Nematode and Acanthocephalan Parasites of Confiscated Sunda pangolins, Manis javanica Desmarest, 1822 (Mammalia: Pholidota: Manidae), with an Updated List of the Parasites of Pangolins
Abstract
1. Introduction
2. Materials and Methods
Molecular Analysis
3. Results
4. Discussion
Helminth Parasites of Pangolins: An Updated List of Species
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IUCN. The IUCN Red List of Threatened Species. Version 2022-1. Available online: https://www.iucnredlist.org (accessed on 8 March 2022).
- Zhang, F.; Yu, J.; Wu, S.; Li, S.; Zou, C.; Wang, Q.; Sun, R. Keeping and breeding the rescued Sunda pangolins (Manis javanica) in captivity. Zoo Biol. 2017, 36, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, R.K.; Panda, S.; Nair, M.V.; Acharjyo, L.N. Check list of parasites and bacteria recorded from pangolins (Manis sp.). J. Parasit. Dis. 2016, 40, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Wicker, L.V.; Lourens, K.; Hai, L.K. Veterinary health of pangolins. In Pangolins: Science, Society and Conservation; Challender, D.W.S., Nash, H.C., Waterman, C., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 461–493. [Google Scholar]
- Hsü, H.F. A study of some parasitic nematodes from Tonkin, Indo-China and of Strongyluris brevicaudata Mueller, 1894 from Hainan Island, South China. Peking Nat. Hist. Bull. 1932, 7, 99–115 + Plates I–III. [Google Scholar]
- Myers, B.J.; Kuntz, R.E. Nematode parasites of mammals (Dermoptera, Primates, Pholidota, Rodentia, Carnivora, and Artiodactyla) from North Borneo (Malaysia). Can. J. Zool. 1969, 47, 419–421. [Google Scholar] [CrossRef]
- Zhai, J.; Wu, Y.; Chen, J.; Zou, J.; Shan, F.; Li, W.; Chen, W.; Zhou, N. Identification of Amblyomma javanense and detection of tick-borne Ehrlichia spp. in confiscated Malayan Pangolins. Int. J. Parasitol. Parasites Wildl. 2021, 14, 107–116. [Google Scholar] [CrossRef]
- Yodsheewan, R.; Sukmak, M.; Sangkharak, B.; Kaolim, N.; Ploypan, R.; Phongphaew, W. First report on detection of Babesia spp. in confiscated Sunda pangolins (Manis javanica) in Thailand. Vet. World 2021, 14, 2380–2385. [Google Scholar] [CrossRef]
- Tuli, M.D.; Li, H.; Li, S.; Zhai, J.; Wu, Y.; Huang, W.; Feng, Y.; Chen, W.; Yuan, D. Molecular detection of a novel Ancylostoma sp. by whole mtDNA sequence from pangolin Manis javanica. Parasites Vectors 2022, 15, 70. [Google Scholar] [CrossRef]
- Baylis, H.A. XLIII.—On some parasitic worms from Java, with remarks on the Acanthocephalan genus Pallisentis. Ann. Mag. Nat. History. Ser. 10. 1933, 12, 443–449. [Google Scholar] [CrossRef]
- Gardiner, C.H.; Werner, R.M. Polypoid gastritis in a scaly anteater caused by larvae of Gendrespirura sp. J. Wildl. Dis. 1984, 20, 68–70. [Google Scholar] [CrossRef]
- Tubangui, M.A.; Masiluñgan, V.A. Nephridiorhynchus palawanensis sp. nov., an acanthocephalan parasites of Manis javanica Desmarest. Philipp. J. Sci. 1938, 66, 1–6. [Google Scholar]
- Amin, O.M.; Van Ha, N.; Heckmann, R.A. New and already known acanthocephalans mostly from mammals in Vietnam, with descriptions of two new genera and species in Archiacanthocephala. J. Parasitol. 2008, 94, 194–201. [Google Scholar] [CrossRef]
- Schmidt, G.D. Revision of the Class Archiacanthocephala Meyer, 1931 (Phylum Acanthocephala), with emphasis on Oligacanthorhynchidae Southwell et Macfie, 1925. J. Parasitol. 1972, 58, 290–297. [Google Scholar] [CrossRef]
- Amin, O.M.; Heckmann, R.A.; Sist, B.; Basso, W.U. A review of the parasite fauna of the black-bellied pangolin, Phataginus tetradactyla Lin. (Manidae), from central Africa with the description of Intraproboscis sanghae n. gen., n. sp. (Acanthocephala: Gigantorhynchidae). J. Parasitol. 2021, 107, 222–238. [Google Scholar] [CrossRef]
- Sist, B.; Basso, W.; Hemphill, A.; Cassidy, T.; Cassidy, R.; Gudehus, M. Case report: Intestinal perforation and secondary peritonitis due to Acanthocephala infection in a black-bellied pangolin (Phataginus tetradactyla). Parasitol. Int. 2021, 80, 102182. [Google Scholar] [CrossRef]
- Blaxter, M.L.; De Ley, P.; Garey, J.R.; Liu, L.X.; Scheldeman, P.; Vierstraete, A.; Vanfleteren, J.R.; Mackey, L.Y.; Dorris, M.; Frisse, L.M.; et al. A molecular evolutionary framework for the phylum Nematoda. Nature 1998, 392, 71–75. [Google Scholar] [CrossRef]
- Littlewood, D.T.J.; Olson, P. Small Subunit rDNA and the Platyhelminthes: Signal, Noise, Conflict and Compromise. In Interrelationships of the Platyhelminthes; Littlewood, D.T.J., Bray, R.A., Eds.; CRC Press: Boca Raton, FL, USA, 2000; pp. 262–278. [Google Scholar]
- Jian, R.; Wang, S.-W.; Zhang, W.-X.; Zhang, L.-P. Morphological and molecular identification of Habronema spp. (Nematoda: Habronematidae) from donkeys in Xinjiang, China, and notes on the taxonomical status of Habronema majus (Creplin, 1849) and H. microstoma (Schneider, 1866). Syst. Parasitol. 2017, 94, 511–525. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Ronquist, F.; Huelsenbeck, J. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef]
- Rambaut, A. FigTree, Version 1.4.3. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 1 December 2020).
- Neveu-Lemaire, M. Protospirura hamospiculata n. sp. Nématode parasite d’un pangolin Africain Manis (Pholidotus) temminckii. Ann. De Parasitol. 1927, 5, 107–113. [Google Scholar] [CrossRef]
- Meyer, A. Neue ceyloische Nematoden aus Säugetieren (Filaria, Strongylus) und aus Julus (Oxyuris). Arch. Für Nat. 1896, 62, 54–82 & plates IV–V. [Google Scholar]
- Kou, C.C. Studies on parasitic nematodes of mammals from Canton. I. Some new species from Paradoxurus minor exitus Schwarj, Paguma larvata larvata (Hamilton Smith) and Manis pentadactyla aurita Hodgson. Acta Zool. Sin. 1958, 10, 60–72+63 plates. [Google Scholar]
- Von Linstow, O. Helminthes from the collection of the Colombo Museum. Spolia Zeylan. 1906, 3, 166–188. [Google Scholar]
- Baylis, H.A. XXIII.—On a nematode parasite of pangolins. Ann. Mag. Nat. Hist. 1931, 8, 191–194. [Google Scholar] [CrossRef]
- Gao, J.F.; Mao, R.-F.; Li, Y.; Sun, Y.-Y.; Gao, Z.-Y.; Zhang, X.-G.; Jin, Z.-H.; An, Q.; Zhang, Z.-H.; Zhang, A.-H.; et al. Characterization of the mitochondrial genome of Tetrameres grusi and insights into the phylogeny of Spirurina. Int. J. Parasitol. Parasites Wildl. 2022, 17, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Mariaux, J.; Georgiev, B.B. Cestode parasites (Neodermata, Platyhelminthes) from Malaysian birds, with description of five new species. Eur. J. Taxon. 2020, 616, 1–35. [Google Scholar] [CrossRef]
- Chabaud, A.G. Essai de classification des Nêmatodes Habronematinae. Ann. De Parasitol. Hum. Et Comp. 1958, 33, 455–508. [Google Scholar]
- Esslinger, J.H. Dipetalonema fausti sp. n. (Filarioidea: Ochocercidae), a filarial parasite of the scaly anteater, Manis pentadactyla L. (Pholidota), from China. J. Parasitol. 1966, 52, 494–497. [Google Scholar] [CrossRef]
- Baylis, H.A. Nematoda. Vol. II. (Filarioidea, Dioctophymoidea and Trichinelloidea). In The Fauna of British India, Including Ceylon and Burma; Sewell, R.B.S., Ed.; Taylor and Francis: London, UK, 1939; pp. 1–274. [Google Scholar]
- Chabaud, A.G. No. 3. Key to the genera of the Order Spirurida. Part 2. Spiruroidea, Habronematoidea and Acuarioidea. In CIH Keys to the Nematode Parasites of Vertebrates; Anderson, R.C., Chabaud, A.G., Willmott, S., Eds.; Commonwealth Agricultural Bureaux: Farnham Royal, UK, 1975; pp. 29–58. [Google Scholar]
- Hodda, M. Phylum Nematoda: A classification, catalogue and index of valid genera, with a census of valid species. Zootaxa 2022, 5114, 1–289. [Google Scholar] [CrossRef]
- Anderson, R. Nematode Parasites of Vertebrates: Their Development and Transmission; CAB International: Wallingford, UK, 1992; p. 578. [Google Scholar]
- Hua, L.; Gong, S.; Wang, F.; Li, W.; Ge, Y.; Li, X.; Hou, F. Captive breeding of pangolins: Current status, problems and future prospects. Zookeys 2015, 507, 99–114. [Google Scholar] [CrossRef]
- Smales, L.R. Multisentis myrmecobius, gen. et. sp. nov. (Acanthocephala: Oligacanthorhynchidae), from the Numbat, Myrmecobius fasciatus, and a key to genera of the Oligacanthorhynchidae. Invertebr. Taxon. 1997, 11, 301–307. [Google Scholar] [CrossRef]
- Near, T.J.; Garey, J.R.; Nadler, S.A. Phylogenetic relationships of the Acanthocephala inferred from 18S ribosomal DNA sequences. Mol. Phylogenetics Evol. 1998, 10, 287–298. [Google Scholar] [CrossRef]
- García-Varela, M.; Pérez-Ponce De León, G.; de la Torre, P.; Cummings, M.P.; Sarma, S.S.S.; Laclette, J.P. Phylogenetic relationships of Acanthocephala based on analysis of 18S ribosomal RNA gene sequences. J. Mol. Evol. 2000, 50, 532–540. [Google Scholar] [CrossRef]
- Telford, M.J.; Holland, P.W.H. The phylogenetic affinities of the Chatognaths: A molecular analysis. Mol. Biol. Evol. 1993, 10, 660–676. [Google Scholar]
- Tuli, M.D.; Li, H.; Pan, X.; Li, S.; Xhai, J.; Wu, Y.; Chen, W.; Huang, W.; Feng, Y.; Xiao, L.; et al. Heteroplasmic mitochondrial genomes of a Raillietina tapeworm in wild Pangolin. Parasites Vectors 2022, 15, 204. [Google Scholar] [CrossRef]
- Chabaud, A.G.; Bain, O. La lignée Dipetalonema. Nouvel essai de classification. Ann. De Parasitol. Hum. Et Comp. 1976, 51, 365–397. [Google Scholar] [CrossRef]
- Chabaud, A.G.; Bain, O.; Puylaert, F. Description de trois nouveaux nématodes Molineinae et considérations sur la systématique et le caractére archaïque de cette sous-famille. Bull. Du Mus. Natl. D’histoire Nat. Ser. 2 1967, 38, 904–920. [Google Scholar]
- Baer, J.G.; Fain, A. Les Cestodes des Pangolins. Bull. De La Société Neuchâteloise Des Sci. Nat. 1955, 78, 37–52. [Google Scholar]
- Yamaguti, S. Systema Helminthum. Volume II. The Cestodes of Vertebrates; Interscience Publishers: New York, NY, USA; London, UK, 1959; p. vii + 860. [Google Scholar]
- Singh, S.N. On a new nematode Leipernema leiperi n.g., n.sp. (Strongyloididae), parasitic in the pangolin Manis pentadactyla from Hyderabad, India. J. Helminthol. 1976, 50, 267–274. [Google Scholar] [CrossRef]
- Singh, S.N. On Trichochenia mucronata n.sp. and a new subfamily Trichocheniinae (Trichostrongylidae Leiper, 1912). J. Helminthol. 1958, 32, 251–258. [Google Scholar] [CrossRef]
- Zhang, F.; Yu, J.; Wu, S.; Li, S.; Zou, C.; Wang, Q.; Sun, R. Keeping and breeding the rescued Sunda pangolins (Manis javanica) in captivity. Zoo Biol. 2017, 36, 387–396. [Google Scholar]
- Baer, J.G. Helminthes Parasites. In Exploratie der Nationale Parken van Belgisch Congo; Baer, J.G., Gerber, W., Eds.; Institut des Parcs Nationaux du Congo Belge: Brussels, Belgium, 1959; p. 159. [Google Scholar]
- Mettrick, D.F. A new tapeworm, Inermicapsifer rhodesiensis sp. nov. from a scaly ant-eater, Manis temminckii, in southern Rhodesia. J. Helminthol. 1959, 33, 273–276. [Google Scholar] [CrossRef]
- Okonkwo, V.O.; Okaka, C.E. An investigation into parasites of the African grand pangolins Manis temminckii (Akabo in Igbo Langage) Ayamelum L.G.A. of Anambra south eastern Nigeria. Int. J. Health Saf. Environ. 2018, 4, 308–314. [Google Scholar]
- Ugiagbe, N.S.; Awharitome, A.O. Parasitic infections in African pangolin (Manis temminckii) from Edo State, southern Nigeria. Zoologist 2015, 13, 17–21. [Google Scholar]
- Chin, T.-H. Trichoskrabinia costata from the pangolin, Manis pentadactyla. Chin. Med. J. 1941, 60, 81–83. [Google Scholar]
- Cameron, T.W.M.; Myers, B.J. Manistrongylus meyeri (Travassos, 1937) gen. nov., and Necator americanus from the pangolin. Can. J. Zool. 1960, 38, 781–786. [Google Scholar] [CrossRef]
- Prod’hon, J. Redescription de Trichochenia conincki Chabaud, Bain et Puylaert, 1967. Cah. De La Maboké 1969, 7, 135–137. [Google Scholar]
- Durette-Desset, M.-C. No. 10. Keys to genera of the superfamily Trichostrongyloidea. In CIH Keys to the Nematode Parasites of Vertebrates; Anderson, R.C., Chabaud, A.G., Eds.; Commonwealth Agricultural Bureaux: Farnham Royal, UK, 1983; p. 86. [Google Scholar]
- Gibbons, L.M. Keys to the Nematode Parasites of Vertebrates. Supplementary Volume; CABI: Wallingford, UK, 2010; p. 416. [Google Scholar]
- IRMNG. Mainspinostrongyius Kalyankar & Palladwar. 1989. Available online: https://irmng.org/aphia.php?p=taxdetails&id=1454988 (accessed on 26 August 2022).
- IRMNG. Trichochenia Kou. 1958. Available online: https://www.irmng.org/aphia.php?p=taxdetails&id=1390094 (accessed on 26 August 2022).
- Mohapatra, R.K.; Banik, A.; Sahu, S.K.; Panda, S.; Dangar, T.K. Parasites and bacteria associated with Indian pangolins Manis crassicaudata (Mammalia: Manidae). Glob. Ecol. Conserv. 2020, 23, e01042. [Google Scholar] [CrossRef]
- Heath, M.E.; Vanderlip, S.L. Biology, husbandry, and veterinary care of captive Chinese pangolins (Manis pentadactyla). Zoo Biol. 1988, 7, 293–312. [Google Scholar] [CrossRef]
- Mariaux, J.; Tkach, V.V.; Vasileva, G.P.; Waeschenbach, A.; Beveridge, I.; Dimitrova, Y.D.; Haukislami, V.; Greiman, S.E.; Littlewood, D.T.J.; Makarikov, A.A.; et al. Cyclophyllidea van Beneden in Braun, 1900. In Planetary Biodiversity Inventory (2008–2017): Tapeworms from Vertebrate Bowels of the Earth; Caira, J.N., Jensen, K., Eds.; The University of Kansas Natural History Museum: Lawrence, KS, USA, 2017; Volume Special Publication No. 25; pp. 77–148. [Google Scholar]
- Rahm, U. Notes on pangolins of the Ivory Coast. J. Mammal. 1956, 37, 531–537. [Google Scholar] [CrossRef]
- Jones, A.; Bray, R.A. Family Davaineidae Braun, 1900. In Keys to the Cestode Parasites of Vertebrates; Khalil, L., Jones, A., Bray, R., Eds.; CAB International: Wallingford, UK, 1994; pp. 407–441. [Google Scholar]
- Sapp, S.; Bradbury, R. The forgotten exotic tapeworms: A review of uncommon zoonotic Cyclophyllidea. Parasitology 2020, 147, 533–558. [Google Scholar] [CrossRef]
- Riley, J. The biology of pentastomids. Adv. Parasitol. 1986, 25, 46–128. [Google Scholar]
- Cantlay, J.; Ingram, D.; Meredith, A. A review of zoonotic infection risks associated with the wild meat trade in Malaysia. EcoHealth 2017, 14, 361–388. [Google Scholar] [CrossRef]
- Bezerra-Santos, M.; Mendoza-Roldan, J.; Thompson, R.; Dantas-Torres, F.; Otranto, D. Illegal wildlife trade: A gateway to zoonotic infectious diseases. Trends Parasitol. 2021, 37, 181–184. [Google Scholar] [CrossRef]

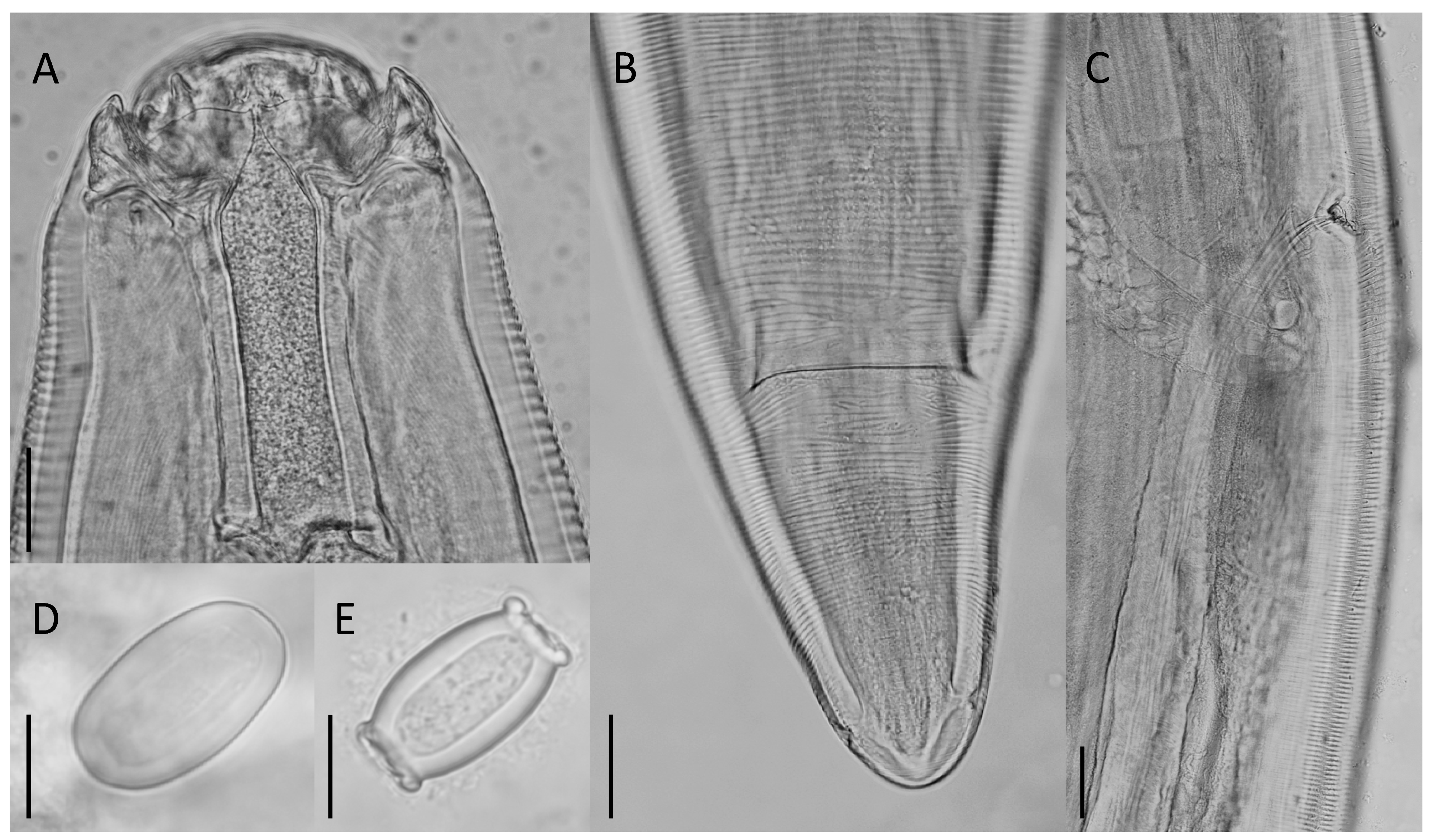
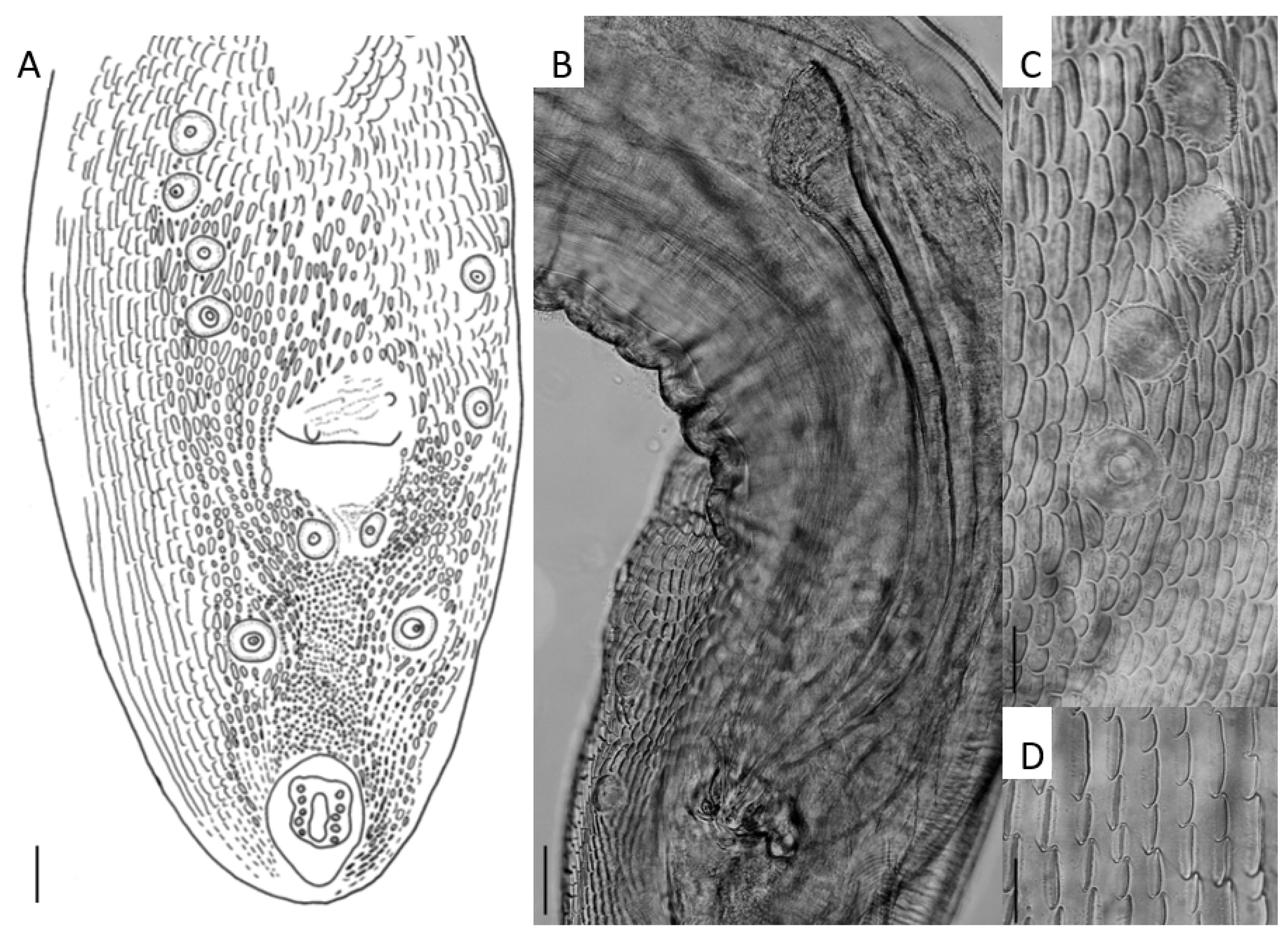
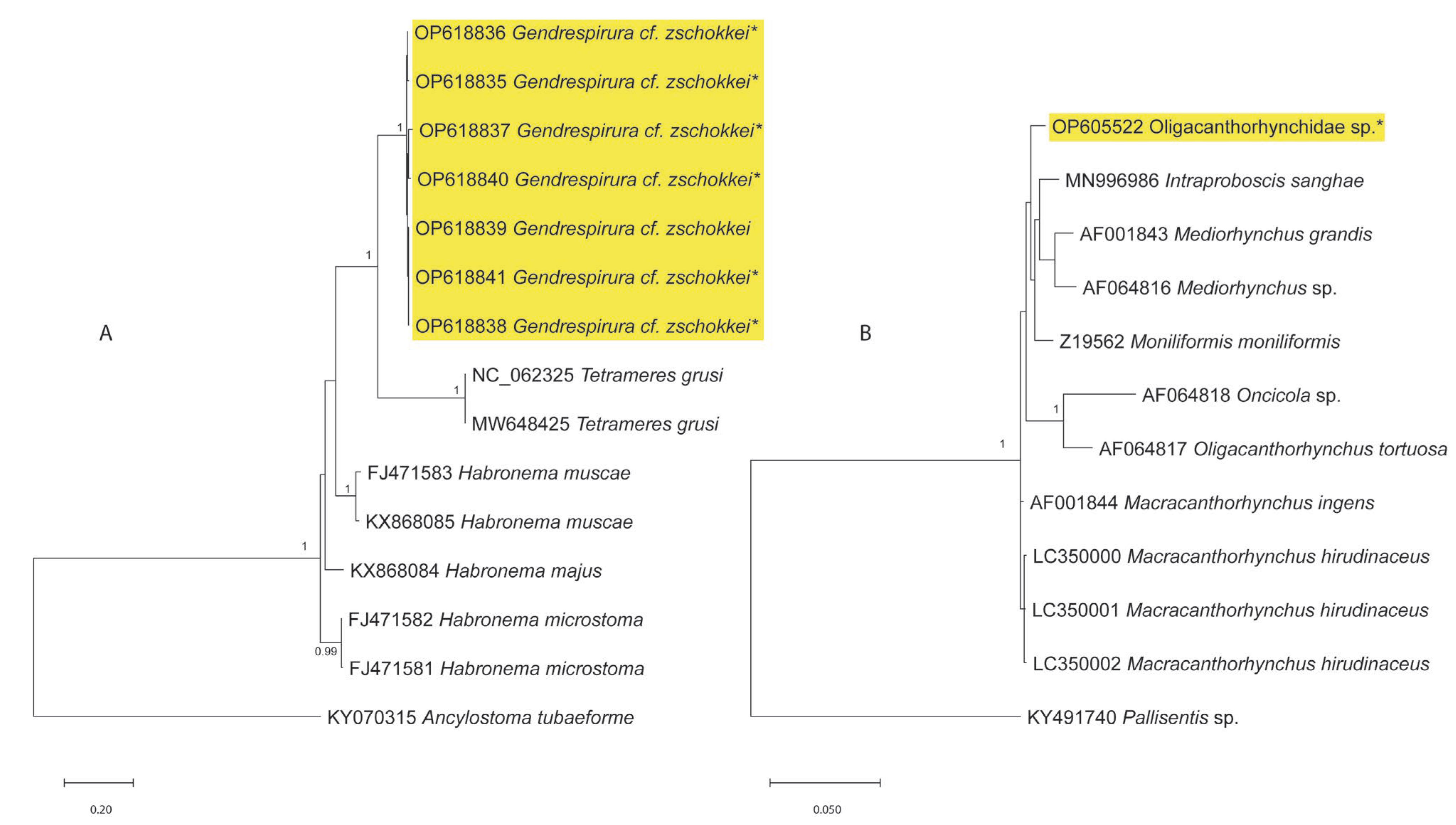
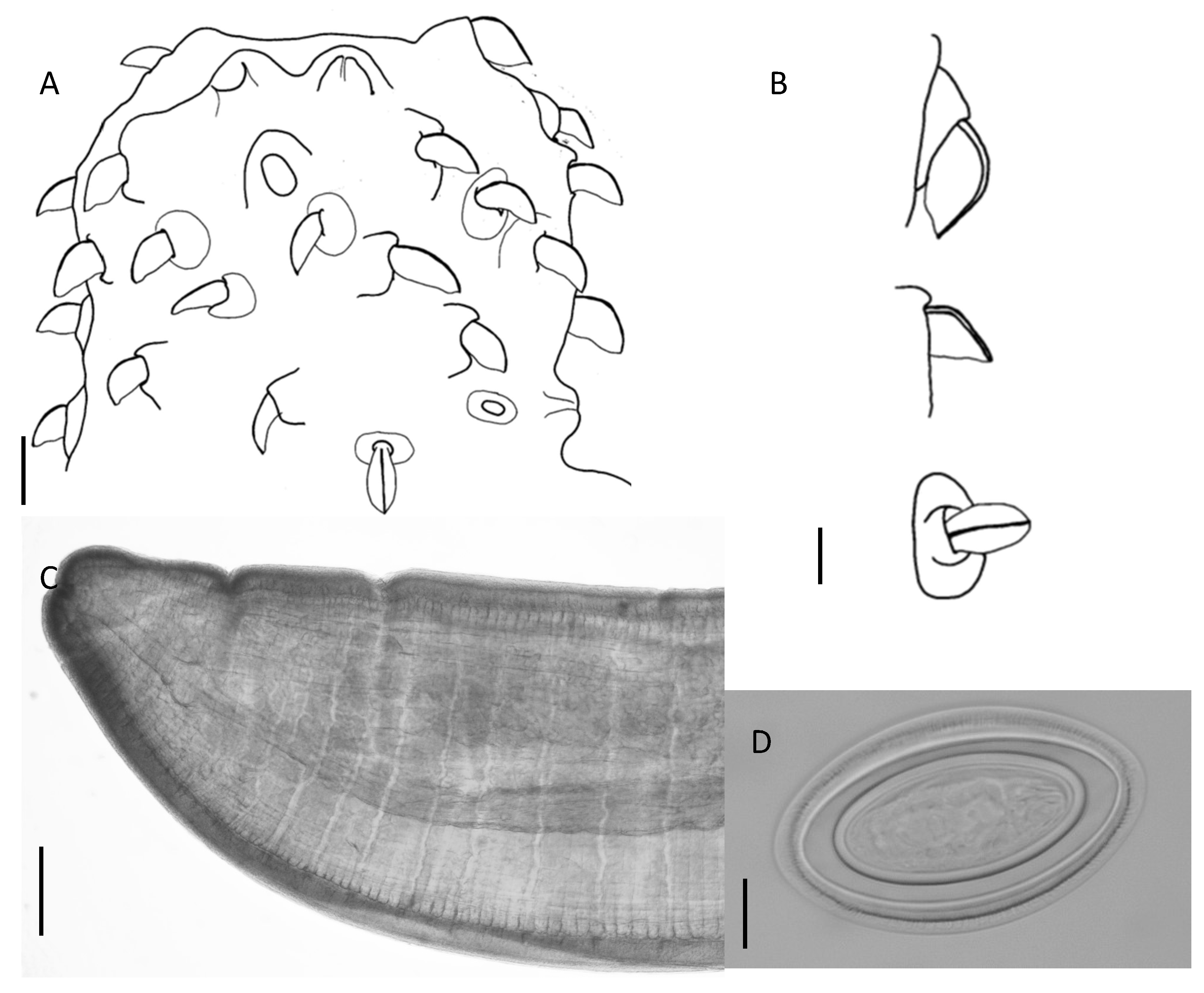
| HKU Number | Geographical Origin a | SPL Number | Total Length | Weight | Sex | Parasites (Number Collected) | Location in Host |
|---|---|---|---|---|---|---|---|
| HKU41-2018 | Borneo | 1292 | 100 cm | 3.2 kg | Female | Nematodes (4) | Embedded in stomach wall |
| HKU46-2018 | Borneo | 1295 | 134.5 cm | 7.6 kg | Male | Acanthocephala (3) | Faecal extrusion |
| HKU51-2018 | Borneo | 1293 | 113.5 cm | 4.16 kg | Female | Nematodes (5) | Stomach |
| HKU74-2018 | Java, Indonesia | 1294 | 95.5 cm | 3.1 kg | Female | Acanthocephala (3) | Small intestine |
| Original Identification | Gendrespirura cf. zschokkei | Habronema hamospiculatum | Protospirura hamospiculata | Filariazschokkei | Chenospirura kwangtungensis | Gendrespirura sp. |
|---|---|---|---|---|---|---|
| Host | Manis javanica | Manis javanica | Smutsia temminckii | Manis crassicaudata | Manis pentadactyla | Manis crassicaudata |
| Location | Malaysia | Vietnam | Central African Republic | Sri Lanka | China | Sri Lanka |
| Reference | This study | Hsü [5] | Neveu-Lemaire [24] | Meyer [25] | Kou [26] | von Linstow [27] |
| Body length | 17,685 (7525–25,375) Mature only: 23,662.5 (21,950–25,375) | 15,700–23,800 | 18,000–23,000 | 32,000 (25,000–35,000) | 25,810–29,510 | 32,000 |
| Body width | 453.1 (337.5–550) | 500–800 | 590–680 | 1300 | 770–885 | 950 |
| No. pairs of teeth on pseudolabia | 3 | 3 | 7 a | - | 7 | Not stated |
| Nerve ring to anterior end | 400 (350–450) | 260–310 | 350–400 | 330–370 | ||
| Cervical papillae to anterior end | 292.5 (230–390) | 265–306 | ||||
| Excretory pore to anterior end | 540 (250–900) | 402 | ||||
| Vestibule length | 120 (105–135) | 150–180 | 130–150 | 114 | 200 | |
| Oesophagus length | 3940 (2430–5100) | 3800–3920 | 3800–4300 | ~6400 (5000–7000) | 4500–5370 | 9143 b |
| Vulva to anterior end | 6243.3 (5180–7850) | 5380 (in 22,000 specimen) | 9000 | 8000 (1/4 body length) | 7350–7750 | Behind mid-body (18,667 b) |
| Egg length × width | Mature: 46.7 (45–47.5) × 25.4 (25–26.25) Immature: 35.6 (32.5–37.5) × 22.8 (21.25 × 25) | 42–43 × 24–26 | 44–46 × 23–24 | 43 × 23–34 | 46–47 × 26–27 | 47 × 29 |
| Tail length | 233.8 (155–305) | 280–440 | 250–300 | 330–380 | 485 b |
| Original Identification | Gendrespirura cf. zschokkei | Habronema hamospiculatum | Protospirura hamospiculata | Filaria zschokkei | Chenospirura kwangtungensis | Gendrespirura sp. |
|---|---|---|---|---|---|---|
| Host | Manis javanica | Manis javanica | Smutsia temminckii | Manis crassicaudata | Manis pentadactyla | Manis crassicaudata |
| Location | Malaysia | Vietnam | Central African Republic | Sri Lanka | China | Sri Lanka |
| Reference | This study | Hsü [5] | Neveu-Lemaire [24] | Meyer [25] | Kou [26] | von Linstow [27] |
| Body length | 13,375 | 11,400–16,100 | 15,000–17,000 | 21,000 (19,000–24,000) | 18,250–22,380 | 25,000 |
| Body width | 500 | 380–590 | 525–570 | 1100 | 664–758 | 710 |
| No. pairs of teeth on pseudolabia | 3 | 3 | 7 a | - | 7 | Not stated |
| Nerve ring to anterior end | 230–250 | 325 | 324–367 | |||
| Cervical papillae to anterior end | 230 | 265–278 | ||||
| Excretory pore to anterior end | 230 | |||||
| Buccal cavity length | 130 | 132–145 | 130 | 114 | 200 | |
| Oesophagus length | 3800 | 3240–3900 | 3300–3700 | ~4200 (3800–4800) | 3337–4651 | 8333 b |
| Left spicule length | 3400 | 3170–3450 | 2300 | 4700–5300 | 3010–3230 | 3740 |
| Right spicule length | 725 | 550–620 | 600 | 1800–2000 | 650–710 | 570 |
| Gubernaculum length | 110 | 70 | ||||
| Tail length | 370 | 360–450 | 370 | 490 | 481 |
| Pangolin Host (Scientific Name) | Parasite Type | Parasite Species | References |
|---|---|---|---|
| Asian pangolins | |||
| Chinese pangolin (Manis pentadactyla) | Acanthocephala | Paraprosthenorchis ornatus | Amin et al. [13] |
| Cestoda | Raillietina sp. | Tuli et al. [42] | |
| Nematoda | Acanthocheilonema (Chenofilaria) fausti (Esslinger, 1966) | Chabaud and Bain [43] | |
| Ancylostoma sp. | Wicker et al. [4] | ||
| Capillaria spp. | Wicker et al. [4] | ||
| Cylicospirura sp. | Mohapatra et al. [3], Wicker et al. [4] | ||
| Gendrespirura zschokkei | Chabaud [31] | ||
| Necator americanus | Mohapatra et al. [3], Wicker et al. [4] | ||
| Strongyle type | Wicker et al. [4] | ||
| Strongyloides spp. | Wicker et al. [4] | ||
| Trichochenia cantonensis | Chabaud et al. [44] | ||
| Trichochenia manisa | Chabaud et al. [44] | ||
| Trichochaenia meyeri | Chabaud et al. [44] | ||
| Trichochenia papillosa | Chabaud et al. [44] | ||
| Indian pangolin (Manis crassicaudata) | Cestoda | Diorchiraillietina contorta | Baer and Fain [45], Yamaguti [46] |
| Echinococcus sp. | Mohapatra et al. [3], Wicker et al. [4] | ||
| Nematoda | Gendrespirura zschokkei | Chabaud [31] | |
| Leipernema leiperi | Singh [47] | ||
| Necator americanus | Mohapatra et al. [3] | ||
| Trichochenia meyeri | Mohapatra et al. [3], Wicker et al. [4] | ||
| Trichochenia mucronata | Singh [48], Chabaud et al. [44] | ||
| Philippine pangolin (Manis culionensis) | Acanthocephala | Nephridiacanthus palawanesis | Tubangui and Masiluñgan [12] |
| Malayan/Sunda pangolin (Manis javanica) | Acanthocephala | Oligacanthorhynchidae gen. sp. | This study |
| Cestoda | Cestode sp. | Zhang et al. [49] | |
| Diorchiraillietina contorta | Baer and Fain [45], Yamaguti [46] | ||
| Raillietina (Paroniella) bovieni | Baer & Fain [45] | ||
| Nematoda | Ancylostoma sp. | Tuli et al. [9] | |
| Brugia malayi | Mohapatra et al. [3], Wicker et al. [4] | ||
| Brugia pahangi | Mohapatra et al. [3], Wicker et al. [4] | ||
| Gendrespirura zschokkei | Chabaud [31], This study | ||
| Gendrespirura sp. | Mohapatra et al. [3] | ||
| Necator americanus | Mohapatra et al. [3], Wicker et al. [4] | ||
| Strongyle type | Wicker et al. [4] | ||
| Pentastomida | Pentastomida sp. | Zhang et al. [49] | |
| African pangolins | |||
| Giant ground pangolin (Smutsia gigantea) | Acanthocephala | Nephridiacanthus gerberi | Baer [50] |
| Cestode | Baerfinia anoplocephaloides (Baer and Fain, 1955) | Baer and Fain [45], Yamaguti [46] | |
| Hymenolepis manidis | Baer and Fain [45] | ||
| Metadavainea aelleni | Baer and Fain [45] | ||
| Nematoda | Ancylostoma sp. | Mohapatra et al. [3], Wicker et al. [4] | |
| Temminck’s ground pangolin (Smutsia temminckii) | Cestoda | Inermicapsifer rhodiensis | Mettrick [51] |
| Metadavainea sp. | Okonkwo and Okaka [52] | ||
| Oochoristica sp. | Ugiagbe and Awharitome [53] | ||
| Nematoda | Gendrespirura hamospiculata | Chabaud [31] | |
| Parastrongyloides sp. | Wicker et al. [4] | ||
| Strongylidae sp. | Ugiagbe and Awharitome [53] | ||
| Strongyle type | Wicker et al. [4] | ||
| Pentastomida | Armillifer sp. | Ugiagbe and Awharitome [53] | |
| Tree/White-bellied pangolin (Phataginus tricuspis) | Acanthocephala | Macracanthorhynchus sp. | Amin et al. [15] |
| Nephridiacanthus manisensis | Amin et al. [15] | ||
| Oncicola sp. | Amin et al. [15] | ||
| Cestoda | Baerfinia anoplocephaloides | Baer and Fain [45], Yamaguti [46] | |
| Cestodes (unidentified) | Chabaud et al. [44] | ||
| Metadavainea aelleni | Baer and Fain [45] | ||
| Metadavainea sp. | Wicker et al. [4] | ||
| Raillietina (Raillietina) rahmi | Baer and Fain [45] | ||
| Nematoda | Ancylostoma sp. | Wicker et al. [4] | |
| Gendrespirura hamospiculata | Chabaud [31] | ||
| Microfilaria lukakae Pais Caeiro, 1959 | Wicker et al. [4] | ||
| Microfilaria lundae Pais Caeiro, 1959 | Wicker et al. [4] | ||
| Microfilaria nobrei Pais Caeiro, 1959 | Wicker et al. [4] | ||
| Microfilaria vilhenae Pais Caeiro, 1959 | Wicker et al. [4] | ||
| Parastrongyloides sp. | Wicker et al. [4] | ||
| Trichochenia armata | Chabaud et al. [44] | ||
| Trichochenia conincki | Chabaud et al. [44] | ||
| Trichochenia manidis | Chabaud et al. [44] | ||
| Trichochenia rousseloti | Chabaud et al. [44] | ||
| Pentastomida | Unidentified pentastome | Wicker et al. [4] | |
| Black-bellied pangolin (Phataginus tetradactyla) | Acanthocephala | Intraproboscis sanghae | Amin et al. [15] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barton, D.P.; Martelli, P.; Worthington, B.M.; Lam, T.T.-Y.; Zhu, X.; Shamsi, S. Nematode and Acanthocephalan Parasites of Confiscated Sunda pangolins, Manis javanica Desmarest, 1822 (Mammalia: Pholidota: Manidae), with an Updated List of the Parasites of Pangolins. Diversity 2022, 14, 1039. https://doi.org/10.3390/d14121039
Barton DP, Martelli P, Worthington BM, Lam TT-Y, Zhu X, Shamsi S. Nematode and Acanthocephalan Parasites of Confiscated Sunda pangolins, Manis javanica Desmarest, 1822 (Mammalia: Pholidota: Manidae), with an Updated List of the Parasites of Pangolins. Diversity. 2022; 14(12):1039. https://doi.org/10.3390/d14121039
Chicago/Turabian StyleBarton, Diane P., Paolo Martelli, Brian M. Worthington, Tommy T.-Y. Lam, Xiaocheng Zhu, and Shokoofeh Shamsi. 2022. "Nematode and Acanthocephalan Parasites of Confiscated Sunda pangolins, Manis javanica Desmarest, 1822 (Mammalia: Pholidota: Manidae), with an Updated List of the Parasites of Pangolins" Diversity 14, no. 12: 1039. https://doi.org/10.3390/d14121039
APA StyleBarton, D. P., Martelli, P., Worthington, B. M., Lam, T. T.-Y., Zhu, X., & Shamsi, S. (2022). Nematode and Acanthocephalan Parasites of Confiscated Sunda pangolins, Manis javanica Desmarest, 1822 (Mammalia: Pholidota: Manidae), with an Updated List of the Parasites of Pangolins. Diversity, 14(12), 1039. https://doi.org/10.3390/d14121039







