Species- and Trait-Based Reconstructions of the Hydrological Regime in a Tropical Peatland (Central Sumatra, Indonesia) during the Holocene Using Testate Amoebae
Abstract
1. Introduction
2. Materials and Methods
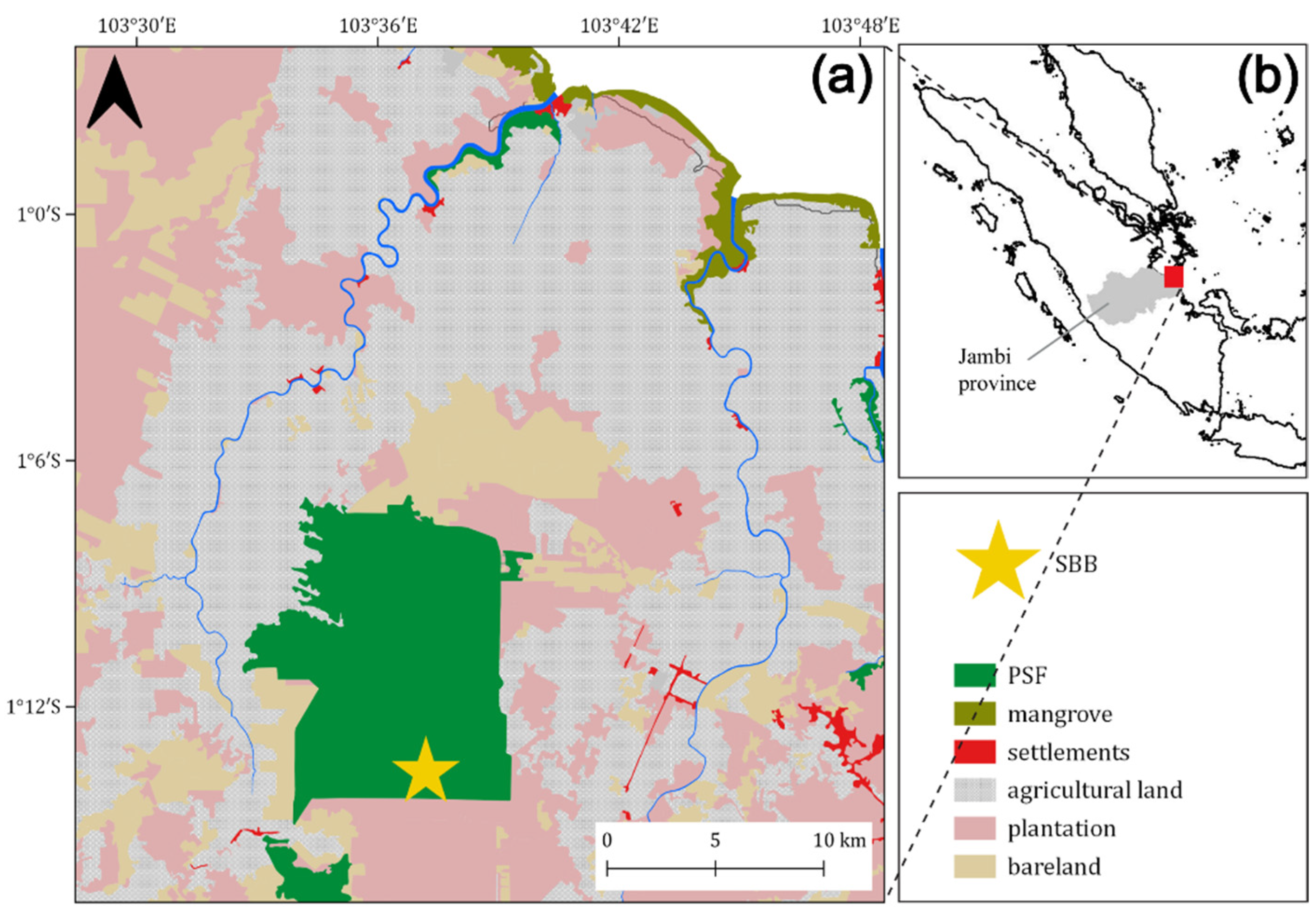
2.1. Study Region
2.2. Study Site
2.3. Peat Sampling
2.4. Testate Amoeba Analysis
2.5. Data Analyses
3. Results
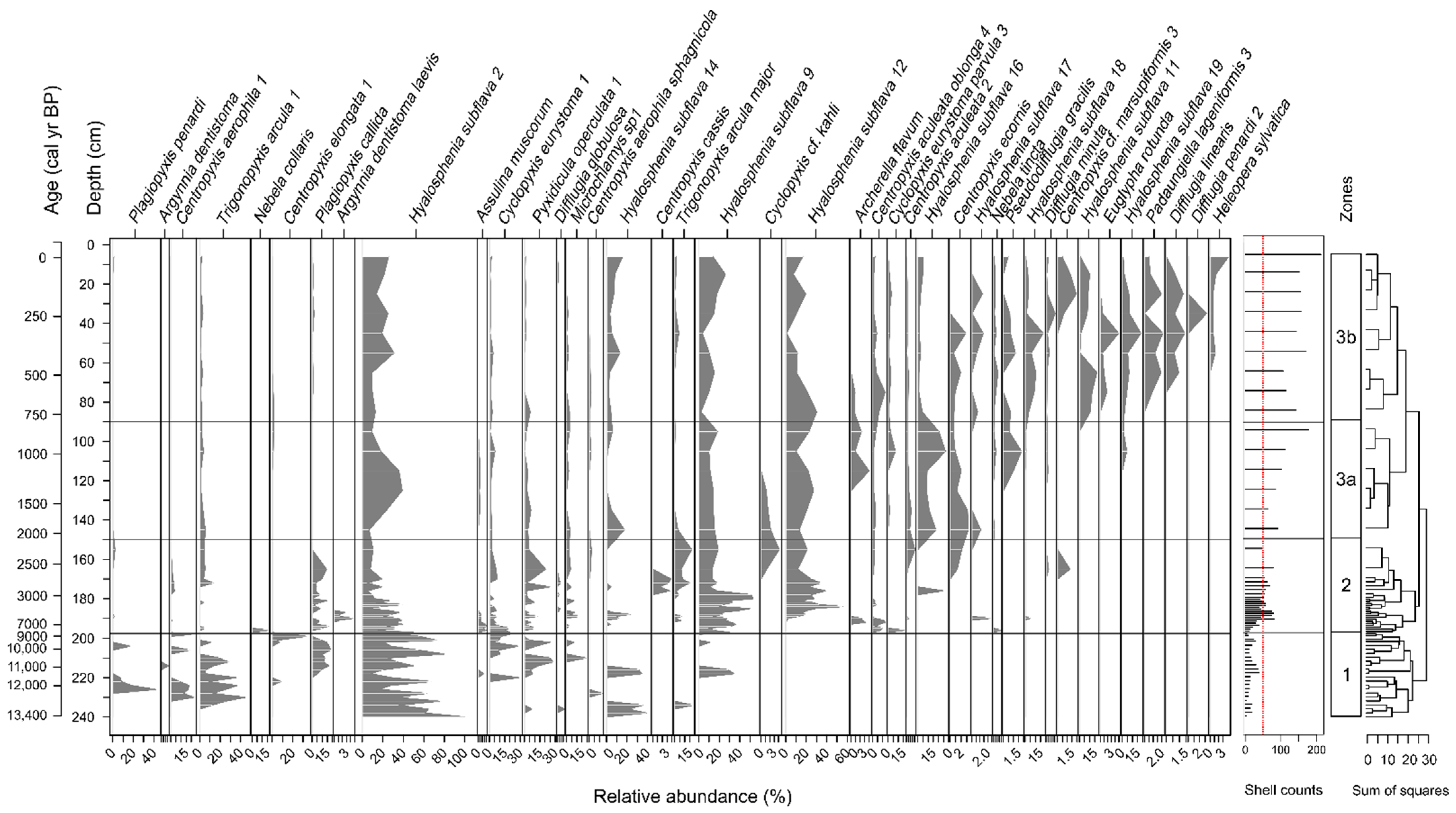
3.1. Overall Characteristics of Testate Amoeba Communities
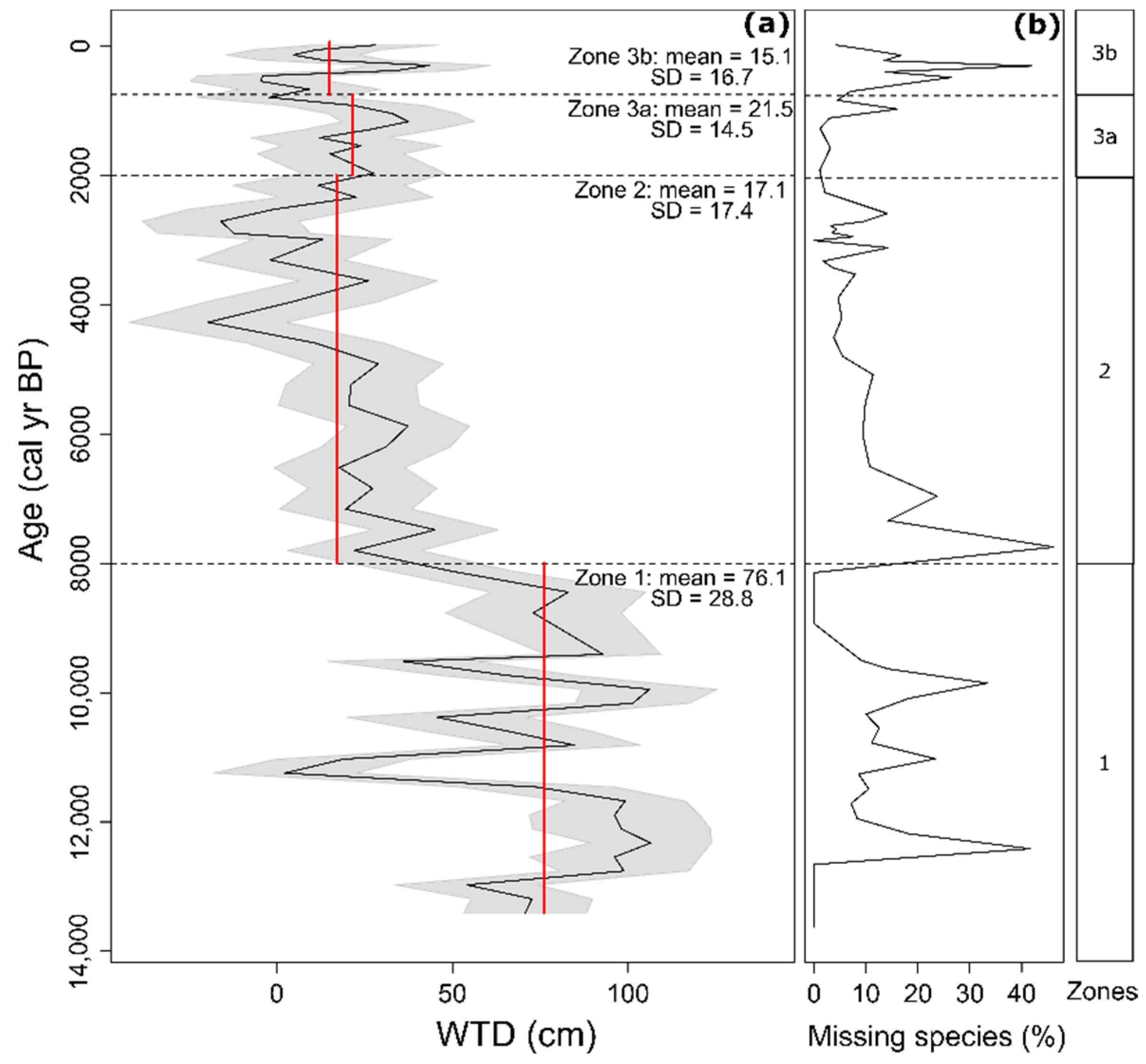
3.2. Morphospecies-Based Zonation of Peat Deposits and Reconstruction of Water Table Depth
3.3. Functional Trait-Based Zonation of Peat Deposits and Reconstruction of Water Table Depth
4. Discussion
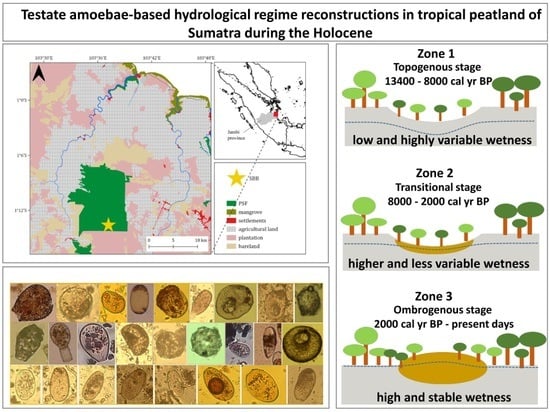
4.1. Initial Stages of Peatland Development and Topogeneous Stage (13,400–8000 cal yr BP)
4.2. Transitional Stage (8000–2000 cal yr BP)
4.3. Ombrogenous Stage (2000–750 cal yr BP)
4.4. Ombrogenous Stage and Anthropogenic Impacts (750 cal yr BP–Present Days)
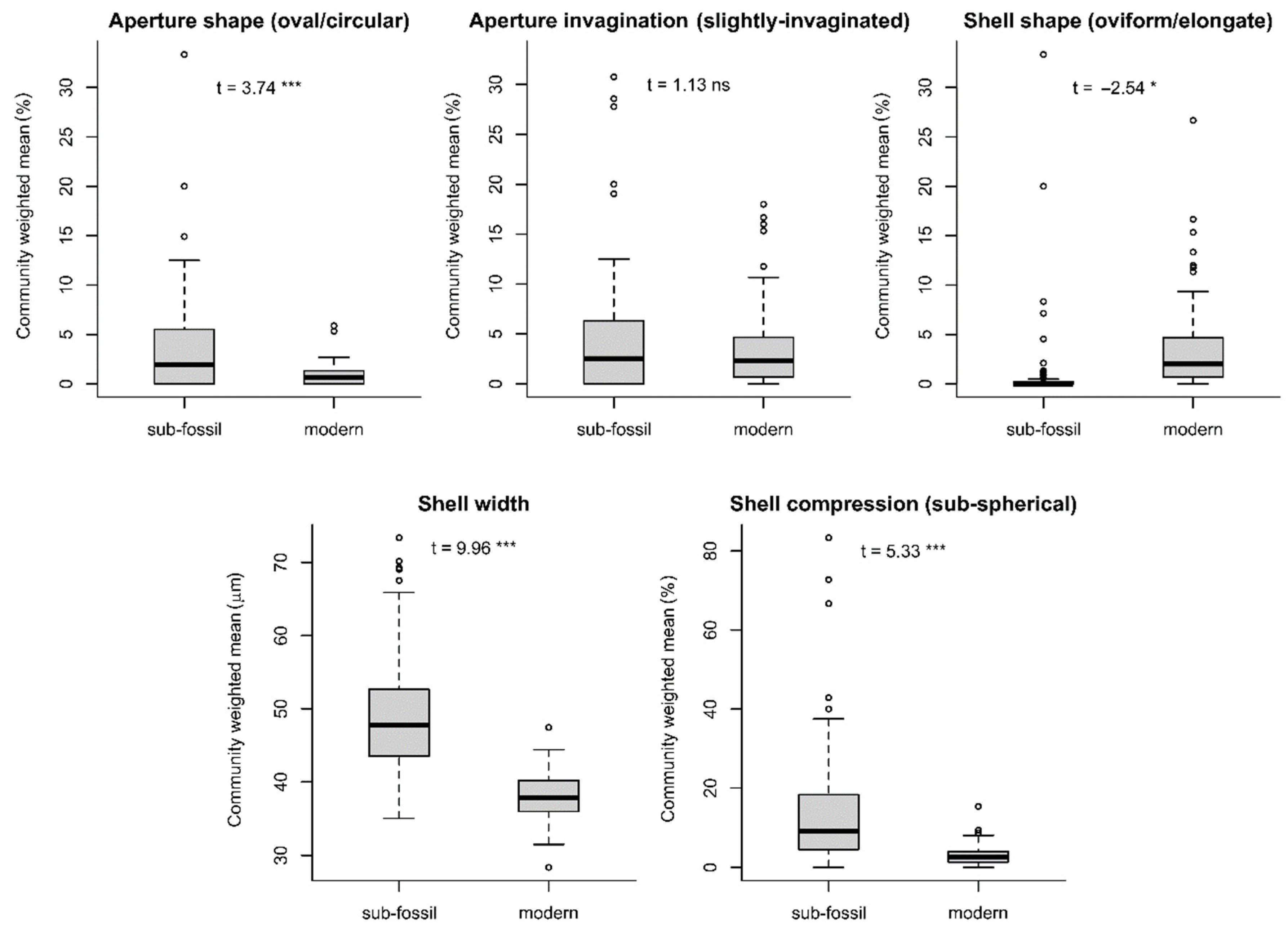
4.5. Morphospecies- vs. Functional-Trait-Based Reconstructions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Strack, M. Peatlands and Climate Change; IPS, International Peat Society: Jyväskylä, Finland, 2008. [Google Scholar]
- Xu, J.; Morris, P.J.; Liu, J.; Holden, J. PEATMAP: Refining Estimates of Global Peatland Distribution Based on a Meta-Analysis. Catena 2018, 160, 134–140. [Google Scholar] [CrossRef]
- Gumbricht, T.; Roman-Cuesta, R.M.; Verchot, L.; Herold, M.; Wittmann, F.; Householder, E.; Herold, N.; Murdiyarso, D. An Expert System Model for Mapping Tropical Wetlands and Peatlands Reveals South America as the Largest Contributor. Glob. Change Biol. 2017, 23, 3581–3599. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, K.; Pacheco, F.S.; Ferreira, J.W.; Sousa-Neto, E.R.; Hastie, A.; Krieger Filho, G.C.; Alvalá, P.C.; Forti, M.C.; Ometto, J.P. Tropical Peatlands and Their Contribution to the Global Carbon Cycle and Climate Change. Glob. Change Biol. 2021, 27, 489–505. [Google Scholar] [CrossRef] [PubMed]
- Anda, M.; Ritung, S.; Suryani, E.; Hikmat, M.; Yatno, E.; Mulyani, A.; Subandiono, R.E. Revisiting Tropical Peatlands in Indonesia: Semi-Detailed Mapping, Extent and Depth Distribution Assessment. Geoderma 2021, 402, 115235. [Google Scholar] [CrossRef]
- Andriesse, J. Nature and Management of Tropical Peat Soils; Food & Agriculture Organization: Rome, Italy, 1988. [Google Scholar]
- Page, S.E.; Rieley, J.O.; Banks, C.J. Global and Regional Importance of the Tropical Peatland Carbon Pool. Glob. Change Biol. 2011, 17, 798–818. [Google Scholar] [CrossRef]
- Dargie, G.C.; Lewis, S.L.; Lawson, I.T.; Mitchard, E.T.A.; Page, S.E.; Bocko, Y.E.; Ifo, S.A. Age, Extent and Carbon Storage of the Central Congo Basin Peatland Complex. Nature 2017, 542, 86–90. [Google Scholar] [CrossRef]
- Draper, F.C.; Roucoux, K.H.; Lawson, I.T.; Mitchard, E.T.A.; Coronado, E.N.H.; Lähteenoja, O.; Montenegro, L.T.; Sandoval, E.V.; Zaráte, R.; Baker, T.R. The Distribution and Amount of Carbon in the Largest Peatland Complex in Amazonia. Environ. Res. Lett. 2014, 9, 124017. [Google Scholar] [CrossRef]
- Hapsari, K.A.; Jennerjahn, T.; Nugroho, S.H.; Yulianto, E.; Behling, H. Sea Level Rise and Climate Change Acting as Interactive Stressors on Development and Dynamics of Tropical Peatlands in Coastal Sumatra and South Borneo since the Last Glacial Maximum. Glob. Change Biol. 2022, 28, 3459–3479. [Google Scholar] [CrossRef]
- Biagioni, S.; Krashevska, V.; Achnopha, Y.; Saad, A.; Sabiham, S.; Behling, H. 8000 Years of Vegetation Dynamics and Environmental Changes of a Unique Inland Peat Ecosystem of the Jambi Province in Central Sumatra, Indonesia. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2015, 440, 813–829. [Google Scholar] [CrossRef]
- Dommain, R.; Couwenberg, J.; Joosten, H. Development and Carbon Sequestration of Tropical Peat Domes in South-East Asia: Links to Post-Glacial Sea-Level Changes and Holocene Climate Variability. Quat. Sci. Rev. 2011, 30, 999–1010. [Google Scholar] [CrossRef]
- Chambers, F.M.; Beilman, D.W.; Yu, Z. Methods for Determining Peat Humification and for Quantifying Peat Bulk Density, Organic Matter and Carbon Content for Palaeostudies of Climate and Peatland Carbon Dynamics. Mires Peat 2011, 7, 1–10. [Google Scholar]
- Fournier, B.; Coffey, E.E.D.; van der Knaap, W.O.; Fernández, L.D.; Bobrov, A.; Mitchell, E.A.D. A Legacy of Human-Induced Ecosystem Changes: Spatial Processes Drive the Taxonomic and Functional Diversities of Testate Amoebae in Sphagnum Peatlands of the Galápagos. J. Biogeogr. 2016, 43, 533–543. [Google Scholar] [CrossRef]
- Barrett, K.D.; Sanford, P.; Hotchkiss, S.C. The Ecology of Testate Amoebae and Cladocera in Hawaiian Montane Peatlands and Development of a Hydrological Transfer Function. J. Paleolimnol. 2021, 66, 83–101. [Google Scholar] [CrossRef]
- Liu, B.; Booth, R.K.; Escobar, J.; Wei, Z.; Bird, B.W.; Pardo, A.; Curtis, J.H.; Ouyang, J. Ecology and Paleoenvironmental Application of Testate Amoebae in Peatlands of the High-Elevation Colombian Páramo. Quat. Res. 2019, 92, 14–32. [Google Scholar] [CrossRef]
- Beyens, L.; Meisterfeld, R. Protozoa: Testate Amoebae. In Tracking Environmental Change Using Lake Sediments: Terrestrial, Algal, and Siliceous Indicators; Smol, J.P., Birks, H.J.B., Last, W.M., Bradley, R.S., Alverson, K., Eds.; Springer: Dordrecht, The Netherlands, 2001; pp. 121–153. ISBN 978-0-306-47668-6. [Google Scholar]
- Krashevska, V.; Bonkowski, M.; Maraun, M.; Scheu, S. Testate Amoebae (Protista) of an Elevational Gradient in the Tropical Mountain Rain Forest of Ecuador. Pedobiologia 2007, 51, 319–331. [Google Scholar] [CrossRef]
- Krashevska, V.; Klarner, B.; Widyastuti, R.; Maraun, M.; Scheu, S. Changes in Structure and Functioning of Protist (Testate Amoebae) Communities Due to Conversion of Lowland Rainforest into Rubber and Oil Palm Plantations. PLoS ONE 2016, 11, e0160179. [Google Scholar] [CrossRef]
- Krashevska, V.; Tsyganov, A.N.; Esaulov, A.S.; Mazei, Y.A.; Hapsari, K.A.; Saad, A.; Sabiham, S.; Behling, H.; Biagioni, S. Testate Amoeba Species- and Trait-Based Transfer Functions for Reconstruction of Hydrological Regime in Tropical Peatland of Central Sumatra, Indonesia. Front. Ecol. Evol. 2020, 8, 225. [Google Scholar] [CrossRef]
- Cailleuax, A. Répartition Géographique Des Espèces de Thécamœbiens. CR Soc. Biogeogr. 1978, 472, 29–39. [Google Scholar]
- Amesbury, M.J.; Swindles, G.T.; Bobrov, A.; Charman, D.J.; Holden, J.; Lamentowicz, M.; Mallon, G.; Mazei, Y.; Mitchell, E.A.D.; Payne, R.J.; et al. Development of a New Pan-European Testate Amoeba Transfer Function for Reconstructing Peatland Palaeohydrology. Quat. Sci. Rev. 2016, 152, 132–151. [Google Scholar] [CrossRef]
- Charman, D.J.; Blundell, A. A New European Testate Amoebae Transfer Function for Palaeohydrological Reconstruction on Ombrotrophic Peatlands. J. Quat. Sci. 2007, 22, 209–221. [Google Scholar] [CrossRef]
- Van Bellen, S.; Mauquoy, D.; Payne, R.J.; Roland, T.P.; Daley, T.J.; Hughes, P.D.M.; Loader, N.J.; Street-Perrott, F.A.; Rice, E.M.; Pancotto, V.A. Testate Amoebae as a Proxy for Reconstructing Holocene Water Table Dynamics in Southern Patagonian Peat Bogs. J. Quat. Sci. 2014, 29, 463–474. [Google Scholar] [CrossRef]
- Tsyganov, A.N.; Babeshko, K.V.; Novenko, E.Y.; Malysheva, E.A.; Payne, R.J.; Mazei, Y.A. Quantitative Reconstruction of Peatland Hydrological Regime with Fossil Testate Amoebae Communities. Russ. J. Ecol. 2017, 48, 191–198. [Google Scholar] [CrossRef]
- Qin, Y.; Li, H.; Mazei, Y.; Kurina, I.; Swindles, G.T.; Bobrov, A.; Tsyganov, A.N.; Gu, Y.; Huang, X.; Xue, J.; et al. Developing a Continental-Scale Testate Amoeba Hydrological Transfer Function for Asian Peatlands. Quat. Sci. Rev. 2021, 258, 106868. [Google Scholar] [CrossRef]
- Swindles, G.T.; Reczuga, M.; Lamentowicz, M.; Raby, C.L.; Turner, T.E.; Charman, D.J.; Gallego-Sala, A.; Valderrama, E.; Williams, C.; Draper, F.; et al. Ecology of Testate Amoebae in an Amazonian Peatland and Development of a Transfer Function for Palaeohydrological Reconstruction. Microb. Ecol. 2014, 68, 284–298. [Google Scholar] [CrossRef] [PubMed]
- Swindles, G.T.; Lamentowicz, M.; Reczuga, M.; Galloway, J.M. Palaeoecology of Testate Amoebae in a Tropical Peatland. Eur. J. Protistol. 2016, 55, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Swindles, G.T.; Kelly, T.J.; Roucoux, K.H.; Lawson, I.T. Response of Testate Amoebae to a Late Holocene Ecosystem Shift in an Amazonian Peatland. Eur. J. Protistol. 2018, 64, 13–19. [Google Scholar] [CrossRef]
- Marcisz, K.; Colombaroli, D.; Jassey, V.E.J.; Tinner, W.; Kołaczek, P.; Gałka, M.; Karpińska-Kołaczek, M.; Słowiński, M.; Lamentowicz, M. A Novel Testate Amoebae Trait-Based Approach to Infer Environmental Disturbance in Sphagnum Peatlands. Sci. Rep. 2016, 6, 33907. [Google Scholar] [CrossRef]
- Van Bellen, S.; Mauquoy, D.; Payne, R.J.; Roland, T.P.; Hughes, P.D.M.; Daley, T.J.; Loader, N.J.; Street-Perrott, F.A.; Rice, E.M.; Pancotto, V.A. An Alternative Approach to Transfer Functions? Testing the Performance of a Functional Trait-Based Model for Testate Amoebae. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2017, 468, 173–183. [Google Scholar] [CrossRef]
- Green, J.L.; Bohannan, B.J.M.; Whitaker, R.J. Microbial Biogeography: From Taxonomy to Traits. Science 2008, 320, 1039–1043. [Google Scholar] [CrossRef]
- Dıíaz, S.; Cabido, M. Vive La Différence: Plant Functional Diversity Matters to Ecosystem Processes. Trends Ecol. Evol. 2001, 16, 646–655. [Google Scholar] [CrossRef]
- Lacourse, T. Environmental Change Controls Postglacial Forest Dynamics through Interspecific Differences in Life-History Traits. Ecology 2009, 90, 2149–2160. [Google Scholar] [CrossRef]
- Butterfield, B.J.; Holmgren, C.A.; Anderson, R.S.; Betancourt, J.L. Life History Traits Predict Colonization and Extinction Lags of Desert Plant Species since the Last Glacial Maximum. Ecology 2019, 100, e02817. [Google Scholar] [CrossRef]
- Marcisz, K.; Jassey, V.E.J.; Kosakyan, A.; Krashevska, V.; Lahr, D.J.G.; Lara, E.; Lamentowicz, Ł.; Lamentowicz, M.; Macumber, A.; Mazei, Y.; et al. Testate Amoeba Functional Traits and Their Use in Paleoecology. Front. Ecol. Evol. 2020, 8, 340. [Google Scholar] [CrossRef]
- Fournier, B.; Malysheva, E.; Mazei, Y.; Moretti, M.; Mitchell, E.A.D. Toward the Use of Testate Amoeba Functional Traits as Indicator of Floodplain Restoration Success. Eur. J. Soil Biol. 2012, 49, 85–91. [Google Scholar] [CrossRef]
- Koenig, I.; Mulot, M.; Mitchell, E.A.D. Taxonomic and Functional Traits Responses of Sphagnum Peatland Testate Amoebae to Experimentally Manipulated Water Table. Ecol. Indic. 2018, 85, 342–351. [Google Scholar] [CrossRef]
- Fournier, B.; Lara, E.; Jassey, V.E.; Mitchell, E.A. Functional Traits as a New Approach for Interpreting Testate Amoeba Palaeo-Records in Peatlands and Assessing the Causes and Consequences of Past Changes in Species Composition. Holocene 2015, 25, 1375–1383. [Google Scholar] [CrossRef]
- Gałka, M.; Tobolski, K.; Lamentowicz, Ł.; Ersek, V.; Jassey, V.E.J.; van der Knaap, W.O.; Lamentowicz, M. Unveiling Exceptional Baltic Bog Ecohydrology, Autogenic Succession and Climate Change during the Last 2000 Years in CE Europe Using Replicate Cores, Multi-Proxy Data and Functional Traits of Testate Amoebae. Quat. Sci. Rev. 2017, 156, 90–106. [Google Scholar] [CrossRef]
- Lamentowicz, M.; Kajukało-Drygalska, K.; Kołaczek, P.; Jassey, V.E.J.; Gąbka, M.; Karpińska-Kołaczek, M. Testate Amoebae Taxonomy and Trait Diversity Are Coupled along an Openness and Wetness Gradient in Pine-Dominated Baltic Bogs. Eur. J. Protistol. 2020, 73, 125674. [Google Scholar] [CrossRef]
- Lamentowicz, M.; Gałka, M.; Lamentowicz, Ł.; Obremska, M.; Kühl, N.; Lücke, A.; Jassey, V.E.J. Reconstructing Climate Change and Ombrotrophic Bog Development during the Last 4000 Years in Northern Poland Using Biotic Proxies, Stable Isotopes and Trait-Based Approach. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2015, 418, 261–277. [Google Scholar] [CrossRef]
- Marcisz, K.; Lamentowicz, M.; Gałka, M.; Colombaroli, D.; Adolf, C.; Tinner, W. Responses of Vegetation and Testate Amoeba Trait Composition to Fire Disturbances in and around a Bog in Central European Lowlands (Northern Poland). Quat. Sci. Rev. 2019, 208, 129–139. [Google Scholar] [CrossRef]
- Aldrian, E.; Dwi Susanto, R. Identification of Three Dominant Rainfall Regions within Indonesia and Their Relationship to Sea Surface Temperature. Int. J. Climatol. 2003, 23, 1435–1452. [Google Scholar] [CrossRef]
- Saji, N.H.; Goswami, B.N.; Vinayachandran, P.N.; Yamagata, T. A Dipole Mode in the Tropical Indian Ocean. Nature 1999, 401, 360–363. [Google Scholar] [CrossRef] [PubMed]
- Nurjanah, S.; Octavia, D.; Kusumadewi, F. Identifikasi Lokasi Penanaman Kembali Ramin (Gonystylus Bancanus Kurz) Di Hutan Rawa Gambut Sumatera Dan Kalimantan; Pusat Penelitian dan Pengembangan Konservasi dan Rehabilitasi, Badan Penelitian dan Pengembangan Kehutanan: Bogor, Indonesia, 2013. [Google Scholar]
- Tata, H.L.; van Noordwijk, M.; Widayati, A. Domestication of Dyera Polyphylla (Miq.) Steenis in Peatland Agroforestry Systems in Jambi, Indonesia. Agrofor. Syst. 2016, 90, 617–630. [Google Scholar] [CrossRef]
- Melati, D.N.; Nengah Surati Jaya, I.; Pérez-Cruzado, C.; Zuhdi, M.; Fehrmann, L.; Magdon, P.; Kleinn, C. Spatio-Temporal Analysis on Land Transformation in a Forested Tropical Landscape in Jambi Province, Sumatra. In Proceedings of the EGU General Assembly Conference Abstracts, Vienna, Austria, 12–17 April 2015; p. 15014. [Google Scholar]
- Hapsari, K.A.; Biagioni, S.; Jennerjahn, T.C.; Reimer, P.M.; Saad, A.; Achnopha, Y.; Sabiham, S.; Behling, H. Environmental Dynamics and Carbon Accumulation Rate of a Tropical Peatland in Central Sumatra, Indonesia. Quat. Sci. Rev. 2017, 169, 173–187. [Google Scholar] [CrossRef]
- Tjoa-Bonatz, M.L.; Neidel, J.D.; Widiatmoko, A. Early Architectural Images from Muara Jambi on Sumatra, Indonesia. Asian Perspect. 2009, 48, 32–55. [Google Scholar] [CrossRef]
- Witrianto, W. Potensi Sejarah Dan Purbakala DAS Batanghari. Anal. Sej. 2014, 5, 68–79. [Google Scholar]
- Hapsari, K.A.; Biagioni, S.; Jennerjahn, T.C.; Reimer, P.; Saad, A.; Sabiham, S.; Behling, H. Resilience of a Peatland in Central Sumatra, Indonesia to Past Anthropogenic Disturbance: Improving Conservation and Restoration Designs Using Palaeoecology. J. Ecol. 2018, 106, 2473–2490. [Google Scholar] [CrossRef]
- Aaby, B.; Digerfeldt, G. Sampling Techniques for Lakes and Bogs. In Handbook for Holocene Palaeoecology and Palaeohydrology; Berglund, B.E., Ed.; John Wiley and Sons Ltd.: Chichester, UK, 1986; pp. 181–194. [Google Scholar]
- Wüst, R.A.J.; Bustin, R.M.; Lavkulich, L.M. New Classification Systems for Tropical Organic-Rich Deposits Based on Studies of the Tasek Bera Basin, Malaysia. Catena 2003, 53, 133–163. [Google Scholar] [CrossRef]
- Hogg, A.G.; Hua, Q.; Blackwell, P.G.; Niu, M.; Buck, C.E.; Guilderson, T.P.; Heaton, T.J.; Palmer, J.G.; Reimer, P.J.; Reimer, R.W.; et al. SHCal13 Southern Hemisphere Calibration, 0–50,000 Years Cal BP. Radiocarbon 2013, 55, 1889–1903. [Google Scholar] [CrossRef]
- Blaauw, M.; Christen, J.A. Flexible Paleoclimate Age-Depth Models Using an Autoregressive Gamma Process. Bayesian Anal. 2011, 6, 457–474. [Google Scholar] [CrossRef]
- Mazei, Y.A.; Chernyshov, V.A. Testate Amoebae Communities in the Southern Tundra and Forest-Tundra of Western Siberia. Biol. Bull. 2011, 38, 789–796. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing, version 4.2.1; R Foundation for Statistical Computing: Vienna, Austria. Available online: https://www.R-project.org/ (accessed on 23 June 2022).
- Laliberté, E.; Legendre, P.; Shipley, B. FD: Measuring Functional Diversity from Multiple Traits, and Other Tools for Functional Ecology, R Package Version 1.0-12.1; R Foundation for Statistical Computing: Vienna, Austria, 2022. Available online: https://cran.r-project.org/web/packages/FD (accessed on 22 May 2022).
- Simpson, G.L.; Oksanen, J. analogue: Analogue and Weighted Averaging Methods for Palaeoecology, R package version 0.17-6. Available online: https://cran.r-project.org/web/packages/analogue/ (accessed on 20 June 2021).
- Juggins, S. Rioja: Analysis of Quaternary Science Data, R Package Version 0.9-26. Available online: https://cran.r-project.org/web/packages/rioja/ (accessed on 28 October 2020).
- Ogden, C.G. The Fine Structure of the Shell of Pyxidicula Operculata, an Aquatic Testate Amoeba (Rhizopoda). Arch. Protistenkd. 1987, 133, 157–164. [Google Scholar] [CrossRef]
- Lamentowicz, M.; Gałka, M.; Milecka, K.; Tobolski, K.; Lamentowicz, Ł.; Fiałkiewicz-Kozieł, B.; Blaauw, M. A 1300-Year Multi-Proxy, High-Resolution Record from a Rich Fen in Northern Poland: Reconstructing Hydrology, Land Use and Climate Change. J. Quat. Sci. 2013, 28, 582–594. [Google Scholar] [CrossRef]
- Bonnet, L. Intérêt Biogéographique et Paléogéographique Des Thécamoebiens Des Sols. Ann. Stn. Biol. Besse En Chandesse 1983, 54, 298–334. [Google Scholar]
- Bonnet, L. Quelques Aspects Des Populations Thecamoebienes Endogées. Bull. Soc. Sci Toulouse 1959, 94, 413–428. [Google Scholar]
- Thomas, R. Les Thécamoebiens Muscicoles et Terricoles: Notions d’écologie Générale et Comparative. P. Soc. Linn. Bordx. 1959, 98, 27–53. [Google Scholar]
- Foissner, W.; Korganova, G.A. The Centropyxis Aerophila Complex (Protozoa: Testacea). Acta Protozool. 2000, 39, 257–273. [Google Scholar]
- Swindles, G.T.; Baird, A.J.; Kilbride, E.; Low, R.; Lopez, O. Testing the Relationship between Testate Amoeba Community Composition and Environmental Variables in a Coastal Tropical Peatland. Ecol. Indic. 2018, 91, 636–644. [Google Scholar] [CrossRef]
- Schönborn, W. The Topophenetic Analysis as a Method to Elucidate the Phylogeny of Testate Amoebae (Protozoa, Testacealobosia and Testaceafilosia). Arch. Für Protistenkd. 1989, 137, 223–245. [Google Scholar] [CrossRef]
- Van Geest, G.J.; Wolters, H.; Roozen, F.C.J.M.; Coops, H.; Roijackers, R.M.M.; Buijse, A.D.; Scheffer, M. Water-Level Fluctuations Affect Macrophyte Richness in Floodplain Lakes. Hydrobiologia 2005, 539, 239–248. [Google Scholar] [CrossRef]
- Keddy, P.A. Wetland Ecology: Principles and Conservation; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Jung, M.; Burt, T.P.; Bates, P.D. Toward a Conceptual Model of Floodplain Water Table Response. Water Resour. Res. 2004, 40, W12409. [Google Scholar] [CrossRef]
- Geyh, M.A.; Streif, H.; Kudrass, H.-R. Sea-Level Changes during the Late Pleistocene and Holocene in the Strait of Malacca. Nature 1979, 278, 441–443. [Google Scholar] [CrossRef]
- Wurtzel, J.B.; Abram, N.J.; Lewis, S.C.; Bajo, P.; Hellstrom, J.C.; Troitzsch, U.; Heslop, D. Tropical Indo-Pacific Hydroclimate Response to North Atlantic Forcing during the Last Deglaciation as Recorded by a Speleothem from Sumatra, Indonesia. Earth Planet. Sci. Lett. 2018, 492, 264–278. [Google Scholar] [CrossRef]
- Branß, T.; Dittrich, A.; Núñez-González, F. Reproducing Natural Levee Formation in an Experimental Flume. In River Flow 2016; CRC Press: St. Louis, MO, USA, 2016; pp. 1122–1128. [Google Scholar]
- Sathiamurthy, E.; Voris, H.K. Maps of Holocene Sea Level Transgression and Submerged Lakes on the Sunda Shelf. Trop. Nat. Hist. 2006, 2, 1–44. [Google Scholar]
- Partin, J.W.; Cobb, K.M.; Adkins, J.F.; Clark, B.; Fernandez, D.P. Millennial-Scale Trends in West Pacific Warm Pool Hydrology since the Last Glacial Maximum. Nature 2007, 449, 452–455. [Google Scholar] [CrossRef]
- Hirano, T.; Jauhiainen, J.; Inoue, T.; Takahashi, H. Controls on the Carbon Balance of Tropical Peatlands. Ecosystems 2009, 12, 873–887. [Google Scholar] [CrossRef]
- Kozlowski, T.T. Physiological-Ecological Impacts of Flooding on Riparian Forest Ecosystems. Wetlands 2002, 22, 550–561. [Google Scholar] [CrossRef]
- Birks, H.J.B.; Lotter, A.F.; Juggins, S.; Smol, J.P. Tracking Environmental Change Using Lake Sediments: Data Handling and Numerical Techniques; Springer Science & Business Media: Berlin, Germany, 2012; Volume 5. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsyganov, A.N.; Malysheva, E.A.; Mazei, Y.A.; Anggi Hapsari, K.; Behling, H.; Sabiham, S.; Biagioni, S.; Krashevska, V. Species- and Trait-Based Reconstructions of the Hydrological Regime in a Tropical Peatland (Central Sumatra, Indonesia) during the Holocene Using Testate Amoebae. Diversity 2022, 14, 1058. https://doi.org/10.3390/d14121058
Tsyganov AN, Malysheva EA, Mazei YA, Anggi Hapsari K, Behling H, Sabiham S, Biagioni S, Krashevska V. Species- and Trait-Based Reconstructions of the Hydrological Regime in a Tropical Peatland (Central Sumatra, Indonesia) during the Holocene Using Testate Amoebae. Diversity. 2022; 14(12):1058. https://doi.org/10.3390/d14121058
Chicago/Turabian StyleTsyganov, Andrey N., Elena A. Malysheva, Yuri A. Mazei, K. Anggi Hapsari, Hermann Behling, Supiandi Sabiham, Siria Biagioni, and Valentyna Krashevska. 2022. "Species- and Trait-Based Reconstructions of the Hydrological Regime in a Tropical Peatland (Central Sumatra, Indonesia) during the Holocene Using Testate Amoebae" Diversity 14, no. 12: 1058. https://doi.org/10.3390/d14121058
APA StyleTsyganov, A. N., Malysheva, E. A., Mazei, Y. A., Anggi Hapsari, K., Behling, H., Sabiham, S., Biagioni, S., & Krashevska, V. (2022). Species- and Trait-Based Reconstructions of the Hydrological Regime in a Tropical Peatland (Central Sumatra, Indonesia) during the Holocene Using Testate Amoebae. Diversity, 14(12), 1058. https://doi.org/10.3390/d14121058