Abstract
French Guiana forests are threatened by increasing human activity such as infrastructure development, facilitating access to the forest and, therefore, logging, mining, farming and hunting. To highlight the impact of human pressure on the forest fauna, dung beetle assemblage was analyzed near Saint-Georges-de-l’Oyapock and compared with other sites in French Guiana, considering the distance to the main city and forest cover loss as proxies of human activities. Hill numbers and beta diversity were calculated. Non-metric multidimensional scaling and redundancy analyses were carried out to disentangle the effect of the distance to the nearest city and forest cover loss as proxies of human pressure, but also temperature and rainfall as proxies of climatic variations on dung beetle assemblage. Species richness increased significantly with the distance to the nearest city and decreasing forest cover loss. Assemblage structure varied among sites mainly with distance to the nearest city but also with rainfall. It varied also with forest cover loss, but not significantly. This study showed that human disturbances and climatic conditions, even if represented by proxies, affected dung beetle assemblage structures in French Guiana forests.
1. Introduction
Dung beetles (Coleoptera: Scarabaeidae: Scarabaeinae) are a diverse and abundant group of insects, inhabiting a wide variety of habitats. Most dung beetles feed and breed on dung, but some species use carrion, rotting fruit, fungi and decaying plant matter as alternative resources [1]. As such, they provide essential ecological services to the ecosystem, e.g., recycling of nutrients, soil bioturbation and secondary seed dispersal [2,3]. In addition, through feeding and nesting, they control the abundance of dung-breeding hematophagic and detrivorous flies and dung-dispersed nematodes and protozoa [2]. Due to their close relationship with vegetation cover, soil types and mammal richness, they are sensitive to environmental disturbance and have been widely used as an indicator of human impact, considering their richness and abundance, but also their ecological function [4,5,6,7,8,9,10,11]. A decline in dung beetle species richness often occurs in areas that have suffered environmental degradation, particularly loss of habitat [12,13] and loss of wildlife [14]. Dung beetle assemblages vary with land use [15,16,17] and forest degradation due to road construction [18]. However, seasonality, mainly rainfall, also strongly influences dung beetle assemblages [15]. For example, rainfall constitutes the second strongest effect on dung beetle species richness in Gebert et al. [19] after temperature. Several studies link the decline of dung beetle fauna to an impoverished mammal community in fragmented forests [20,21,22,23,24,25,26,27,28,29]. In Panama Andresen and Laurance [25] reported that species richness and abundance of dung beetles declined with decreasing mammal abundance due to hunting pressure. Barlow et al. [30] showed a positive relationship between large mammal activity and dung beetle abundance, independently of isolation and forest structure in Amazonian forests. Still, the effects of anthropogenic pressures on dung beetle assemblage vary depending on which environmental factors are analyzed. Disentangling the drivers that affect dung beetle assemblage is crucial to prevent their decline and provide solutions for conservation purposes [31].
The forests of the Guiana shield represent approximately 26% of the Amazonian forests and remain poorly disturbed compared to the other parts of the Amazonian forest [32]. Yet Guianese forests are under growing pressure, mainly because of the rapid increase of human population and its resulting activities such as logging, bush meat hunting and mining exploitation [33]. Such activities are facilitated by the development of infrastructures. As an example, the road RN2 connecting Regina to Saint-Georges-de-l’Oyapock (hereafter SGO) at the border between French Guiana and Brazil was asphalted in 2003 and was followed by the construction and the opening of a bridge crossing the Oyapock River near SGO in 2017. Such recent infrastructure developments have therefore increased traffic and facilitated access to the forest along the road in the Western part of French Guiana. The road itself destroys the habitat of dung beetles, as shown for example in the Ecuadorian Amazon [18]. Still, the road opening also enables access to hunters, disturbing vertebrate communities and thus dung beetle assemblages which are closely linked with vertebrates for their trophic resources. Decreasing hunting pressure along with increasing distance to the city was observed during a hunting survey conducted in 2014 by ONCFS (C. Richard Hansen, pers. comm.). The road also facilitates access for logging and mining activities and therefore decreases the habitat quality of the fauna, dung beetles included. Although the Guianese forests are rather well studied, information is lacking about their conservation status and their degradation rate [34].
To measure the short-term impact of human disturbances on dung beetle assemblage, we analyzed dung beetle assemblage in several forest sites in French Guiana, characterized by different degrees of anthropogenic pressure and climatic conditions, represented by four proxies: distance to the nearest city, forest cover loss, rainfall and temperature. We hypothesized a decrease in the diversity and richness of dung beetle assemblage with increasing human disturbances. We also hypothesized that reducing diversity would induce nested beta diversity between sites, with a variation in the structure of dung beetle assemblage among sites due to human disturbance.
2. Materials and Methods
2.1. Study Sites
The climate in French Guiana is equatorial, with a wet season from December to July and a slight decrease in rainfall in March, while the dry season occurs from August to November. Annual rainfall ranges from 2000 to 4000 mm with monthly variations from 50 mm (driest month in dry season) to 450 mm (wettest month in rainy season). Rainfall is usually higher on the coast than inside the country. The region between Regina and SGO along the National Road 2 (RN2) is near the coast and receives heavy rainfall (3312 mm/year). The mean annual temperature in French Guiana is 26 °C with low variation between seasons (±2 °C). The coast is usually warmer than the inland.
Ecological corridors were settled along the RN2 when constructed near SGO to minimize the effects of the road (see [35]). These corridors consist of small portions of the road (200–300 m) where the forest cover was conserved on each side to allow the connection of the tree canopy and to facilitate road crossing by animals. For the purpose of this study, four forest sites (SGO1, SGO2, SGO4 and SGO7) were selected in the vicinity of the corridors, based on their distance to Saint Georges, knowing that several corridors are too close and too similar to be compared (Figure 1). SGO1, the closest to SGO, is the most disturbed with forest fragmentation due to cattle farming (2 km away), slash-and-burn agroforest and logging. SGO2 remains disturbed by logging activities, but it is not as fragmented as SGO1 and without cattle farming. SGO4 and SGO7 are the farthest and probably the less disturbed. They are included in the Regional Natural Park. The forest flora in these sites is similar to that of Nouragues [36] (P.-M. Forget, pers. Obs.), where plant species richness and composition of the uphill forest are different from that of downhill forest [37]. The forest types encountered in these corridors are a mosaic of ecotone and mixed forest types, mainly characterized by the abundance of Fabaceae, Sapotaceae, Chrysobalanaceae, Burseraceae and Lecythidaceae (P.-M. Forget, pers. obs).
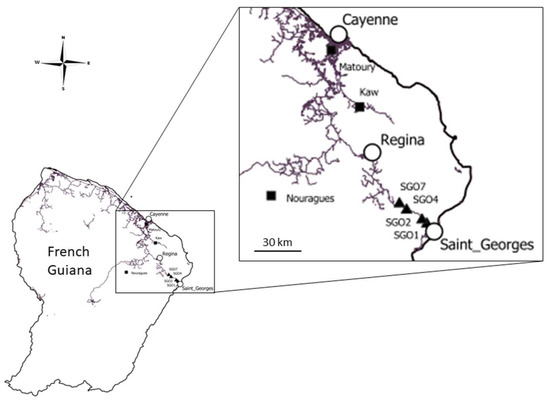
Figure 1.
Sampling locations. Dung beetles have been sampled at SGO1, SGO2, SGO4 and SGO7, located at a distance of 7.8, 11.8, 25.6 and 32 km from Saint-Georges-de-l’Oyapock, respectively. Black triangles represent the original sample of this study and black squares represent sample sites of the study of Feer & Boissier [8]. White circles are the main cities along the RN2 road. Lines represent roads.
Three other sites where dung beetles were sampled and studied by Feer & Boissier [8] were compared with the one presented formerly. They showed different environmental conditions than that of SGO in terms of human pressure and spatial scale. The most protected forest site is located in the 105,800 ha Nouragues National Nature Reserve (hereafter Nouragues). The site is located 100 km upriver from the village of Régina (830 inhabitants) and is free of human activity. Montagne de Kaw (hereafter Kaw) is a 40 km long ridge (309 m above sea level), 45 km from the city of Cayenne. Mont Grand Matoury National Nature Reserve (hereafter Matoury) is in the vicinity of Cayenne, 2 km from the center of Matoury city. It is a moderately disturbed primary forest isolated on a hill (234 m above sea level), surrounded by a secondary forest on the slopes. The forest is almost surrounded by an agricultural landscape.
The sites were characterized by forest cover loss and the distance to the nearest city as proxies of human disturbance. Forest cover loss represents mining exploitation, livestock farming and logging and distance to the city represents hunting pressure, since it is challenging to quantify hunting pressure [25,29,33]. We measured the distance between sites and cities using Qgis 3.14 (Free Software Foundation, Boston, MA, USA). Forest cover loss was estimated based on Hansen’s global forest change data from 2000 to 2019 [38]. A buffer area of 1 km diameter was defined around the GPS point of sampling sites. The percentage of forest cover loss was estimated using Qgis 3.14. Temperature and rainfall were used as proxies of climatic conditions. MétéoFrance provided monthly cumulated rainfall and monthly mean temperature for each site for the sampling period (Table 1).

Table 1.
Temperature (°C), monthly rainfall (mm), forest cover loss (%) and distance to the nearest city (km) by sampling period for all sites.
2.2. Dung Beetle Sampling
Dung beetles in SGO sites were sampled using pitfall traps (10 cm diameter, 15 cm depth), baited with 10 g of fresh human feces hanging above. Three pitfall traps were placed at each sampling site, 20 m apart. The specimens were collected every two days for ten days and stored in 95% alcohol. Sampling was conducted three times. The first sampling survey took place from 30 January to 8 February 2018 and the second from 31 January to 9 February 2019, both during the rainy season. The third sampling period was conducted from 25 November to 4 December 2019, during the dry-to-wet season transition. Dung beetles from Feer & Boissier’s [8] sites were sampled in the same way, although 10 to 12 traps were set up 40 m apart.
2.3. Data Analysis
All the collected specimens were sorted, counted and identified at the genus and species levels when possible, otherwise at morphotype level. Identification was made using the Museum’s collections (especially François Feer’s collection) as a reference, the identification key to Guianese Scarabaeinae genera [39], the identification key to species of Guianese Phanaeini [40], Deltochilum Eschscholtz, 1822 [41] and Eurysternus Dalman, 1824 [42,43]. As the two genera Ateuchus Weber, 1801 and Canthidium Erichson, 1847 are being taxonomically revised, we only quoted morphospecies.
Forest structure at the sites considered here is known not to have notably changed during the time lapse between Feer & Boissier’s [8] study (2007–2012) and SGO surveys (2018–2019). Therefore, we considered no significant changes between the years of sampling concerned. Only samples corresponding to the same seasonal period were retained, as assemblage structure may vary according to the season. Counts from the different traps were pooled together as a single observation for each site at each season. Abundances were weighted by using the mean number of a random sampling according to the number of traps used for sampling in each site to ensure the same representativeness of samples. Hill numbers (Species richness, Shannon and Simpson indexes) were compared among sites using the Kruskal-Wallis test, as data were not normally distributed. For the same reason, we tested the correlation between distances to the city and forest cover loss with the former metrics using Spearman’s correlation test. We first performed a non-metric multidimensional scaling (NMDS) to visualize the difference between dung beetle assemblages among sites. To assess the extent to which the variation of assemblage structure could be related to temperature, rainfall, distance to the city and forest cover loss, we performed a redundancy analysis (RDA) as described in Borcard et al. [44]. Collinearity between explanatory variables was tested before using the variance inflation factor (VIF). It was low, except between rainfall and temperature. (VIF values for distance–forest_loss, 1.210; distance–temperature, 1.093; distance–rainfall, 1.002; forest_loss–temperature, 1.008; forest_loss–rainfall, 1.005; rainfall–temperature, 2.291). Therefore, the temperature has been removed from the RDA. Hellinger transformation was used to lower the weight of common absences in the dataset. Species represented by less than a total of 10 individuals were considered to have negligible statistical weight on the analysis and thus were discarded. A permanova was performed to assess the significance of constraints. We also conducted a beta-diversity analysis to test the turnover and nestedness of the assemblage at the different sites using the Jaccard dissimilarity index, using the ‘betapart’ R package. An Anova was used to test beta-diversity significance. A plot representing extinction probability as calculated with the temperature matrix (using the ‘nestedtemp’ function in the ‘vegan’ R package) was used to summarize the data. Environmental conditions induced by human activities and climate change may have changed through time. Monte Carlo permutation (999 permutations) was used for testing the significance of the environmental variables considered. All statistical analyses were performed using R software version 1.2.5033 (Foundation for Statistical Computing, Vienna, Austria) [45], using the packages ade4 [46]), betapart [47], dplyr [48], tibble [49] and vegan [50].
3. Results
A total of 2666 individuals belonging to 70 different species or morphospecies were sampled and identified during the survey in SGO sites (Table S1). Hill numbers were calculated for the dung beetle assemblages of SGO, Matoury, Kaw and Nouragues and by pooling all the sampling periods by site (Table 2). Shannon and Simpson indexes are similar for Nouragues, Kaw and SGO, but show the lowest values for Matoury (Figure 2). However, no significant differences were found for Shannon index (Kruskal-Wallis value = 10.345, df = 6, p-value = 0.1109) and Simpson index (Kruskal-Wallis value = 10.304, df = 6, p-value = 0.1124). Conversely, Richness is significantly different between sites (Kruskal-Wallis value = 14.066, df = 6, p-value = 0.0289). The Kaw assemblage has the highest species richness while SGO7 has the highest Shannon and Simpson indexes. Species richness and diversity indexes increase significantly with the distance to the nearest city, but only species richness decreases significantly with increasing forest cover loss (Table 3). Beta diversity is high (0.904) and significantly different between sites (F value = 2.81, df = 6, p-value = 0.046). This is mainly due to the turnover (0.861) while nestedness is low (0.044). Nestedness temperature is high (34.738) and reflects a high turnover.

Table 2.
Total abundance, total species richness, Simpson and Shannon indexes for each site at SGO at each period (Data for the other sites are available in Feer & Boisier [8]).
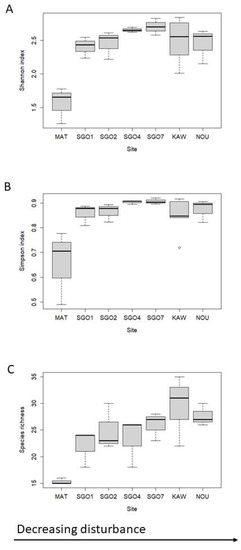
Figure 2.
Boxplots of (A) species richness, (B) Shannon and (C) Simpson indexes at SGO sites and sites in Feer & Boissier (2015), i.e., Kaw (KAW), Matoury (MAT) and Nouragues (NOU).

Table 3.
Spearman Rank Correlation test of species richness, Shannon and Simpson indexes with distance to the city (Distance) and forest cover loss (Forest) for the four SGO sites and the other sites in the Feer & Boisier [8] study, i.e., Nouragues, Kaw and Matoury.
Dung beetle assemblages differ among sites (Stress = 0.0421) as shown by the two first axes of the second NMDS (S1). The Matoury assemblage stays apart from the other sites on the first axis, while the SGO and Matoury assemblages differ from Kaw and Nouragues on the second axis. The Kaw and Nouragues assemblages are very similar.
The resulting figure of the RDA (Figure 3) shows the first two axes representing 17.95% and 7.61% of the variance, respectively. Distance to the nearest city explains more than 99% of the first axis, while rainfall explains 94% of the second axis. Forest cover loss represents 81% of the third axis. Distance to the city and rainfall significantly contribute to the variance with a p-value of 0.002 and 0.043, respectively, while forest cover loss does not (p-value: 0.138). Matoury is isolated from the other sites at the right of the first axis, due to higher forest cover loss and proximity to the city. Matoury also shows higher rainfall than the other sites. Nouragues and Kaw are the farthest locations with no forest cover loss and high rainfall compared to SGO. Although rainfall is usually greater near the coast than inland, especially at Kaw, this is not obvious in the RDA analysis. SGO sites are displayed in the upper part of the second axis, representing drier sampling periods, compared to the other sites. SGO4 and SGO7 are closer to Kaw and Nouragues on the first axis as they are the farthest sites to the city, while SGO1 and SGO2 are closer to Matoury as they are the nearest sites to the city. The third season in the SGO sites is at the top of the second axis, as it was the driest.
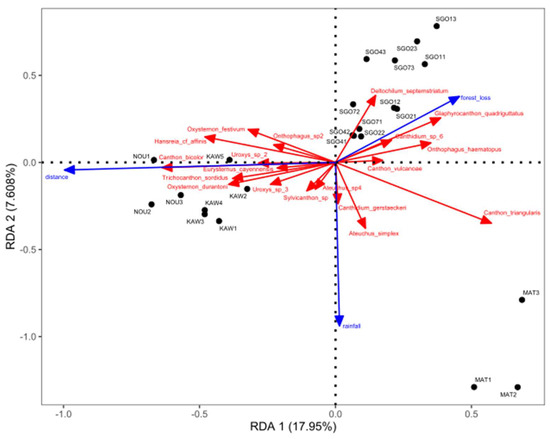
Figure 3.
Bi–dimensional representation of the redundancy analysis (RDA) of sampling sites (black points), species (red) and explanatory variables (blue), considering dung beetle assemblage in the four sites near SGO (P1 stands for Jan. 2018, P2 for Jan. 2019 and P3 for Nov. 2019) and the sites from Feer & Boissier [8]. Numbers after KAW, MAT and NOU stand for the sampling period (for example KAW1 refers to the period of Feb. 2009, see Table 1). (Scaling= 1).
The dung beetle species Canthon triangularis (Drury) and Ateuchus simplex characterize the Matoury assemblage. Canthidium gerstaeckeri Harold is correlated with high rainfall. Oxysternon durantoni, Canthon bicolor, Trichocanthon sordidus (Harlod), Eurysternus cayennensis Castelnau and Uroxys sp2 and sp3 also characterize Nouragues and Kaw. They are correlated with high distances from the city and low forest cover loss. Deltochilum septemstriatum characterizes SGO, with a trend to prefer a dry period and high forest cover loss. Glaphyrocanthon quadriguttatus, Onthophagus haematopus Harold and Canthidium sp6 also characterize SGO and are correlated with high forest cover loss.
4. Discussion
Species richness and diversity of dung beetle assemblage increased significantly along a gradient of distance to the nearest city and with decreasing forest cover loss, but not significantly. Proxies of human pressure are more influential than climatic conditions; however, rainfall also affected dung beetle assemblages. Beta diversity showed that most of the differences in dung beetle assemblages between sites were due to turnover rather than nestedness. Dung beetle assemblages in poorer sites are not subsets of assemblages in richer sites. There might be a gain in species adapted to different environmental conditions. Disturbance in sites would induce the replacement of species rather than species loss. This suggests that, in disturbed forests, highly sensitive species are replaced by species relatively tolerant to human activities. Although human pressures were not explicitly measured in this study, forest cover loss and distance to the city used as proxies showed that human disturbances affect dung beetle assemblage richness and structure. In addition, such impact was observed in forest sites, not differing in terms of landscape or land use, even if a pasture was present 2 km away from SGO1. Navarrete & Halffter [16] stated that difference in assemblages within forest could be due to canopy coverage. These observations suggest that the distance to the city is a good proxy for human disturbance, also shown by the negative correlation of species richness with forest cover loss. These proxies allow comparison of different forest sites, while most of the studies on dung beetle assemblages focus on different landscapes and land uses such as plantations and pasture [31,51]. However, a doubt remains about the effect of regional and time scales on assemblage differences, as SGO sites remain close together in terms of distance and sampling date, compared to the three other sites. Our analysis does not allow to disentangle such scale effect.
Farms and pastures are known to affect dung beetle assemblage structure [16,52]. However, the presence of a cattle farm near SGO1 did not impact the assemblage, as dung beetle assemblages remain quite similar to that of the other sites near SGO.
In our study, several species of dung beetle remain undescribed and the ecology of those already described is unknown, nor are their trophic preferences. We cannot provide any information about their tolerance of human activities, nor can we provide indicators of disturbed areas. Some species were abundant in all sites (Glaphyrocanthon quadriguttatus, Hansreia affinis, Trichocanthon sordidus and Onthophagus haematopus), suggesting that these species are relatively tolerant. Others were absent from Matoury (Oxysternon durantoni, O. festivum, Canthon bicolor, Eurysternus hypocrita and E. ventricosus Gill), suggesting that they are sensitive to human pressure, as Matoury forest has been highly disturbed in the past. All these species occur on the Guiana Shield, some being restricted to it.
In addition to the lack of knowledge about species ecology, there may be a sampling bias, as most of the studies on dung beetle assemblage used pitfall traps baited with human feces. Trap and bait types attract species differently [53,54]. The use of complementary methods such as interception traps would improve the results, as suggested by Ong et al. [54]. However, such a strategy needs to be standardized to enable comparison between studies.
The sampling was concentrated in short periods and species’ phenology is unknown. Dung beetle assemblage may vary through the year, as suggested by Batista et al. [15]. In our case, rainfall contributed significantly to assemblage differences, but not temperature. Gebert et al. [19] found species richness of dung beetles is mainly influenced by temperature rather than rainfall. However, they dealt with an altitudinal gradient where the temperature varies considerably compared to rainfall. This was the contrary in French Guiana forests, where the temperature is globally stable while rainfall varies greatly geographically and among seasons. Williamson et al. [55] highlighted the influence of temperature on dung beetle assemblage. However, according to several authors, dung beetles highly depend on rainfall and reach their highest activity in the rainy season [56,57,58]. Batista et al. [15] found much higher abundances during rainy season, but not a significant difference in richness. Despite higher rainfall in Matoury, its richness in terms of Hill numbers was the lowest because of an anthropic pressure higher than in the other sites studied.
Several studies along gradients of increasing defaunation stress the link between dung beetles and mammals as resource availability [31,59,60]. These studies suggest that the cascading effects of mammal defaunation lead to a decline in dung beetle richness and abundance. As such, they are suspected to be affected by differences in the structure of the vertebrate communities. Mammal composition, either individually or in combination with habitat structure, explains 40% of the total variation in the dung beetle assemblage analysis in Bogoni et al. [59]. A first hunting survey conducted in 2014 by ONCFS in French Guiana (C. Richard Hansen, pers. comm.) suggests such an impact. In addition, a recent study highlighted the impact of the road RN2 on vertebrate community near SGO [61]. However, studies dealing with dung beetle and mammal co-occurrences are scarce [62]. In addition, trophic relationships between the two assemblages remain unknown and difficult to establish [63]. The inclusion of an indicator of hunting pressure would help in disentangling the effect of the different human activities impacting dung beetle assemblages.
5. Conclusions
Despite the increasing impact of hunting and other sources of human disturbance, the forest along the RN2 road was classified as quite well preserved ten years ago, compared to the Nouragues forest. De Thoisy et al. [33] gave a footprint value (representing human impact) of 4 in the forest between Regina and SGO (where our study sites are located), compared with 0 for Nouragues forest and 24 for SGO city. A trend of increasing human impact, represented by the distance to the nearest city as a proxy, was detected here, even if climatic variations affected dung beetle assemblage structure. Many other environmental parameters influence dung beetle assemblages, but using proxies is a first step toward a better understanding of how assemblages are affected by human disturbances and climatic variations. Disentangling the effects of human pressure, as pointed out by Fuzessy et al. [31], but also integrating natural environmental variations, should help in understanding how dung beetle assemblages vary and react to their changing environment. Given their implication in essential ecological processes, understanding how dung beetle assemblage composition and structure are affected by anthropogenic disturbances is essential to allow appropriate conservation strategies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d14121059/s1, Table S1: Results of identification and counting of dung beetles in the four sampling sites near SGO. P1 to P3 refer to periods of sampling. P1 is January 2018, P2 is January 2019 and P3 is November 2019; Figure S1: Figure showing the fist two axes of the NMDS analysis, considering dung beetles assemblage in the four sites near St_Georges and the sites from Feer & Boissier [8], i.e. Kaw, Matoury, and Nouragues.
Author Contributions
Conceptualization, E.G., O.M. and P.-M.F.; methodology, E.G., O.M. and P.-M.F.; formal analysis, E.G. and O.A.; investigation, E.G., O.A., O.C., O.M. and P.-M.F.; writing—original draft preparation, E.G. and O.A.; writing—review and editing, E.G., O.A., O.C., O.M. and P.-M.F.; project administration, E.G. and P.-M.F.; funding acquisition, E.G., O.M. and P.-M.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Labex DRIIHM—OHM Oyapock (USR mixte LEEISA) project in 2018 and 2019.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Original data used in this study are provided in Supplementary Materials.
Acknowledgments
This study was funded by the Labex DRIIHM—OHM Oyapock project in 2018 and 2019. We warmly thank Damien Davy for supporting this project, the French Office of Biodiversity (OFB) at Cayenne for providing information on hunting pressure in French Guiana, François Feer and Olivier Boissier for sharing their results and experience and anonymous reviewers for improving the manuscript.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Hanski, I.; Cambefort, Y. Dung Beetle Ecology; Princeton University Press: Princeton, NJ, USA, 1991; 520p. [Google Scholar]
- Nichols, E.; Spector, S.; Louzada, J.; Larsen, T.; Amezquita, S.; Favila, M.E. Ecological functions and ecosystem services provided by Scarabaeinae dung beetles. Biol. Conserv. 2008, 141, 1461–1474. [Google Scholar] [CrossRef]
- Feer, F.; Ponge, J.F.; Jouard, S.; Gomez, D. Monkey and dung beetle activities influence soil seed bank structure. Ecol. Res. 2013, 28, 93–102. [Google Scholar] [CrossRef]
- Barragán, F.; Moreno, C.E.; Escobar, F.; Halffter, G.; Navarrete, D. Negative impacts of human land use on dung beetle functional diversity. PLoS ONE 2011, 6, e17976. [Google Scholar] [CrossRef] [PubMed]
- Braga, R.F.; Korasaki, V.; Andresen, E.; Louzada, J. Dung beetle community and functions along a habitat-disturbance gradient in the Amazon: A rapid assessment of ecological functions associated to biodiversity. PLoS ONE 2013, 8, e57786. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.J.; Holloway, J.D.; Huijbregts, H.; Krikken, J.; Kirk-Spriggs, A.H.; Sutton, S.L. Dung beetles as indicators of change in the forests ofnorthern Borneo. J. Appl. Ecol. 2001, 38, 593–616. [Google Scholar] [CrossRef]
- Edwards, F.A.; Edwards, D.P.; Larsen, T.H.; Hsu, W.W.; Benedick, S.; Chung, A.; Khen, C.V.; Wilcove, D.S.; Hamer, K.C. Does logging and forest conversion to oil palm agriculture alter functional diversity in a biodiversity hotspot? Anim. Conserv. 2014, 17, 163–173. [Google Scholar] [CrossRef]
- Feer, F.; Boissier, O. Variations in dung beetle assemblages across a gradient of hunting in a tropical forest. Ecol. Indic. 2015, 57, 164–170. [Google Scholar] [CrossRef]
- Nichols, E.; Gardner, T.A.; Peres, C.A.; Spector, S. Co-declining mammals and dung beetles: An impending ecological cascade. Oiko 2009, 118, 481–487. [Google Scholar] [CrossRef]
- Nichols, E.; Gardner, T.A. Dung beetles as a candidate study taxon in applied biodiversity conservation research. In Ecology and Evolution of Dung Beetles, 1st ed.; Simmons, L.W., Ridsdill-Smith, T.J., Eds.; Blackwell Publishing Ltd.: Oxford, UK, 2011; pp. 267–291. [Google Scholar]
- Noriega, J.A.; March-Salas, M.; Castillo, S.; Garcia-Q, H.; Hortal, J.; Santos, A.M.C. Human perturbations reduce dung beetle diversity and dung removal ecosystem function. Biotropica 2021, 53, 753–766. [Google Scholar] [CrossRef]
- Barlow, J.; Gardner, T.A.; Araujo, I.S.; Ávila-Pires, T.C.; Bonaldo, A.B.; Costa, J.E.; Esposito, M.C.; Ferreira, L.V.; Hawes, J.; Hernandez, M.I.M.; et al. Quantifying the biodiversity value of tropical primary, secondary, and plantation forests. Proc. Natl. Acad. Sci. USA 2007, 104, 18555–18560. [Google Scholar] [CrossRef]
- Barlow, J.; Lennox, G.D.; Ferreira, J.; Berenguer, E.; Lees, A.C.; Mac Nally, R.; Thomson, J.R.; de Ferraz, S.F.; Louzada, J.; Oliveira, V.H.F.; et al. Anthropogenic disturbance in tropical forests can double biodiversity loss from deforestation. Nature 2016, 535, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Goh, T.G.; Hashim, R. Trait responses of Peninsular Malaysian dung beetles (Scarabaeidae: Scarabaeinae) to the loss of megafauna dung. J. Trop. Ecol. 2020, 36, 39–41. [Google Scholar] [CrossRef]
- Batista, M.C.; Lopes, G.S.; Marques, L.J.P.; Teodoro, A.V. The dung beetle assemblage (Coleoptera: Scarabaeinae) is differently affected by land use and seasonality in northeastern Brazil. Entomotropica 2016, 31, 95–104. [Google Scholar]
- Navarrete, D.; Halffter, G. Dung beetle (Coleoptera: Scarabaeidae: Scarabaeinae) diversity in continuous forest, forest fragments and cattle pastures in a landscape of Chiapas, Mexico: The effects of anthropogenic changes. Biodivers. Conserv. 2008, 17, 2869–2898. [Google Scholar] [CrossRef]
- Carrión-Paladines, V.; Fries, A.; Muñoz, A.; Castillo, E.; García-Ruiz, R.; Marín-Armijos, D. Effects of Land-Use Change on the Community Structure of the Dung Beetle (Scarabaeinae) in an Altered Ecosystem in Southern Ecuador. Insects 2021, 12, 306. [Google Scholar] [CrossRef]
- Carpio, C.; Donoso, D.A.; Ramón, G.; Dangles, O. Short term response of dung beetle communities to disturbance by road construction in the Ecuadorian Amazon. Ann. Soc. Entomol. Fr. 2009, 45, 455–469. [Google Scholar] [CrossRef]
- Gebert, F.; Steffan-Dewenter, I.; Moretto, P.; Peters, M.K. Climate rather than dung resources predict dung beetle abundance and diversity along elevational and land use gradients on Mt. Kilimanjaro. J. Biogeogr. 2019, 47, 371–381. [Google Scholar] [CrossRef]
- Klein, B.C. Effects of forest fragmentation on dung and carrion beetle communities in Central Amazonia. Ecology 1989, 70, 1715–1725. [Google Scholar] [CrossRef]
- Estrada, A.; Coates-Estrada, R.; Meritt, D., Jr.; Montiel, S.; Curiel, D. Patterns of frugivorous species richness and abundance in forest islands and in agricultural habitats at Los Tuxtlas, Mexico. Vegetatio 1993, 107, 245–257. [Google Scholar] [CrossRef]
- Estrada, A.; Anzures, A.; Coates-Estrada, R. Tropical rain forest fragmentation, howler monkeys (Alouatta palliata), and dung beetles at Los Tuxtlas, Mexico. Am. J. Primatol. 1999, 48, 253–262. [Google Scholar] [CrossRef]
- Vulinec, K. Dung beetles (Coleoptera: Scarabaeidae), monkeys, and conservation in Amazonia. Fla. Entomol. 2000, 83, 229–241. [Google Scholar] [CrossRef]
- Andresen, E. Effect of forest fragmentation on dung beetle communities and functional consequences for plant regeneration. Ecography 2003, 26, 87–97. [Google Scholar] [CrossRef]
- Andresen, E.; Laurance, S.G.W. Possible indirect effects of mammal hunting on Dung beetle assemblages in Panama. Biotropica 2007, 39, 141–146. [Google Scholar] [CrossRef]
- Feer, F.; Hingrat, Y. Effects of forest fragmentation on a dung beetle community in French Guiana. Conserv. Biol. 2005, 19, 1103–1112. [Google Scholar] [CrossRef]
- Otavo, S.E.; Parrado-rosselli, A.; Noriega, J.A. Superfamilia Scarabaoidea (Insecta: Copeoptera) como elemento bioindicador de perturbacion antropogénica en un parque nacional amazonico. Rev. Biol. Trop. 2013, 61, 735–752. [Google Scholar] [CrossRef]
- Galetti, M.; Moleón, M.; Jordano, P.; Pires, M.M.; Guimarães, P.R., Jr.; Pape, T.; Nichols, E.; Hansen, D.; Olesen, J.M.; Munk, M.; et al. Ecological and evolutionary legacy of megafauna extinctions. Biol. Rev. 2018, 93, 845–862. [Google Scholar] [CrossRef] [PubMed]
- Raine, E.H.; Gray, C.L.; Mann, D.J.; Slade, E.M. Tropical dung beetle morphological traits predict functional traits and show intraspecific differences across land uses. Ecol. Evol. 2018, 8, 8686–8696. [Google Scholar] [CrossRef]
- Barlow, J.; Louzada, J.; Parry, L.; Hernández, M.I.M.; Hawes, J.; Peres, C.A.; Vaz-de-Mello, F.Z.; Gardner, T.A. Improving the design and management of forest strips in human-dominated tropical landscapes: A field test on Amazonian dung beetles: Dung beetles in Amazonian forest strips. J. Appl. Ecol. 2010, 47, 779–788. [Google Scholar] [CrossRef]
- Fuzessy, F.L.; Benítez-López, A.; Slade, E.M.; Bufalo, F.S.; Magro-de-Souza, G.C.; Pereira, L.A.; Culot, L. Identifying the anthropogenic drivers of declines in tropical dung beetle communities and functions. Biol. Conserv. 2021, 256, 109063. [Google Scholar] [CrossRef]
- Bovolo, C.I.; Wagner, T.; Parkin, G.; Hein-Griggs, D.; Pereira, R.; Jones, R. The Guiana Shield rainforests-overlooked guardians of South American climate. Environ. Res. Lett. 2018, 13, 074029. [Google Scholar] [CrossRef]
- De Thoisy, B.; Richard-Hansen, C.; Goguillon, B.; Joubert, P.; Obstancias, J.; Winterton, P.; Brosse, S. Rapid evaluation of threats to biodiversity: Human footprint score and large vertebrate species responses in French Guiana. Biodivers. Conserv. 2010, 19, 1567–1584. [Google Scholar] [CrossRef]
- De Thoisy, B.; Brosse, S.; Dubois, M.A. Assessment of large-vertebrate species richness and relative abundance in Neotropical forest using line-transect censuses: What is the minimal effort required? Biodivers. Conserv. 2008, 17, 2627–2644. [Google Scholar] [CrossRef]
- Nicolle, S.; d’Hautefeuille, M.B. Anticiper la route: Étude de cas dans l’est de la Guyane française. VertigO 2014, 14, 1–21. [Google Scholar] [CrossRef]
- Poncy, O.; Sabatier, D.; Prévost, M.F.; Hardy, I. The lowland high rainforest: Structure and tree species diversity. In Nouragues. Dynamics and Plant-Animal Interactions in a Neotropical Rainforest; Bongers, F., Charles-Dominique, P., Forget, P.M., Théry, M., Eds.; Kluwer Academic Publisher: Dordrecht, The Netherlands, 2001; pp. 31–46. [Google Scholar]
- Forget, P.M.; Hammond, D.S. Rainforest Vertebrates and Food Plant Diversity in the Guiana Shield. In Tropical Forests of the Guiana Shield: Ancient Forests in a Modern World; Hammond, D.S., Ed.; Cabi: Wallington, UK, 2005; pp. 233–294. [Google Scholar]
- Hansen, M.C.; Potapov, P.V.; Moore, R.; Hancher, M.R.; Turubanova, S.A.; Tyukavina, A.; Thau, D.; Stehman, S.V.; Goetz, S.J.; Loveland, T.R.; et al. High-Resolution Global Maps of 21st-Century Forest Cover Change. Science 2013, 342, 850–853. [Google Scholar] [CrossRef] [PubMed]
- Boilly, O.; Vaz-De-Mello, F.Z. Les Scarabaeinae de Guyane: Clé illustrée des genres. Coléoptériste Coléoptères Guyane 2013, 7, 103–112. [Google Scholar]
- Boilly, O.; Lapèze, J.; Dalens, P.H.; Giuglaris, J.L.; Touroult, J. Les Phanaeini de Guyane: Liste commentée, clés et iconographie. ACOREP-Fr. Coléoptères Guyane 2016, 10, 86–96. [Google Scholar]
- Boilly, O. Les Deltochilum de Guyane (Coleoptera, Scarabaeidae). ACOREP-Fr. Coléoptères Guyane 2015, 9, 82–88. [Google Scholar]
- Génier, F. Le Genre Eurysternus Dalman, 1824 Révision Taxonomique et Clés de Détermination Illustrées; Pensoft Series Faunistica: Sofia, Bulgary, 2009; Volume 85, pp. 1–430. [Google Scholar]
- Boilly, O. Les Eurysternus Dalman, 1824 de Guyane, une clef illustrée des espèces. Contrib. À L’étude Coléoptères Guyane 2018, 12, 33–39. [Google Scholar]
- Borcard, D.; Gillet, F.; Legendre, P. Numerical Ecology with R, 2nd ed.; Springer: Berlin, Germany, 2018; pp. 308–309. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing [Software]; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Dray, S.; Dufour, A. The ade4 Package: Implementing the Duality Diagram for Ecologists. J. Stat. Softw. 2007, 22, 1–20. [Google Scholar] [CrossRef]
- Baselga, A.; David, C.; Orme, L. Betapart: An R package for the study of beta diversity. Meth. Ecol. Evol. 2012, 3, 808–812. [Google Scholar] [CrossRef]
- Wickham, H.; François, R.; Henry, L.; Müller, K. dplyr: A Grammar of Data Manipulation. R Package Version 1.0.5. 2021. Available online: https://dplyr.tidyverse.org/ (accessed on 1 October 2021).
- Müller, K.; Wickham, H. Tibble: Simple Data Frames. R Package Version 3.1.0. 2021. Available online: https://tibble.tidyverse.org/ (accessed on 1 October 2021).
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; et al. Vegan: Community Ecology Package. R Package Version 2.5-7. 2020. Available online: https://cran.r-project.org/web/packages/vegan/vegan.pdf (accessed on 1 October 2021).
- Nichols, E.; Larsen, T.; Spector, S.; Davis, A.L.; Escobar, F.; Favila, M.; Vuline, K. Global dung beetle response to tropical forest modification and fragmentation: A quantitative literature review and meta-analysis. Biol. Conserv. 2007, 137, 1–19. [Google Scholar] [CrossRef]
- Correa, C.M.A.; Peres, N.D.; Holdbrook, R. Patterns of alimentary resource use by dung beetles in introduced Brazilian pastures: Cattle versus sheep dung. Entomol. Sci. 2020, 23, 271–279. [Google Scholar] [CrossRef]
- Raine, E.H.; Mikich, S.B.; Lewis, O.T.; Slade, E.M. Interspecific and intraspecific variation in diet preference in five Atlantic forest dung beetle species. Ecol. Entomol. 2019, 44, 436–439. [Google Scholar] [CrossRef]
- Ong, X.R.; Hemprich-Bennett, D.; Gray, C.L.; Kemp, V.; Chung, A.Y.C.; Slade, E.M. Trap type affects dung beetle taxonomic and functional direversity in Bornean tropical forest. Austral Ecol. 2022, 47, 68–78. [Google Scholar] [CrossRef]
- Williamson, J.; Slade, E.M.; Luke, S.H.; Swinfield, T.; Chung, A.Y.C.; Coomes, D.A.; Heroin, H.; Jucker, T.; Lewis, O.T.; Vairappan, C.S.; et al. Riparian buffers act as micro climatic refugia in oil palm landscapes. J. Appl. Ecol. 2021, 58, 431–442. [Google Scholar] [CrossRef]
- Davis, A.L.V.; Dewhurst, C.F. Climatic and biogeographical associations of Kenyan and northern Tanzanian dung beetles (Coleoptera: Scarabaeidae). Afr. J. Ecol. 1993, 31, 290–305. [Google Scholar] [CrossRef]
- Davis, A.L.V.; Scholtz, C.H.; Chown, S.L. Species turnover, community boundaries and biogeographical composition of dung beetle assemblages across an altitudinal gradient in South Africa. J. Biogeogr. 1999, 26, 1039–1055. [Google Scholar] [CrossRef]
- Noriega, J.A.; Santos, A.M.C.; Calatayud, J.; Chozas, S.; Hortal, J. Short- and long-term temporal changes in the assemblage structure of Amazonian dung beetles. Oecologia 2021, 195, 719–736. [Google Scholar] [CrossRef]
- Bogoni, J.A.; Graipel, M.E.; de Castilho, P.V.; Fantacini, F.M.; Kuhnen, V.V.; Luiz, M.R.; Maccarini, T.B.; Marcon, C.B.; de Souza Pimentel Teixeira, C.; Tortato, M.A.; et al. Contributions of the mammal community, habitat structure, and spatial distance to dung beetle community structure. Biodivers. Conserv. 2016, 25, 1661–1675. [Google Scholar] [CrossRef]
- Culot, L.; Bovy, E.; Vaz-de-Mello, F.Z.; Guevara, R.; Galetti, P. Selective defaunation affects dung beetle communities in continuous Atlantic rainforest. Biol. Conserv. 2013, 163, 79–89. [Google Scholar] [CrossRef]
- Coutant, O.; Boissier, O.; Ducrettet, M.; Albert-Daviaud, A.; Bouiges, A.; Dracxler, C.M.; Feer, F.; Mendoza, I.; Guilbert, E.; Forget, P.M. Roads Disrupt Frugivory and Seed Removal in Tropical Animal-Dispersed Plants in French Guiana. Front. Ecol. Evol. 2022, 10, 805376. [Google Scholar] [CrossRef]
- Raine, E.H.; Slade, E.M. Dung beetle–mammal associations: Methods, research trends and future directions. Proc. R. Soc. B 2019, 286, 20182002. [Google Scholar] [CrossRef] [PubMed]
- Gillett, C.P.D.T.; Johnson, A.J.; Barr, I.; Hulcr, J. Metagenomic sequencing of dung beetle intestinal contents directly detects and identifies mammalian fauna. BioRxiv 2016. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).