours. Mar. Biodivers. 2020, 50, 48. [Google Scholar] [CrossRef]
- Abbott, R.J. Plant invasions, interspecific hybridization and the evolution of new plant taxa. Trends Ecol. Evol. 1992, 7, 401–405. [Google Scholar] [CrossRef]
- Preston, C.D.; Pearman, D.A. Plant hybrids in the wild: Evidence from biological recording. Biol. J. Linn. Soc. 2015, 115, 555–572. [Google Scholar] [CrossRef]
- Ainouche, M.L.; Fornute, M.P.; Salmon, A.; Parisod, C.; Grandbastien, M.-A.; Fukunaga, K.; Ricou, M.; Misset, M.-T. Hybridization, polyploidy and invasion: Lessons from Spartina (Poaceae). Biol. Invasions 2009, 11, 1159–1173. [Google Scholar] [CrossRef]
- Ferreira de Carvalho, J.; Poulain, J.; Da Silva, C.; Wincker, P.; Michon-Coudouel, S.; Dheilly, A.; Naquin, D.; Boutte, J.; Salmon, A.; Ainouche, M. Transcriptome de novo assembly from next-generation sequencing and comparative analyses in the hexaploid salt marsh species Spartina maritima and Spartina alterniflora (Poaceae). Heredity 2013, 110, 181–193. [Google Scholar] [CrossRef]
- Mobberley, D. Taxonomy and distribution of the genus Spartina. Iowa State Coll J. Sci. 1956, 30, 471–574. [Google Scholar]
- Baumel, A.; Rousseau-Gueutin, M.; Sapienza-Bianchi, C.; Gareil, A.; Duong, N.; Rousseau, H.; Coriton, O.; Amirouche, R.; Sciandrello, S.; Duarte, B.; et al. Spartina versicolor Fabre: Another case of Spartina trans-Atlantic introduction? Biol. Invasions 2016, 18, 2123–2135. [Google Scholar] [CrossRef]
- Patrício, J.; Little, S.; Mazik, K.; Papadopoulou, K.N.; Smith, C.J.; Teixeira, H.; Hoffmann, H.; Uyarra, M.C.; Solaun, O.; Zenetos, A. European marine biodiversity monitoring networks: Strengths, weaknesses, opportunities and threats. Front. Mar. Sci. 2016, 3, 161. [Google Scholar] [CrossRef]
- Mauritzen, C.; Morel, Y.; Paillet, J. On the influence of Mediterranean Water on the Central Waters of the North Atlantic Ocean. Deep Sea Res. Part I Oceanogr. Res. Pap. 2001, 48, 347–381. [Google Scholar] [CrossRef]
- Carrasco, J.F. Excepcional presencia de Cymbium papillatum Schumacher (Grastropoda, Volutidae) en la costa catalana. Butll. Centre d’Est. Natura B-N 2000, 5, 67–68. [Google Scholar]
- Hidalgo, J. Fauna malacológica de España, Portugal y las Baleares: Moluscos Testáceos Marinos. Trab. del Mus. Nac. Ciencias Nat. Ser. Zool. 1917, 30, 1–752. [Google Scholar]
- Bachelet, G.; Cazaux, C.; Gantès, H.; Labourg, P. Contribution à l’étude de la faune marine de la région d’Arcachon, IX. Bull. Cent. Etud. Rech. Sci. Biarritz 1980, 13, 45–64. [Google Scholar]
- Simon-Bouhet, B.; Garcia-Meunier, P.; Viard, F. Multiple introductions promote range expansion of the mollusc Cyclope neritea (Nassariidae) in France: Evidence from mitochondrial sequence data. Mol. Ecol. 2006, 15, 1699–1711. [Google Scholar] [CrossRef]
- Sauriau, P.-G. Spread of Cyclope neritea (Mollusca: Gastropoda) along the north-eastern Atlantic coasts in relation to oyster culture and to climatic fluctuations. Mar. Biol. 1991, 109, 299–309. [Google Scholar] [CrossRef]
- Boissin, E.; Neglia, V.; Baksay, S.; Micu, D.; Bat, L.; Topaloglu, B.; Todorova, V.; Panayotova, M.; Kruschel, C.; Milchakova, N.; et al. Chaotic genetic structure and past demographic expansion of the invasive gastropod Tritia neritea in its native range, the Mediterranean Sea. Sci. Rep. 2020, 10, 21624. [Google Scholar] [CrossRef]
- Couceiro, L.; López, L.; Ruiz, J.M.; Barreiro, R. Population structure and range expansion: The case of the invasive gastropod Cyclope neritea in northwest Iberian Peninsula. Int. Zoo. 2012, 7, 286–298. [Google Scholar] [CrossRef]
- Gouillieux, B.; Ariyama, H.; Costa, A.C.; Daffe, G.; Marchini, A.; Micael, J.; Ulman, A. New records of Ericthonius didymus Krapp-Schickel, 2013 (Crustacea: Amphipoda: Ischyroceridae) in European waters with a focus in Arcachon Bay, France and key to Ericthonius species. J. Mar. Biol. Assoc. UK 2020, 100, 401–412. [Google Scholar] [CrossRef]
- Zenetos, A.; Gratsia, E.; Cardoso, A.; Tsiamis, K. Time lags in reporting of biological invasions: The case of Mediterranean Sea. Meditter. Mar. Sci. 2019, 20, 469–475. [Google Scholar] [CrossRef]
- Outinen, O.; Bailey, S.A.; Broeg, K.; Chasse, J.; Clarke, S.; Daigle, R.M.; Gollasch, S.; Kakkonen, J.E.; Lehtiniemi, M.; Normant-Saremba, M.; et al. Exceptions and exemptions under the ballast water management convention–Sustainable alternatives for ballast water management? J. Environ. Manag. 2021, 293, 112823. [Google Scholar] [CrossRef] [PubMed]
- EEA Trends in Marine Non-Indigenous Species. European Environment Agency. 2019. Available online: https://www.eea.europa.eu/data-and-maps/indicators/trends-in-marine-alien-species-mas-3/assessment (accessed on 1 September 2022).
- Lehtiniemi, M.; Outinen, O.; Puntila-Dodd, R. Citizen science provides added value in the monitoring for coastal non-indigenous species. J. Environ. Manag. 2020, 267, 110608. [Google Scholar] [CrossRef] [PubMed]
- Jalkanen, J.-P.; Johansson, L.; Wilewska-Bien, M.; Granhag, L.; Ytreberg, E.; Eriksson, K.M.; Yngsell, D.; Hassellöv, I.-M.; Magnusson, K.; Raudsepp, U.; et al. Modelling of Discharges from Baltic Sea Shipping. Ocean Sci. 2021, 17, 699–728. [Google Scholar] [CrossRef]
- Robbins, J.R.; Bouchet, P.J.; Miller, D.L.; Evans, P.G.; Waggitt, J.; Ford, A.T.; Marley, S.A. Shipping in the north-east Atlantic: Identifying spatial and temporal patterns of change. Mar. Pollut. Bull. 2022, 179, 113681. [Google Scholar] [CrossRef]
- OSPAR NIS3: Trends in New Records of Non-Indigenous Species Introduced by Human Activities, in: OSPAR (Ed.), OSPAR Intermediate Assessment OSPAR London UK. Available online: https://oap.ospar.org/en/ospar-assessments/intermediateassessment-2017/pressures-human-activities/non-indigenous/ (accessed on 10 August 2022).
- Bishop, J.; Wood, C.A.; Lévêque, L.; Yunnie, A.L.E.; Viard, F. Repeated rapid assessment surveys reveal contrasting trends in occupancy of marinas by non-indigenous species on opposite sides of the western English Channel. Mar. Pollut. Bull. 2015, 95, 699–706. [Google Scholar] [CrossRef]
- Ibabe, A.; Miralles, L.; Carleos, C.E.; Soto-López, V.; Menéndez-Teleña, D.; Bartolomé, M.; Montes, H.J.; González, M.; Dopico, E.; Garcia-Vazquez, E.; et al. Building on gAMBI in ports for a challenging biological invasions scenario: Blue-gNIS as a proof of concept. Mar. Environ. Res. 2021, 169, 105340. [Google Scholar] [CrossRef]
- Miralles, L.; Ibabe, A.; González, M.; García-Vázquez, E.; Borrell, Y.J. “If you know the enemy and know yourself”: Addressing the problem of biological invasions in ports through a new NIS invasion threat score, routine monitoring, and preventive action plans. Front. Mar. Sci. 2021, 8, 633118. [Google Scholar] [CrossRef]
- Pejovic, I.; Ardura, A.; Miralles, L.; Arias, A.; Borrell, Y.J.; Garcia-Vazquez, E. DNA barcoding for assessment of exotic molluscs associated with maritime ports in northern Iberia. Mar. Bio. Res. 2015, 12, 168–176. [Google Scholar] [CrossRef]
- Miralles, L.; Ardura, A.; Arias, A.; Borrell, Y.J.; Clusa, L.; Dopico, E.; de Rojas, A.; Lopez, B.; Muñoz-Colmenero, M.; Roca, A.; et al. Barcodes of marine invertebrates from north Iberian ports: Native diversity and resistance to biological invasions. Mar. Pollut. Bull. 2016, 112, 183–188. [Google Scholar] [CrossRef]
- Viard, F.; Roby, C.; Turon, X.; Bouchemousse, S.; Bishop, J. Cryptic diversity and database errors challenge non-indigenous species surveys: An illustration with Botrylloides spp. in the English Channel and Mediterranean Sea. Front. Mar. Sci. 2019, 6, 615. [Google Scholar] [CrossRef]
- Duarte, S.; Vieira, P.E.; Lavrador, A.S.; Costa, F.O. Status and prospects of marine NIS detection and monitoring through (e)DNA metabarcoding. Sci. Total Environ. 2021, 751, 141729. [Google Scholar] [CrossRef]
- Darling, J.A.; Pochon, X.; Abbott, C.L.; Inglis, G.J.; Zaiko, A.; Leroy, B. The risks of using molecular biodiversity data for incidental detection of species of concern. Divers. Distrib. 2020, 26, 1116–1121. [Google Scholar] [CrossRef]
- Pezy, J.-P.; Baffreau, A.; Raoux, A.; Rusig, A.-M.; Mussio, I.; Dauvin, J.-C. Non-indigenous species in marine and brackish waters along the Normandy coast. BioInvasions Rec. 2021, 10, 755–774. [Google Scholar] [CrossRef]
- Bishop, J.D.D.; Roby, C.; Yunnie, A.L.E.; Wood, C.A.; Leveque, L.; Turon, X.; Viard, F. The southern hemisphere ascidian Asterocarpa humilis is unrecognised but widely established in NW France and Great Britain. Biol. Invasions 2013, 15, 253–260. [Google Scholar] [CrossRef][Green Version]
- Zenetos, A.; Albano, P.G.; Garcia, E.L.; Stern, N.; Tsiamis, K.; Galanidi, M. Corrigendum to the Review Article. Meditter. Mar. Sci. 2022, 23, 196–212, Erratum in 2022, 23, 876–878. [Google Scholar] [CrossRef]
- Zenetos, A.; Galanidi, M. Mediterranean non indigenous species at the start of the 2020s: Recent changes. Mar. Biodivers. Rec. 2020, 13, 10. [Google Scholar] [CrossRef]
- Raitsos, D.E.; Beaugrand, G.; Georgopoulos, D.; Zenetos, A.; Pancucci-Papadopoulou, A.M.; Theocharis, A.; Papathanassiou, E. Global climate change amplifies the entry of tropical species into the Eastern Mediterranean Sea. Limnol. Oceanogr. 2010, 55, 1478–1484. [Google Scholar] [CrossRef]
- Zenetos, A. Mediterranean Sea: 30 years of biological invasions (1988–2017). In Proceedings of the 1st Mediterranean Symposium on the Non-Indigenous Species, Antalya, Turkey, 17–18 January 2019; Langar, H., Ouerghi, A., Eds.; SPA/RAC Publiction: Tunis, Tunis, 2019. 116p. [Google Scholar]
- Mavrič, B.; Orlando-Bonaca, M.; Fortič, A.; Francé, J.; Mozetič, P.; Slavinec, P.; Pitacco, V.; Trkov, D.; Vascotto, I.; Zamuda, L.L.; et al. Monitoring Species Diversity and Abundance of Non-Indigenous Species in the Slovenian Sea (in Slovenian). Final Project report. Marine Biology Station Piran, National Institute of Biology, Report 195, p. 83. Available online: http://www.ribiski-sklad.si/f/docs/Dokumenti_1/koncno_porocilo_NIS_2018-2021_s_CIP.pdf (accessed on 15 May 2022).
- Ferrario, J.; Caronni, S.; Occhipinti-Ambrogi, A.; Marchini, A. Role of commercial harbours and recreational marinas in the spread of non-indigenous fouling species. Biofouling 2017, 33, 651–660. [Google Scholar] [CrossRef]
- Ulman, A.; Ferrario, J.; Occhipinti-Ambrogi, A.; Arvanitidis, C.; Bandi, A.; Bertolino, M.; Bogi, C.; Chatzigeorgiou, G.; Çiçek, B.A.; Deidun, A.; et al. A massive update of non-indigenous species records in Mediterranean marinas. PeerJ 2017, 5, 1–59. [Google Scholar]
- Bulgurkov, K. Occurrence of Callinectes sapidus Rathbun (Crustacea—Decapoda) in Black Sea. Bull. Natl.Inst. Ocean. Fish Varna 1968, 9, 97–99. (In Bulgarian) [Google Scholar]
- Stefanov, T. Recent expansion of the alien invasive blue crab Callinectes sapidus (Rathbun, 1896)(Decapoda, Crustacea) along the Bulgarian coast of the Black Sea. Hist. Nat. Bulg. 2021, 42, 49–53. [Google Scholar] [CrossRef]
- Gücü, A.C.; Ünal, V.; Ulman, A.; Morello, E.B.; Bernal, M. Management responses to non-indigenous species in response to climate change. In Adaptive Management to Fisheries FAO Fisheries and Aquaculture Technical Paper 667; Bahri, T., Vasconcellos, M., Welch, D.J., Johnson, J., Perry, R.I., Ma, X., Eds.; FAO: Rome, Italy, 2021; pp. 161–176. [Google Scholar]
- Maltsev, V.I.; Kulish, A.V.; Beletskaya, M.A. The First Find of the Marbled Spinefoot Siganus rivulatus (Siganidae) in the Black Sea. J. Ichthyol. 2022, 62, 514–516. [Google Scholar] [CrossRef]
- Boltachova, N.A.; Lisitskaya, E.V.; Podzorova, D.V. Distribution of alien polychaetes in biotopes of the northern part of the Black Sea. Russ. J. Biol. Invasions 2021, 12, 11–26. [Google Scholar] [CrossRef]
- Teacă, A.; Begun, T.; Menabit, S.; Mureșan, M. The First Record of Marenzelleria neglecta and the Spread of Laonome xeprovala in the Danube Delta–Black Sea Ecosystem. Diversity 2022, 14, 423. [Google Scholar] [CrossRef]
- Lavesque, N.; Daffe, G.; Bonifácio, P.; Hutchings, P. A new species of the Marphysa sanguinea complex from French waters (Bay of Biscay, NE Atlantic) (Annelida, Eunicidae). Zookeys 2017, 716, 1–17. [Google Scholar] [CrossRef]
- Lavesque, N.; Hutchings, P.; Abe, H.; Daffe, G.; Gunton, L.M.; Glasby, C.J. Confirmation of the exotic status of Marphysa victori Lavesque, Daffe, Bonifácio & Hutchings, 2017 (Annelida) in French waters and synonymy of Marphysa bulla Liu, Hutchings & Kupriyanova. Aquat. Invasions 2018, 15, 355–366. [Google Scholar] [CrossRef]
- O’Donoghue, C.H.; White, K.M. A collection of marine molluscs, mainly opisthobranchs, from Palestina. Proc. Malacol. Soc. Lond. 1940, 24, 92–96. [Google Scholar]
- Bariche, M.; Al-Mabruk, S.A.; Ates, M.A.; Büyük, A.; Crocetta, F.; Dritsas, M.; Edde, D.; Fortic, A.; Gavriil, E.; Gerovasileiou, V.; et al. New Alien Mediterranean Biodiversity Records Meditter. Mar. Sci. 2020, 21, 129–145. [Google Scholar] [CrossRef]
- Tillier, L.; Bavay, A. Les mollusques testacés du Canal de Suez. Bull. de la Soc. Zool. de France 1905, 30, 170–181. [Google Scholar]
- Zenetos, A.; Konstantinou, F.; Konstantinou, G. Towards homogenization of the Levantine alien biota: Additions to the alien molluscan fauna along the Cypriot coast. Mar. Biodivers. Rec. 2009, 2, E156. [Google Scholar] [CrossRef]
- Olenin, S.; Narščius, A.; Gollasch, S.; Lehtiniemi, M.; Marchini, A.; Minchin, D.; Srėbalienė, G. New arrivals: An indicator for non-indigenous species introductions at different geographical scales. Front. Mar. Sci. 2016, 3, 208. [Google Scholar] [CrossRef]
- UNEP/MAP. Integrated Monitoring and Assessment Programme of the Mediterranean Sea and Coast and Related Assessment Criteria; UN Environment/MAP: Athens, Greece, 2017; Available online: https://wedocs.unep.org/bitstream/handle/20.500.11822/17012/imap_2017_eng.pdf?sequence=5&isAllowed=y (accessed on 28 October 2021).
- UNEP(DEPI)/MED. Decision IG.21/3 on the Ecosystems Approach Including Adopting Definitions of Good Environmental Status (GES) and Targets; UNEP(DEPI)/MED IG.21/9, Annex II—Thematic Decisions; UNEP(DEPI)/MED: Istanbul, Turkey, 2013. [Google Scholar]
- Vasilakopoulos, P.; Palialexis, A.; Boschetti, S.T.; Cardoso, A.C.; Druon, J.-N.; Konrad, C.; Kotta, M.; Magliozzi, C.; Palma, M.; Piroddi, C.; et al. Marine Strategy Framework Directive, Thresholds for MSFD Criteria: State of Play and Next Steps; EUR 31131 EN; Publications Office of the European Union: Luxembourg, 2022; ISBN 978-92- 76-53689-5. [Google Scholar] [CrossRef]
- Cavallo, M.; Elliott, M.; Quintino, V.; Touza, J. Can National Management Measures Achieve Good Status across International Boundaries? A Case Study of the Bay of Biscay and Iberian Coast Sub-Region. Ocean Coast. Manag. 2018, 160, 93–102. [Google Scholar] [CrossRef]
- WG-AS; Gittenberger, A. Trilateral Wadden Sea Alien Species Management and Action Plan; Busch, J.A., Lüerssen, G., de Jong, F., Eds.; Common Wadden Sea Secretariat (CWSS): Wilhelmshaven, Germany, 2018. [Google Scholar]

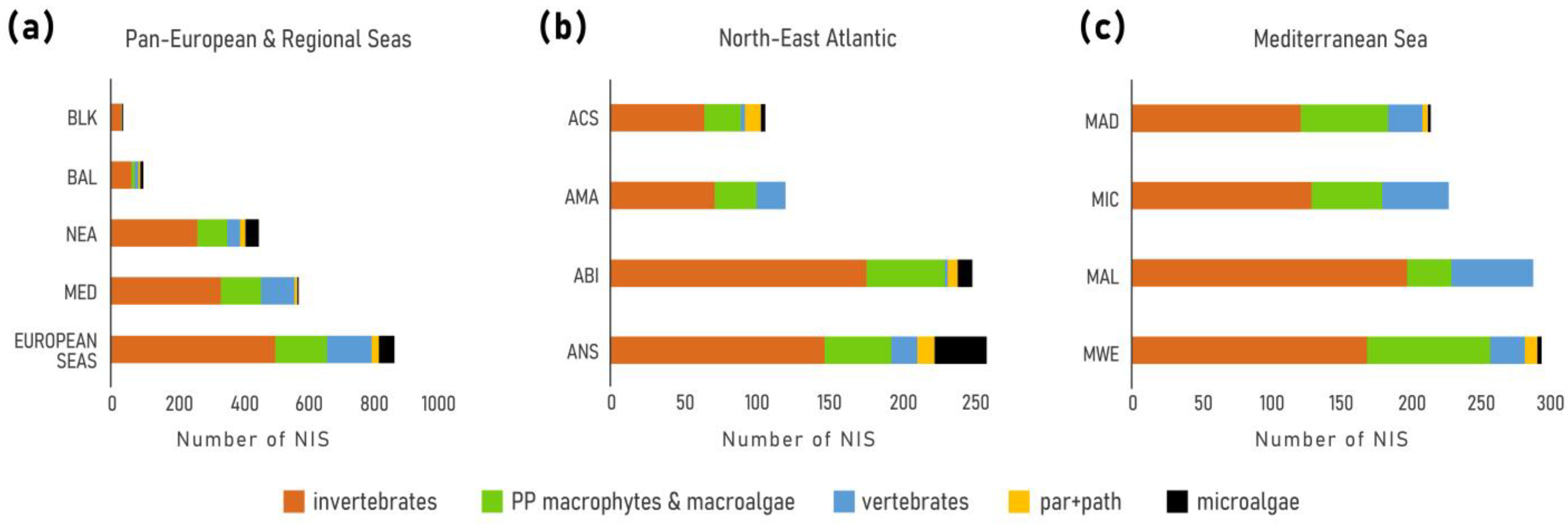
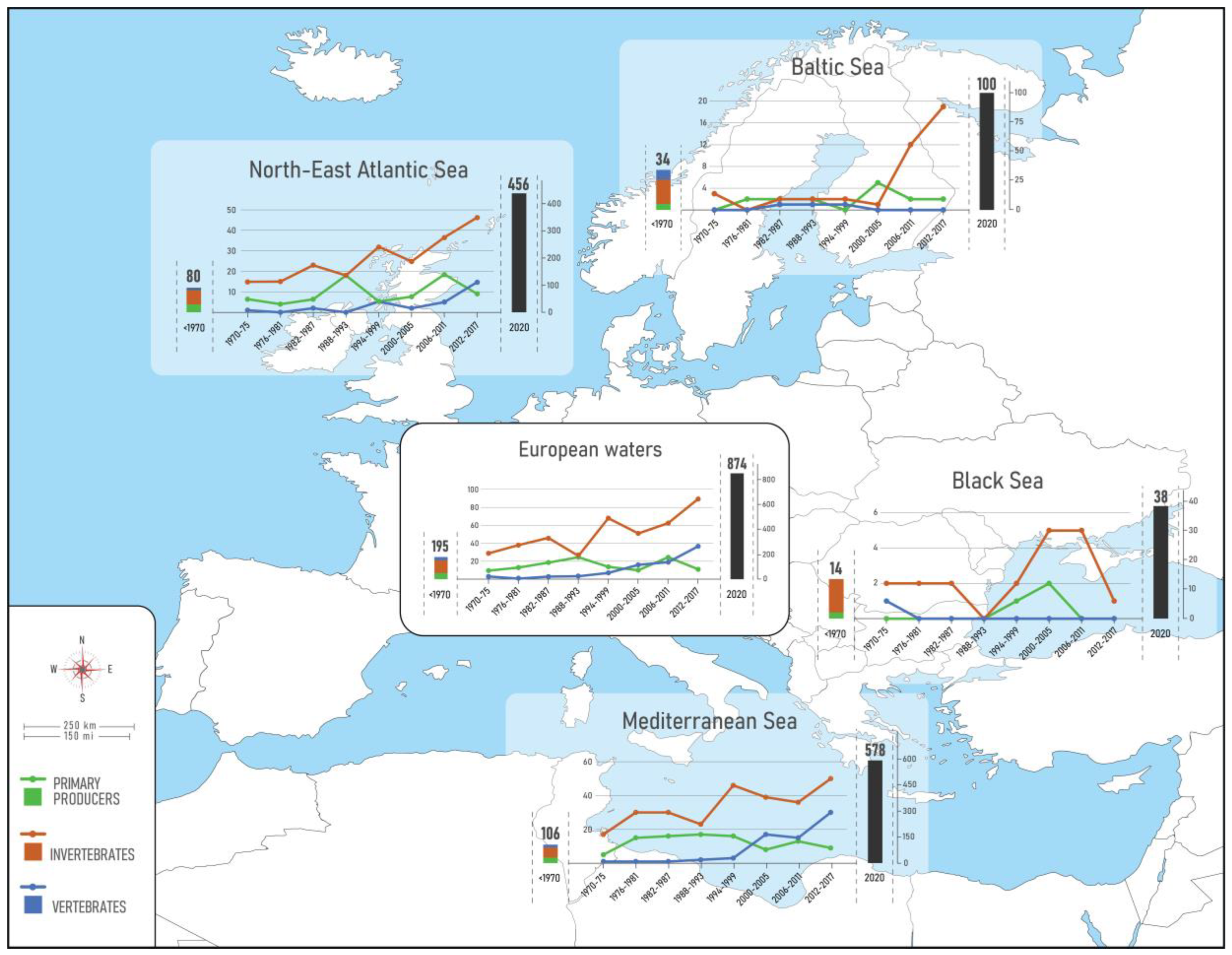
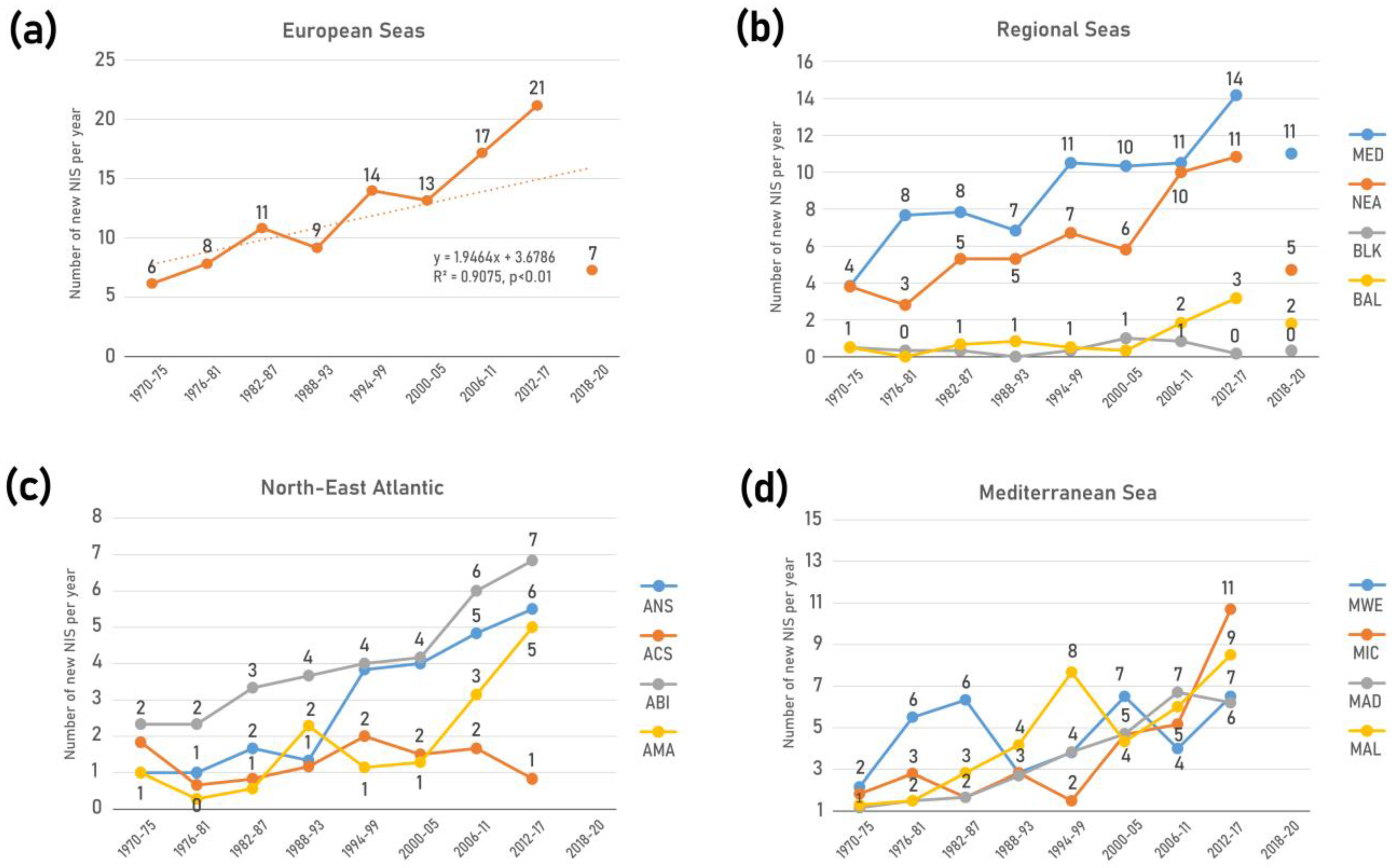
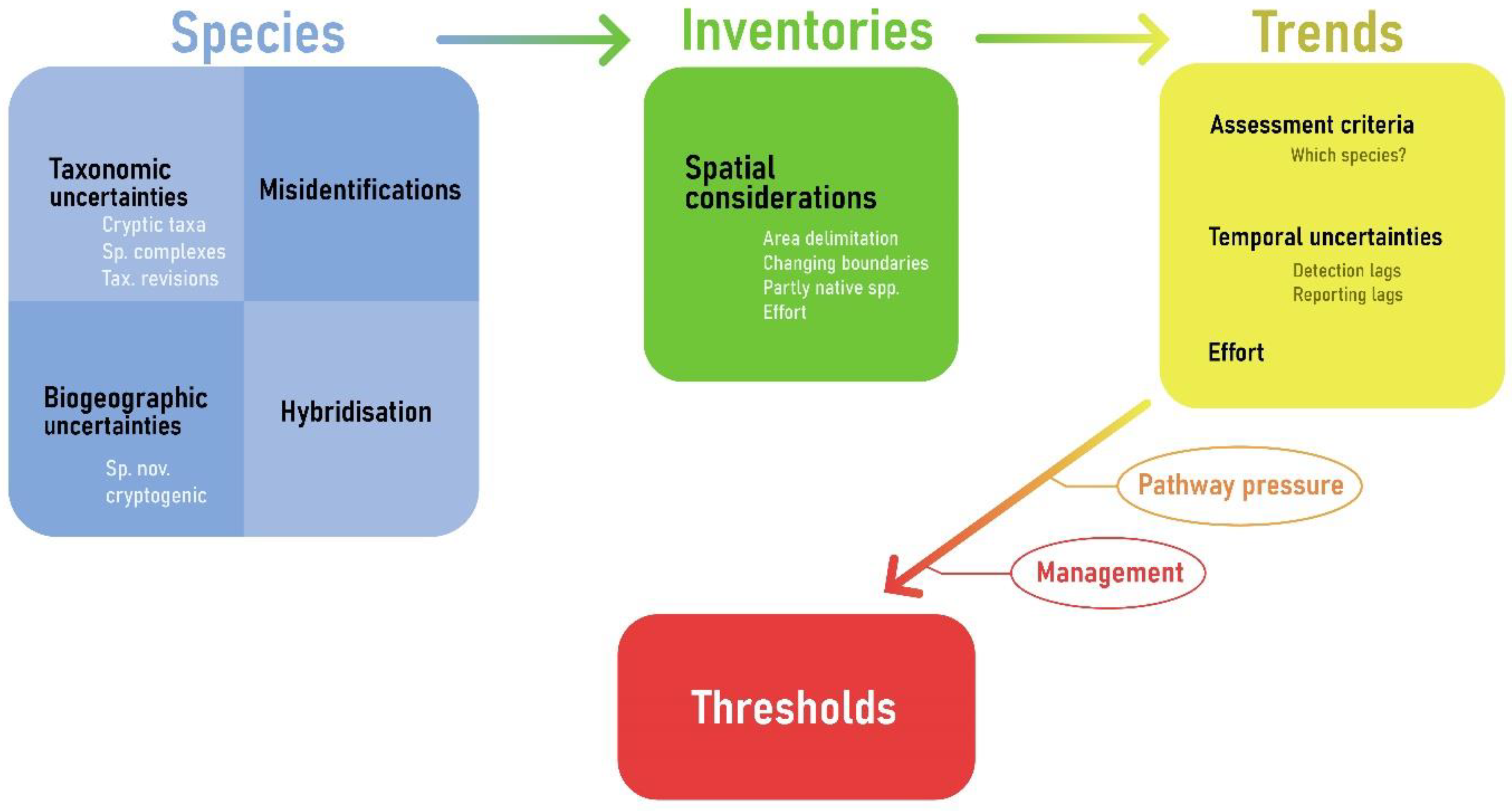
| Regional Level | Subregional Level | |||
|---|---|---|---|---|
| Baltic Sea (BAL) | BAL Denmark (In the Sound area of the Kattegat, the border follows the Øresund/Öresund bridge between Denmark and Sweden and in Copenhagen harbor, the border is defined by a lock just north of the bridge. On the west side of Sjælland, the border follows the OSPAR Convention boundary connecting Gniben Point on Sjællands Odde with Hasenore Head on the coast of Jutland), Estonia, Finland, Germany (Baltic Sea-side), Latvia, Lithuania, Poland, Sweden (Baltic Sea-side) | |||
| North-East Atlantic Ocean (NEA) | ANS France (including Eastern English Channel, and a small area of the Western English Channel), Belgium, Netherlands, Germany, Denmark, Sweden, Norway up to 62° N (EEA country). | ACS Ireland and France (Western English Channel) | ABI Spain (mainland), Portugal (mainland), and France. | AMA Portugal (Azores, and Madeira) Spain (Canary Islands) |
| Mediterranean Sea (MED) | WME Spain, France, and Western Italy | MIC Western Greece (Ionian Sea), Ionian coasts of Italy, and Malta | MAD Adriatic coasts of Italy, Slovenia, Croatia, and Albania and Montenegro (EU candidates) | MAL Cyprus and Eastern Greece |
| Black Sea (BLK) | BLKBulgaria and Romania | |||
| Group | Species | Region/Subregion Native | Country/Region Introduced |
|---|---|---|---|
| Dinoflagellates | Prorocentrum lima (Ehrenberg) F.Stein, 1878 | NEA | Denmark/NEA |
| Macroalgae | Asperococcus scaber Kuckuck, 1899 | NEA/ANS | Netherlands |
| Macroalgae | Fucus distichus subsp. evanescens (C.Agardh) H.T.Powell | NEA/ANS CRY in Norway | Sweden/NEA |
| Crustacea | Necora puber (Linnaeus, 1767) | NEA, MED | Sweden/NEA |
| Crustacea | Pseudomyicola spinosus spinosus (Raffaele & Monticelli, 1885) | NEA, MED | France/NEA |
| Crustacea | Pilumnus spinifer H. Milne Edwards, 1834 | NEA, MED | Sweden/NEA |
| Mollusca | Calliostoma zizyphinum (Linnaeus, 1758) | NEA, MED | Netherlands |
| Mollusca | Cymbium olla (Linnaeus, 1758) | NEA/ABI | Spain/MED |
| Mollusca | Tritia corniculum (Olivi, 1792) | NEA, MED | Spain/NEA |
| Mollusca | Tritia neritea (Linnaeus, 1758) | MED, partly in ABI | France/NEA |
| Cnidaria | Cereus pedunculatus (Pennant, 1777) | NEA/ANS | Denmark/NEA |
| Porifera | Suberites massa Nardo, 1847 | NEA/ANS | Netherlands |
| Porifera | Haliclona (Haliclona) urceolus (Rathke & Vahl, 1806) | NEA/ANS | Netherlands |
| Porifera | Haliclona (Reniera) cinerea (Grant, 1826) | NEA/ANS | Netherlands |
| Bryozoa | Reptadeonella violacea (Johnston, 1847) | NEA | Portugal |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zenetos, A.; Tsiamis, K.; Galanidi, M.; Carvalho, N.; Bartilotti, C.; Canning-Clode, J.; Castriota, L.; Chainho, P.; Comas-González, R.; Costa, A.C.; et al. Status and Trends in the Rate of Introduction of Marine Non-Indigenous Species in European Seas. Diversity 2022, 14, 1077. https://doi.org/10.3390/d14121077
Zenetos A, Tsiamis K, Galanidi M, Carvalho N, Bartilotti C, Canning-Clode J, Castriota L, Chainho P, Comas-González R, Costa AC, et al. Status and Trends in the Rate of Introduction of Marine Non-Indigenous Species in European Seas. Diversity. 2022; 14(12):1077. https://doi.org/10.3390/d14121077
Chicago/Turabian StyleZenetos, Argyro, Konstantinos Tsiamis, Marika Galanidi, Natacha Carvalho, Cátia Bartilotti, João Canning-Clode, Luca Castriota, Paula Chainho, Robert Comas-González, Ana C. Costa, and et al. 2022. "Status and Trends in the Rate of Introduction of Marine Non-Indigenous Species in European Seas" Diversity 14, no. 12: 1077. https://doi.org/10.3390/d14121077
APA StyleZenetos, A., Tsiamis, K., Galanidi, M., Carvalho, N., Bartilotti, C., Canning-Clode, J., Castriota, L., Chainho, P., Comas-González, R., Costa, A. C., Dragičević, B., Dulčić, J., Faasse, M., Florin, A.-B., Gittenberger, A., Jakobsen, H., Jelmert, A., Kerckhof, F., Lehtiniemi, M., ... Outinen, O. (2022). Status and Trends in the Rate of Introduction of Marine Non-Indigenous Species in European Seas. Diversity, 14(12), 1077. https://doi.org/10.3390/d14121077










